Ischemic stroke, characterized by the abrupt loss of cerebral blood flow due to an occlusion, precipitates a cascade of pathophysiological events culminating in extensive neuronal damage (1–3). The complexity of these events, encompassing excitotoxicity, oxidative stress, inflammation, and apoptosis, presents a formidable challenge for therapeutic intervention (4–8). Traditional pharmacological treatments, while invaluable, often offer limited windows of efficacy and are frequently accompanied by substantial side effects. In this context, electroacupuncture (EA) presents a unique multimodal approach, purportedly targeting multiple pathways implicated in ischemic injury.
Electroacupuncture, a modern adaptation of the ancient Chinese acupuncture technique, involves the application of electrical stimulation to traditional acupuncture points (9, 10). Its potential neuroprotective effects, mediated through various biological and physiological mechanisms, offer a promising avenue for post-stroke rehabilitation (11, 12). Preliminary investigations into EA’s efficacy have reported promising outcomes, including improved cerebral perfusion, reduced infarct volume, and enhanced neurological function (13–15). These effects are hypothesized to stem from EA’s ability to modulate neurovascular and pathways, promote neurogenesis, and inhibit (16–20). However, the diversity of experimental designs, acupuncture protocols, and outcome measures across studies necessitates a comprehensive analysis to distill these findings into actionable clinical insights.
This research seeks to bridge this gap through a systematic review and meta-analysis of the existing literature on EA’s effects on ischemic stroke models. By aggregating data from diverse studies, this work aims to quantify EA’s impact on key outcomes such as cerebral infarction volume, neuronal apoptosis rates, and functional recovery scores. Furthermore, this study will employ advanced statistical techniques to explore the heterogeneity among findings and assess the robustness of the evidence. In doing so, it will not only provide a consolidated overview of EA’s efficacy but also identify knowledge gaps and directions for future research.
In addressing these objectives, this study will contribute to the field by offering a rigorous, evidence-based assessment of EA’s role in ischemic stroke rehabilitation. It will also propose a conceptual framework for understanding the complex interplay of biological processes involved in EA-mediated neuroprotection, thereby paving the way for targeted clinical applications and further scientific inquiry.
2 Methods and materialsThe methods used in this study followed the meta-analysis criteria in the PRISMA guidelines. The meta-analysis did not need to follow ethical review and was also approved by Chengdu University of Traditional Chinese Medicine.
2.1 Retrieval strategyWe conducted comprehensive searches across four major databases: PubMed, Web of Science, Embase, and the Cochrane Library, covering the period from their inception up to December 31st, 2023. Our search strategy was specifically formulated. The search method is: “electroacupuncture” and “ischemic stroke,” to ensure the retrieval of relevant studies pertaining to the efficacy of electroacupuncture in the treatment of ischemic stroke.
2.2 Eligibility criteriaThe inclusion criteria were pre-specified as follows: (1) Established experimental animal models of ischemic stroke; (2) The intervention group received electroacupuncture treatment; (3) The model group received sham acupuncture intervention or no treatment; (4) There were no restrictions on animal species, gender, age, weight, and sample size; (5) The primary outcome measures included neurological function scores, infarct size or ratio, and all markers indicative of ischemic stroke.
The exclusion criteria for the documents were rigorously defined as follows: (1) Exclusion of reviews, in vitro studies, and trials; (2) Studies not involving electroacupuncture for prevention; (3) Research focusing on electroacupuncture in the treatment of diseases other than ischemic stroke; (4) Elimination of duplicate publications; (5) Exclusion of unpublished literature.
2.3 Data extractionThe data extraction table was independently completed by two authors to ensure accuracy. Additionally, the literature table includes the following information: (1) the first author’s name and the year of study publication; (2) detailed information about the animal model, such as species, sex, and weight; (3) the induction method of ischemic stroke in the animal model; (4) detailed information of the intervention group, including selected acupoints and details of electroacupuncture stimulation; (5) information on sham acupuncture if used in the model group; (6) the main conclusions of the study; (7) outcome indicators. If experimental animals received varying stimulation intensities or intervention durations, only the data from the highest intensity or longest duration were taken. If data were presented in graph form, they were measured using digital ruler software. We attempted to obtain further information by contacting the authors when primary data were incomplete.
2.4 Risk of biasTo assess the quality of literature on electroacupuncture treatment in ischemic stroke animal studies, the CAMARADES checklist with 10 criteria proposed by Macleod was employed. The assessment included: (1) Sample size calculation; (2) Random sequence generation; (3) Blinded induction; (4) Blinded outcome assessment; (5) Use of anesthetics without significant neuroprotective activity; (6) Appropriateness of animal models; (7) Temperature control statement; (8) Publication in peer-reviewed journals; (9) Compliance with animal welfare laws; (10) Declaration of potential conflicts of interest. Each study was scored on a scale of 10, with one point for each criterion. The Risk of Bias Assessment was independently conducted by two researchers, involving a third in cases of discrepancy.
2.5 Quantitative synthesis and other statistical analysesFor the meta-analysis of animal studies, a quantitative synthesis was adopted. Outcomes, quantified as standardized mean differences, were accompanied by 95% confidence intervals. Statistical significance was determined at p < 0.05 for contrasts between experimental and control groups. Heterogeneity assessment employed the I-square statistic, with I2 values above 50% denoting considerable heterogeneity. Given the investigative nature of these studies, random effects models were uniformly utilized, independent of the I2 value. In addition, we performed a sensitivity analysis by sequentially excluding individual studies to assess the robustness and stability of the results of the meta-analysis.
3 Results 3.1 Research screeningFirstly, a total of 771 relevant records were retrieved from four databases through a detailed search strategy. The records were then imported into Endnote X9 and 312 records were excluded due to duplication and other reasons. Upon title and abstract screening, 89 were excluded for not meeting inclusion criteria, leaving 370 full-text articles for detailed review, subsequently, 359 articles were excluded for reasons including in vitro experiments, no use of electroacupuncture therapy, etc. Finally, a total of 11 eligible English studies were included in this meta-analysis (13, 15, 21–29) (The detailed document scheduling process is shown in Figure 1).
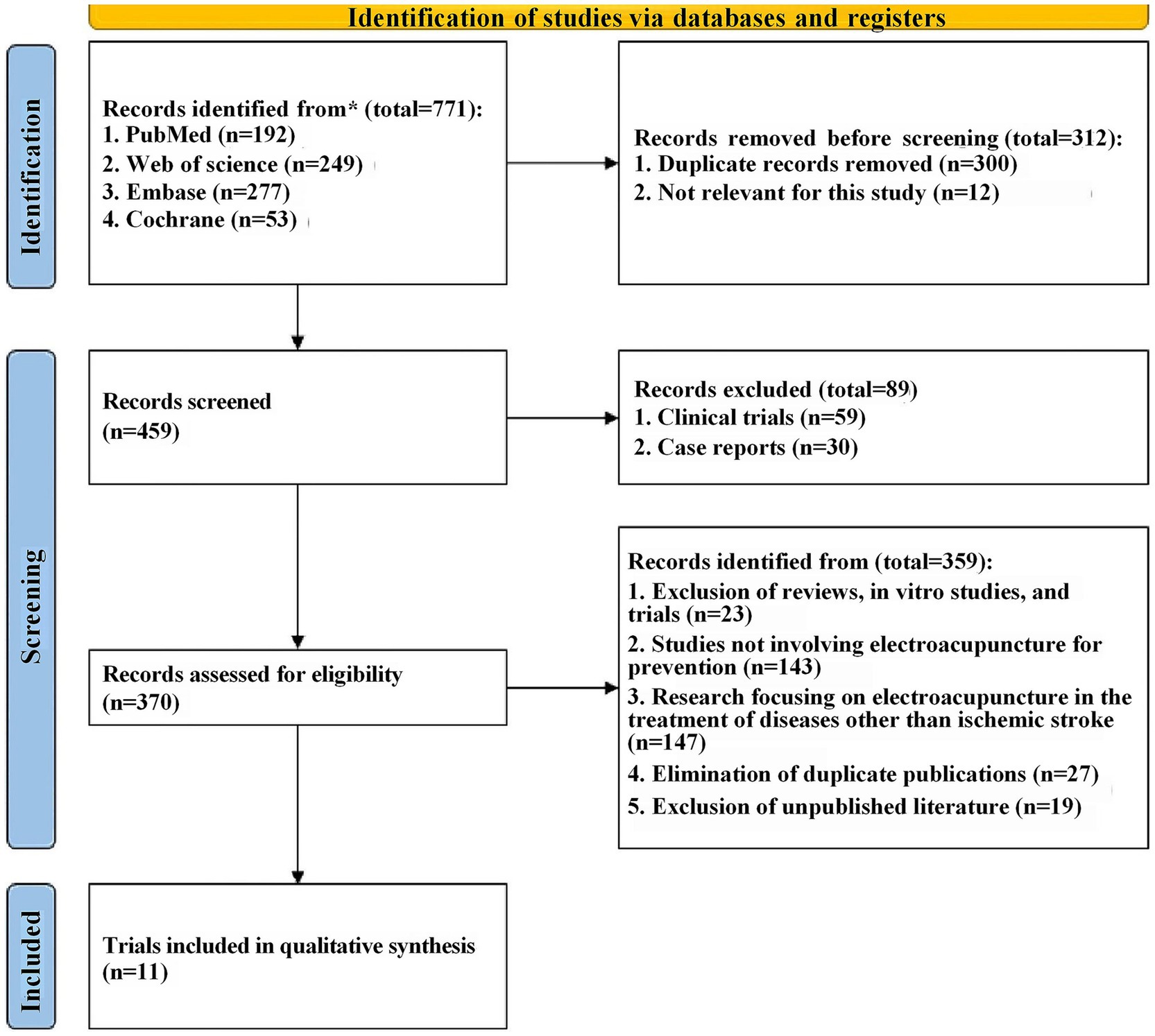
Figure 1. PRISMA-type flow diagram.
3.2 Features of included researchesThe 11 articles included a total of 302 animals, of which the experimental group (n = 151) was receiving EA treatment, and the control group (n = 151) was treated with sham EA. All experiments were carried out on male rodents, predominantly Sprague–Dawley rats (96.03%) and the rest were C57BL/6 mice. The rats weighed between 150 and 320 g, while the mice weighed between 16 and 18 g. Regarding the use of anesthetic in experimental animals, chloral hydrate is used in 72.73% (8/11) articles, and isoflurane is used in 27.27% (3/11) articles.
In terms of electroacupuncture treatment, 81.82% (9/11) of the articles used electroacupuncture for 30 min each time, whereas two articles EA treatments for durations of both 15 and 20 min, respectively (Details are in Table 1).
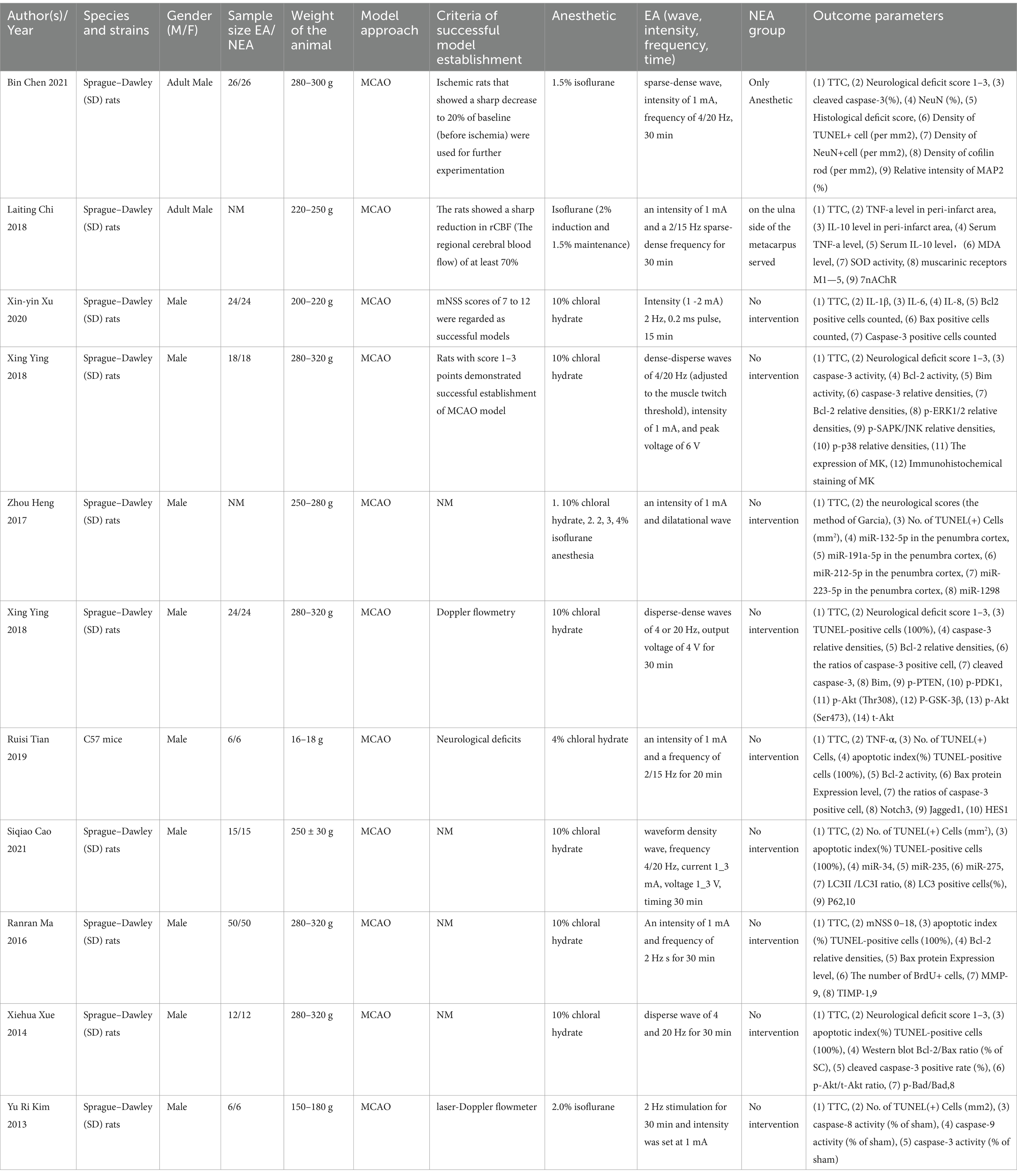
Table 1. List of key characteristics in all 11 studies based on full-text assessment.
3.3 Quality evaluationThe rigor of our selected studies was upheld by consistent baseline characteristics and the absence of incomplete data, minimizing reporting bias. However, the lack of explicit blinding of outcome assessors in four studies introduces a potential limitation. Other aspects such as allocation concealment, randomization, and blinding procedures varied, introducing elements of uncertainty in bias evaluation. All the details are shown in Figure 2.
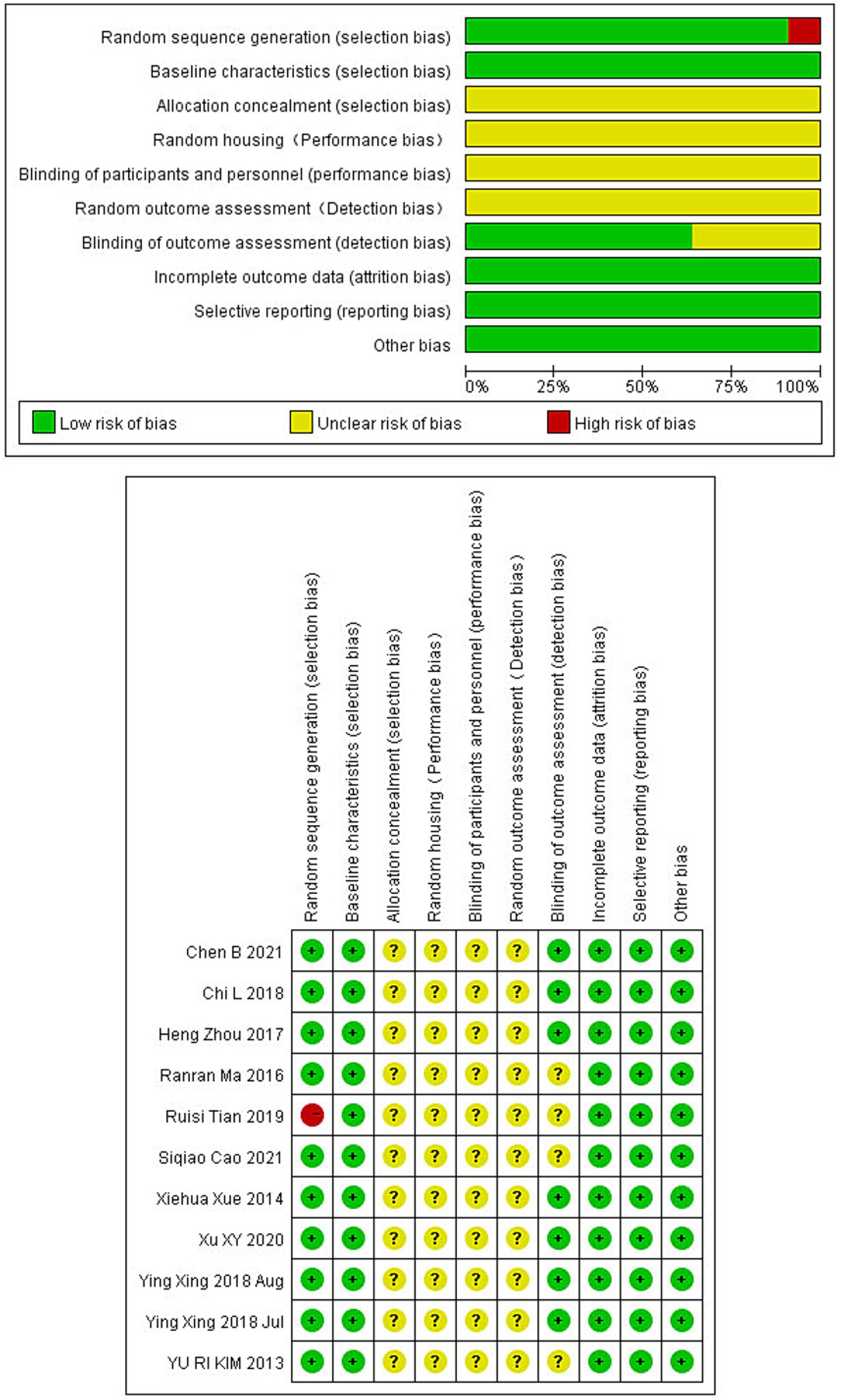
Figure 2. Bias risk in studies on electroacupuncture for ischemic stroke treatment.
3.4 Pathological analysisThe pathological results can intuitively demonstrate the therapeutic effect of EA on ischemic stroke animals, so the pathological results of the included studies were qualitatively analyzed. Triphenyl tetrazolium chloride (TTC) staining was used in all the 11 included articles, and the results showed that the infarct size of the EA group was smaller than that in the control group, and the difference was statistically significant. Notably, EA treatment exhibited a robust neuroprotective effect, particularly by reducing middle brain region damage. Hematoxylin–eosin (H&E) staining (n = 1) revealed that EA treatment could effectively improve neuronal necrosis and pathological tissue disorder in cerebral infarction tissue. Immunohistochemical staining (n = 8) indicated that ischemic stroke could significantly increase the number of caspase-3 positive cells, while EA treatment could reduce the expression of apoptosis-related proteins, such as caspase-3, and it also inhibit the expression of a7nAChR mRNA, preventing the damage of central cholinergic system. Additionally, the number of BrdU+ /GFAP+(red) cells were significantly decreased by EA treatment. One article found that there was autophagosome accumulation in the electroacupuncture treatment group by transmission electron microscopy. However, compared with the control group, the electron density of the autophagy structure was lower and the autophagy structure was different.
3.5 Neuroprotection and brain injury reduction 3.5.1 Cerebral infarction volumeDue to the high heterogeneity among the 11 included studies (I2 = 94%, p < 0.01), a random effect model was used for data analysis, and the meta-analysis revealed statistically significant result: Compared with the control group, EA treatment could significantly reduce the cerebral infarction volume in the ischemic stroke animals [MD = −15.78, 95%CI (−21.40, −10.16), p < 0.05] (Figure 3A). This substantial decrease underscores EA’s neuroprotective efficacy, aligning with the mechanistic insights suggesting EA’s role in modulating inflammatory responses and enhancing cerebral blood flow. Considering the subjectivity of the funnel plot (Supplementary Figure 2A), Egger’s test was used to explore publication bias, and the result was (t = −0.30, p = 0.77 > 0.05) indicates no publication bias (Figure 3B), indicating that it had little impact on our results. In the sensitivity analysis, we calculated the 95% confidence intervals of the relevant parameters. According to the results of the analysis, the 95% confidence intervals for the relevant variables are [−2.46, −1.60] (Supplementary Figure 3).
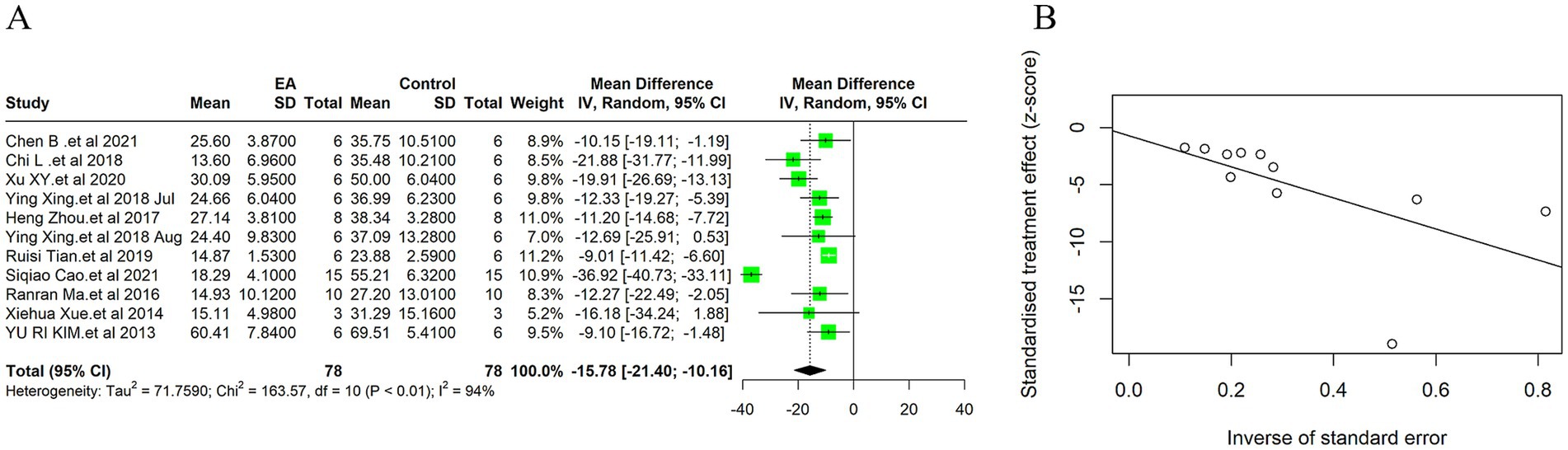
Figure 3. Meta-analytic assessment of electroacupuncture’s efficacy in reducing cerebral infarction volume. (A) The forest plot. (B) Egger’s test funnel plot.
3.6 Apoptosis indicators 3.6.1 TUNEL positive cellsDue to the high heterogeneity of the 3 included studies (I2 = 99%, p < 0.01), a random effect model was used for data analysis, and the meta-analysis revealed statistically significant result: Compared with the control group, EA treatment could significantly reduce the TUNEL positive cells in the ischemic stroke animals [MD = −26.46, 95%CI (−40.40, −12.51), p < 0.05] (Figure 4A). Indicates EA’s role in reducing cell death by apoptosis, a key aspect of neuroprotection. The funnel plot was made to explore publication bias, and the results showed that there was a slight publication bias (Supplementary Figure 2B). EA’s influence on cellular apoptosis was evident, with a significant reduction in TUNEL positive cells in treated subjects, further validating EA’s neuroprotective role.
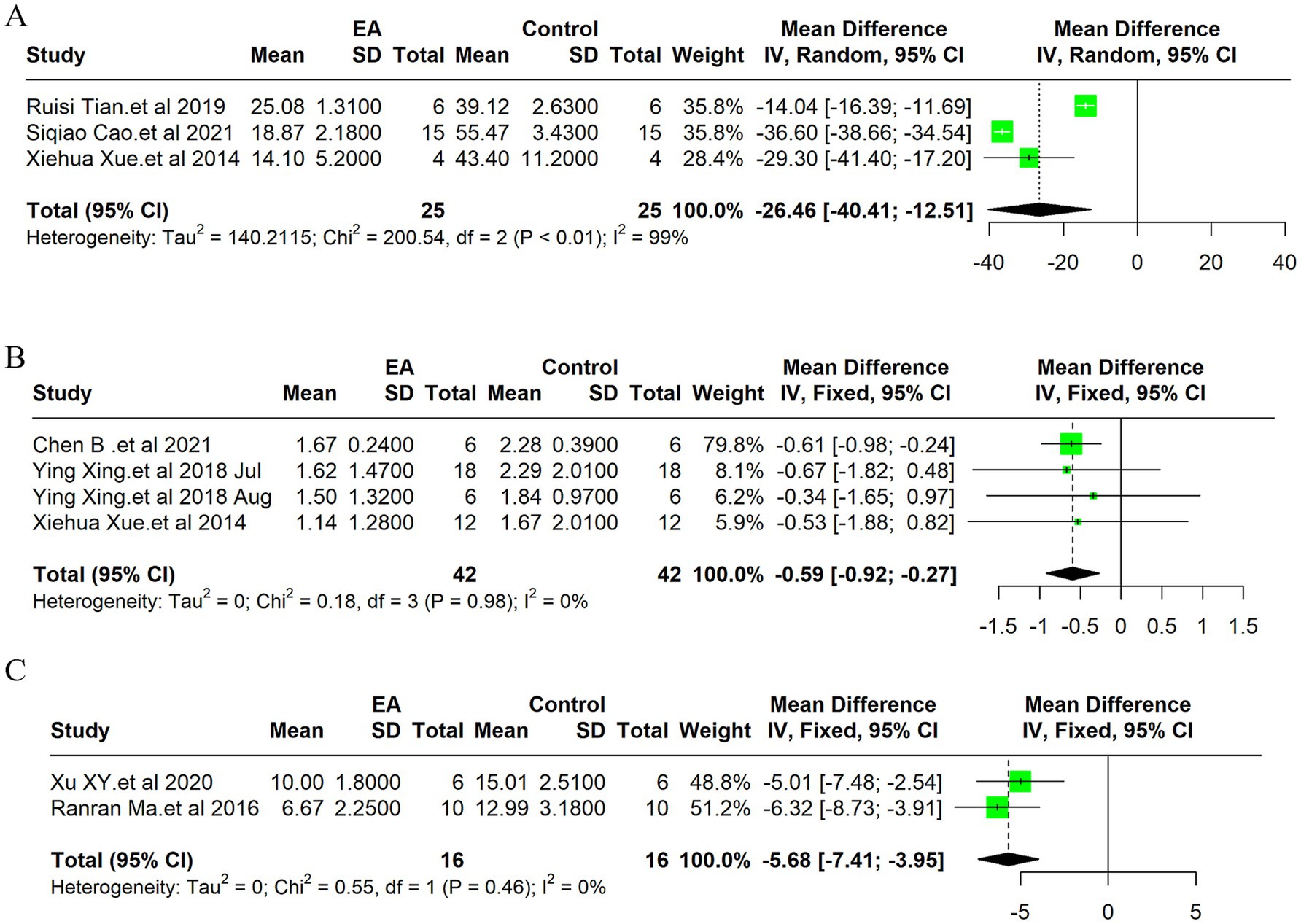
Figure 4. Meta-analysis of electroacupuncture’s efficacy in ischemic stroke models: forest plots of (A) TUNEL positive cells, (B) Neurological deficit scores (C) the modified neurological severity scores (mNSS).
3.6.2 Caspase-3 relative densitiesDue to the high heterogeneity of the 2 included studies (I2 = 76%, p < 0.05), a random effect model was used for data analysis, and the meta-analysis revealed this result: there was no statistically significant difference in the caspase-3 relative densities between the EA treatment group and control group, EA treatment could not reduce the caspase-3 relative densities in the animals with ischemic stroke [MD = −0.39, 95%CI (−0.79, 0.02), p > 0.05] (Supplementary Figure 1A). This suggests that while EA exhibits neuroprotective properties, its mechanism may not primarily involve the modulation of caspase-3 mediated apoptotic pathways. The funnel plot was made to explore publication bias, and the results showed that there was a slight publication bias (Supplementary Figure 2C).
3.6.3 Bax protein expression levelDue to the high heterogeneity of the 2 included studies (I2 = 99%, p < 0.01), a random effect model was used for data analysis, and the meta-analysis revealed this result: there was no statistically significant difference in the Bax protein expression level between the EA treatment group and control group, EA treatment could not reduce the Bax protein expression level in the animals with ischemic stroke [MD = −0.73, 95%CI (−1.68, 0.21), p > 0.05] (Supplementary Figure 1B). The funnel plot was made to explore publication bias, and the results showed that there was a significant publication bias (Supplementary Figure 2E).
3.6.4 Apoptotic indexDue to the high heterogeneity of the 2 included studies (I2 = 67%, p < 0.05), a random effect model was used for data analysis, and the meta-analysis revealed statistically significant result: Compared with the control group, EA treatment could significantly reduce the apoptotic index in the ischemic stroke animals [MD = −22.14, 95%CI (−34.72, −9.56), p < 0.05] (Supplementary Figure 1C). The funnel plot was made to explore publication bias, and the results showed that there was a slight publication bias (Supplementary Figure 2F).
3.7 Neurological scores 3.7.1 Neurological deficit scoreDue to the low heterogeneity of the 4 included studies (I2 = 0%, p > 0.05), a fixed effect model was used for data analysis, and the meta-analysis revealed statistically significant result: Compared with the control group, EA treatment could significantly reduce the neurological deficit score in the ischemic stroke animals [MD = −0.59, 95%CI (−0.92, −0.27), p < 0.05] (Figure 4B). This improvement underscores EA’s efficacy not only in reducing cellular and molecular markers of ischemic damage but also in enhancing functional recovery post-stroke. The funnel plot was made to explore publication bias, and the results showed that there was a slight publication bias (Supplementary Figure 2G).
3.7.2 Neurological function score of mNSSDue to the low heterogeneity of the 2 included studies (I2 = 0%, p > 0.05), a fixed effect model was used for data analysis, and the meta-analysis revealed statistically significant result: Compared with the control group, EA treatment could significantly reduce the modified Neurological Severity Scores (mNSS) scores in the ischemic stroke animals [MD = −5.68, 95%CI (−7.41, −3.95), p < 0.05] (Figure 4C). Consistent with the improvement in neurological deficit scores, the mNSS were significantly lower in the EA-treated groups. This finding indicates a notable enhancement in neurological functions, aligning with EA’s proposed benefits in stroke rehabilitation. The funnel plot was made to explore publication bias, and the results showed that there was no publication bias (Supplementary Figure 2H).
4 DiscussionThis meta-analysis elucidates the considerable neuroprotective potential of EA in the rehabilitation of ischemic stroke, highlighted by a marked reduction in cerebral infarction volume and significant improvements in neurological functions (See Table 2 for details). The substantial decrease in TUNEL-positive cells we observed underscores a notable reduction in apoptosis within ischemic cerebral territories, aligning with contemporary research that explores EA’s impact on apoptotic mechanisms, suggesting a multifaceted neuroprotective strategy encompassing both apoptosis modulation and cerebral perfusion enhancement (11, 26, 30–32).
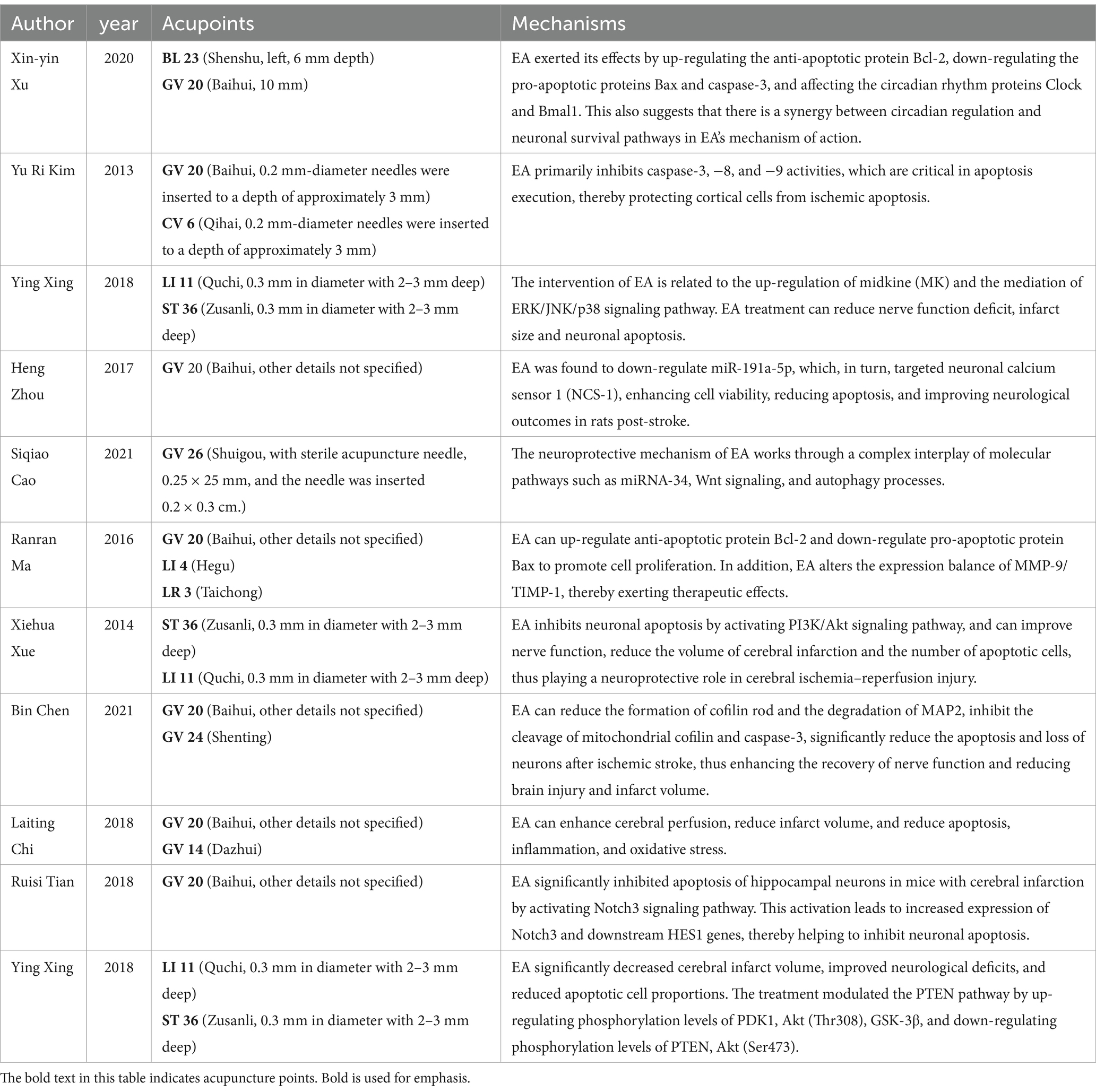
Table 2. A comprehensive overview of the mechanism, with attention to acupoint selection.
4.1 Neuroprotective mechanisms of EACurrent literature suggests that EA’s therapeutic efficacy in cerebral ischemia is driven by complex, multifactorial mechanisms (33). Our synthesis of data from 302 subjects across 11 studies highlights EA’s significant neuroprotective influence, particularly evidenced by the pronounced reduction in cerebral infarction volume [MD = −15.78, 95%CI (−21.40, −10.16), p < 0.05]. This observation resonates with Kim et al.’s findings, suggesting that EA’s protective effects might involve local blood flow modulation and neuronal metabolic regulation (22). Moreover, the enhancements in neurological function echo Chi's et al. (13) findings, attributing such improvements to EA’s stimulation of the parasympathetic nervous system, further underscoring the diverse mechanisms of EA’s action. Additionally, the modulation of the MMP-9/TIMP-1 expression balance by EA, crucial for maintaining extracellular matrix integrity and preventing excessive proteolytic activity, suggests another dimension of EA’s neuroprotective mechanism (23, 34).
The notable reduction in TUNEL positive cells observed in our analysis suggests a substantial attenuation of cellular apoptosis within ischemic cerebral regions. This is consistent with the molecular insights provided by Kim et al. (22) and Xing et al. (25, 26), where EA was shown to influence apoptotic pathways, underscoring its potential in curtailing neuronal loss post-stroke. EA’s mechanistic underpinnings, embracing the modulation of apoptosis-centric pathways alongside cerebral hemodynamic enhancements, have been the focal point of preceding inquiries (35–39). But this is not consistent with the results of our analysis. Investigations delineate EA’s modulatory effect over the PTEN signaling cascade, the PI3K/Akt pathway, and the miRNA-34/Wnt/Autophagy axis, implicating these in the apoptotic and autophagic processes within ischemic cerebral detriment (21, 25, 28). Furthermore, EA’s engagement with cofilin—a pivotal apoptotic mediator—through mitochondrial translocation, attenuates ischemic cerebral injury, modulating death receptor (DR) 5 expression and upregulating the inhibitor of apoptosis (IAP) family members cIAP-1 and -2 (15, 22).
The reduction in TUNEL-positive cells, alongside the increase in Bcl-2 relative densities observed in our analysis, suggests a shift toward anti-apoptotic signaling, further evidenced by the significant decrease in the apoptotic index (22, 26, 27, 40). This is in line with literature demonstrating EA’s modulation of key cell survival and apoptosis regulatory pathways, such as the PTEN/PI3K/Akt pathway (21, 28).
EA’s broad-spectrum neuroprotection can be attributed to multifaceted mechanisms, including apoptosis pathway regulation and cerebral perfusion enhancement. The reduction in apoptosis, particularly through the downregulation of caspase-3 and upregulation of Bcl-2, suggests a pivotal role of anti-apoptotic signaling in EA’s neuroprotective effects. It’s noteworthy, however, that our findings regarding caspase-3 relative densities did not exhibit significant differences between the EA and sham-EA groups, hinting at the possibility that EA’s neuroprotective mechanisms may transcend the caspase-3 mediated apoptotic pathways, potentially involving alternative or complementary pathways like those involving Bcl-2, as our analysis indicated a significant increase in Bcl-2 relative densities in the EA treatment group. This is consistent with the findings of Kim et al. (22) and Ma et al. (23), which reported similar modulations in apoptotic regulators following EA treatment. However, the absence of significant differences in caspase-3 densities and Bax levels indicates that EA’s neuroprotective mechanisms extend beyond simple modulation of apoptotic pathways, warranting further investigation into alternative pathways and mechanisms, such as neuroinflammation, autophagy, and neurogenesis (23, 25).
The notable improvements in neurological deficit scores post-EA treatment point toward its efficacy in facilitating neural recovery and functional restoration, consistent with hypotheses suggesting EA’s role in neurorehabilitation via neuroinflammatory response modulation, neurogenesis, and angiogenesis (41, 42). The enhancements in motor, sensory, reflex, and balance functions, as indicated by the significant reduction in mNSS scores, align with Xue's et al. (28) findings on EA’s potential to upregulate neurotrophic factors and enhance synaptic plasticity. These findings suggest EA’s integral role in comprehensive stroke rehabilitation strategies aimed at restoring neurological functions and improving stroke survivors’ quality of life. The improvement in neurological deficit scores and mNSS further accentuates EA’s potential in enhancing functional recovery, a finding that echoes the work of Xu et al. (27), where EA facilitated neural repair and functional rehabilitation through mechanisms that might involve neurogenesis and synaptic plasticity enhancement.
In this study, we included a relatively small number of studies in strict adherence to the inclusion exclusion criteria, and the strict inclusion and exclusion criteria were developed to ensure that the synthesized evidence was of the highest relevance and quality, consistent with the specific objectives of our review. By adhering to these criteria, we aimed to minimize bias and increase the reliability of study results. The limited number of studies may also indicate a lack of high-quality research in this particular area. This suggests the need for more rigorous, well-designed trials to examine the role of electroacupuncture in preventing ischemic stroke.
4.2 InnovationIn this study, we systematically integrated the animal experimental data to provide a reference for future clinical study design. In addition, the effect size of electroacupuncture treatment was assessed by quantitative analysis to clarify the influence of different experimental conditions on the effect. Moreover, identifying the potential mechanisms of electroacupuncture effects may provide some basis for advancing basic research and clinical translation.
4.3 Clinical implications and future directionsThe clinical implications of these findings are profound, suggesting that EA could serve as a viable non-pharmacological intervention in ischemic stroke rehabilitation. The potential for EA to improve functional recovery and reduce neuronal loss opens new avenues for integrative approaches to stroke treatment (43–45). However, these findings hold profound clinical implications, positioning EA as a promising non-pharmacological intervention in ischemic stroke rehabilitation. However, translating these animal model findings to clinical practice necessitates careful consideration of EA protocols, including electroacupuncture points selection, frequency, duration of treatment, and patient-specific factors to optimize therapeutic outcomes.
4.4 Limitations and considerationsWhile this analysis provides robust evidence of EA’s neuroprotective effects, it is not devoid of limitations. The inherent variability in experimental designs and outcome measures across the included studies calls for standardized methodological approaches in future research. Furthermore, addressing the heterogeneity among studies and the predominance of male rodent models will be crucial in refining the evidence base for EA’s role in stroke recovery. Future research should aim to elucidate the molecular and cellular mechanisms underlying EA’s neuroprotective effects further, exploring the potential synergies between EA and conventional stroke therapies.
5 ConclusionThis systematic review and meta-analysis highlight the significant neuroprotective effects of electroacupuncture in ischemic stroke models, suggesting its potential as a therapeutic intervention. The primary findings, including the reduction in cerebral infarction volume and apoptosis, coupled with improvements in neurological function, underscore EA’s multifaceted neuroprotective mechanisms. Future research should aim to further elucidate these mechanisms and explore the synergistic effects of EA with conventional stroke therapies, paving the way for innovative integrative treatment strategies in stroke rehabilitation.
Data availability statementThe original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Author contributionsYG: Conceptualization, Data curation, Formal analysis, Visualization, Writing – original draft. SH: Conceptualization, Data curation, Formal analysis, Visualization, Writing – original draft. SL: Data curation, Visualization, Writing – original draft. LT: Data curation, Methodology, Writing – original draft. YT: Visualization, Writing – original draft. FZ: Conceptualization, Supervision, Writing – review & editing.
FundingThe author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This work was supported by Sichuan Provincial Administration of Traditional Chinese Medicine Science and Technology Research Special Project: 2021 ms144; Scientific research project of Sichuan Provincial Key Research Base of Humanities and Social Sciences in Universities (No. ZYYWH1812); Chengdu University of Traditional Chinese Medicine Affiliated Hospital Science and Technology Development Fund (19ZL11) and Personal academic points of the Affiliated Hospital of Chengdu University of Traditional Chinese Medicine (22JF113).
AcknowledgmentsThe authors gratefully acknowledge the reviewers and all the authors of the references.
Conflict of interestThe authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s noteAll claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary materialThe Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2025.1491132/full#supplementary-material
Abbreviations95% CI, 95% confidence Interval; Akt, protein kinase B; Bax protein, bcl-2-associated X protein; Bcl, b-cell lymphoma; BrdU, bromodeoxyuracil; C, control; CAMARADES, collaborative approach to meta-analysis and review of animal data from experimental studies; cIAP, cellular inhibitor of apoptosis protein; DR, death receptor; EA, electroacupuncture; ERK, extracellular regulated protein kinases; GFAP, glial fibrillary acidic protein; GSK-3β, glycogen synthase kinase-3β; H&E, hematoxylin–eosin; I, intervention; I2, I-square; IAP, inhibitor of apoptosis; JNK, jun n-terminal kinase; MAP2, microtubule-associated protein 2; MCAO, middle cerebral artery occlusion; miR, microRNA; MK, midkine; MMP-9, matrix metalloproteinase-9; mNSS, modified neurological severity scores; N, number; NCS, neuronal calcium sensor; NM, not mentioned; O, outcome; P, population; PDK1, pyruvate dehydrogenase kinase 1; PI3K, phosphatidylinositol 3-kinase; PNS, panax notoginseng saponins; PTEN, phosphatase and tensin homolog deletedon chromosome ten; Qu, quercetin; Ranran Ma, Wingless/Integrated; SCI, science citation index; SMD, standardized mean difference; TIMP-1, tissue inhibitor of metalloproteinase-1; TTC, triphenyl tetrazolium chloride; TUNEL, the terminal deoxynucleotidyl transferase (TdT) dUTP nick-end labeling; α7nAChR, alpha-7 nicotinic acetylcholine receptor.
References1. Huang, G, Zang, J, He, L, Zhu, H, Huang, J, Yuan, Z, et al. Bioactive Nanoenzyme reverses oxidative damage and endoplasmic reticulum stress in neurons under ischemic stroke. ACS Nano. (2021) 16:431–52. doi: 10.1021/acsnano.1c07205
PubMed Abstract | Crossref Full Text | Google Scholar
2. Zhao, Y, Zhang, X, Chen, X, and Wei, Y. Neuronal injuries in cerebral infarction and ischemic stroke: from mechanisms to treatment (review). Int J Mol Med. (2021) 49:70. doi: 10.3892/ijmm.2021.5070
PubMed Abstract | Crossref Full Text | Google Scholar
3. Candelario-Jalil, E, Dijkhuizen, RM, and Magnus, T. Neuroinflammation, stroke, blood-brain barrier dysfunction, and imaging modalities. Stroke. (2022) 53:1473–86. doi: 10.1161/STROKEAHA.122.036946
PubMed Abstract | Crossref Full Text | Google Scholar
4. Kanai, Y, Clémençon, B, Simonin, A, Leuenberger, M, Lochner, M, Weisstanner, M, et al. The SLC1 high-affinity glutamate and neutral amino acid transporter family. Mol Asp Med. (2013) 34:108–20. doi: 10.1016/j.mam.2013.01.001
PubMed Abstract | Crossref Full Text | Google Scholar
6. Qin, C, Yang, S, Chu, Y-H, Zhang, H, Pang, X-W, Chen, L, et al. Signaling pathways involved in ischemic stroke: molecular mechanisms and therapeutic interventions. Signal transduction and targeted. Therapy. (2022) 7:215. doi: 10.1038/s41392-022-01064-1
PubMed Abstract | Crossref Full Text | Google Scholar
7. Orellana-Urzúa, S, Rojas, I, Líbano, L, and Rodrigo, R. Pathophysiology of ischemic stroke: role of oxidative stress. Curr Pharm Des. (2020) 26:4246–60. doi: 10.2174/1381612826666200708133912
PubMed Abstract | Crossref Full Text | Google Scholar
8. Zhang, Y, Chen, Q, Chen, D, Zhao, W, Wang, H, Yang, M, et al. SerpinA3N attenuates ischemic stroke injury by reducing apoptosis and neuroinflammation. CNS Neurosci Ther. (2021) 28:566–79. doi: 10.1111/cns.13776
PubMed Abstract | Crossref Full Text | Google Scholar
9. Fan, AY. Anti-inflammatory mechanism of electroacupuncture involves the modulation of multiple systems, levels and targets and is not limited to "driving the vagus-adrenal axis". J Integr Med. (2023) 21:320–3. doi: 10.1016/j.joim.2023.06.001
Crossref Full Text | Google Scholar
10. Xie, L, Ng, DQ, Heshmatipour, M, Acharya, M, Coluzzi, P, Guerrero, N, et al. Electroacupuncture for the management of symptom clusters in cancer patients and survivors (EAST). BMC Complement Med Ther. (2023) 23:92. doi: 10.1186/s12906-023-03926-9
PubMed Abstract | Crossref Full Text | Google Scholar
12. Jiang, T, Wu, M, Zhang, Z, Yan, C, Ma, Z, He, S, et al. Electroacupuncture attenuated cerebral ischemic injury and neuroinflammation through α7nAChR-mediated inhibition of NLRP3 inflammasome in stroke rats. Mol Med. (2019) 25:22. doi: 10.1186/s10020-019-0091-4
PubMed Abstract | Crossref Full Text | Google Scholar
13. Chi, L, Du, K, Liu, D, Bo, Y, and Li, W. Electroacupuncture brain protection during ischemic stroke: a role for the parasympathetic nervous system. J Cereb Blood Flow Metab. (2017) 38:479–91. doi: 10.1177/0271678X17697988
Crossref Full Text | Google Scholar
14. Sha, R, Zhang, B, Han, X, Peng, J, Zheng, C, Zhang, F, et al. Electroacupuncture alleviates ischemic brain injury by inhibiting the miR-223/NLRP3 pathway. Med Sci Monit. (2019) 25:4723–33. doi: 10.12659/MSM.917213
PubMed Abstract | Crossref Full Text | Google Scholar
15. Chen, B, Lin, W-q, Li, Z-f, Zhong, X-y, Wang, J, You, X-f, et al. Electroacupuncture attenuates ischemic brain injury and cellular apoptosis via mitochondrial translocation of Cofilin. Chin J Integr Med. (2021) 27:705–12. doi: 10.1007/s11655-021-3335-4
PubMed Abstract | Crossref Full Text | Google Scholar
16. Lan, X, Zhang, X, Zhou, G-P, Wu, C-X, Li, C, and Xu, X-H. Electroacupuncture reduces apoptotic index and inhibits p38 mitogen-activated protein kinase signaling pathway in the hippocampus of rats with cerebral ischemia/reperfusion injury. Neural Regen Res. (2017) 12:409–16. doi: 10.4103/1673-5374.202944
PubMed Abstract | Crossref Full Text | Google Scholar
17. Shi, L, Cao, H-M, Li, Y, Xu, S-X, Zhang, Y, Zhang, Y, et al. Electroacupuncture improves neurovascular unit reconstruction by promoting collateral circulation and angiogenesis. Neural Regen Res. (2017) 12:2000–6. doi: 10.4103/1673-5374.221156
PubMed Abstract | Crossref Full Text | Google Scholar
18. Xue, N-Y, Ge, D-Y, Dong, R-J, Kim, H-H, Ren, X-J, and Tu, Y. Effect of electroacupuncture on glial fibrillary acidic protein and nerve growth factor in the hippocampus of rats with hyperlipidemia and middle cerebral artery thrombus. Neural Regen Res. (2021) 16:137–42. doi: 10.4103/1673-5374.286973
PubMed Abstract | Crossref Full Text | Google Scholar
19. Shen, S-Y, Yu, R, Li, W, Liang, L-F, Han, Q-Q, Huang, H-J, et al. The neuroprotective effects of GPR55 against hippocampal neuroinflammation and impaired adult neurogenesis in CSDS mice. Neurobiol Dis. (2022) 169:105743. doi: 10.1016/j.nbd.2022.105743
PubMed Abstract | Crossref Full Text | Google Scholar
20. Sun, L, Yong, Y, Wei, P, Wang, Y, Li, H, Zhou, Y, et al. Electroacupuncture ameliorates postoperative cognitive dysfunction and associated neuroinflammation via NLRP3 signal inhibition in aged mice. CNS Neurosci Ther. (2022) 28:390–400. doi: 10.1111/cns.13784
Crossref Full Text | Google Scholar
21. Cao, S, Yang, Y, Yu, Q, Shu, S, and Zhou, S. Electroacupuncture alleviates ischaemic brain injury by regulating the miRNA-34/Wnt/autophagy axis. Brain Res Bull. (2021) 170:155–61. doi: 10.1016/j.brainresbull.2021.02.002
PubMed Abstract | Crossref Full Text | Google Scholar
22. Kim, YR, Kim, HN, Jang, JY, Park, C, Lee, JH, Shin, HK, et al. Effects of electroacupuncture on apoptotic pathways in a rat model of focal cerebral ischemia. Int J Mol Med. (2013) 32:1303–10. doi: 10.3892/ijmm.2013.1511
PubMed Abstract | Crossref Full Text | Google Scholar
23. Ma, R, Yuan, B, Du, J, Wang, L, Ma, L, Liu, S, et al. Electroacupuncture alleviates nerve injury after cerebra ischemia in rats through inhibiting cell apoptosis and changing the balance of MMP-9/TIMP-1 expression. Neurosci Lett. (2016) 633:158–64. doi: 10.1016/j.neulet.2016.09.033
PubMed Abstract | Crossref Full Text | Google Scholar
24. Tian, R, and Wang, S. Electroacupuncture reduced apoptosis of hippocampal neurons in mice with cerebral infarction by regulating the Notch3 signaling pathway. J Mol Neurosci. (2019) 67:456–66. doi: 10.1007/s12031-018-1253-5
PubMed Abstract | Crossref Full Text | Google Scholar
25. Xing, Y, Wang, M-M, Feng, Y-S, Dong, F, and Zhang, F. Possible involvement of PTEN signaling pathway in the anti-apoptotic effect of Electroacupuncture following ischemic stroke in rats. Cell Mol Neurobiol. (2018) 38:1453–63. doi: 10.1007/s10571-018-0615-4
PubMed Abstract | Crossref Full Text | Google Scholar
26. Xing, Y, Zhang, M, Li, W-B, Dong, F, and Zhang, F. Mechanisms involved in the neuroprotection of Electroacupuncture therapy for ischemic stroke. Front Neurosci. (2018) 12:929. doi: 10.3389/fnins.2018.00929
PubMed Abstract | Crossref Full Text | Google Scholar
27. Xu, X-y, Fang, Q, Huang, W, Li, B-c, Zhou, X-h, Zhou, Z-y, et al. Effect of Electroacupuncture on neurological deficit and activity
留言 (0)