Japanese encephalitis (JE) is a mosquito-borne zoonotic disease caused by Japanese encephalitis virus (JEV) (Dong et al., 2023). JE is mainly harmful to children and adolescents, causing central nervous system lesions (Kumar, 2020). The mortality rate caused by JE is about 10% - 30%, and up to 50% of survivors are left neurological defects (Campbell et al., 2011; Tolsa-Garcia et al., 2023). According to WHO statistics (https://www.who.int/data/collections), there are about 68,000 clinical cases of JE each year, with more than 3 billion people at risk of infection. Mainland China used to be a high-epidemic area for JE, but the incidence of JE has declined rapidly since the end of the 20th century (Tong et al., 2023; Yan et al., 2024). However, in recent year, JE outbreaks have been reported in central and western regions of China including Sichuan Province (Shi et al., 2022).
Currently, the main measures to control the epidemic of JE include vaccination and mosquito control. Although vaccination is the most economical and reliable measure for JE control in China, mosquito control remains an important means to prevent and control JE due to the change of the dominant JEV genotype and incomplete vaccination coverage in China (Cao et al., 2016; Dong et al., 2023; Yan et al., 2024), especially when the outbreak of JE.
While more than 20 species of mosquitoes are possible vector of JEV in China, Culex tritaeniorhynchus is the most important one (Yan et al., 2024). The breeding areas of Cx. tritaeniorhynchus are mostly rice fields, which are heavily exposed to agricultural insecticides. Due to the continuous use of insecticides in agriculture and the short generation and high fertility of Cx. tritaeniorhynchus, field populations of this mosquito species have developed resistance to organophosphate (OP), carbamate (CB) and pyrethroid insecticides in different regions of China (Wu et al., 2016; Shi et al., 2019; Wu et al., 2021). For example, Cx. tritaeniorhynchus collected from four different provinces of China were resistant to deltamethrin, beta-cypermethrin, permethrin, dichlorvos, and propoxur (Wu et al., 2016).
Two major mechanisms of insecticide resistance have been documented in mosquitoes, i.e. metabolic resistance and target resistance (Bandibabone et al., 2021; Li et al., 2021; Wang et al., 2023). Metabolic resistance is caused by enhanced insecticide detoxification, and target resistance is resulted from reduced sensitivity of insecticidal targets including acetylcholinesterase, nerve axon sodium ion channel, and γ-aminobutyric acid receptor chloride ion channel. As the target of organochlorine and pyrethroid insecticides, the voltage-gated sodium channel (VGSC) can regulate the sodium ions of cardiomyocytes, neurons and other cells, depolarization and repolarization process, and determine the state of excitable cells (Orjuela et al., 2019). Insensitivity to dichloro-diphenyl-trichloroethane (DDT) and pyrethroids caused by sodium channel point mutations is called knockdown resistance (kdr) (Roca-Acevedo et al., 2023). The first reported kdr mutation is a change of Leu to Phe at site 1014 of the Musca domestica sodium channel gene (VGSC) (Rinkevich et al., 2012). Acetylcholinesterase (AChE), as a key enzyme in biological nerve conduction, is the target site of OP and CB insecticides (Vale and Lotti, 2015). Several mutations of AChE related to insecticide resistance have been reported in mosquitoes (reviewed in Liu, 2015), including the F455W substitution identified in Cx. tritaeniorhynchus (Nabeshima et al., 2004). Cyclopentadiene insecticides (e.g. lindane and dieldrin) and other chemicals (e.g. fipronil) act on Gamma-aminobutyric acid (GABA) receptor in insects (Chebib et al., 2001; Chen et al., 2006; Nakao, 2017). A point mutation leading to a substitution of Ala 296 to Ser/Gly (A296S/G) in the GABA receptor Rdl subuint (Rdl) was reported to be responsible for dieldrin resistance in several species of mosquitoes (Liu, 2015).
Mianyang City is located in the northwest of Sichuan Basin of China within the north subtropical mountainous humid monsoon climate zone. The climate in Mianyang is mild throughout the year and rainfall is abundant. These natural and social factors are suitable for the spread of mosquito-borne infectious diseases. As the global climate warms and population mobility increases, Mianyang City is facing an increasing threat of these mosquito-borne diseases.
Given that resistance will undermine the effectiveness of current insecticide based JE vector control interventions, it is of great significance to carry out regular monitoring of vector resistance to commonly used chemical insecticides. Unfortunately, except a recent study on genetic mutations in populations of Cx.tritaeniorhynchus in southeast of Sichuan Basin of China (Liu et al., 2023), there is no other report on the status of pesticide resistance in Cx. tritaeniorhynchu field populations in Sichuan Province of China to our knowledge. In this study, as the first effort in this regard, we investigated the possible presence of the resistance conferring mutations in three genes encoding insecticide targets in six representative Cx. tritaeniorhynchoides populations in Mianyang City of Sichuan Province.
2 Methods2.1 SamplesThe sampling sites are located in Anzhou (AZ), Beichuan (BC), Jiangyou (YJ), Pingwu (PW), Santai (ST) and Youxian (YX) of Mianyang City as shown in Figure 1. Around these sites, rice is the main crop and Cx. tritaeniorhynchus is one of the dominant mosquito species. In these rice fields, pyrethroid and organophosphate insecticides have been commonly used.
Figure 1. Sampling locations in the Mianyang City, Sichuan Province of China.
The adults of Cx. tritaeniorhynchus were captured using 1-3 mosquito light traps around pig houses or cattle sheds during 20:00 to 8:00 at each site for 1 to 4 days from August to September in 2023. The specimens were morphologically identified and individually stored in 75% or 95% ethanol at 4°C. The species identification of samples used in this study was confirmed molecularly based on the sequences of mitochondrial COI gene.
2.2 Genomic DNA extractionThe genomic DNA of individual mosquitoes was isolated according to the protocol described in Rinkevich et al. (2006) with a few modifications. Briefly, individual adults were washed with sterile water for several times. The head and thorax were placed in a 1.5 mL EP tube with 50 µL of lysis buffer (100mM Tris-Hcl, pH8.0, 10 mM EDTA, 50 mM NaCl and 1% SDS), ground using TGrinder OSE-Y30 (Tiangen, China), and then supplemented with additional 300 µL of lysis buffer. 5 µL protease K (20 mg/mL) was added into the tube, and the mixture was mixed and incubated at 60°C for 1 hour. After that, 40 µL of 8 M potassium acetate solution was added into the tube, and leaved on ice for 10 min. After the mixture was centrifuged 14000 g for 30 min, 320 µL of supernatant was taken to a new centrifuge tube. Then, 640 µL of chilled anhydrous ethanol were added into the supernatant, and the samples were kept at room temperature for 20 min after mixing, followed by centrifugation at 14000 g for 20 min. The supernatant was discarded, then pellet was resuspected using 600 µL of 70% ethanol, and centrifuged at 8000 g for 15 min. The supernatant was discarded, and pellet was air-dried and dissolved in ~30 µL of sterile water. The DNA samples were stored at 4°C or -20°C.
2.3 PCR amplification of a fragment of the VGSC, Rdl and Ace genePrimer pairs TRI5 (CTTCACCGACTTCATGCACTC, Wu et al., 2016) and Ct-kdr-R1 (TAGAAATATTGTAACCACACTGAAC, this study), Rdl-F (CAGTTTGTACGTTCGATGGGT) and Rdl-R (GGCAAATACCATGACGAAGCA), and Ct-Ace-F (GTCTAGCCGAAGCCGTCAA) and Ct-Ace-R (TTGGG ATTGCCGGTTTTGGCAAAA) were used to amplify a target-site-containing fragment of the VGSC, Rdl and Ace gene respectively. The PCR mixture (20 µL) consisted of 10 µL of 2×Es Taq MasterMix (CWBIO, Beijing, China), 0.5 µL of each primer (10 µM), and 1 µL of genomic DNA (50-200 ng). The PCR was run as: 94°C for 2 min, 35 cycles of 94°C for 30s, 56°C (for VGSC), or 58°C (for Rdl) for 30 s, 72°C for 15 s, and 72°C for 2 min. For Ace, the PCR mixture included 10 µL of 2×EZ3 Mix (Dakewe Biotech Co., Ltd, China), 0.75 µL of each primer, and 1 µL of genomic DNA (50-200 ng). The PCR procedure was as follows: 95°C for 3 min, 35 cycles (95°C for 10 s, 55°C for 15 s, 72°C for 30 s), 72°C for 5 min.
2.4 DNA sequencing and gene sequence analysisPCR products were detected by agarose gel electrophoresis and Sanger sequenced after purification by BGI Company (Beijing, China). DNA sequences obtained by Sanger sequencing were manually checked and both-end trimmed. All confirmed sequences were aligned by Muscle 3.8 (Edgar, 2004) to identify polymorphic sites. Haplotypes were identified by directly reading from homozygotes. Basic population genetic analysis was conducted using the online software Genepop 4.7.5 (https://genepop.curtin.edu.au/).
3 Results3.1 Distribution and frequency of the VGSC L1014F mutationTen nucleotide polymorphic sites were identified from the 165-bp sequences of the VGSC gene, with 2 polymorphic sites existing in the exon region and 8 polymorphic sites in the intron region. The only nonsynonymous variation (A to T) was the nucleotide 103 that led to the amino acid replacement (Leu to Phe) corresponding to the insecticide-related amino acid residue 1014 of the VGSC. Four different haplotypes (H1 to H4), including two wild types and two resistant types, were identified from homozygotes in our samples (Figure 2).
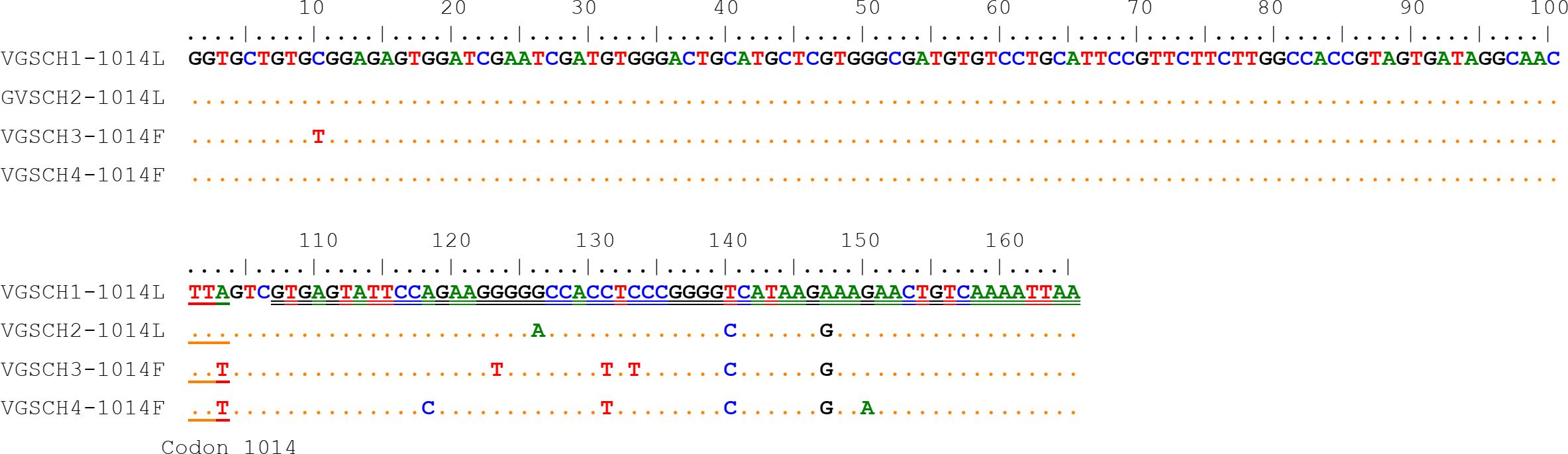
Figure 2. Nucleotide sequence alignment of the four VGSC haplotypes of the Cx. tritaeniorhynchus identified in this study. The codon 1014 and the intron region are indicated by single underline and double underline respectively.
At position 1014 of the VGSC (VGSC-1014), all three possible genotypes were detected, namely wild homozygote (TTA), resistant heterozygote (TTA/TTT), and resistant homozygote (TTT) (Table 1). The frequency of resistant homozygotes ranged from 0 (BC) to 6.25% (YX). Overall, all populations did not deviate from HWE (probability test, P>0.05), and there was no significant difference in genotype frequency among populations (exact G test, P=0.2913). The frequency of resistant allele (1014F) was in the range between 16.98% (PW) and 27.78% (ST) (Figure 3A).
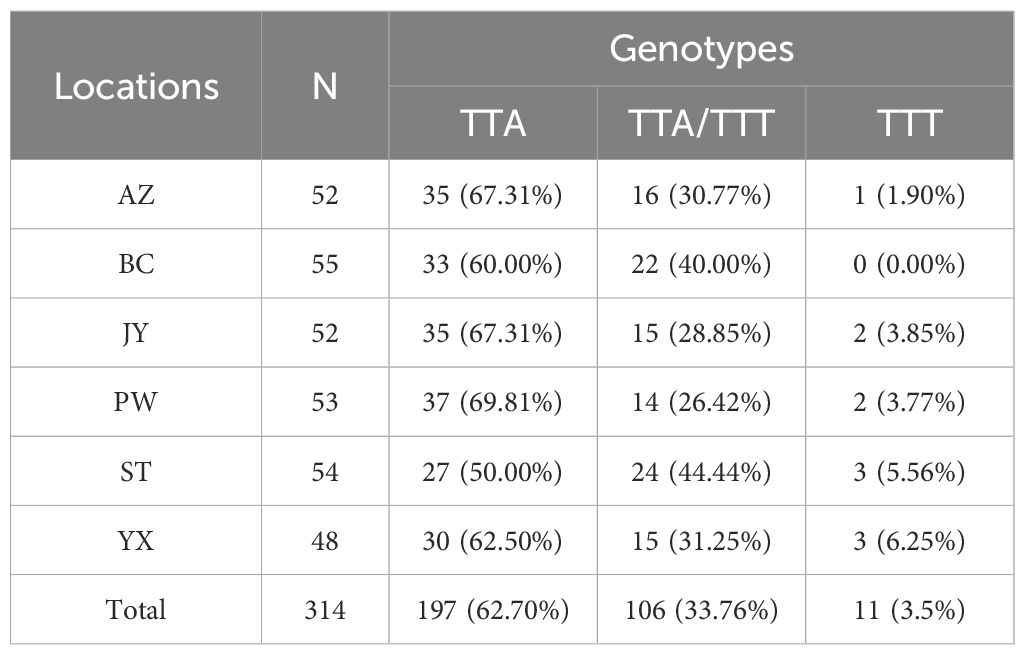
Table 1. Frequency of individual genotypes of the VGSC gene at position 1014 in six Cx. tritaeniorhynchus populations in Mianyang City, Sichuan Province of China.
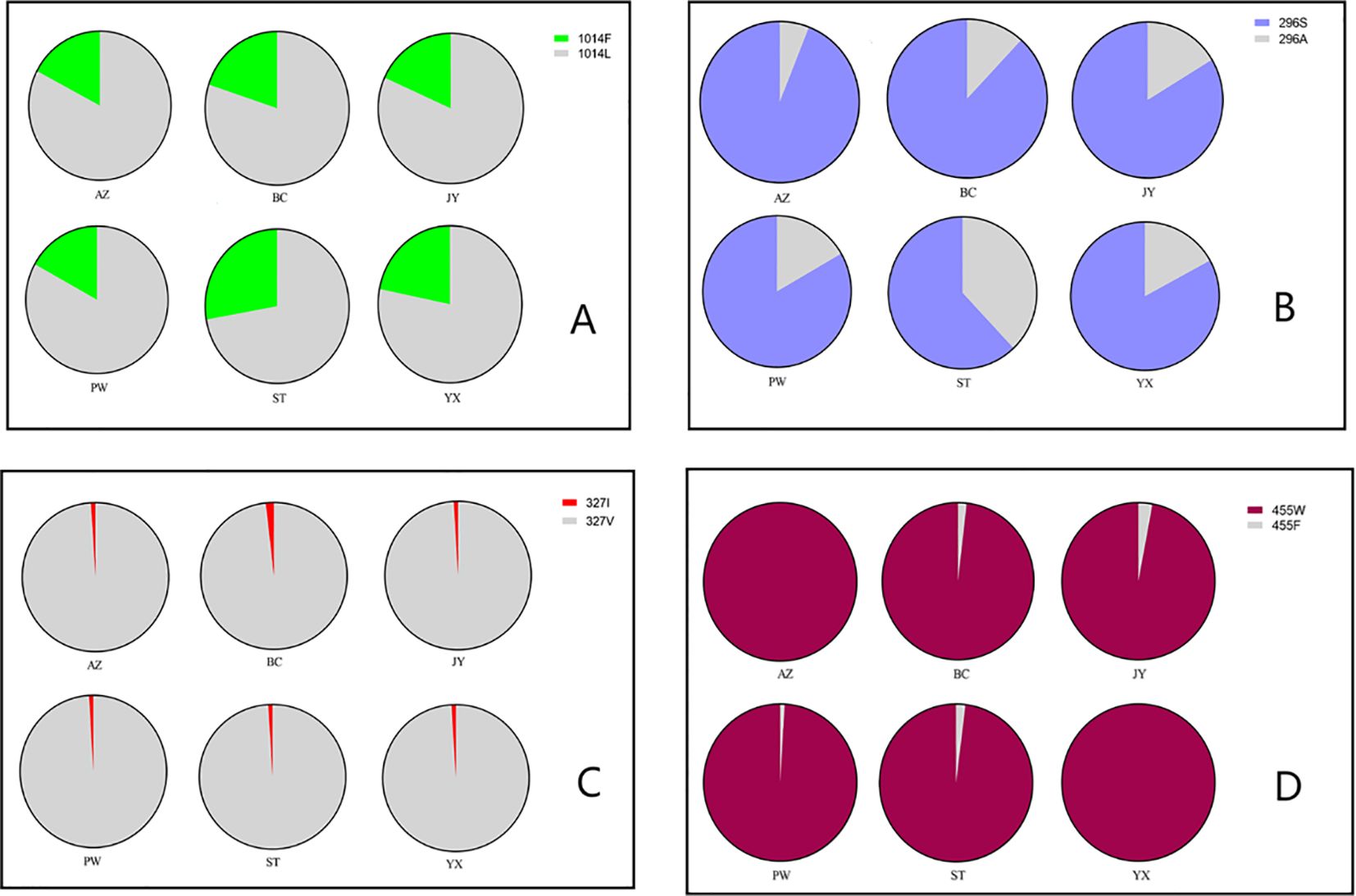
Figure 3. Distribution and frequency of alleles in six populations of the Cx. tritaeniorhynchus in Mianyang City, Sichuan Province of China. (A) VGSC-1014; (B) Rdl-296; (C) Rdl-327; (D) Ace-455.
3.2 Distribution and frequency of the Rdl A296S and V327I mutationsFrom the 182-bp sequences that cover the codons 296 and 327 of the Rdl gene, five haplotypes with five nucleotide polymorphic sites in total were identified (Figure 4). The nucleotide variation at nucleotides 75 (T to G) and 168 (G to A) resulted in amino acid changes from Ala to Ser (A296S) and from Val to Ile (V327I), respectively. Other three nucleotide differences were synonymous. Among the five phased haplotypes, one was the wild (RdlH1-AV), three were single-site (296S) mutant, and the RdlH5-SI carried two mutations (196S+327I).
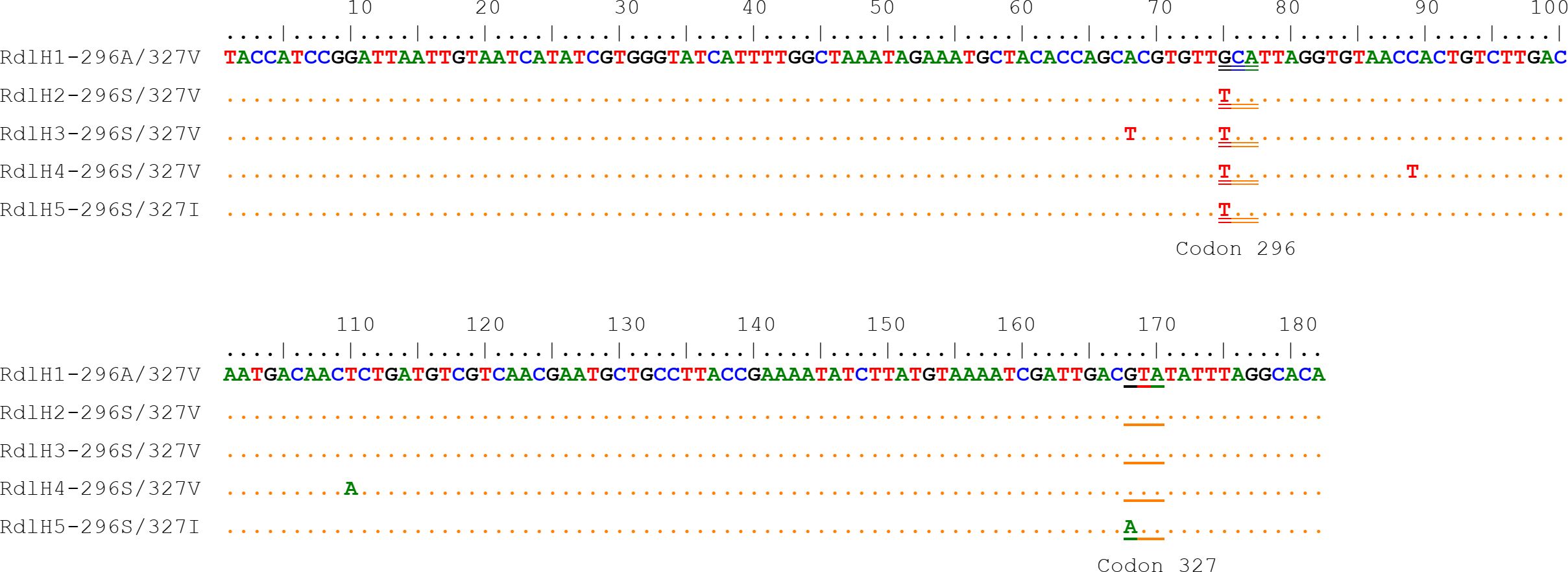
Figure 4. Nucleotide sequence alignment of the five Rdl haplotypes of the Cx. tritaeniorhynchus identified in this study. The codon 327 and codon 296 are indicated by single underline and double underline respectively.
All the possible genotypes for the resistance-related Rdl residue at position 296 (Rdl-296) were detected in our samples (Table 2). Except for YX (P=0.016), the Rdl A296S mutation in other five populations were within HWE (P>0.05). Significant differences in genotype frequencies were observed among populations (P < 1.19e-07). The frequency of resistant homozygote and that of resistance allele (296S) ranged from 44.44% (ST) to 90.00% (AZ), and from 62.04% (ST) to 94.00% (AZ) respectively (Table 2; Figure 3B). Inter-population comparison showed that the frequency of the A296S mutation in ST was significantly lower than that in the other 5 populations (P<0.01).
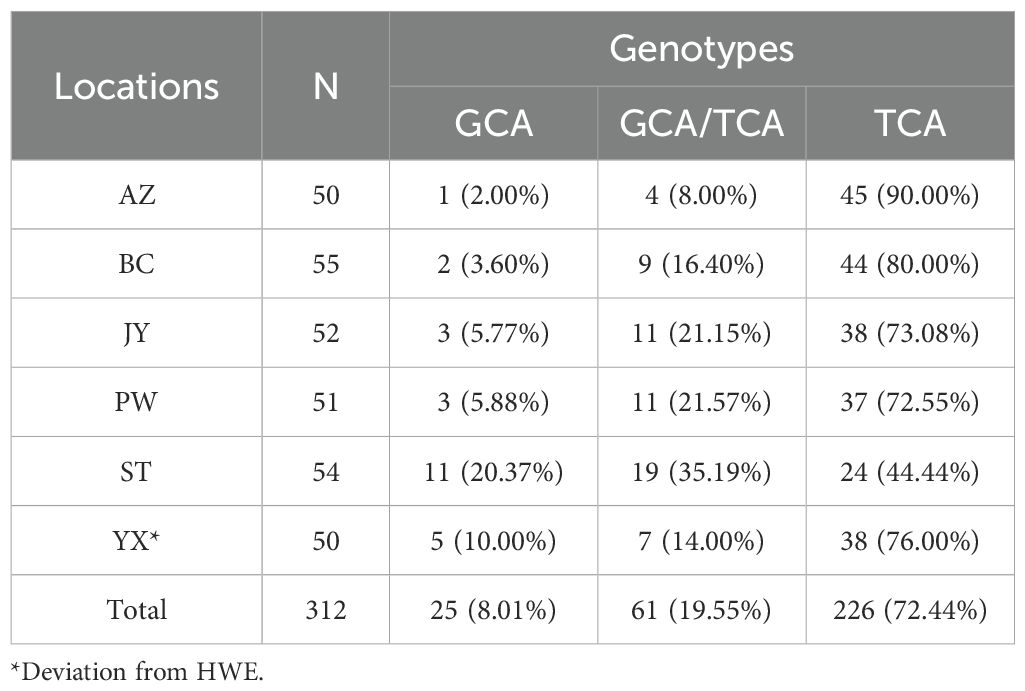
Table 2. Frequency of individual genotypes of the Rdl gene at position 296 in six Cx. tritaeniorhynchus populations in Mianyang City, Sichuan Province of China.
Focusing on the amino acid residue of Rdl at position 327 (Rdl-327), the wild homozygote (GTA) and heterozygote (GTA/ATA) were detected, while no resistant homozygote was observed in our samples (Figure 5; Table 3). Most individuals (> 95%) were wild homozygotes, and the frequency of the resistant allele (327I) was less than 2% (Figure 3C). No significant difference in the frequency of the Rdl V327I mutation was detected among populations.
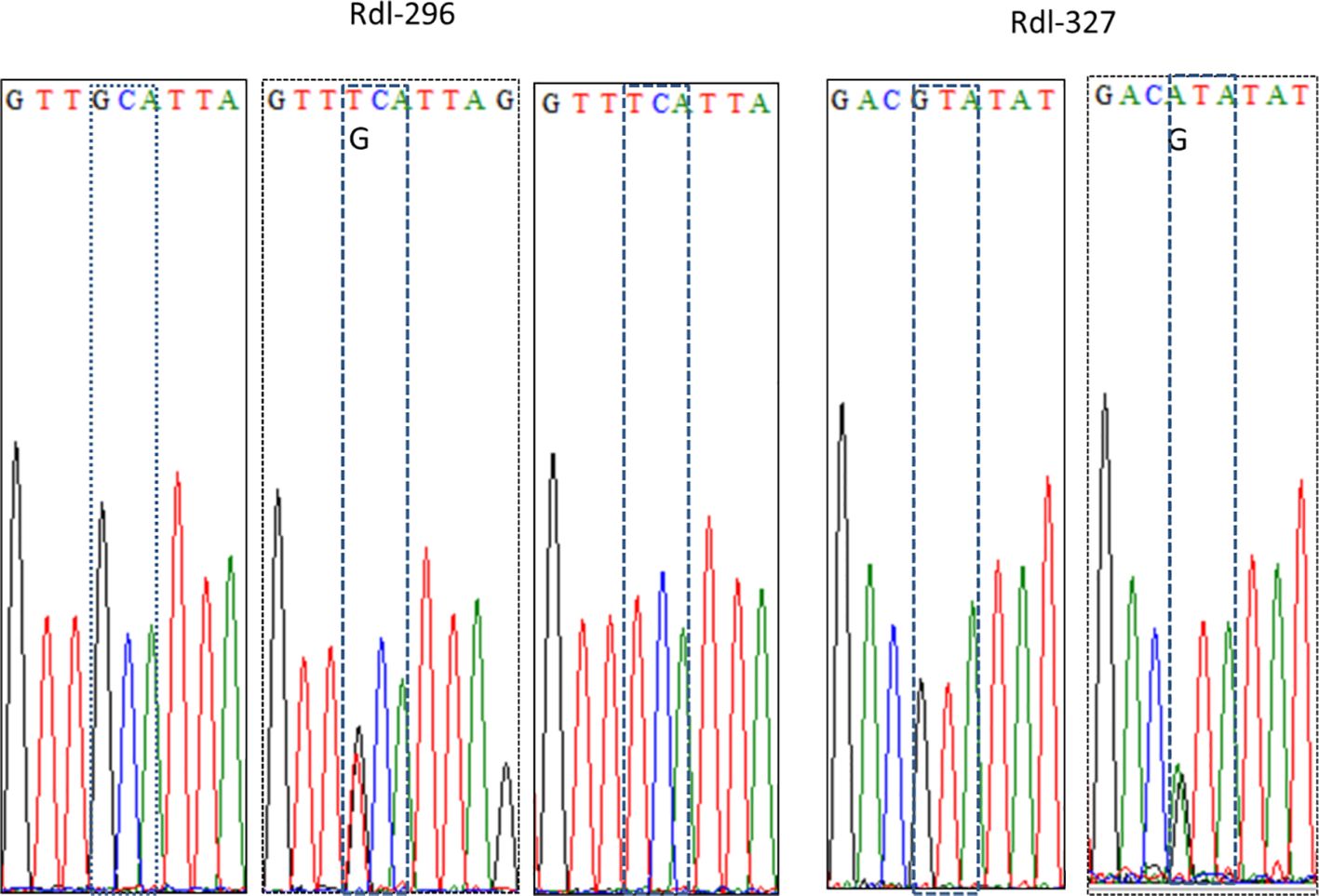
Figure 5. Representative DNA sequencing chromatogram of partial Rdl gene of the Cx. tritaeniorhynchus. The codons encoding the amino acid residues at position 296 and 327 are dash-boxed.
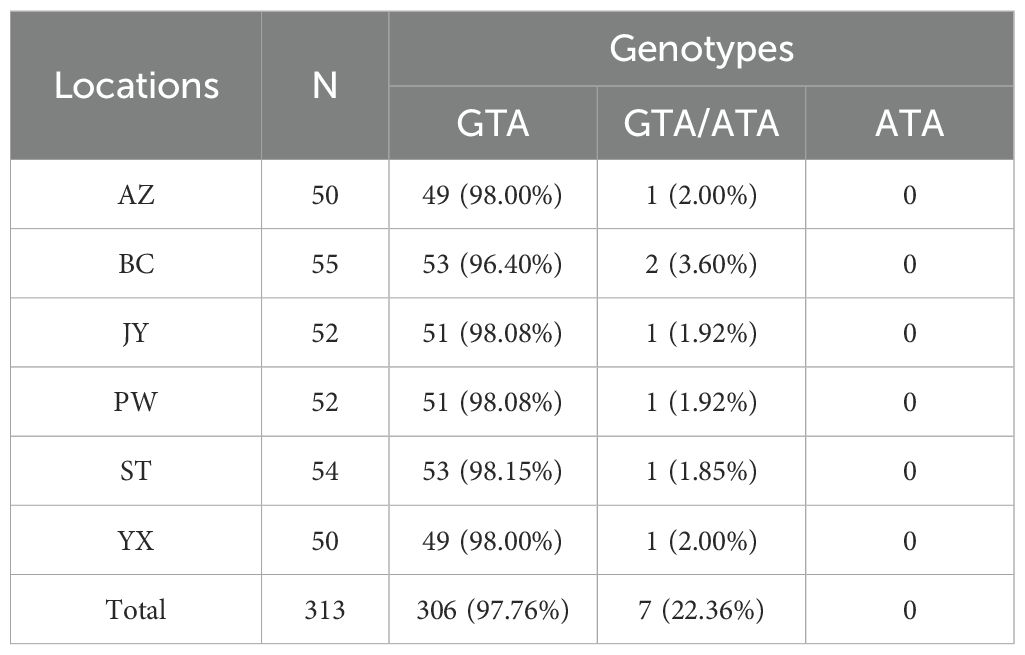
Table 3. Frequency of individual genotypes of the Rdl gene at position 327 in six Cx. tritaeniorhynchus populations in Mianyang City, Sichuan Province of China.
3.3 Distribution and frequency of the AChE F455W mutationA total of 18 nucleotide polymorphic sites were observed in the 633-bp fragments of the Ace gene (Figure 6). Except for nucleotides 1364 and 1365 that lead to an amino acid substitution, other variations were synonymous. Two haplotypes (the wild Ace-455F and the resistant Ace-455W) were identified from our samples (Figure 6).
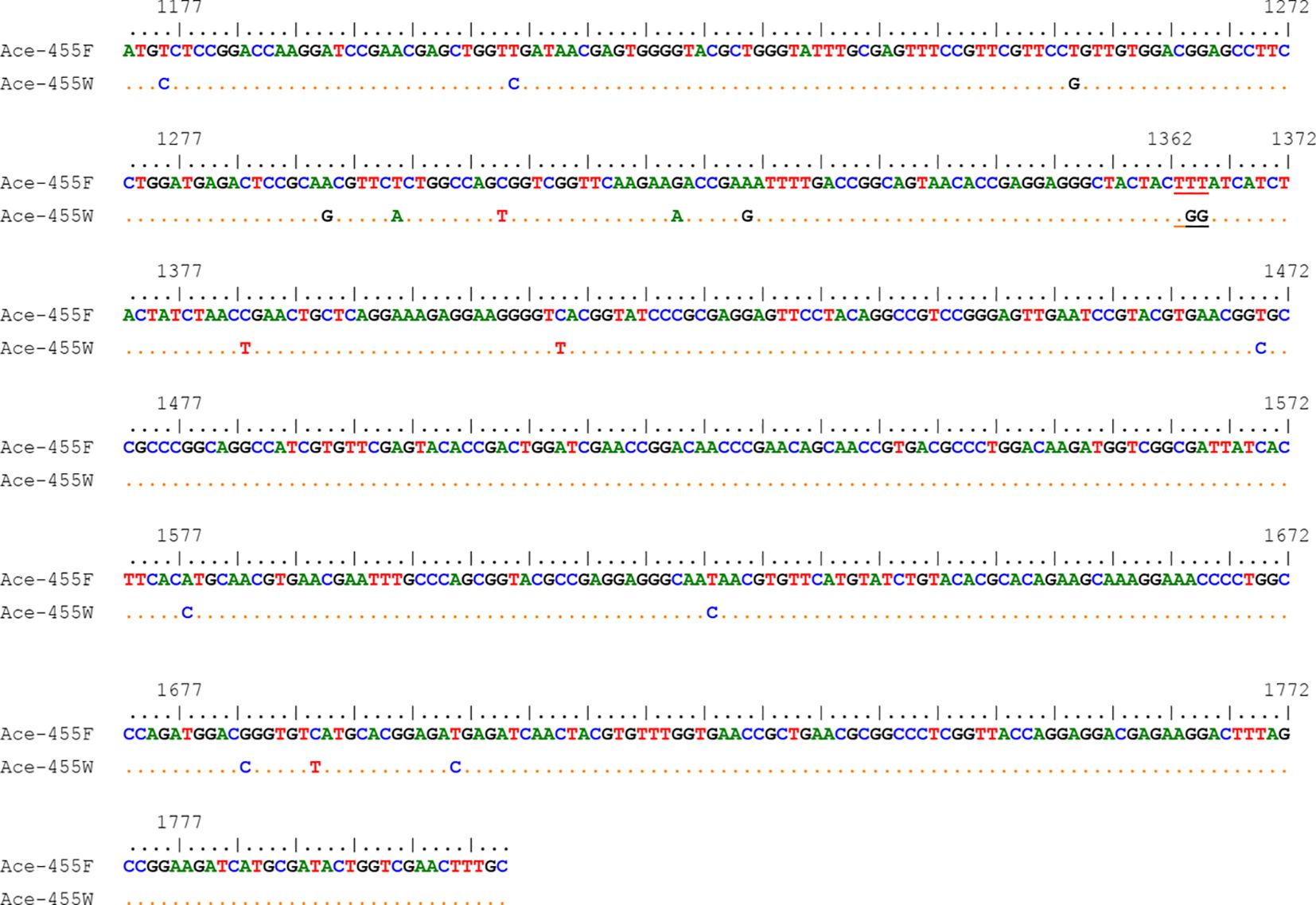
Figure 6. Nucleotide sequence alignment of the two Ace haplotypes of the Cx. tritaeniorhynchus identified in this study. The codon 455 is indicated by single underline. The numbers above the sequences are corresponding to the positions of the coding sequence (GenBank accession No. AB122152.1).
The frequency of resistant (Ace-455W) homozygote was above 96% in all populations (Table 4). The presence of wild homozygote was at a very low frequency (1.96%), and only detected in JY. Ace-455F/W heterozygotes were distributed in 4 out of the 6 populations, but its frequency was very low (< 5%) (Figure 3D). Notably, the frequency of resistance allele (Ace-455W) was more than 97%, and the 455W mutation was fixed in AZ and YX populations (Figure 3D). Overall, there was no significant difference in the frequency of the AChE F455W mutation among different populations (P= 0.381230).
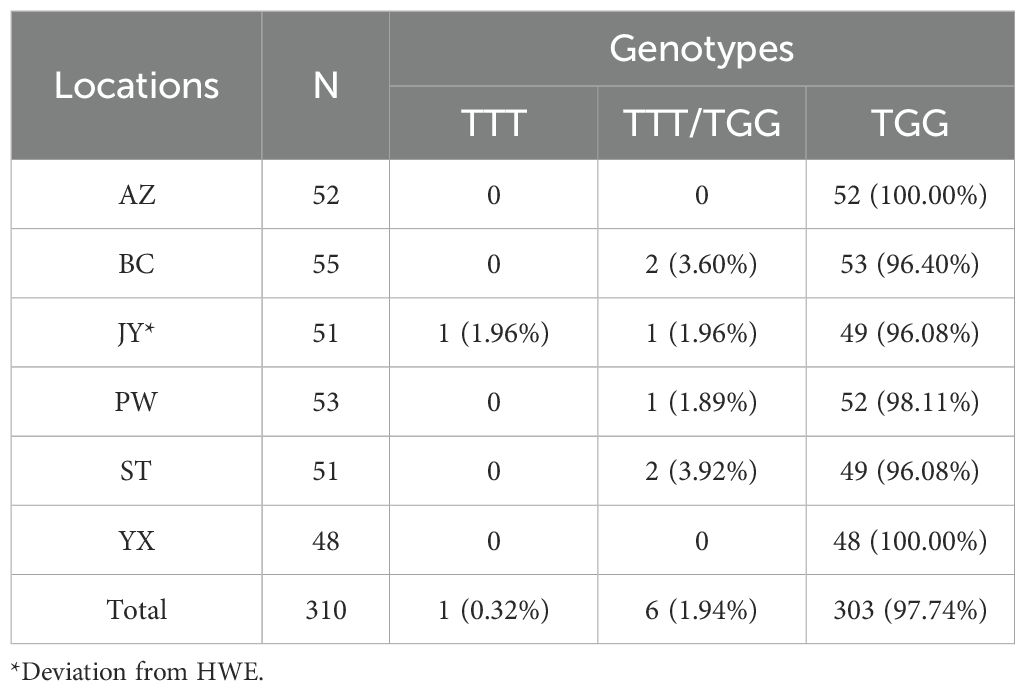
Table 4. Frequency of individual genotypes of the Ace gene at position 455 in six Cx. tritaeniorhynchus populations in Mianyang City, Sichuan Province of China.
3.4 Distribution and frequency of triple−locus genotype combinationsFrom the examined samples, 14 triple-locus genotype combinations were documented (Table 5). Type A (LL-SS-WW) and B (LF-SS-WW) were the two dominant combinations, which were distributed across Mianyang City. Notably, both Type A and Type B were resistant homozygotes for both Rdl and Ace. In addition, there were resistant individuals homozygous for the three target genes (Type N) in four sites (AZ,JY,ST,YX) despite low frequencies.
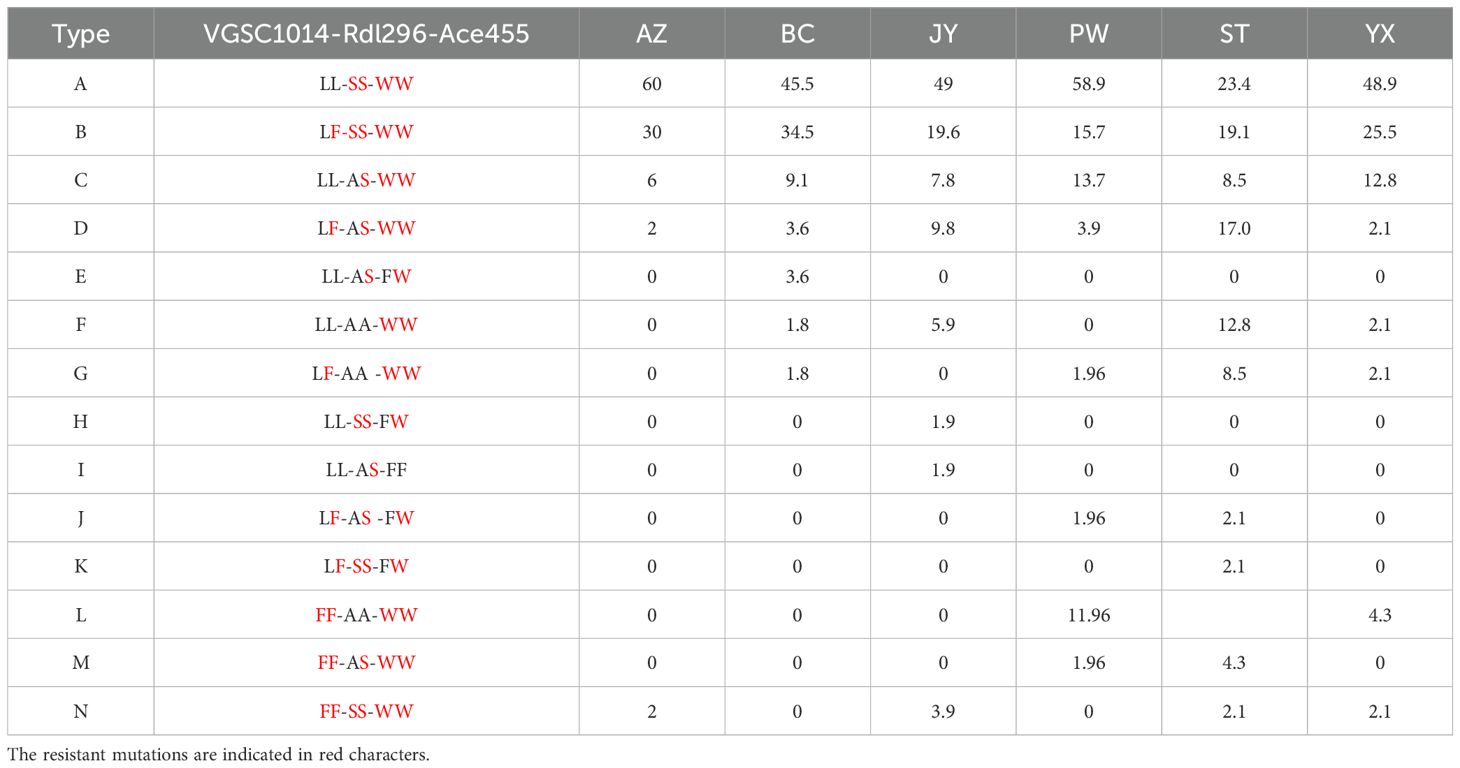
Table 5. Distribution and frequency (%) of triple-locus genotype combinations in six Cx. tritaeniorhynchus populations in Mianyang City, Sichuan Province of China.
4 DiscussionAlthough other measures help, chemical insecticides have long been used to prevent and control mosquito biting and mosquito-borne diseases. Consequently, many mosquito populations have developed resistance to commonly used insecticides (Pai et al., 2023; Tungu et al., 2023). Considering the facts that Cx. tritaeniorhynchus is the dominant mosquito species, and little is known about the status and involved mechanism of insecticide resistance in Cx. tritaeniorhynchus populations in Sichuan Province including Mianyang City, we investigated the occurrence of insecticide resistance-related genetic mutations.
Previous studies have well demonstrated that the L1014F mutation in VGSC is commonly present in several species of mosquitoes including Cx. tritaeniorhynchus (Liu, 2015; Wu et al., 2016; Liu et al., 2023). As expected, this mutation was also detected in Cx. tritaeniorhynchus samples collected from Mianyang City. The frequency of resistant allele (1014F) ranged from 16.98% (PW) to 27.78% (ST), which is similar to that reported in Cx. tritaeniorhynchus samples in Neijiang City of Sichuan Province (Liu et al., 2023), and in 12 sites in other four provinces of China (Wu et al., 2016).
The point mutation resulting in a substitution of Ala 296 to Ser (A296S) in the channel-lining region within the Rdl molecule has been documented for dieldrin resistance in several species of mosquitoes (Liu, 2015). In Sichuan, the A296S mutation is present at high frequencies in many populations of Anopheles sinensis (Qian et al., 2021). In this study, we found that the conserved A296S mutation was distributed in Cx. tritaeniorhynchus populations in Mianyang. To our knowledge, this is the first record of Rdl A296S mutation in this mosquito species. Notably, The A296S mutation was present at high frequencies (62.04% to 94.00%). In addition to the V327I mutation which has been reported to coexist with A296S in dieldrin-resistant An. funestus (Wondji et al., 2011) and in An. sinensis (Qian et al., 2021), a low frequency (0.93% to 1.80%) of the same V327I mutation was detected for the first time in Cx. tritaeniorhynchus in this study. Interestingly, the V327I was found only in individuals that harbored the A296S mutation. Until now, the impact of the V327I mutation on Rdl sensitivity remains unknown and is worthy of further investigation.
The Gly-119-Ser (G119S) substitution in AChE is commonly detected in mosquitoes (Liu, 2015), but not reported in Cx. tritaeniorhynchus. Instead, the F455W mutation leading to insensitivity of AChE to OP and CB has been identified in Cx. tritaeniorhynchus from Japan (Nabeshima et al., 2004), India (Misra and Gore, 2015), and China (Wu et al., 2016; Liu et al., 2023). The distribution scenario of the AChE F455W mutation observed in this study further supports the notion that the resistance Ace-455W allele is widely distributed at a very high frequency in China (Wu et al., 2016; Liu et al., 2023).
5 ConclusionsThe results revealed the prevalent existence of resistance-conferring mutations in multiple insecticide target proteins of Cx. tritaeniorhynchus in Mianyang City. The co-occurrence of Ace- F455W and Rdl-296S at high levels warns a risk of failure in the control of Cx. tritaeniorhynchus using chemical insecticides targeting the AChE and Rdl in this region. It is highly recommended to monitor phenotypic resistance in Cx. tritaeniorhynchus on a regular basis.
Data availability statementThe original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Ethics statementThe manuscript presents research on animals that do not require ethical approval for their study.
Author contributionsHX: Investigation, Writing – original draft, Writing – review & editing. MT: Methodology, Writing – original draft, Writing – review & editing. HS: Investigation, Writing – original draft. ZH: Investigation, Writing – original draft. MD: Investigation, Writing – review & editing. XW: Investigation, Writing – original draft, Writing – review & editing.
FundingThe author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by financial assistance for vector control in Mianyang City. The authors would like to express our special thanks to Professor Xinghui Qiu (Institute of Zoology, Chinese Academy of Sciences) for his great guidance and support.
Conflict of interestThe authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s noteAll claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
ReferencesBandibabone, J., McLoughlin, C., N'Do, S., Bantuzeko, C., Byabushi, V., Jeanberckmans, M., et al. (2021). Investigating molecular mechanisms of insecticide resistance in the Eastern Democratic Republic of the Congo. Malar J. 20, 464. doi: 10.1186/s12936-021-04002-8
PubMed Abstract | Crossref Full Text | Google Scholar
Campbell, G. L., Hills, S. L., Fischer, M., Jacobson, J. A., Hoke, C. H., Hombach, J. M., et al. (2011). Estimated global incidence of Japanese encephalitis: a systematic review. Bull. World Health Organ 1; 89, 766–74, 774A-774E. doi: 10.2471/BLT.10.085233
PubMed Abstract | Crossref Full Text | Google Scholar
Cao, L., Fu, S., Gao, X., Li, M., Cui, S., Li, X., et al. (2016). Low protective efficacy of the current Japanese encephalitis vaccine against the emerging genotype 5 Japanese encephalitis virus. PloS Negl. Trop. Dis. 10, e0004686. doi: 10.1371/journal.pntd.0004686
PubMed Abstract | Crossref Full Text | Google Scholar
Chebib, M., Duke, R. K., Allan, R. D., Johnston, G. A. (2001). The effects of cyclopentane and cyclopentene analogues of GABA at recombinant GABA(C) receptors. Eur. J. Pharmacol. 430, 185–192. doi: 10.1016/S0014-2999(01)01390-5
PubMed Abstract | Crossref Full Text | Google Scholar
Chen, L., Durkin, K. A., Casida, J. E. (2006). Structural model for gamma-aminobutyric acid receptor noncompetitive antagonist binding: widely diverse structures fit the same site. Proc. Natl. Acad. Sci. U S A. 103, 5185–5190. doi: 10.1073/pnas.0600370103
PubMed Abstract | Crossref Full Text | Google Scholar
Dong, N., Zhang, X., Zhang, H., Zheng, J., Qiu, Y., Li, Z., et al. (2023). Genotype Change in circulating JEV strains in Fujian Province, China. Viruses. 15, 1822. doi: 10.3390/v15091822
PubMed Abstract | Crossref Full Text | Google Scholar
Li, Y., Zhou, G., Zhong, D., Wang, X., Hemming-Schroeder, E., David, R. E., et al. (2021). Widespread multiple insecticide resistance in the major dengue vector Aedes albopictus in Hainan Province, China. Pest Manag Sci. 77, 1945–1953. doi: 10.1002/ps.v77.4
PubMed Abstract | Crossref Full Text | Google Scholar
Liu, J., Wang, Y., Liu, P., Yu, X., Tan, A., Zeng, J., et al. (2023). Detection of target site mutations in the acetylcholinesterase and voltage-gated sodium channel in field populations of Culex quinquefasciatus and Culex tritaeniorhynchus from southern Sichuan region of China. J. Am. Mosq. Control Assoc. 39, 57–60. doi: 10.2987/22-7093
PubMed Abstract | Crossref Full Text | Google Scholar
Misra, B. R., Gore, M. (2015). Malathion resistance status and mutations in acetylcholinesterase gene (Ace) in Japanese encephalitis and filariasis vectors from endemic area in India. J. Med. Entomol. 52, 442–446. doi: 10.1093/jme/tjv015
PubMed Abstract | Crossref Full Text | Google Scholar
Nabeshima, T., Mori, A., Koazki, T., Iwata, Y., Hidoh, O., Harada, S., et al. (2004). An amino acid substitution attributable to insecticide-insensitivity of acetylcholinesterase in Japanese encephalitis vector mosquito Culex tritaeniorhynchus. Biochem. Biophys. Res. Commun. 313, 794–801. doi: 10.1016/j.bbrc.2003.11.141
PubMed Abstract | Crossref Full Text | Google Scholar
Orjuela, L. I., Alvarez-Diaz, D. A., Morales, J. A., Grisales, N., Ahumada, M. L., Venegas, H. J., et al. (2019). Absence of knockdown mutations in pyrethroid and DDT resistant populations of the main malaria vectors in Colombia. Malar J. 18, 384. doi: 10.1186/s12936-019-3034-1
PubMed Abstract | Crossref Full Text | Google Scholar
Pai, H. H., Chang, C. Y., Lin, K. C., Hsu, E. L. (2023). Rapid insecticide resistance bioassays for three major urban insects in Taiwan. Parasit Vectors. 16, 447. doi: 10.1186/s13071-023-06055-x
PubMed Abstract | Crossref Full Text | Google Scholar
Qian, W., Liu, N., Yang, Y., Liu, J., He, J., Chen, Z., et al. (2021). A survey of insecticide resistance-conferring mutations in multiple targets in Anopheles sinensis populations across Sichuan, China. Parasit Vectors 14, 169. doi: 10.1186/s13071-021-04662-0
PubMed Abstract | Crossref Full Text | Google Scholar
Rinkevich, F. D., Hedtke, S. M., Leichter, C. A., Harris, S. A., Su, C., Brady, S. G., et al. (2012). Multiple origins of kdr-type resistance in the house fly, Musca domestica. PloS One 7, e52761. doi: 10.1371/journal.pone.0052761
PubMed Abstract | Crossref Full Text | Google Scholar
Rinkevich, F. D., Zhang, L., Hamm, R. L., Brady, S. G., Lazzaro, B. P., Scott, J. G. (2006). Frequencies of the pyrethroid resistance alleles of Vssc1 and CYP6D1 in house flies from the eastern United States. Insect Mol. Biol. 15, 157–167. doi: 10.1111/j.1365-2583.2006.00620.x
PubMed Abstract | Crossref Full Text | Google Scholar
Shi, T., Meng, L., Li, D., Jin, N., Zhao, K., Zhang, X., et al. (2022). Effect of different vaccine strategies for the control of Japanese encephalitis in Chinese mainland from 1961 to 2020: A quantitative analysis. Vaccine 40, 6243–6254. doi: 10.1016/j.vaccine.2022.09.030
PubMed Abstract | Crossref Full Text | Google Scholar
Shi, Q., Song, X., Lv, Y., Huang, X., Kou, J., Wang, H. W., et al. (2019). Potential risks associated with Japanese Encephalitis prevalence in Shandong Province, China. Vector Borne Zoonotic Dis. 19, 640–645. doi: 10.1089/vbz.2018.2416
PubMed Abstract | Crossref Full Text | Google Scholar
Tolsa-Garcia, M. J., Wehmeyer, M. L., Luhken, R., Roiz, D. (2023). Worldwide transmission and infection risk of mosquito vectors of West Nile, St. Louis encephalitis, Usutu and Japanese encephalitis viruses: a systematic review. Sci. Rep. 13, 308. doi: 10.1038/s41598-022-27236-1
PubMed Abstract | Crossref Full Text | Google Scholar
Tong, Y., Jiang, H., Xu, N., Wang, Z., Xiong, Y., Yin, J., et al. (2023). Global Distribution of Culex tritaeniorhynchus and impact factors. Int. J. Environ. Res. Public Health 20, 4701. doi: 10.3390/ijerph20064701
PubMed Abstract | Crossref Full Text | Google Scholar
Tungu, P., Kabula, B., Nkya, T., Machafuko, P., Sambu, E., Batengana, B., et al. (2023). Trends of insecticide resistance monitoring in mainland Tanzania 2004-2020. Malar J. 22, 100. doi: 10.1186/s12936-023-04508-3
PubMed Abstract | Crossref Full Text | Google Scholar
Wang, Y., Wang, X., Brown, D. J., An, M., Xue, R. D., Liu, N. (2023). Insecticide resistance: Status and potential mechanisms in Aedes aEgypti. Pestic Biochem. Physiol. 195, 105577. doi: 10.1016/j.pestbp.2023.105577
PubMed Abstract | Crossref Full Text | Google Scholar
Wondji, C. S., Dabire, R. K., Tukur, Z., Irving, H., Djouaka, R., Morgan, J. C. (2011). Identifcation and distribution of a GABA receptor mutation conferring dieldrin resistance in the malaria vector Anopheles funestus in Africa. Insect Biochem. Mol. Biol. 41, 484–491. doi: 10.1016/j.ibmb.2011.03.012
PubMed Abstract | Crossref Full Text | Google Scholar
Wu, Z., Chu, H., Wang, G., Zhu, X., Guo, X., Zhang, Y., et al. (2016). Multiple-insecticide resistance and classic gene mutations to Japanese encephalitis vector culex tritaeniorhynchus from China. J. Am. Mosq. Control Assoc. 32, 144–151. doi: 10.2987/moco-32-02-144-151.1
PubMed Abstract | Crossref Full Text | Google Scholar
Wu, Z., Yin, Q., Tian, Y., Chu, H. (2021). Resistnace of Culex tritaeniorhynchus to pyrethroid in southeast coastal areas of China. Acta Parasitol.Med. Entomol.Sin. 28, 218–223.
Yan, W., Li, J., Guo, Y., Yue, Y., Liu, X., Song, X., et al. (2024). Research progress on the epidemic characteristics and virus genotypes of Japanese encephalitis in China. Chin. J. Vector Biol. Control 35, 1–6.
Yang, C., Huang, Z., Li, M., Feng, X., Qiu, X. (2017). RDL mutations predict multiple insecticide resistan
留言 (0)