H. pylori is a Gram-negative bacterium that is closely associated with chronic gastritis, peptic ulcers, gastric cancer, and gastric mucosal tissue-related lymphoma. It is classified as a Class I carcinogen by the World Health Organization (Peek, 2013). Approximately 50% of people worldwide are infected with H. pylori (Mesquita et al., 2022; Katelaris et al., 2023), and the infection and recurrence rates in developing countries are higher than those in developed countries (Sun and Zhang, 2019). In China, the total prevalence rate is 44.2%, with an estimated 589 million people infected with H. pylori (Ren et al., 2022).Currently, the mainstream method for eradicating H. pylori is triple or quadruple therapy, including antibiotics, proton pump inhibitors, and bismuth agents (Helicobacter pylori Study Group, C. S. o. G and Chinese Medical Association, 2022). The triple therapy proposed at the first Maastricht Conference, including PPI, clarithromycin and amoxicillin, is currently the most common therapy (Malfertheiner et al., 2012).However, with the abuse of antibiotics and the increase in drug resistance rates, the effectiveness of this traditional method is gradually decreasing, with many adverse effects such as abdominal pain, diarrhea, aggravation of nausea or vomiting and intolerance (Flores-Treviño et al., 2018).The consensus states that if the resistance rate of clarithromycin in a region is greater than 15%, the triple therapy of PPI, clarithromycin and amoxicillin should be abandoned and replaced with PPI, metronidazole and amoxicillin, or a quadruple therapy containing bismuth: bismuth, PPI, tetracycline and metronidazole. The consensus also mentioned that S. boulardii has a certain effect on improving H. pylori infection.
S. boulardii is a yeast that has been isolated from the peel of tropical fruits and is commonly used to treat gastrointestinal diseases such as diarrhea (Kaźmierczak-Siedlecka et al., 2020; Pais et al., 2020). In recent years, domestic and international studies have shown that S. boulardii has a positive effect on the eradication of H. pylori (Szajewska et al., 2015; Seddik et al., 2019; Yang et al., 2022). In 2017, Yang et al. found that S. boulardii could inhibit the formation of gastric lymphatic follicles caused by H. pylori infection, thereby reducing the incidence of adverse effects (Lazo-Vélez et al., 2018). Previous studies have also indicated that S. boulardii can directly destroy H. pylori cells, resulting in cell destruction and damage. It produces substances, such as short chain fatty acids, that inhibit H. pylori growth (McFarland, 2010).Moreover, although S. boulardii cannot completely eradicate H. pylori, it can reduce the colonization of H. pylori in the intestines of children (Namkin et al., 2016). The possible mechanism for this is S. boulardii’s expression of neuraminidase activity selective for α2,3-linked sialic acid, which can inhibit the adhesion of H. pylori to duodenal epithelial cells (Sakarya and Gunay, 2014). Also S. boulardii has been shown to stabilize the tight junction of gastric mucosal epithelial cells, stimulating SIgA response and strengthening the gastric mucosal barrier (Buts et al., 1990; Dahan et al., 2003).Moreover, some theories suggest that S. boulardii can reduce the abundance of antibiotic-resistance genes and reduce the unsatisfactory traditional treatment effects caused by antibiotic resistance (Cifuentes et al., 2022). To further clarify the role of S. boulardii in the eradication of H. pylori, we conducted a retrospective meta-analysis of randomized controlled trials published between January 2002 and January 2023 and evaluated the impact of adding S. boulardii to traditional methods for the eradication of H. pylori infection.
Materials and methodsData sources and literature searchWe systematically searched the PubMed and Web of Science databases for literature published from January 2002 to January 2023, using the search terms “H. pylori” and “S. boulardii.” In total, 125 articles were detected.
Study selection criteriaThe inclusion criteria were as follows: (1) randomized controlled trials; (2) H. pylori infection patients positive for H. pylori antigens or with a positive 14C breath test; (3) a control group consisting of standard triple therapy, quadruple therapy, sequential therapy, or the addition of a placebo to the standard therapy; (4) experimental group treated with the addition of S. boulardii to the standard therapy; and (5) outcomes including the eradication rate of H. pylori, overall adverse effects, abdominal pain, diarrhea, bloating, nausea and vomiting. The exclusion criteria were as follows: (1) therapy consisting of a combination of S. boulardii and other non-S. boulardii probiotics; (2) animal experiments; (3) meta-analyses, reviews, and conference abstracts; (4) repetitive literature published in different publications; (5) non-randomized controlled trials; and (6) inability to obtain full text or incomplete data. Two reviewers independently screened the studies by reading the titles and abstracts and obtained the full texts of the relevant articles. Any differences were resolved by consensus.
Data extractionThe following data were extracted from each eligible study: first author, year of publication, study location, patient age, number of participants, eradication protocol, S. boulardii protocol, and follow-up duration. Two reviewers independently extracted data from each selected study and any differences were resolved by consensus.
Evidence quality and bias testA Cochrane quality evaluation form (Figure 1) was developed to evaluate the quality of the studies. Using the Egger test, we found that the selected studies had no publication bias, indicating that the conclusions of this study were accurate and reliable.
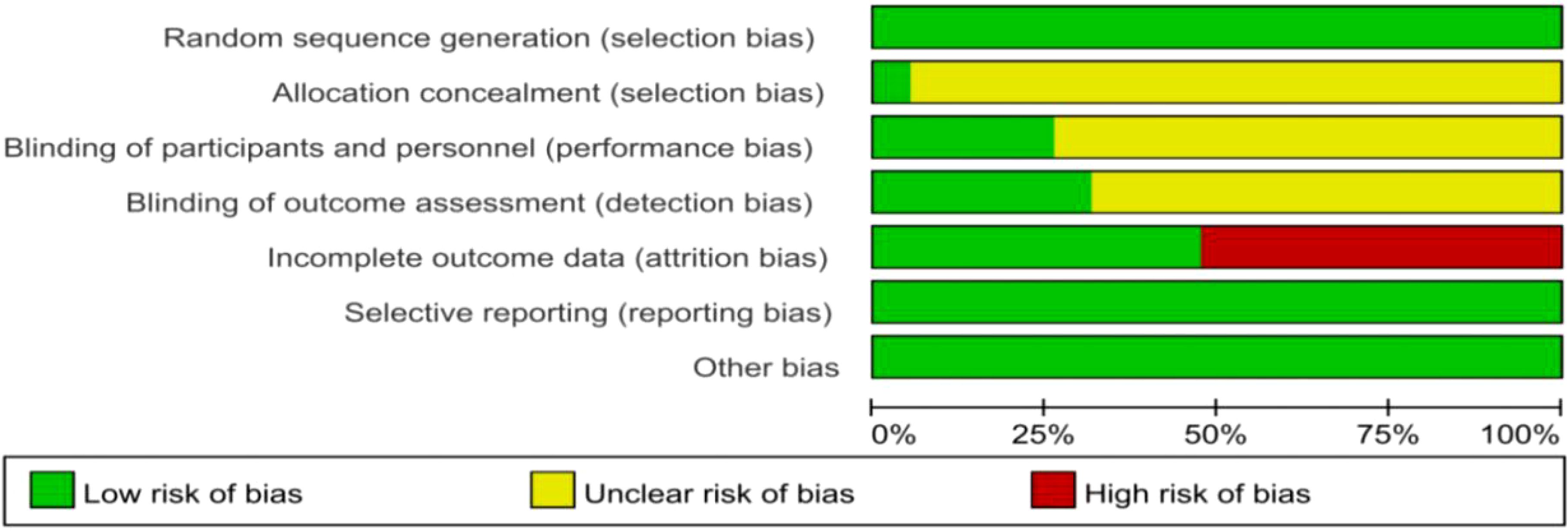
Figure 1. Cochrane Quality Evaluation Form. Studies were analyzed for a variety of bias using the tools in Review Manager software (Version 5.4).
Meta-analysis and statistical analysisAll statistical analyses were conducted using Review Manager software (version 5.4; Cochrane Collaboration, Oxford, UK), and the results were expressed as the risk ratio (RR) with a 95% confidence interval (CI). Statistical significance was set at P<0.05.
We used I2 statistics to evaluate heterogeneity, with I2 values of 0%–25%, 26%–50%, 51%–75%, and >75% indicating no heterogeneity, low heterogeneity, medium heterogeneity, and high heterogeneity, respectively. For studies without heterogeneity, a fixed-effects model was used to calculate the RRs. In contrast, for studies with heterogeneity, a random-effects model was used to calculate the RRs, and sensitivity and subgroup analyses were performed.
ResultsLiterature search process and resultsA total of 149 studies published between January 2002 and January 2023 were included. After removing duplicate and unrelated records, 57 full-text articles were evaluated, of which 38 studies were excluded based on the selection criteria. Therefore, the final meta-analysis included 19 studies (Cremonini et al., 2002; Duman et al., 2005; Cindoruk et al., 2007; Hurduc et al., 2009; Song et al., 2010; Zojaji et al., 2013; Zhao et al., 2014; Bin et al., 2015; Szajewska et al., 2015; Wang and Yu, 2015; Chotivitayatarakorn et al., 2017; Zhu et al., 2017; Zhu et al., 2018; He et al., 2019; He et al., 2019; Seddik et al., 2019; Chang et al., 2020; Zhao et al., 2021; Naghibzadeh et al., 2022). A PRISMA flowchart of the study selection process is shown in Figure 2.
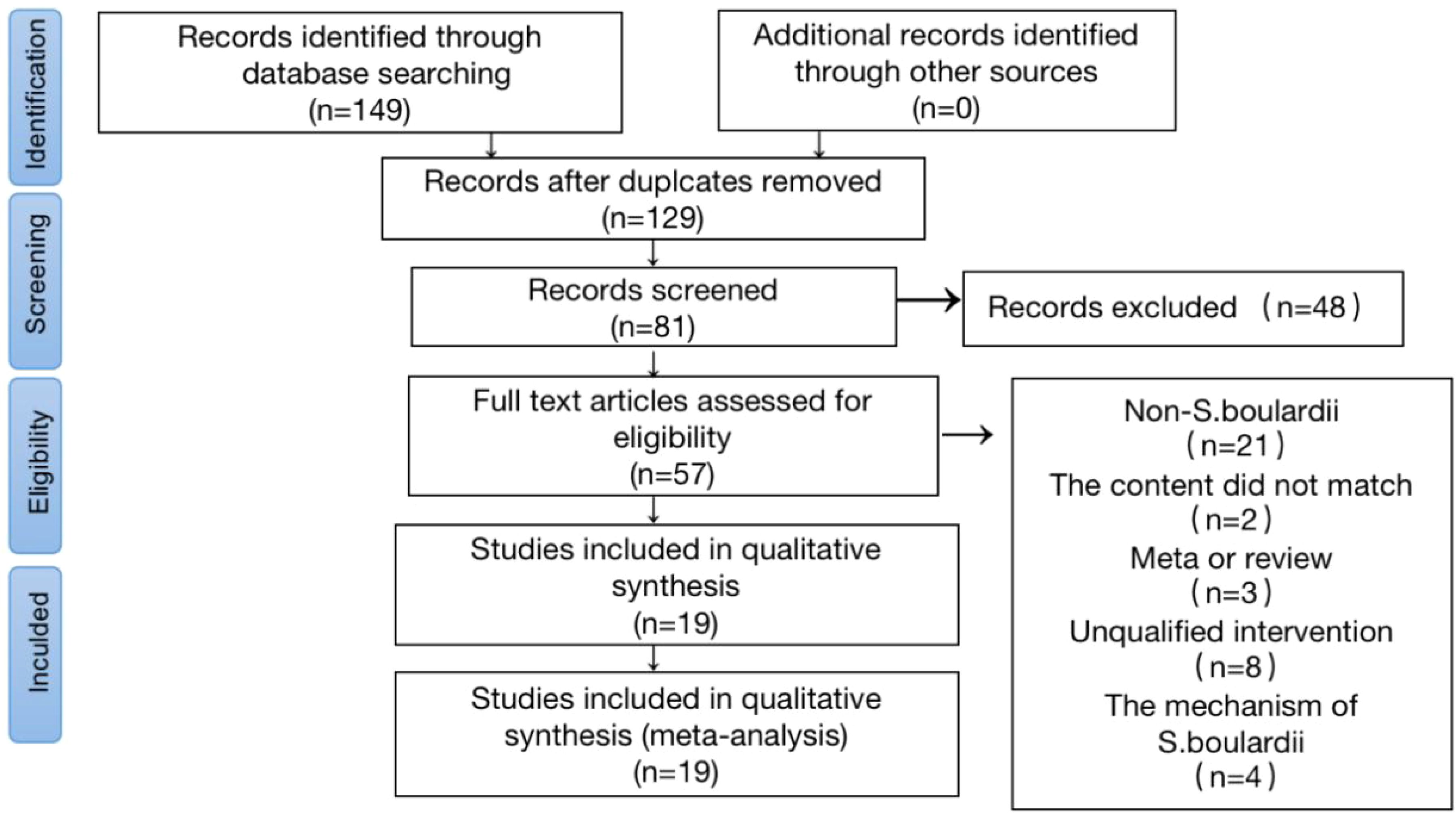
Figure 2. Flow Chart of Literature Screening. A flow diagram of articles retrieved and inclusion progress through the stage of meta-analysis.
Characteristics of the included studiesA total of 19 randomized controlled trials involving 5,036 patients (Table 1) were included, of which 9 were domestic studies and 10 were foreign studies; 15 were adult studies and 4 were child studies; and 13 used triple therapy, 5 used quadruple therapy, and 1 used sequential therapy. Moreover, regarding the duration of therapy, four studies used 7-day therapy, 13 studies used 14-day therapy, one study collected data on both 7-day and 14-day therapy, and one study used 10-day therapy.
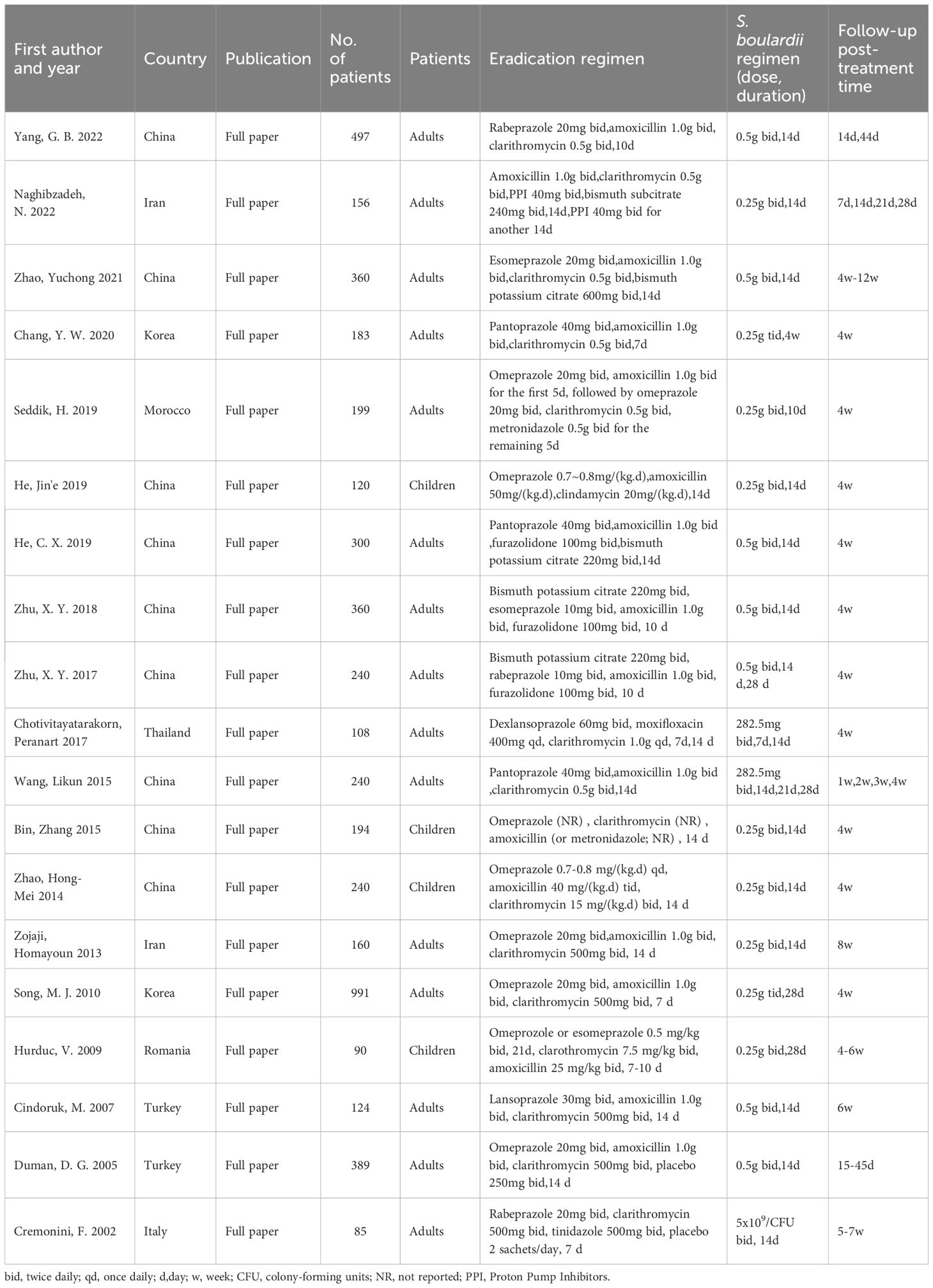
Table 1. Basic characteristics of included study.
Primary outcome: H. pylori eradication rateEradication rateAmong the 19 included articles, 18 statistically analyzed the eradication rate of H. pylori. We performed ITT analysis on 18 articles (Figure 3) and the results showed no heterogeneity (I2 = 23%, P=0.18). Using a fixed-effects model, we found that the eradication rate was increased by 11% when using S. boulardii supplementation (RR=1.11, 95% Cl: 1.08–1.15, P<0.0001), with no observed bias (Egger’s test: P=0.068), proving that supplementation with S. boulardii can improve the eradication rate of the standard protocol.
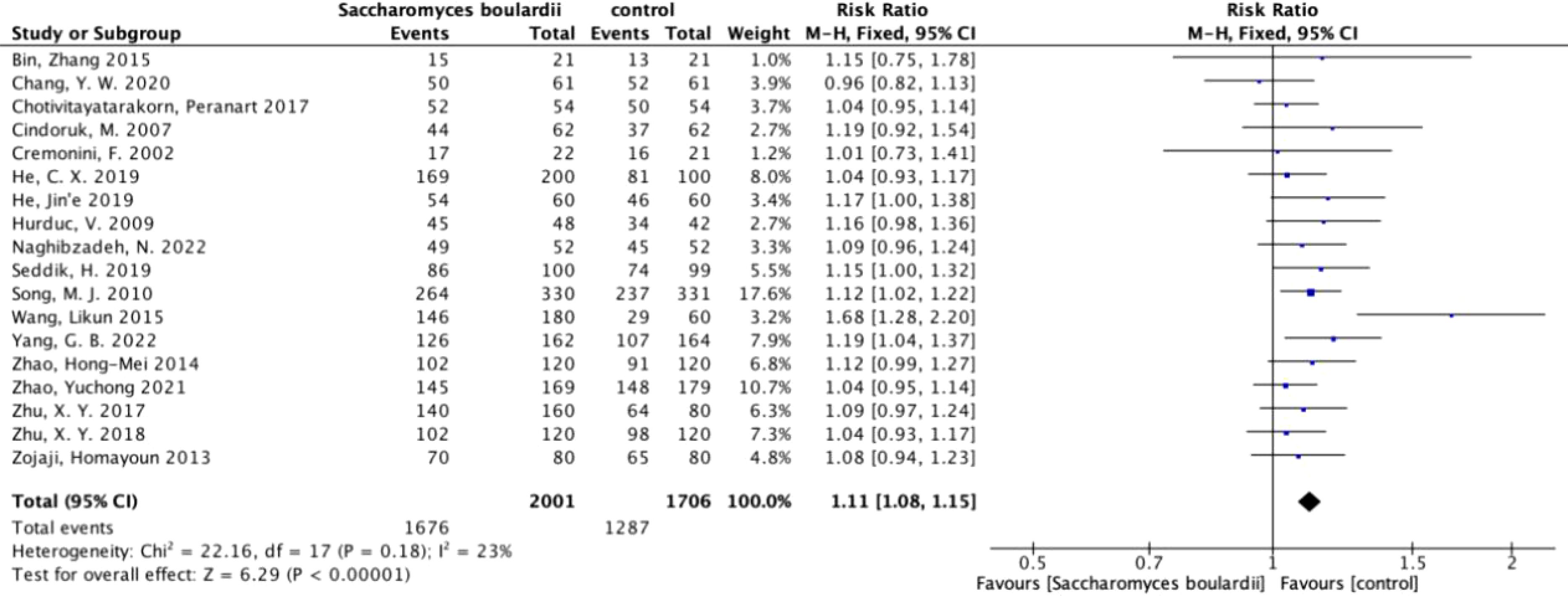
Figure 3. Forest plot of eradication rate. Forest plot analyzing the eradication rate of S. boulardii as an adjuvant therapy for H. pylori.
Layered analysisSubgroup analysis stratified by patient age indicated that the eradication rate in children (RR=1.14, 95% CI: 1.05–1.25, P=0.002) was 5% higher than that in adults (RR=1.09, 95% CI: 1.04–1.14, P=0.0002).
Subgroup analysis stratified by study location revealed that the eradication rate in China (RR=1.12, 95% CI: 1.04–1.19, P=0.001) was 2% higher than that in foreign countries (RR=1.10, 95% CI: 1.05–1.15, P=0.0001).
Subgroup analysis stratified by the standard eradication protocol showed that the eradication rate with quadruple therapy (RR=1.12, 95% CI: 1.06–1.19, P=0.0002) increased by 7% when compared with triple therapy (RR=1.05, 95% CI: 1.00–1.11, P=0.04). Moreover, the eradication rate of sequential therapy increased by 3% when compared with quadruple therapy.
Subgroup analysis stratified by medication duration showed that there was no significant difference between the 7-day therapy (RR=1.09, 95% CI: 1.03–1.17, P=0.006) and the 14-day therapy (RR=1.10, 95% CI: 1.04–1.16, P=0.0007), with only a 1% difference. However, the addition of S. boulardii to the 7-day therapy did not increase the eradication rate at 4 weeks (Table 2).
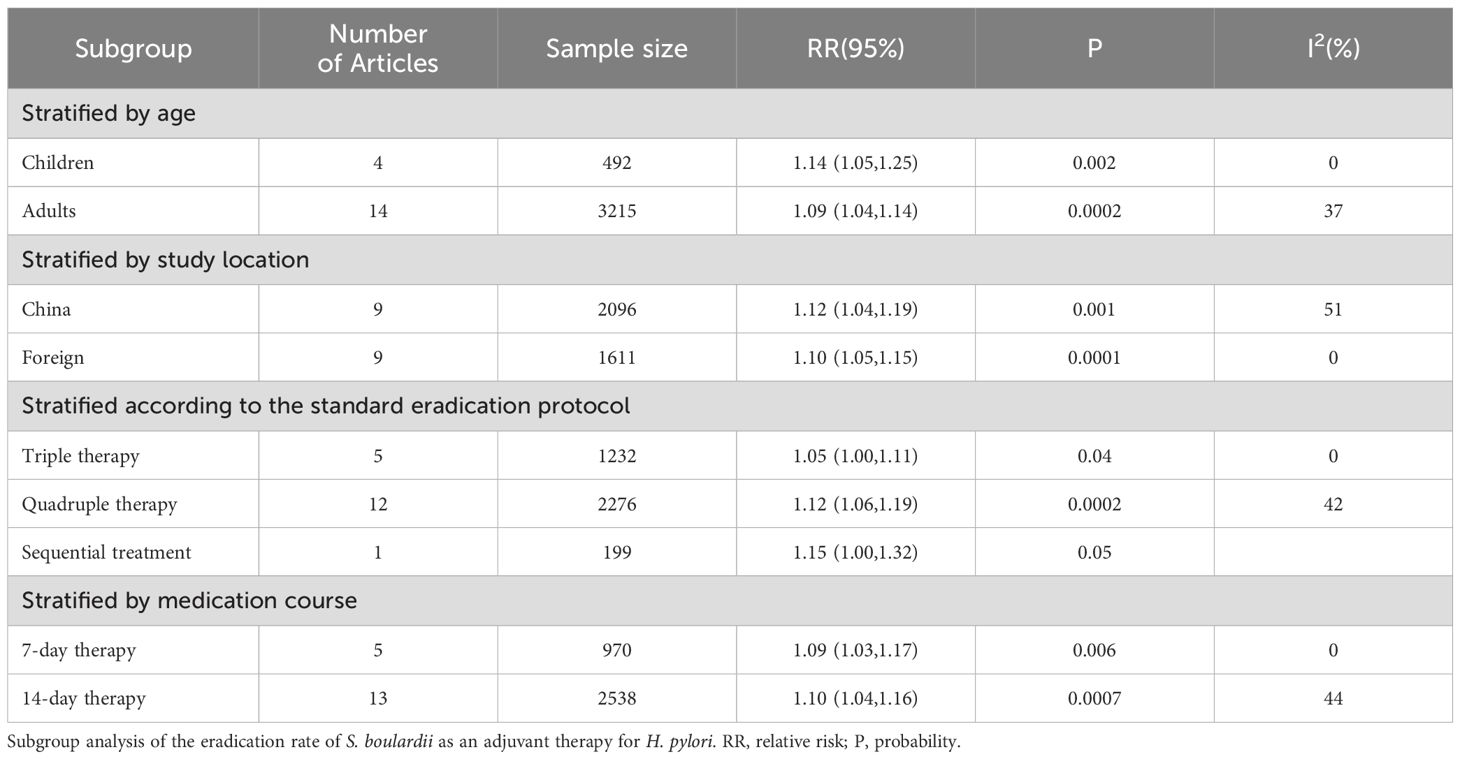
Table 2. Layered analysis of eradication rate.
Secondary outcome: adverse effectsTotal adverse effectsAmong the 19 included articles, 9 analyzed the differences in overall adverse effects after the addition of S. boulardii (Figure 4), and these studies showed high heterogeneity (I2 = 79%, P<0.00001). In total, the overall adverse effects decreased by 51% (RR=0.49, 95% CI: 0.37–0.66, P<0.00001) when using random effects combined with effect measures.
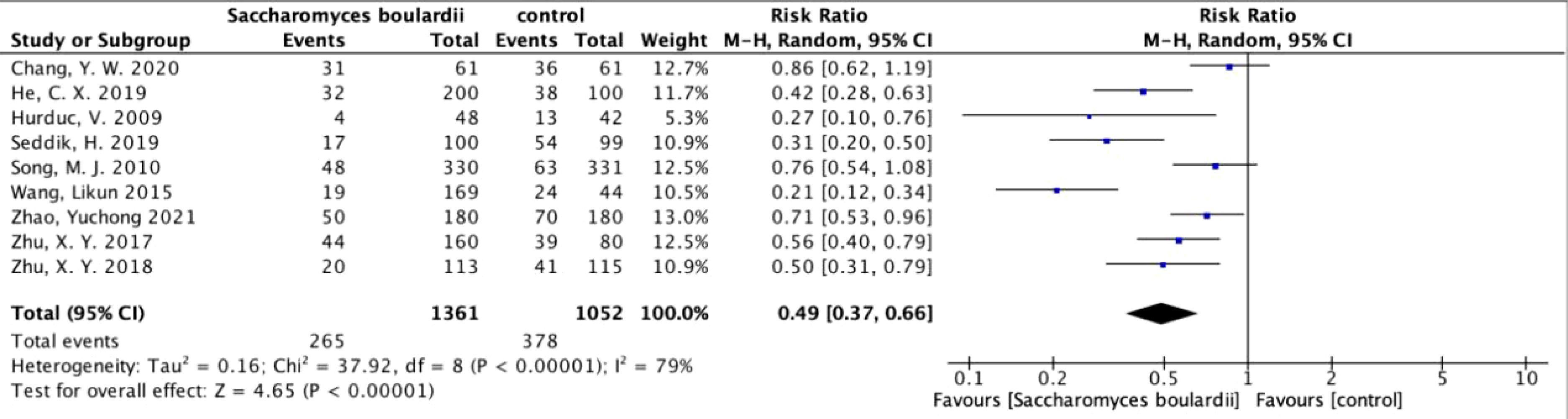
Figure 4. Forest plot analyzing the total adverse effects of S. boulardii as an adjuvant therapy for H. pylori.
Other adverse effectsDiarrheaWe performed a meta-analysis on 16 articles reporting the incidence of diarrhea. These studies had moderate heterogeneity (I2 = 58%, P=0.002). Using random effects combined with effect measures, we found that the incidence of diarrhea decreased by 64% (RR=0.36, 95% CI: 0.26–0.48, P<0.0001) with S. boulardii supplementation.
Abdominal painA meta-analysis was conducted on nine articles reporting the incidence of abdominal pain, and we found that these studies had no heterogeneity (I2 = 0%, P=0.79). Using a fixed-effects model, we found no significant difference in the incidence of abdominal pain between the S. boulardii and control groups (RR=0.78, P=0.10). Therefore, the addition of S. boulardii did not reduce the incidence of abdominal pain.
Abdominal distensionWe conducted a meta-analysis on seven articles with mild heterogeneity (I2 = 43%, P=0.10) reporting the incidence of abdominal distension. Random effects were used to merge the effects, and we found that the abdominal distension rate decreased by 51% (RR=0.49, 95% CI: 0.33–0.72, P=0.0004) with S. boulardii supplementation, without bias (Egger’s test: P=0.994).
ConstipationA meta-analysis was conducted on six articles reporting the incidence of constipation. These studies had no heterogeneity (I2 = 0%, P=0.48). Therefore, we used a fixed-effects model and found that the addition of S. boulardii significantly reduced the incidence of constipation (RR=0.38, 95% CI: 0.26–0.57, P<0.00001). The results were unbiased (Egger’s test: P=0.973).
NauseaWe performed a meta-analysis on 12 articles with mild heterogeneity (I2 = 48%, P=0.03) reporting the incidence of nausea. Random effects were used to merge the effects, and we found that the nausea rate decreased by 50% (RR=0.50, 95%: 0.37–0.68, P<0.00001) with S. boulardii supplementation. The results were statistically significant and unbiased (Egger’s test: P=0.095).
VomitingA meta-analysis was conducted on eight articles reporting the incidence of vomiting. These articles had mild heterogeneity (I2 = 38%, P=0.12). Random effects were used to merge the effects, and we found that the results were not statistically significant (RR=0.71, P=0.07). Therefore, the addition of S. boulardii did not reduce the incidence of vomiting.
Taste disordersWe conducted a meta-analysis on seven articles that studied the incidence of taste disorders and found moderate heterogeneity (I2 = 64%, P=0.01). Random effects were used to merge the effects, and the results were not statistically significant (RR=0.82, P=0.4). Therefore, the addition of S. boulardii did not reduce the incidence of taste disorders (Table 3).
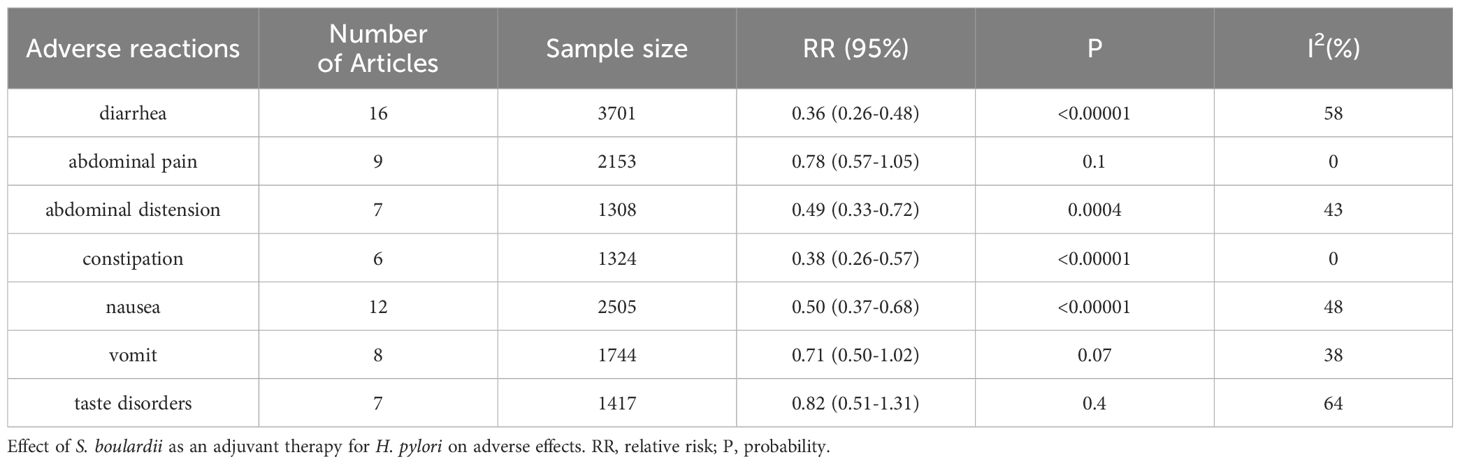
Table 3. Incidence rate of other adverse reactions.
DiscussionIn this meta-analysis, we investigated changes in the rates of H. pylori eradication after the addition of S. boulardii to eradication therapy using ITT analysis. In the ITT analysis, we found that the addition of S. boulardii increased the eradication rate by 11%, with statistically significant and non-heterogeneous results consistent with those of a previous meta-analysis published by Zhou et al. in 2019 (Zhou et al., 2019). In the two latest RCTs published in 2022—one of which was a triple-protocol study by Yang et al (Yang et al., 2022).including 497 patients—the eradication rate increased by 12.6% after the addition of S. boulardii, which is consistent with our results. Another RCT performed by Naghibzadeh et al. (2022) found that the addition of S. boulardii increased the eradication rate by 7.7% in 156 patients undergoing quadruple therapy; however, this increase was not statistically significant. This might have been due to various factors such as the inclusion of a placebo in the experiment, the fact that quadruple therapy itself improved the eradication effect compared to triple therapy, or an insufficient dosage of S. boulardii.
When we studied the total adverse effects reported by the included studies, we found a high degree of heterogeneity in their results. Further investigation of L’Abbé and radial plots confirmed this high degree of heterogeneity. Upon conducting a sensitivity analysis of these studies, we found that deleting any articles did not cause a significant change in the results. Additionally, the sensitivity analysis did not identify the source of heterogeneity. Therefore, we conducted a subgroup analysis from multiple dimensions; however, we did not find any sources of heterogeneity, indicating a reliable 51% reduction in total adverse effects. This conclusion is consistent with the results of a previous meta-analysis by Zhou et al (Zhou et al., 2019), showing that the addition of S. boulardii can reduce the total adverse effects of eradication therapy.
When studying the incidence of diarrhea, we examined the L’Abbé and radial plots again and found moderate heterogeneity. A sensitivity analysis was conducted on these studies, and it was found that the study by Seddik et al. (2019) had a significant impact on heterogeneity. After excluding this study, the I2 decreased to <50%. This study was the only treatment that used sequential therapy; therefore, we concluded that this study might have been a source of heterogeneity. Owing to antibiotic resistance and the adverse effects of bismuth, sequential therapy can serve as a replacement for traditional triple or quadruple therapy failures (Yang et al., 2014). A study by Zullo et al. (2003) including 1,749 patients showed that sequential therapy had a higher eradication rate than traditional therapy (92% vs. 74%). Therefore, sequential therapy may become a new-generation first-line treatment option in the future; however, further experimental studies are required to confirm its efficacy and safety.
In this meta-analysis, we concluded that the addition of S. boulardii to eradication therapy significantly reduced the incidence of abdominal distension, constipation, and nausea, with no heterogeneity or only mild heterogeneity. Similarly, a recent triple-protocol RCT in China (Yang et al., 2022) showed a significant improvement in abdominal distension with S. boulardii supplementation. Our findings are also consistent with the results of a previous meta-analysis by Zhou et al. (2019) that investigated the incidence of diarrhea, bloating, constipation, and nausea with eradication therapy. These results confirm that the addition of S. boulardii can reduce the occurrence of these adverse reactions, providing a theoretical basis for clinical practice.
After conducting a stratified analysis of 18 articles, we found that the eradication rate in the child group increased by 5% when compared with that in the adult group. This is consistent with Zhou et al.’s (Zhou et al., 2019) finding that the eradication rate in children increased by 3.25% when compared with that in adults. Additionally, we found that the eradication rate of quadruple therapy was higher than that of triple therapy, with an increase of 7%. However, sequential therapy had the highest eradication rate, with an increase of 9% when compared with quadruple therapy. Notably, sequential therapy has several advantages over traditional therapy, with some studies reporting that sequential therapy can increase the eradication rate by 18% when compared with traditional therapy (Zullo et al., 2003). This presents new possibilities for clinical treatment. In this study, we also compared the eradication rates at different study locations and medication durations; however, we found no significant differences in these factors.
The present meta-analysis evaluated the incidence of other adverse effects such as abdominal pain, vomiting, and taste disorders. Although the incidence of these adverse effects decreased with the addition of S. boulardii, these results were not statistically significant. Consistent with our results, Naghibzadeh et al. (2022) found no significant improvement in the incidence of vomiting and taste disorders with the addition of S. boulardii. Since there is currently limited clinical research regarding whether the addition of S. boulardii can decrease these adverse effects, these results may still hold some significance for clinical practice. However, further studies are required to confirm this hypothesis.
Previous studies have also shown that other probiotics may increase the eradication rate of H. pylori and reduce adverse effects. For example, Naghibzadeh et al. (2022) studied a group of treatment regimens using L. reuteri in combination with quadruple therapy and found a small improvement in the eradication rate; however, these results were not statistically significant. Similarly, Zhu et al. (2018) studied a treatment regimen using L. reuteri and found a 2.5% increase in the eradication rate, which was also not statistically significant. Previous research has shown that the colonization of lactobacilli in the stomach can reduce the production of gastritis, promote mucus regeneration, downregulate the expression of cag pathogenicity island genes (García et al., 2012), and prevent the colonization of H. pylori through specific adhesives. Another prospective trial statistically analyzed the efficacy of combining four probiotics (Lactobacillus acidophilus, Lactiplantacillus plantarum, Bifidobacterium lactis, and S. boulardii) and found that combining multiple probiotics can improve eradication rates and reduce the incidence of adverse effects (Viazis et al., 2022). However, further randomized trials are required to confirm the efficacy of these probiotics.
Previous researchers have also studied patients’ compliance with the addition of S. boulardii to H. pylori eradication therapy. Seddik et al. (2019) found that adding S. boulardii to the sequential treatment increased treatment compliance by 3.8% (95.0% vs. 91.2%, P<0.001). Similarly, in an RCT involving children, Bin et al. (2015) found that no patient in the treatment group terminated H. pylori treatment prematurely, whereas six patients in the control group stopped treatment without medical advice, with a statistically significant difference (p=0.027). However, due to the small sample size of their study, further investigation is required to determine whether S. boulardii can improve patient compliance with eradication therapy.
ConclusionWe found that adding S. boulardii to traditional eradication treatment methods can improve the H. pylori eradication rate and reduce the total adverse effects and incidence of diarrhea, bloating, constipation, and nausea. However, it did not reduce the incidence of abdominal pain, vomiting, and taste disorders.
Data availability statementThe original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author/s.
Author contributionsML: Writing – original draft, Writing – review & editing. YX: Writing – review & editing.
FundingThe author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was supported by the Doctoral Start-up Foundation of Liaoning Province (Contract No. 2022-BS-127).
AcknowledgmentsIn the process of writing this meta-analysis, we acknowledge everyone who provided a lot of help in the structure and writing standards of the article.
Conflict of interestThe authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s noteAll claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
ReferencesBin, Z., Ya-Zheng, X., Zhao-Hui, D., Bo, C., Li-Rong, J., Vandenplas, Y. (2015). The Efficacy of Saccharomyces boulardii CNCM I-745 in Addition to Standard Helicobacter pylori Eradication Treatment in Children. Pediatr. Gastroenterol. Hepatol. Nutr. 18, 17–22. doi: 10.5223/pghn.2015.18.1.17
PubMed Abstract | Crossref Full Text | Google Scholar
Buts, J. P., Bernasconi, P., Vaerman, J. P., Dive, C. (1990). Stimulation of secretory IgA and secretory component of immunoglobulins in small intestine of rats treated with Saccharomyces Boulardii. Dig Dis. Sci. 35, 251–256. doi: 10.1007/BF01536771
PubMed Abstract | Crossref Full Text | Google Scholar
Chang, Y. W., Park, Y. M., Oh, C. H., Oh, S. J., Cho, J. H., Kim, J. W., et al. (2020). Effects of probiotics or broccoli supplementation on Helicobacter pylori eradication with standard clarithromycin-based triple therapy. Korean J. Intern. Med. 35, 574–581. doi: 10.3904/kjim.2019.139
PubMed Abstract | Crossref Full Text | Google Scholar
Chotivitayatarakorn, P., Mahachai, V., Vilaichone, R. K. (2017). Effectiveness of 7-day and 14-day moxifloxacin-dexlansoprazole based triple therapy and probiotic supplement for helicobacter pylori eradication in Thai patients with non-ulcer dyspepsia: A double-blind randomized placebo-controlled study. Asian Pac J. Cancer Prev. 18, 2839–2843. doi: 10.22034/apjcp.2017.18.10.2839
PubMed Abstract | Crossref Full Text | Google Scholar
Cifuentes, S. G., Prado, M. B., Fornasini, M., Cohen, H., Baldeón, M. E., Cárdenas, P. A. (2022). Saccharomyces boulardii CNCM I-745 supplementation modifies the fecal resistome during Helicobacter pylori eradication therapy. Helicobacter 27, e12870. doi: 10.1111/hel.12870
PubMed Abstract | Crossref Full Text | Google Scholar
Cindoruk, M., Erkan, G., Karakan, T., Dursun, A., Unal, S. (2007). Efficacy and safety of Saccharomyces boulardii in the 14-day triple anti-Helicobacter pylori therapy: a prospective randomized placebo-controlled double-blind study. Helicobacter 12, 309–316. doi: 10.1111/j.1523-5378.2007.00516.x
PubMed Abstract | Crossref Full Text | Google Scholar
Cremonini, F., Di Caro, S., Covino, M., Armuzzi, A., Gabrielli, M., Santarelli, L., et al. (2002). Effect of different probiotic preparations on anti-helicobacter pylori therapy-related side effects: a parallel group, triple blind, placebo-controlled study. Am. J. Gastroenterol. 97, 2744–2749. doi: 10.1111/j.1572-0241.2002.07063.x
PubMed Abstract | Crossref Full Text | Google Scholar
Dahan, S., Dalmasso, G., Imbert, V., Peyron, J. F., Rampal, P., Czerucka, D. (2003). Saccharomyces boulardii interferes with enterohemorrhagic Escherichia coli-induced signaling pathways in T84 cells. Infect. Immun. 71, 766–773. doi: 10.1128/IAI.71.2.766-773.2003
PubMed Abstract | Crossref Full Text | Google Scholar
Duman, D. G., Bor, S., Ozütemiz, O., Sahin, T., Oğuz, D., Iştan, F., et al. (2005). Efficacy and safety of Saccharomyces boulardii in prevention of antibiotic-associated diarrhoea due to Helicobacterpylori eradication. Eur. J. Gastroenterol. Hepatol. 17, 1357–1361. doi: 10.1097/00042737-200512000-00015
PubMed Abstract | Crossref Full Text | Google Scholar
Flores-Treviño, S., Mendoza-Olazarán, S., Bocanegra-Ibarias, P., Maldonado-Garza, H. J., Garza-González, E. (2018). Helicobacter pylori drug resistance: therapy changes and challenges. Expert Rev. Gastroenterol. Hepatol. 12, 819–827. doi: 10.1080/17474124.2018.1496017
PubMed Abstract | Crossref Full Text | Google Scholar
García, A., Sáez, K., Delgado, C., González, C. L. (2012). Low co-existence rates of Lactobacillus spp. and Helicobacter pylori detected in gastric biopsies from patients with gastrointestinal symptoms. Rev. Esp Enferm Dig 104, 473–478. doi: 10.4321/s1130-01082012000900005
PubMed Abstract | Crossref Full Text | Google Scholar
He, C. X., Kong, F. T., Liang, F., Wang, K. X., Li, H., Liu, Y. L., et al. (2019). Influence of different timing of Saccharomyces boulardii combined with bismuth quadruple therapy for Helicobacter pylori eradication. Zhonghua Yi Xue Za Zhi 99, 1731–1734. doi: 10.3760/cma.j.issn.0376-2491.2019.22.010
PubMed Abstract | Crossref Full Text | Google Scholar
He, J. e., Wang, W., Gao, C. (2019). Saccharomyces boulardii combined with triple therapy in treatment of Helicobacter pylori infection in children: A clinical observation. Chin. J. Microecology 31, 171–173,178. doi: 10.13381/j.cnki.cjm.201902011
Crossref Full Text | Google Scholar
Helicobacter pylori Study Group, C. S. o. G, Chinese Medical Association (2022). Sixth Chinese National Consensus report on management of Helicobacter pylori infection (treatment rxcluded). Chin J Gastroenterol. 27, 289–304.
Hurduc, V., Plesca, D., Dragomir, D., Sajin, M., Vandenplas, Y. (2009). A randomized, open trial evaluating the effect of Saccharomyces boulardii on the eradication rate of Helicobacter pylori infection in children. Acta Paediatr. 98, 127–131. doi: 10.1111/j.1651-2227.2008.00977.x
PubMed Abstract | Crossref Full Text | Google Scholar
Katelaris, P., Hunt, R., Bazzoli, F., Cohen, H., Fock, K. M., Gemilyan, M., et al. (2023). Helicobacter pylori world gastroenterology organization global guideline. J. Clin. Gastroenterol. 57, 111–126. doi: 10.1097/mcg.0000000000001719
PubMed Abstract | Crossref Full Text | Google Scholar
Kaźmierczak-Siedlecka, K., Ruszkowski, J., Fic, M., Folwarski, M., Makarewicz, W. (2020). Saccharomyces boulardii CNCM I-745: A non-bacterial microorganism used as probiotic agent in supporting treatment of selected diseases. Curr. Microbiol. 77, 1987–1996. doi: 10.1007/s00284-020-02053-9
PubMed Abstract | Crossref Full Text | Google Scholar
Lazo-Vélez, M. A., Serna-Saldívar, S. O., Rosales-Medina, M. F., Tinoco-Alvear, M., Briones-García, M. (2018). Application of Saccharomyces cerevisiae var. boulardii in food processing: a review. J. Appl. Microbiol. 125, 943–951. doi: 10.1111/jam.14037
PubMed Abstract | Crossref Full Text | Google Scholar
Malfertheiner, P., Megraud, F., O’Morain, C. A., Atherton, J., Axon, A. T., Bazzoli, F., et al. (2012). Management of Helicobacter pylori infection–the Maastricht IV/Florence Consensus Report. Gut 61, 646–664. doi: 10.1136/gutjnl-2012-302084
PubMed Abstract | Crossref Full Text | Google Scholar
Mesquita, A., Rocha-Castro, C., Guimarães, D., Costa, J., Soutinho, J., Taveira-Gomes, T. (2022). Multicentric study to assess helicobacter pylori incidence, patient reported adverse events, compliance and effectiveness, in real-world setting. Int. J. Environ. Res. Public Health 19, 2. doi: 10.3390/ijerph191912847
PubMed Abstract | Crossref Full Text | Google Scholar
Naghibzadeh, N., Salmani, F., Nomiri, S., Tavakoli, T. (2022). Investigating the effect of quadruple therapy with Saccharomyces boulardii or Lactobacillus reuteri strain (DSMZ 17648) supplements on eradication of Helicobacter pylori and treatments adverse effects: a double-blind placebo-controlled randomized clinical trial. BMC Gastroenterol. 22, 107. doi: 10.1186/s12876-022-02187-z
PubMed Abstract | Crossref Full Text | Google Scholar
Namkin, K., Zardast, M., Basirinejad, F. (2016). Saccharomyces boulardii in helicobacter pylori eradication in children: A randomized trial from Iran. Iran J. Pediatr. 26, e3768. doi: 10.5812/ijp.3768
PubMed Abstract | Crossref Full Text | Google Scholar
Ren, S., Cai, P., Liu, Y., Wang, T., Zhang, Y., Li, Q., et al. (2022). Prevalence of Helicobacter pylori infection in China: A systematic review and meta-analysis. J. Gastroenterol. Hepatol. 37, 464–470. doi: 10.1111/jgh.15751
PubMed Abstract | Crossref Full Text | Google Scholar
Sakarya, S., Gunay, N. (2014). Saccharomyces boulardii expresses neuraminidase activity selective for α2,3-linked sialic acid that decreases Helicobacter pylori adhesion to host cells. Apmis 122, 941–950. doi: 10.1111/apm.12237
PubMed Abstract | Crossref Full Text | Google Scholar
Seddik, H., Boutallaka, H., Elkoti, I., Nejjari, F., Berraida, R., Berrag, S., et al. (2019). Saccharomyces boulardii CNCM I-745 plus sequential therapy for Helicobacter pylori infections: a randomized, open-label trial. Eur. J. Clin. Pharmacol. 75, 639–645. doi: 10.1007/s00228-019-02625-0
PubMed Abstract | Crossref Full Text | Google Scholar
Song, M. J., Park, D. I., Park, J. H., Kim, H. J., Cho, Y. K., Sohn, C. I., et al. (2010). The effect of probiotics and mucoprotective agents on PPI-based triple therapy for eradication of Helicobacter pylori. Helicobacter 15, 206–213. doi: 10.1111/j.1523-5378.2010.00751.x
PubMed Abstract | Crossref Full Text | Google Scholar
Szajewska, H., Horvath, A., Kołodziej, M. (2015). Systematic review with meta-analysis: Saccharomyces boulardii supplementation and eradication of Helicobacter pylori infection. Aliment Pharmacol. Ther. 41, 1237–1245. doi: 10.1111/apt.13214
PubMed Abstract | Crossref Full Text | Google Scholar
Viazis, N., Argyriou, K., Kotzampassi, K., Christodoulou, D. K., Apostolopoulos, P.,
留言 (0)