Gout is a common metabolic disorder caused by chronically elevated serum uric acid levels (1). Monosodium urate crystals primarily deposit in the peripheral joints of the extremities, where the temperature and blood circulation are too low to form gout tophi (2–4). Reports of spinal gout are rare, and an intradural gout tophus in the spinal dura is even rarer (5). The presentation of gout tophi within the spinal dura without any systemic manifestation of gout is extremely rare, with only two cases reported in the literature, and is significantly misleading and difficult for doctors to diagnose (6, 7). Herein, we report a case of gouty tophi within the spinal dura without systemic gout manifestations in a patient with degenerative lumbar scoliosis.
2 Case description 2.1 Patient informationThe patient was a 70-year-old woman who had experienced lower back pain for 2 years, which was relieved with oral non-steroidal anti-inflammatory drugs. Two months prior, the pain suddenly worsened and radiated to the right buttock and lateral right lower extremity, accompanied by numbness in the perineal area. The symptoms were aggravated by coughing and sneezing. The patient had no history of spinal trauma or gout. She was 147 cm tall and weighed 38 kg, with a BMI of 17.6 kg/m2.
2.2 Diagnostic assessmentOn physical examination, we noted the absence of lumbar lordosis, considerable percussion and tenderness at the L2–L5 levels, and a right supine straight leg raise angle of 55°. The pyriformis stretch and femoral nerve traction test results were negative. The patient exhibited decreased superficial sensation in the perineum and normal muscle power in the extremities. No swelling or nodes were observed in the joint examination.
Radiographs of the full-length spine revealed thoracolumbar degenerative scoliosis and a pseudoslip of the L4 vertebra (Figures 1a,b). Computed tomography (CT) and magnetic resonance imaging (MRI) revealed a calcified intradural lesion at the L3 vertebral level and L3-S1 disc herniation secondary to lumbar spinal stenosis, predominantly at the L4/5 level (Figure 2). Abdominal color Doppler ultrasonography revealed a small number of urinary salt crystals in the left kidney. However, this finding was not brought to the attention of the doctors as being linked to gout.
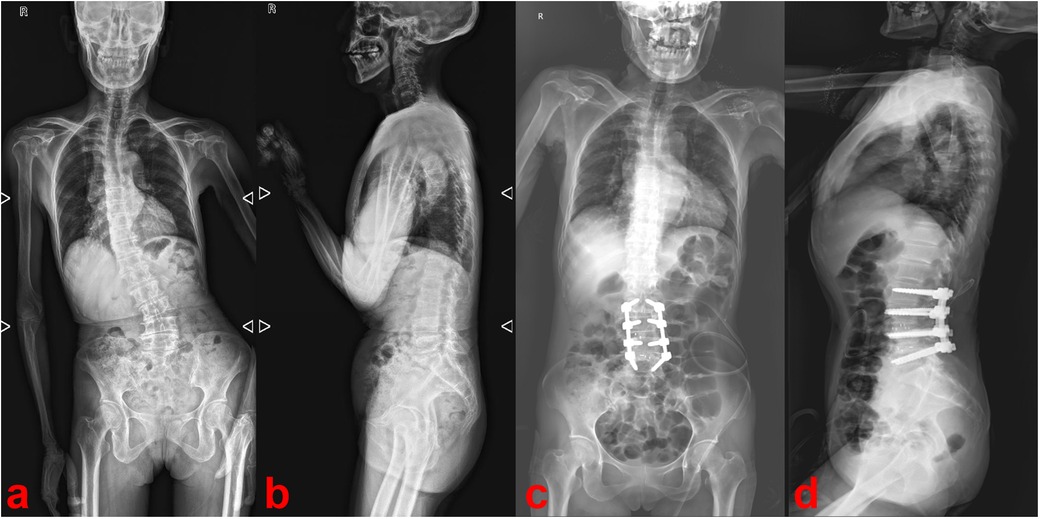
Figure 1. Preoperative (a,b) and postoperative (c,d) radiographs of the full-length spine.
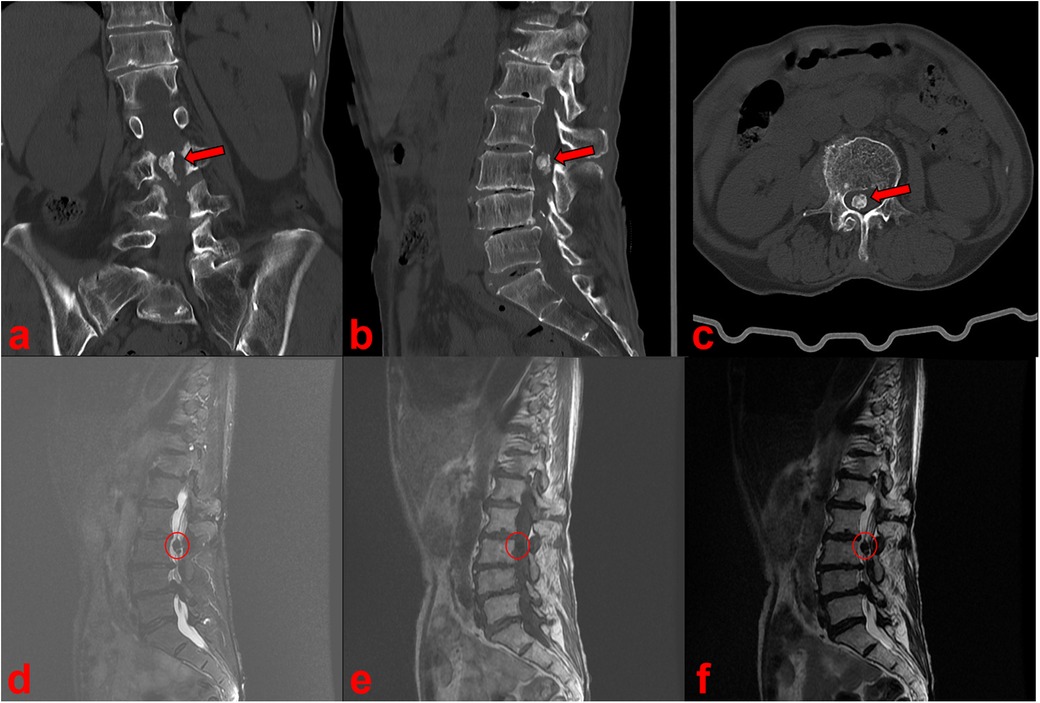
Figure 2. CT (a–c) and MRI (d–f) scan showed a calcified intradural lesion at the L3 vertebral level.
On laboratory examination, we found that her serum uric acid level was 146 µmol/L (normal range: 155–357 µmol/L) and her serum creatinine level was 45 µmol/L (normal range: 57–97 µmol/L), both of which were low. Her glomerular filtration rate (GFR) was calculated to be 61.6 ml/min, suggesting mild renal impairment. Infection markers, such as C-reactive protein, erythrocyte sedimentation rate, and leukocyte count, were within normal ranges. The level of urea was also within the normal range.
As the patient was a woman with no history of gout and no elevated blood uric acid, and no other gout tophi were found on physical examination, the calcified intradural mass was not diagnosed as a gout tophus preoperatively but was considered more likely to be a calcified nerve sheath tumor, calcified chordoma, calcified disc prolapse, or calcified ventricular meningioma.
2.3 Therapeutic interventionDuring surgery, we removed the spinous processes and lamina at the L2–L5 levels to expose the spinal canal. Upon opening the dura, the lesion was completely excised, revealing a white, irregular, crystalline mass (Figures 3a,b). Subsequently, we corrected the lumbar scoliosis deformity and released the lumbar spinal stenosis using posterior lumbar interbody fusion. The pathological examination revealed a white irregular crystalline mass consisting of urate crystals surrounded by calcified, degenerated, and necrotic nerve cells (Figure 3c).
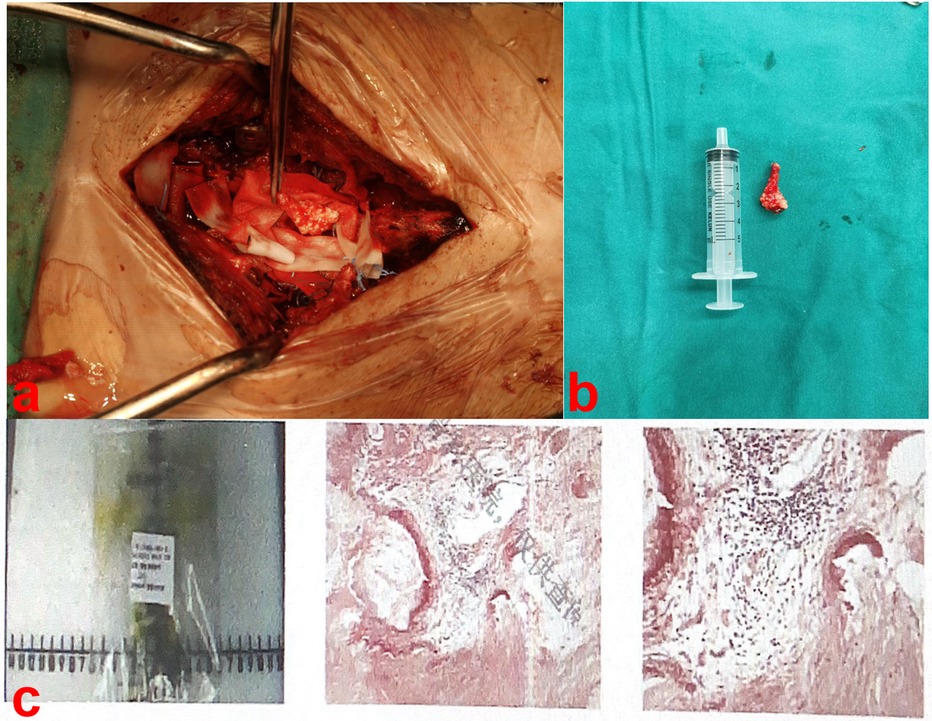
Figure 3. Upon opening the dura, the lesion was a white irregular crystalline mass (a,b). Pathological examination showed the mass consisted of urate crystals surrounded by calcified, degenerated and necrotic nerve tissue (c).
2.4 Follow-up and outcomesPostoperatively, the patient's lower back and radicular pain improved significantly and the pain intensity dropped from 7 to 3 on a 10-point visual analog scale (VAS), but numbness in the perineal area persisted. Postoperative radiographs showed the disappearance of calcification in the spinal canal and correction of the degenerative lumbar scoliosis (Figures 1c,d). The numbness in the perineal area disappeared by the sixth postoperative month.
3 DiscussionA global epidemiological study on gout showed that its incidence ranges from 0.3 to 6 cases per 1,000 person-years, with a prevalence ranging from 0.1% to approximately 10% (8). The primary manifestation of gout is intensely painful peripheral joint synovitis, which eventually leads to joint damage, deformity, and subcutaneous gout tophus deposition (9–11). It has been well established that hyperuricemia is the primary etiology of gout (3, 12), with risk factors including genetics, cardiovascular and inflammatory comorbidities, exposure, medications, and dietary factors (8).
Although gout can affect all the segments of the spine, it is uncommon, with only 315 cases reported as of 2023 (13, 14). The prevalence of axial gout in patients with gout is estimated to range from 14% to 35% (15–18). The prevalence of intraspinal gout has been suggested to be much higher than that previously reported as most cases are asymptomatic, resulting in insidious onset and easy misdiagnosis (19–21). In addition, the majority of gout tophi are located at the lumbar level, and men are more likely to suffer from spinal gout and have a much lower average age than women (13). Back pain is the most common symptom of spinal gout, followed by radiating pain. The treatment of choice for patients with spinal gout is usually conservative treatment with anti-inflammatory medication and urate-lowering therapy, with 88.9% of the patients improving after receiving conservative treatment (13). Surgical resection and decompression are usually initiated if a patient develops new neurological symptoms or progressive neurological damage, with 92% of patients showing improvement after surgery (13).
In 1950, Kersley reported the first case of spinal gout (22). The number of reported cases of axial gout has increased from 69 in 2009 to 315 in 2023 (13). However, only two of the 315 cases involved a gouty tophus occurring within the spinal dura (6, 7). In 2000, Paquette et al. (6) reported an intradural gout tophus in the filum terminale at the L3 vertebral level in an elderly man without systemic gout. In 2015, Willner et al. (7) similarly reported an elderly woman without systemic gout who had intradural gout tophi attached to the intradural nerve root at the L2 vertebral level. We report the third documented case of gout tophi occurring within the spinal dura at the L3 vertebral level. In the present study, the elderly woman also did not have hyperuricemia or systemic gout.
The three patients with intradural gouty tophi shared several features: (1) all complained of lower back pain, radiating pain, and progressive neurological damage; (2) all intradural gout tophi occurred in the lumbar spine and were concentrated at the L2/L3 vertebral level; (3) none had a history of gout, detectable hyperuricemia, or systemic gout manifestations; and (4) all the patients underwent surgical treatment to remove the gout tophi and recovered well after surgery.
In general, the formation of gout tophi is caused by long-term elevated and fluctuating uric acid, which causes monosodium urate crystals to deposit in the peripheral joints of the extremities where the temperature and circulation are low. However, the origin of an intradural gout tophus remains uncertain and may originate from the cerebrospinal fluid or the supply of blood vessels around the nerve root. From the analysis of the only three case reports so far, both we and Paquette found many inflammatory blood vessels and a small amount of necrotic, degenerated neural tissue surrounding the intradural gout tophi. But Willner et al. reported that their gout tophi were attached to a dorsal sensory fascicle flowing from the nerve root without any blood vessel attachment. The patient reported in this case had mild renal impairment leading to reduced uric acid excretion, which may also contribute to the development of an intradural gout tophus.
4 ConclusionTo the best of our knowledge, this is the third reported case of a gout tophus occurring within the spinal dura without hyperuricemia or systemic gout. Owing to the significant pain and progressive neurological damage, we performed timely surgery and the patient recovered well after surgery. As spinal gout (especially intradural gout), is difficult to diagnose, increasing awareness of intradural gout among physicians is essential to optimize treatment and outcomes for these patients. It is hoped that new imaging techniques will be available in the future to improve the diagnosis of intradural gout.
Data availability statementThe raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statementEthical approval was not required for the study involving humans in accordance with the local legislation and institutional requirements. Written informed consent to participate in this study was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and the institutional requirements. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributionsPQ: Writing – original draft, Writing – review & editing, Funding acquisition. SX: Writing – original draft, Writing – review & editing, Funding acquisition. CF: Software, Writing – original draft, Writing – review & editing. HX: Writing – original draft, Writing – review & editing, Investigation. SZ: Writing – original draft, Writing – review & editing, Resources. LZ: Writing – original draft, Writing – review & editing, Methodology. HD: Writing – original draft, Writing – review & editing, Visualization. YW: Writing – original draft, Writing – review & editing, Funding acquisition. YH: Funding acquisition, Writing – original draft, Writing – review & editing.
FundingThe author(s) declare financial support was received for the research, authorship, and/or publication of this article. This research was supported by the Sichuan Provincial Administration of Traditional Chinese Medicine (Nos. 2023ZD025 and 2021MS150) and the Health Commission of Mianyang City (Nos. 202230 and 2021Y005).
Conflict of interestThe authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's noteAll claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References1. Dehlin M, Jacobsson L, Roddy E. Global epidemiology of gout: prevalence, incidence, treatment patterns and risk factors. Nat Rev Rheumatol. (2020) 16(7):380–90. doi: 10.1038/s41584-020-0441-1
PubMed Abstract | Crossref Full Text | Google Scholar
2. Yokota S, Imagawa T, Mori M, Miyamae T, Takei S, Iwata N, et al. Longterm safety and effectiveness of the anti-interleukin 6 receptor monoclonal antibody tocilizumab in patients with systemic juvenile idiopathic arthritis in Japan. J Rheumatol. (2014) 41(4):759–67. doi: 10.3899/jrheum.130690
PubMed Abstract | Crossref Full Text | Google Scholar
6. Paquette S, Lach B, Guiot B. Lumbar radiculopathy secondary to gouty tophi in the filum terminale in a patient without systemic gout: case report. Neurosurgery. (2000) 46(4):986–8.10764275
PubMed Abstract | Google Scholar
7. Willner N, Monoranu CM, Stetter C, Ernestus RI, Westermaier T. Gout tophus on an intradural fascicle: a case description. Eur Spine J. (2016) 25(Suppl 1):162–6. doi: 10.1007/s00586-015-4309-z
PubMed Abstract | Crossref Full Text | Google Scholar
8. Kuo CF, Grainge MJ, Zhang W, Doherty M. Global epidemiology of gout: prevalence, incidence and risk factors. Nat Rev Rheumatol. (2015) 11(11):649–62. doi: 10.1038/nrrheum.2015.91
PubMed Abstract | Crossref Full Text | Google Scholar
9. Zhang W, Feng Q, Gu J, Liu H. Carpal tunnel syndrome caused by tophi deposited under the epineurium of the median nerve: a case report. Front Surg. (2022) 9:942062. doi: 10.3389/fsurg.2022.942062
PubMed Abstract | Crossref Full Text | Google Scholar
13. Harlianto NI, Harlianto ZN. Patient characteristics, surgical treatment, and outcomes in spinal gout: a systematic review of 315 cases. Eur Spine J. (2023) 32(11):3697–703. doi: 10.1007/s00586-023-07942-8
PubMed Abstract | Crossref Full Text | Google Scholar
15. de Mello FM, Helito PV, Bordalo-Rodrigues M, Fuller R, Halpern AS. Axial gout is frequently associated with the presence of current tophi, although not with spinal symptoms. Spine. (2014) 39(25):E1531–6. doi: 10.1097/BRS.0000000000000633
PubMed Abstract | Crossref Full Text | Google Scholar
16. Konatalapalli RM, Lumezanu E, Jelinek JS, Murphey MD, Wang H, Weinstein A. Correlates of axial gout: a cross-sectional study. J Rheumatol. (2012) 39(7):1445–9. doi: 10.3899/jrheum.111517
PubMed Abstract | Crossref Full Text | Google Scholar
18. Jin HJ, Son ES, Kim DH. The frequency of axial deposition in Korean patients with gout at a tertiary spine center. Front Med (Lausanne). (2020) 7:339. doi: 10.3389/fmed.2020.00339
PubMed Abstract | Crossref Full Text | Google Scholar
19. Levin MH, Lichtenstein L, Scott HW. Pathologic changes in gout; survey of eleven necropsied cases. Am J Pathol. (1956) 32(5):871–95.13354714
PubMed Abstract | Google Scholar
20. Hou LC, Hsu AR, Veeravagu A, Boakye M. Spinal gout in a renal transplant patient: a case report and literature review. Surg Neurol. (2007) 67(1):65–73; discussion. doi: 10.1016/j.surneu.2006.03.038
PubMed Abstract | Crossref Full Text | Google Scholar
22. Kersley GD, Mandel L, Jeffrey MR. Gout; an unusual case with softening and subluxation of the first cervical vertebra and splenomegaly. Ann Rheum Dis. (1950) 9(4):282–304. doi: 10.1136/ard.9.4.282
留言 (0)