Intracerebral hemorrhage (ICH), which is caused by the rupture of blood vessels in the brain, leads to blood accumulation within the brain parenchyma (1, 2). The resultant hematoma creates mechanical damage, and inflammation triggered by blood degradation products can rapidly induce neuronal cell death, brain swelling, white matter injury, and compromise of the blood–brain barrier, all of which contribute to the elevated rates of mortality and disability associated with ICH (3–6). ICH accounts for approximately 27% of all newly reported stroke incidents, with an annual global incidence of 42 cases per 100,000 population (7). In 2019, ICH was the second most common cause of death worldwide and the third most common cause of death or disability (8). As the global population ages and use of antiplatelet therapies increases, the incidence of ICH also increases (7), posing a major challenge for healthcare systems worldwide.
Bilirubin, a product of hemoglobin degradation, is frequently used in clinical practice as a biomarker of hepatic diseases (9). Recent studies have indicated its importance in neurological conditions. It has been reported that bilirubin worsens brain injury in mouse models of cerebral infarction via the TRPM2 pathway (10). Moreover, a notable association has been demonstrated between serum bilirubin levels and mortality rates in individuals with traumatic brain injury (11). Another study found a linear correlation between serum bilirubin levels and the severity of ischemic stroke (p for nonlinearity = 0.55) (12). In patients with ICH, a positive correlation was observed between serum bilirubin levels and ICH severity at presentation. High bilirubin levels were associated with poorer Glasgow Coma Scale scores on admission (rs = −0.17, p = 0.011). The levels of total bilirubin (TBIL), direct bilirubin, and albumin were all significantly related to discharge outcomes (p = 0.036, p = 0.014, and p = 0.016, respectively) (13). Several studies have indicated that bilirubin and its oxidized forms may increase the severity of cerebral hemorrhage (14–16). Additionally, it has been proposed that serum bilirubin levels may predict hematoma growth after ICH (17). Despite indications that bilirubin levels are related to ICH severity, the underlying mechanisms remain unclear, particularly regarding the association between serum bilirubin levels and mortality due to ICH.
This retrospective study therefore investigated the association between serum bilirubin levels and ICH-associated mortality. Our goal was to increase understanding of this association and further clarify the relationship between serum bilirubin levels and the outcomes and prognosis of ICH.
Materials and methods Data accessThe information used in this study was obtained from the Medical Information Mart for Intensive Care IV (MIMIC-IV) database version 3.1. This extensive dataset includes the de-identified records of patients admitted to either the emergency department or intensive care unit (ICU) of the Beth Israel Deaconess Medical Center (Boston, MA, USA) between 2008 and 2022 (18). The collection of patient information and establishment of the database were reviewed by the Institutional Review Board of the Beth Israel Deaconess Medical Center, which waived the requirement for informed consent and approved the data-sharing initiative; no additional ethical approval was required for the present study (18, 19). Dachang Qiu, the primary author of this study, completed the requisite training and examinations mandated by the US National Institutes of Health and was given authorized access to the MIMIC-IV database for the purposes of data extraction (record ID: 65770488).
Eligibility criteriaResearchers with significant expertise in clinical studies defined the inclusion and exclusion criteria for the study. Adult patients with a primary diagnosis of non-traumatic ICH in the ICU were included. The exclusion criteria were as follows: (1) repeated admittance to hospital or ICU, (2) died within 24 h of admission, (3) fewer than two serum bilirubin level tests performed after admission, and (4) essential clinical information missing.
Core variables and outcomesThe primary variable in this study was the average serum TBIL level. The research endpoint was defined as mortality within 28 days of admission. The 28-day all-cause mortality rate was defined as the proportion of total deaths attributable to any cause within a 28-day hospital admission compared to the average population of that demographic during the equivalent timeframe.
Clinical data collectionClinical data on the following were collected from the database: (1) demographic factors such as age, sex, and ethnicity; (2) comorbidities such as hypertension, type 2 diabetes, hyperlipidemia, cancer, heart failure, ischemic heart disease, pneumonia, chronic bronchitis, kidney disease, and hepatic disorders; (3) physical assessments of respiration rate, heart rate, systolic average blood pressure, diastolic average blood pressure, and body weight; (4) laboratory analysis of average TBIL, initial TBIL, blood potassium, aspartate transaminase, alanine transaminase, albumin, creatinine, lactate dehydrogenase, blood glucose, triglyceride, serum sodium, and total serum calcium levels, hematocrit, leukocyte count, erythrocyte count, platelet count, and hemoglobin levels; and (5) disease severity measured using the Glasgow Coma Scale, Simplified Acute Physiology Score II, and Oxford Acute Severity of Illness Score.
Statistical analysisDecisionLinnc. software version 1.0. was used to conduct statistical analyses (20). This platform merges various programming language environments and streamlines data handling, analysis, and machine-learning processes via a graphical interface. Variables with more than 25% of values missing were discarded. Multiple imputation was used to fill in missing data if between 5 and 25% of values were missing. Mean imputation was applied if <5% of values were missing. The normality of the data was analyzed after sorting; the results are shown in Supplementary Table S1. Continuous variables displaying skewed distributions are described as median (Q1 – Q3); those with normal distributions are expressed as mean ± standard deviation. Categorical variables are reported as number and frequency. An independent samples t-test was used to compare the differences between two groups of normally distributed continuous variables. The Wilcoxon rank-sum test was applied to compare two groups of non-normally distributed continuous variables, whereas the chi-square test was used to compare two groups of categorical variables. Spearman’s correlation analysis was performed to evaluate the relationship between serum TBIL levels and patient survival. Restricted cubic splines were used to assess the nonlinear association between serum bilirubin levels and 28-day all-cause mortality. The participants were divided into two groups based on median TBIL level, and Kaplan–Meier survival analysis was performed to investigate differences in survival rates between the two groups. Univariate Cox regression analysis was conducted to identify risk factors associated with 28-day mortality. Least absolute shrinkage and selection operator (LASSO) regression was then performed on the identified risk factors to determine the variables with the greatest influence. Multivariable Cox regression was used to examine the association between serum bilirubin levels and 28-day mortality. Furthermore, subgroup analyses were conducted according to predetermined group classifications, and tests for interactions were performed. Statistical significance was defined as a two-tailed p < 0.05.
Results Patient selection and clinical characteristicsThe patient selection process is depicted in Figure 1. A total of 914 participants were finally included in the analysis. Patients were divided into two groups based on whether death occurred within 28 days of admission. Of the 914 patients, 698 survived for 28 days after admission and 216 died during this period. The median ages of the deceased and survivor groups were 70 and 67 years, respectively (p < 0.001). Significant differences were also observed between the two groups in terms of the mean systolic blood pressure (p < 0.001) and average diastolic blood pressure (p < 0.001). The average serum TBIL level in the survivor group was 0.54 (0.36–0.83) mg/dL, which was significantly lower than that of the deceased group, at 0.70 (0.45–1.33) mg/dL (p < 0.001). The overall median serum TBIL level in all patients was 0.55 mg/dL. Patient characteristics are summarized in Table 1.
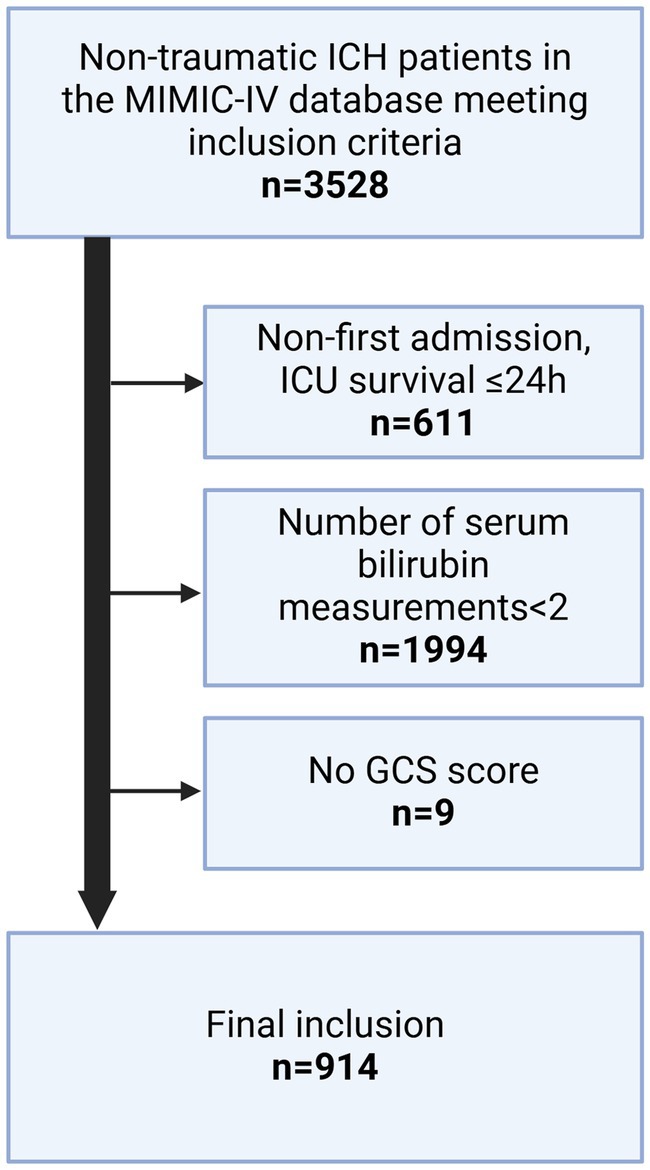
Figure 1. Process flowchart for inclusion/exclusion of study.
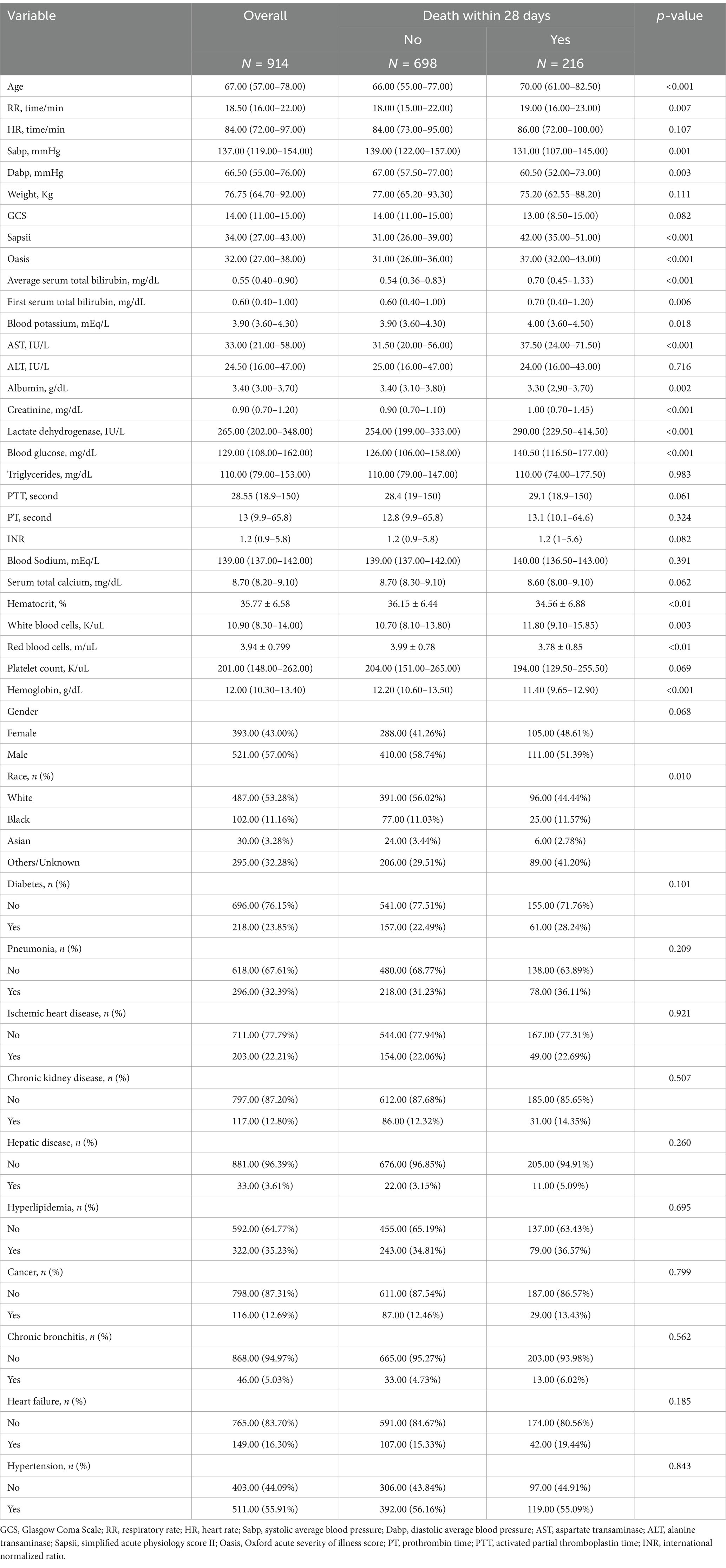
Table 1. Characteristics of the participants.
Serum bilirubin levels correlate with 28-day all-cause mortality in patients with ICHSpearman’s correlation analysis (Figure 2A) demonstrated a significant negative correlation between serum bilirubin levels and 28-day survival in patients with ICH (p < 0.001, correlation = −0.174). Furthermore, restricted cubic spline curve analysis showed a distinct nonlinear correlation between serum bilirubin levels and 28-day all-cause mortality (p for overall = 0, p for nonlinear = 0.001), as shown in Figure 2B. The patients were categorized into two groups according to the median serum TBIL level of 0.55 mg/dL. Kaplan–Meier survival analysis (Figure 3A) showed that the group with higher serum TBIL levels exhibited a more rapid decline in survival over time compared with that of the group with lower serum TBIL levels (p = 0.00016). Restricted mean survival time analysis (Figure 3B) revealed that the group with higher serum bilirubin levels had a significantly reduced mean survival time compared with that of the group with lower serum TBIL levels (p < 0.001).
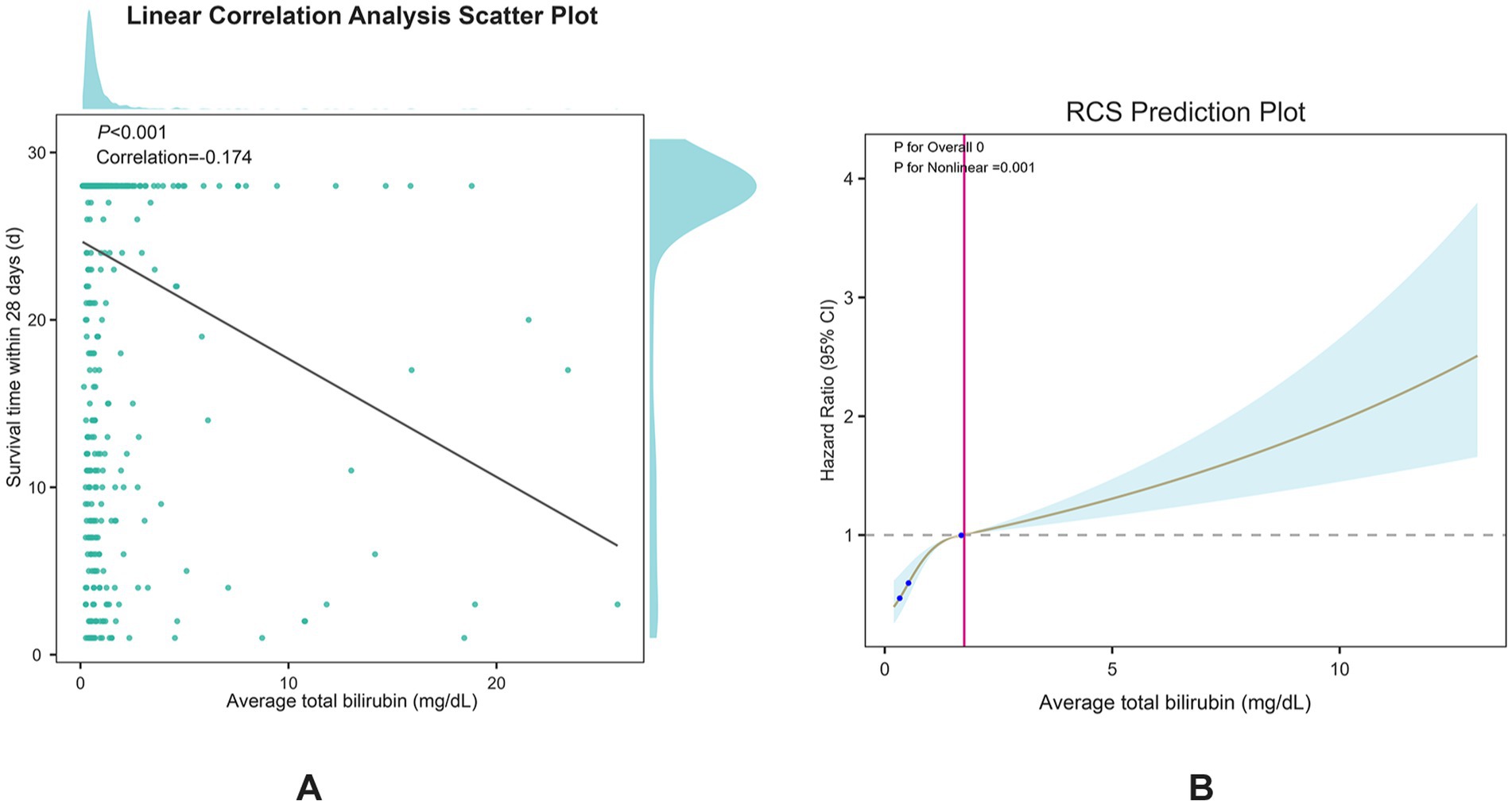
Figure 2. (A) spearman correlation test between serum TBIL and 28-day survival time; (B) Restricted cubic spline plots for serum TBIL and 28-day all-cause mortality.
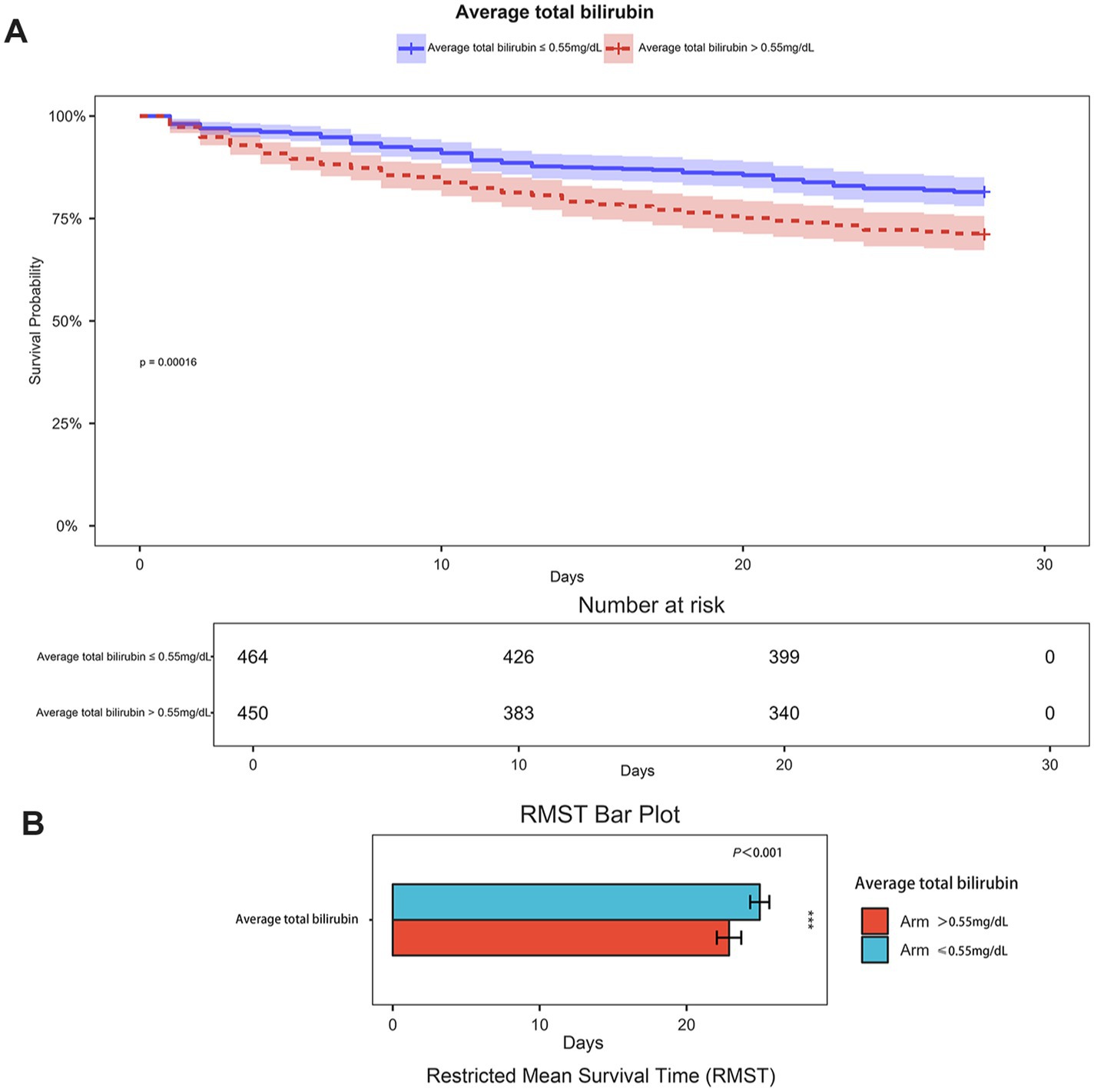
Figure 3. (A) Kaplan–Meier curves curve of two groups of patients(grouped by serum TBIL 0.55 mg/dL cutoff); (B) Restricted mean survival time bar plot of two groups of patients (grouped by serum TBIL 0.55 mg/dL cutoff).
Serum bilirubin level is a significant risk factor for 28-day all-cause mortality in patients with ICHA preliminary univariate Cox regression analysis identified 15 variables associated with 28-day mortality (Supplementary Table S2), including serum TBIL levels (hazard ratio [95% confidence interval] = 1.114 [1.08–1.149], p < 0.001). Subsequently, LASSO regression was performed to filter out possible confounders (Table 2 and Figure 4) and evaluate the influence of these variables on 28-day all-cause mortality. This identified nine variables that had the most significant impact on 28-day all-cause mortality, including average serum TBIL levels (coefficient lambda = 0.0944). These nine variables were further analyzed using multivariate Cox regression (Table 2 and Figure 5). Following adjustment, serum bilirubin level remained a significant risk factor for 28-day mortality in patients with ICH (hazard ratio [95% confidence interval] = 1.121 [1.063–1.182], p < 0.001).
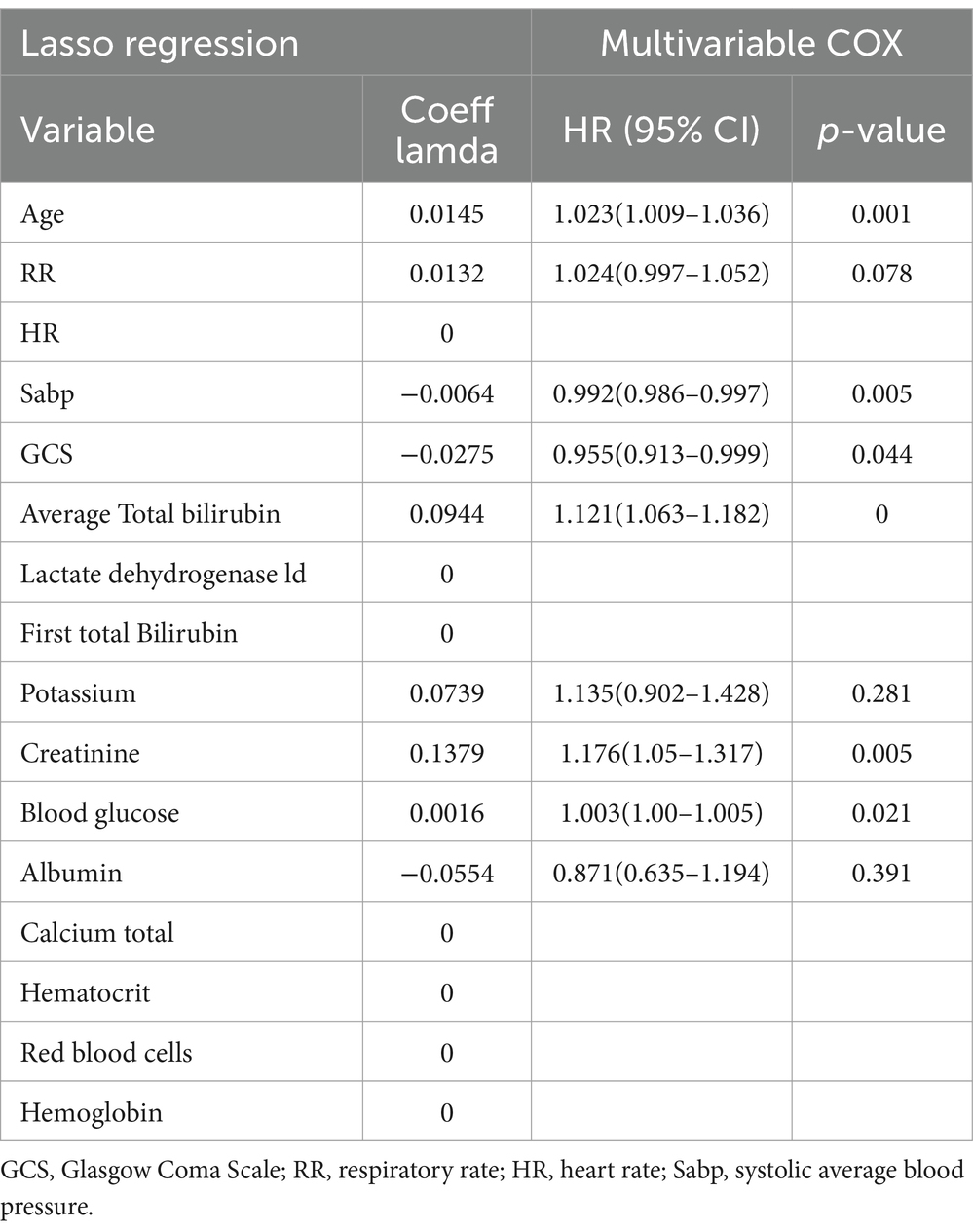
Table 2. Multivariate COX regression on variables screened by lasso regression.
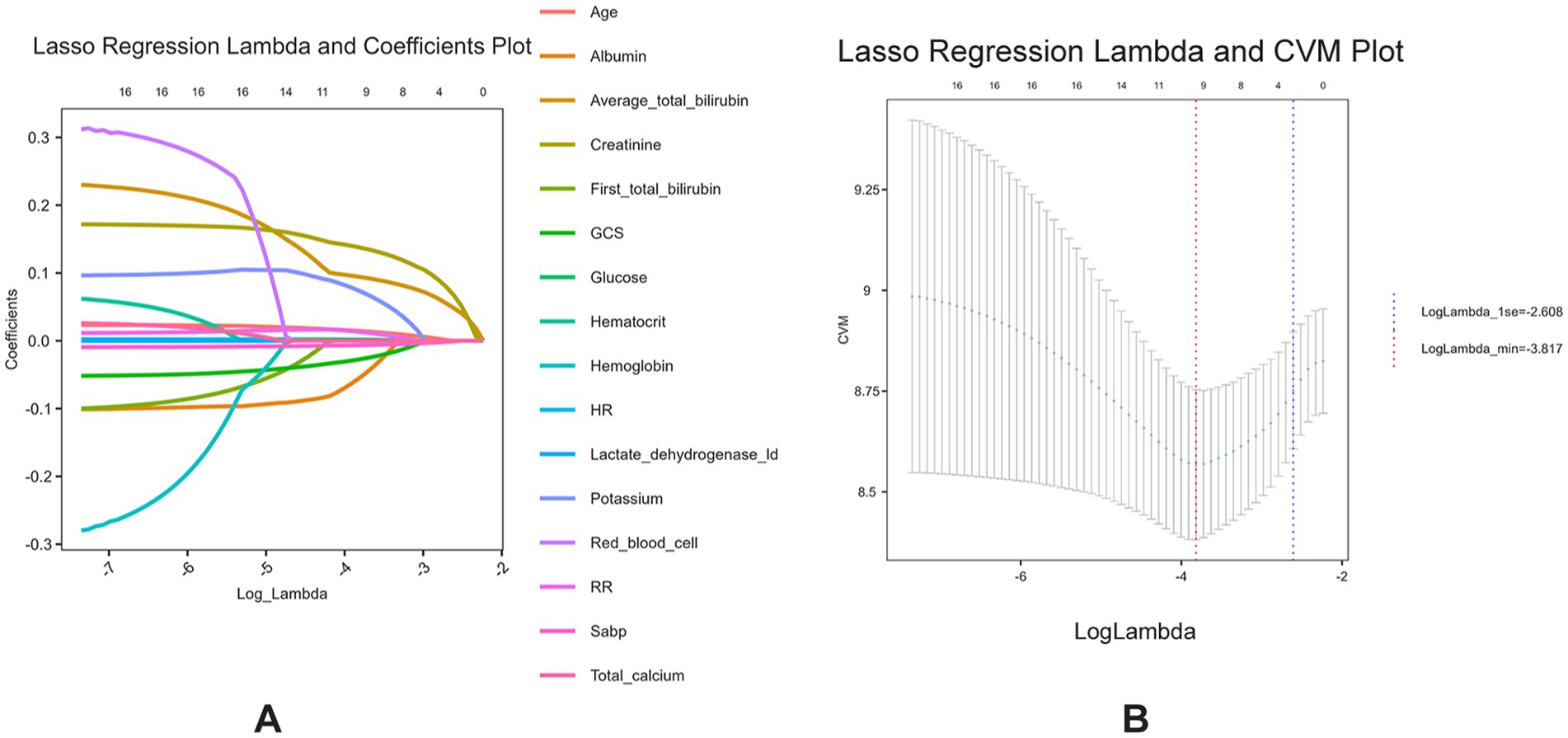
Figure 4. (A) Lasso regression lambda value mean square error plot; (B) Lasso regression lambda value coefficient plot.
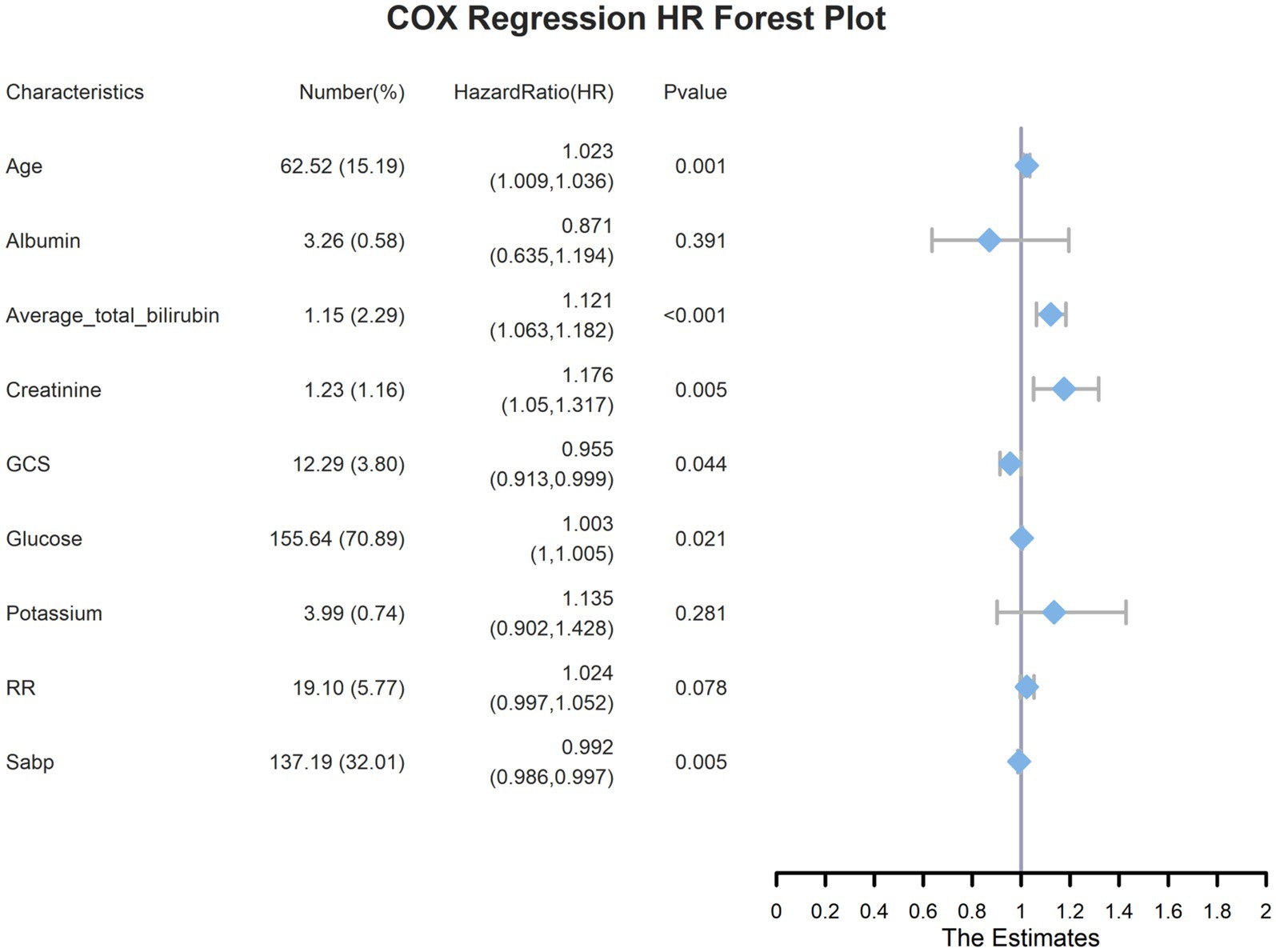
Figure 5. Multivariate COX regression forest plots for the highest weighted influential variables screened by lasso regression.
Subgroup analysisTo further investigate the relationship between serum bilirubin levels and 28-day mortality in patients with ICH, subgroup analyses were conducted (Figure 6). Subgroups were formed based on six factors: age, sex, hypertension, diabetes, hyperlipidemia, and hepatic disease. None of these factors had a significant effect on the association between serum bilirubin levels and 28-day mortality in patients with ICH.
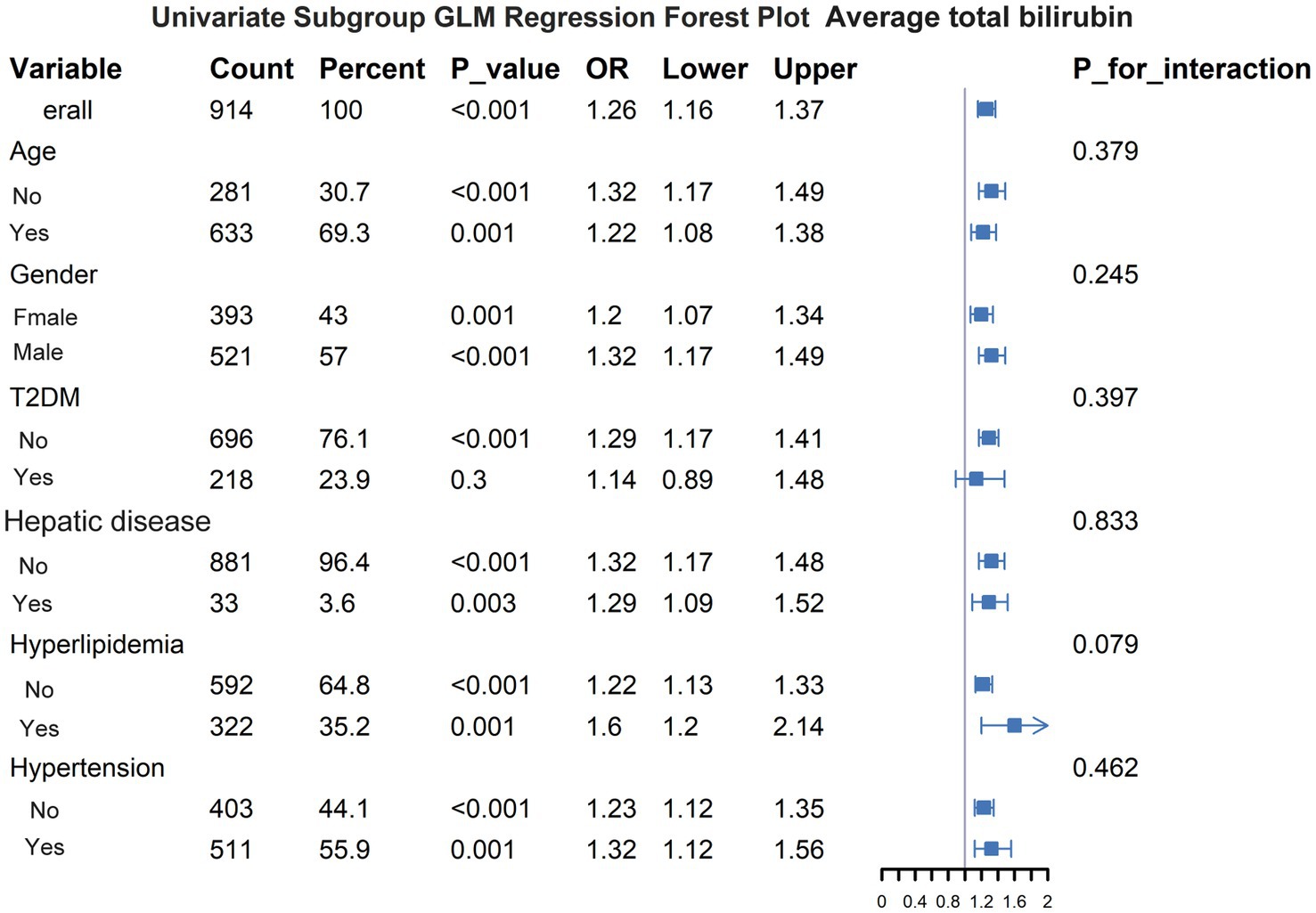
Figure 6. Subgroup analysis of the effect of other variables on the relationship between serum bilirubin and 28-day all-cause mortality.
DiscussionThe results of this retrospective study indicate that serum bilirubin levels are associated with 28-day all-cause mortality in patients with ICH. Elevated serum bilirubin levels were inversely correlated with both survival time and rate, with 28 days serving as the study endpoint. High serum bilirubin levels were a significant risk factor for 28-day all-cause mortality.
Serum bilirubin, a byproduct of heme metabolism and breakdown (21), is used as a diagnostic biomarker in clinical settings (22). Evidence indicates that slightly elevated bilirubin levels may protect against certain pathological conditions (23); however, excessively high bilirubin levels can lead to severe encephalopathy (24). Recent research has revealed an increasingly complex relationship between serum bilirubin levels and stroke. Certain studies have suggested that serum bilirubin can diminish stroke risk (25, 26); however, others have linked high bilirubin levels to greater stroke severity and worse outcomes (27, 28). Furthermore, various studies have illustrated the neurotoxic effects of bilirubin in patients with ICH (29–31).
Blood accumulation in the brain parenchyma can enable serum bilirubin to penetrate the brain directly, and additional bilirubin is generated by hemoglobin breakdown within the hematoma. Oxidative processes in the ICH environment can lead to the degradation of bilirubin into oxidized products, which may lead to unfavorable outcomes in patients with ICH (15). However, few studies have assessed the potential of bilirubin as a therapeutic target in the treatment of neurological disorders (9, 22, 32). Our findings suggest that the prognosis of patients with ICH may be improved by reducing serum bilirubin levels.
The association between serum bilirubin levels and the outcomes and prognosis of hemorrhagic stroke is not yet clearly understood. Only one previous study has been performed, a single-center investigation with a small sample size that demonstrated an association of serum bilirubin with ICH severity and prognosis (13). In 276 patients with ICH, higher direct bilirubin levels were associated with increased initial stroke severity and worse clinical outcomes. However, serum TBIL levels did not show a similar pattern, possibly because of the limited number of participants involved. Our study aimed to clarify this issue and discovered a significant negative relationship between serum TBIL levels and 28-day survival, along with a positive correlation with mortality. This finding is consistent with the results of a study that detected higher levels of serum heme oxygenase-1 (HO-1), which converts heme into bilirubin, TBIL, and indirect bilirubin in patients with ICH (33). However, no direct association between the increase in total and indirect bilirubin levels and the increase in HO-1 levels was determined. Based on these findings, we sought to further explore the interrelationships among total, direct, and indirect bilirubin levels in ICH. Unfortunately, more than 90% of the direct and indirect bilirubin level data were missing from the database.
Bilirubin may also have predictive value in patients with ICH. The results of a single-center retrospective study conducted by Jia et al. (17) over an eight-year period suggested that bilirubin could predict hematoma development in patients with ICH. Our findings indirectly indicated that serum bilirubin may predict the outcomes of patients with ICH. As an easily and routinely measured blood component, serum bilirubin is an attractive candidate as a biomarker for the diagnosis and management of ICH.
The typical cutoff value for defining hyperbilirubinemia is 1.2 mg/dL (34). The levels of TBIL levels in the present study were mostly below this value, even in patients who did not survive, which indicates that there was no or little association between ICH mortality and hyperbilirubinemia. This may be due to the fact that, in order to more accurately assess the relationship between ICH and serum TBIL levels, we excluded patients with severe complications related to hepatobiliary system diseases. We regarded serum TBIL levels and hyperbilirubinemia as two distinct entities, and our findings indicate that serum bilirubin level is an independent risk factor for ICH mortality.
This study has some limitations that should be acknowledged. First, the frequency of serum TBIL level measurement varied; some patients were tested only twice, whereas others underwent more than ten assessments. Although we used mean value as a measure, bias was unavoidable. Additionally, the absence of data on direct and indirect bilirubin levels precluded us from exploring the connections between TBIL, direct bilirubin, and indirect bilirubin and the prognosis of patients with ICH. Certain key covariates such as ICH volume, intraventricular hemorrhage, and subventricular location were also absent from the data, which may have introduced bias into the results. Multicenter, large-scale prospective studies are required to address these issues and confirm our findings.
ConclusionHigher serum bilirubin levels are associated with a greater risk of mortality within 28 days in individuals with ICH; lower serum bilirubin levels are associated with prolonged survival. Additional studies are necessary to establish a causal relationship between total serum bilirubin levels and outcomes in patients with ICH.
Data availability statementPublicly available datasets were analyzed in this study. The datasets generated and/or analyzed during the current study are available in the MIMIC-IV datebase (https://physionet.org/content/mimiciv/3.1/).
Ethics statementThis project was approved by the Institutional Review Boards of Beth Israel Deaconess Medical Center (Boston, MA) and the Massachusetts Institute of Technology (Cambridge, MA). The studies were conducted in accordance with the local legislation and institutional requirements. The Ethics Committee/Institutional Review Board also waived the requirement of written informed consent for participation from the participants or the participants’ legal guardians/next of kin because the project did not impact clinical care and all protected health information was deidentified.
Author contributionsDQ: Data curation, Formal analysis, Investigation, Methodology, Software, Validation, Visualization, Writing – original draft, Writing – review & editing. GL: Methodology, Supervision, Writing – review & editing. YD: Conceptualization, Funding acquisition, Methodology, Resources, Supervision, Visualization, Writing – review & editing.
FundingThe author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
AcknowledgmentsFigure 1 was created with BioRender (Agreement number: RP27I4MUST).
Conflict of interestThe authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statementThe author(s) declare that no Gen AI was used in the creation of this manuscript.
Publisher’s noteAll claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary materialThe Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2025.1529415/full#supplementary-material
References1. Al-Kawaz, MN, Hanley, DF, and Ziai, W. Advances in therapeutic approaches for spontaneous intracerebral hemorrhage. Neurotherapeutics. (2020) 17:1757–67. doi: 10.1007/s13311-020-00902-w
PubMed Abstract | Crossref Full Text | Google Scholar
2. Qiu, D, Li, G, Hu, X, Wang, L, and Dong, Y. Preclinical evaluation on human platelet lysate for the treatment of secondary injury following intracerebral hemorrhage. Brain Res Bull. (2025) 220:111153. doi: 10.1016/j.brainresbull.2024.111153
PubMed Abstract | Crossref Full Text | Google Scholar
3. Cadena, AJ, and Rincon, F. Hypothermia and temperature modulation for intracerebral hemorrhage (ICH): pathophysiology and translational applications. Front Neurosci. (2024) 18:1289705. doi: 10.3389/fnins.2024.1289705
PubMed Abstract | Crossref Full Text | Google Scholar
4. Chen, S, Li, L, Peng, C, Bian, C, Ocak, PE, Zhang, JH, et al. Targeting oxidative stress and inflammatory response for blood-brain barrier protection in intracerebral hemorrhage. Antioxid Redox Signal. (2022) 37:115–34. doi: 10.1089/ars.2021.0072
PubMed Abstract | Crossref Full Text | Google Scholar
5. Jia, P, Peng, Q, Fan, X, Zhang, Y, Xu, H, Li, J, et al. Immune-mediated disruption of the blood-brain barrier after intracerebral hemorrhage: insights and potential therapeutic targets. CNS Neurosci Ther. (2024) 30:e14853. doi: 10.1111/cns.14853
PubMed Abstract | Crossref Full Text | Google Scholar
6. Wan, Y, Holste, KG, Hua, Y, Keep, RF, and Xi, G. Brain edema formation and therapy after intracerebral hemorrhage. Neurobiol Dis. (2023) 176:105948. doi: 10.1016/j.nbd.2022.105948
PubMed Abstract | Crossref Full Text | Google Scholar
7. Puy, L, Parry-Jones, AR, Sandset, EC, Dowlatshahi, D, Ziai, W, and Cordonnier, C. Intracerebral haemorrhage. Nat Rev Dis Primers. (2023) 9:14. doi: 10.1038/s41572-023-00424-7
Crossref Full Text | Google Scholar
8. Feigin, VL, Stark, BA, Johnson, CO, Roth, GA, Bisignano, C, and Abady, GG. Global, regional, and national burden of stroke and its risk factors, 1990-2019: a systematic analysis for the global burden of disease study 2019. Lancet Neurol. (2021) 20:795–820. doi: 10.1016/s1474-4422(21)00252-0
PubMed Abstract | Crossref Full Text | Google Scholar
10. Liu, HW, Gong, LN, Lai, K, Yu, XF, Liu, ZQ, Li, MX, et al. Bilirubin gates the TRPM2 channel as a direct agonist to exacerbate ischemic brain damage. Neuron. (2023) 111:1609–1625.e6. doi: 10.1016/j.neuron.2023.02.022
PubMed Abstract | Crossref Full Text | Google Scholar
12. Song, Y, Zhang, X, Li, C, Xu, S, Zhou, B, and Wu, X. Is bilirubin associated with the severity of ischemic stroke? A dose response Meta-analysis. J Clin Med. (2022) 11:262. doi: 10.3390/jcm11123262
PubMed Abstract | Crossref Full Text | Google Scholar
13. Fu, K, Garvan, CS, Heaton, SC, Nagaraja, N, and Doré, S. Association of Serum Bilirubin with the severity and outcomes of intracerebral hemorrhages. Antioxidants. (2021) 10:346. doi: 10.3390/antiox10091346
PubMed Abstract | Crossref Full Text | Google Scholar
14. Clark, JF, Loftspring, M, Wurster, WL, Beiler, S, Beiler, C, Wagner, KR, et al. Bilirubin oxidation products, oxidative stress, and intracerebral hemorrhage. Acta Neurochir Suppl. (2008) 105:7–12. doi: 10.1007/978-3-211-09469-3_2
PubMed Abstract | Crossref Full Text | Google Scholar
15. Lakovic, K, Ai, J, D'Abbondanza, J, Tariq, A, Sabri, M, Alarfaj, AK, et al. Bilirubin and its oxidation products damage brain white matter. J Cereb Blood Flow Metab. (2014) 34:1837–47. doi: 10.1038/jcbfm.2014.154
PubMed Abstract | Crossref Full Text | Google Scholar
16. Loftspring, MC, Johnson, HL, Feng, R, Johnson, AJ, and Clark, JF. Unconjugated bilirubin contributes to early inflammation and edema after intracerebral hemorrhage. J Cereb Blood Flow Metab. (2011) 31:1133–42. doi: 10.1038/jcbfm.2010.203
PubMed Abstract | Crossref Full Text | Google Scholar
17. Jia, Y, Ye, X, Song, G, Li, X, Ye, J, Yang, Y, et al. Direct bilirubin: a predictor of hematoma expansion after intracerebral hemorrhage. Am J Emerg Med. (2023) 71:150–6. doi: 10.1016/j.ajem.2023.06.042
PubMed Abstract | Crossref Full Text | Google Scholar
18. Johnson, AEW, Bulgarelli, L, Shen, L, Gayles, A, Shammout, A, Horng, S, et al. MIMIC-IV, a freely accessible electronic health record dataset. Sci Data. (2023) 10:1899. doi: 10.1038/s41597-022-01899-x
PubMed Abstract | Crossref Full Text | Google Scholar
19. Johnson, A., Bulgarelli, L., Pollard, T., Gow, B., Moody, B., and Horng, S. (2024). Mimic-iv (version 3.1). PhysioNet. doi: 10.13026/kpb9-mt58
Crossref Full Text | Google Scholar
21. Creeden, JF, Gordon, DM, Stec, DE, and Hinds, TD Jr. Bilirubin as a metabolic hormone: the physiological relevance of low levels. Am J Physiol Endocrinol Metab. (2021) 320:E191–e207. doi: 10.1152/ajpendo.00405.2020
PubMed Abstract | Crossref Full Text | Google Scholar
22. Vasavda, C, Kothari, R, Malla, AP, Tokhunts, R, Lin, A, Ji, M, et al. Bilirubin links Heme metabolism to neuroprotection by scavenging superoxide. Cell Chem Biol. (2019) 26:1450–60. doi: 10.1016/j.chembiol.2019.07.006
PubMed Abstract | Crossref Full Text | Google Scholar
24. Qian, S, Kumar, P, and Testai, FD. Bilirubin encephalopathy. Curr Neurol Neurosci Rep. (2022) 22:343–53. doi: 10.1007/s11910-022-01204-8
Crossref Full Text | Google Scholar
25. Perlstein, TS, Pande, RL, Creager, MA, Weuve, J, and Beckman, JA. Serum total bilirubin level, prevalent stroke, and stroke outcomes: NHANES 1999-2004. Am J Med. (2008) 121:781–8. doi: 10.1016/j.amjmed.2008.03.045
PubMed Abstract | Crossref Full Text | Google Scholar
26. Zhong, P, Wu, D, Ye, X, Wang, X, Zhou, Y, Zhu, X, et al. Association of circulating total bilirubin level with ischemic stroke: a systemic review and meta-analysis of observational evidence. Ann Transl Med. (2019) 7:335. doi: 10.21037/atm.2019.06.71
PubMed Abstract | Crossref Full Text | Google Scholar
27. Arsalan Ismail, M, Khattak, MB, Khan, F, Anwar, MJ, and Murtaza, Z. Prognostic significance of serum bilirubin in stroke. J Ayub Med Coll Abbottabad. (2011) 23:104–7.
28. Sagheb Asl, E, Taheraghdam, A, Rahmani, F, Javadrashid, R, Golzari, SEJ, Ghaemian, N, et al. Determination of the predictive value of serum bilirubin in patients with ischemic stroke: a prospective descriptive analytical study. Adv Pharm Bull. (2018) 8:715–9. doi: 10.15171/apb.2018.080
PubMed Abstract | Crossref Full Text | Google Scholar
29. Cayabyab, R, and Ramanathan, R. High unbound bilirubin for age: a neurotoxin with major effects on the developing brain. Pediatr Res. (2019) 85:183–90. doi: 10.1038/s41390-018-0224-4
PubMed Abstract | Crossref Full Text | Google Scholar
30. Grojean, S, Koziel, V, Vert, P, and Daval, JL. Bilirubin induces apoptosis via activation of NMDA receptors in developing rat brain neurons. Exp Neurol. (2000) 166:334–41. doi: 10.1006/exnr.2000.7518
PubMed Abstract | Crossref Full Text | Google Scholar
31. Zhang, L, Liu, W, Tanswell, AK, and Luo, X. The effects of bilirubin on evoked potentials and long-term potentiation in rat hippocampus in vivo. Pediatr Res. (2003) 53:939–44. doi: 10.1203/01.Pdr.0000061563.63230.86
PubMed Abstract | Crossref Full Text | Google Scholar
32. Thakkar, M, Edelenbos, J, and Doré, S. Bilirubin and ischemic stroke: rendering the current paradigm to better understand the protective effects of bilirubin. Mol Neurobiol. (2019) 56:5483–96. doi: 10.1007/s12035-018-1440-y
PubMed Abstract | Crossref Full Text | Google Scholar
33. Li, X, Li, C, Hou, L, He, M, Song, G, Ren, S, et al. Higher level of serum Heme Oxygenase-1 in patients with intracerebral hemorrhage. Int Surg. (2015) 100:1220–4. doi: 10.9738/intsurg-d-14-00086.1
PubMed Abstract | Crossref Full Text | Google Scholar
34. Ramírez-Mejía, MM, Castillo-Castañeda, SM, Pal, SC, Qi, X, and Méndez-Sánchez, N. The multifaceted role of bilirubin in liver disease: a literature review. J Clin Transl Hepatol. (2024) 12:939–948. doi: 10.14218/jcth.2024.00156
留言 (0)