Lichenoid amyloidosis (LA), a subtype of primary cutaneous amyloidosis (PCA), is characterized by intensely pruritic, hyperkeratotic papules typically distributed on the extensor surfaces, i.e., calves, back, forearm, and thigh (1, 2). Traditional treatments, including topical corticosteroids, tarcolimus, vitamin D3 analogues, oral anti-histamines, retinoides, cyclosporine, phototherapy, do not work well (3). Therefore, new therapeutic options are urgently needed.
Dupilumab, a human monoclonal antibody targeting for interleukin (IL)-4/13 receptor α chain, is widely applied in type 2 inflammation diseases treatment, i.e., atopic dermatitis (AD), nodular prurigo, and asthma (4, 5). Herein, we reported two cases of LA treated with dupilumab due to poor response to conventional treatments and achieved satisfactory response.
Case presentation Case 1A 66-year-old man presented to dermatology clinic with a 30-year history of generalized rashes with severe itching [Pruritus Numeric Scale Score (NRS): 10; Investigator Global Assessment (IGA): 4; Dermatology Life Quality Index (DLQI): 16, Modified Eczema area and severity index (m-EASI) (6): 30.3]. Physical examination revealed red papules, patches, and plaques symmetrically distributed on the trunk and limbs (Figure 1A). He was otherwise healthy and denied family history of similar symptoms. Laboratory examinations were unremarkable, except slightly elevated IgE levels (Serum IgE: 97 IU/mL, reference range < 87 IU/mL). A skin biopsy taken from the leg showed hyperkeratosis, dyskeratosis, acanthosis, and eosinophilic material deposition on the dermal papilla, which was positive for Congo red and CK5/6 staining (Figure 1A). Accordingly, he was diagnosed with LA. After the failure of phototherapy, topical corticosteroids, and oral anti-histamines, the patient was started with dupilumab on April 21th, 2024 at a dosage of 600 mg for first injection and 300 mg every 2 weeks thereafter. The pruritus was relieved after 1 week and the skin lesions began to improve at 2 weeks and significant subsided at 10 weeks (Figure 1B). During the treatment, all scores continued to decline (NRS:0; IGA:2; DLQI: 1; m-EASI: 5.6 at 10 weeks) (Figure 1C), without adverse reactions.
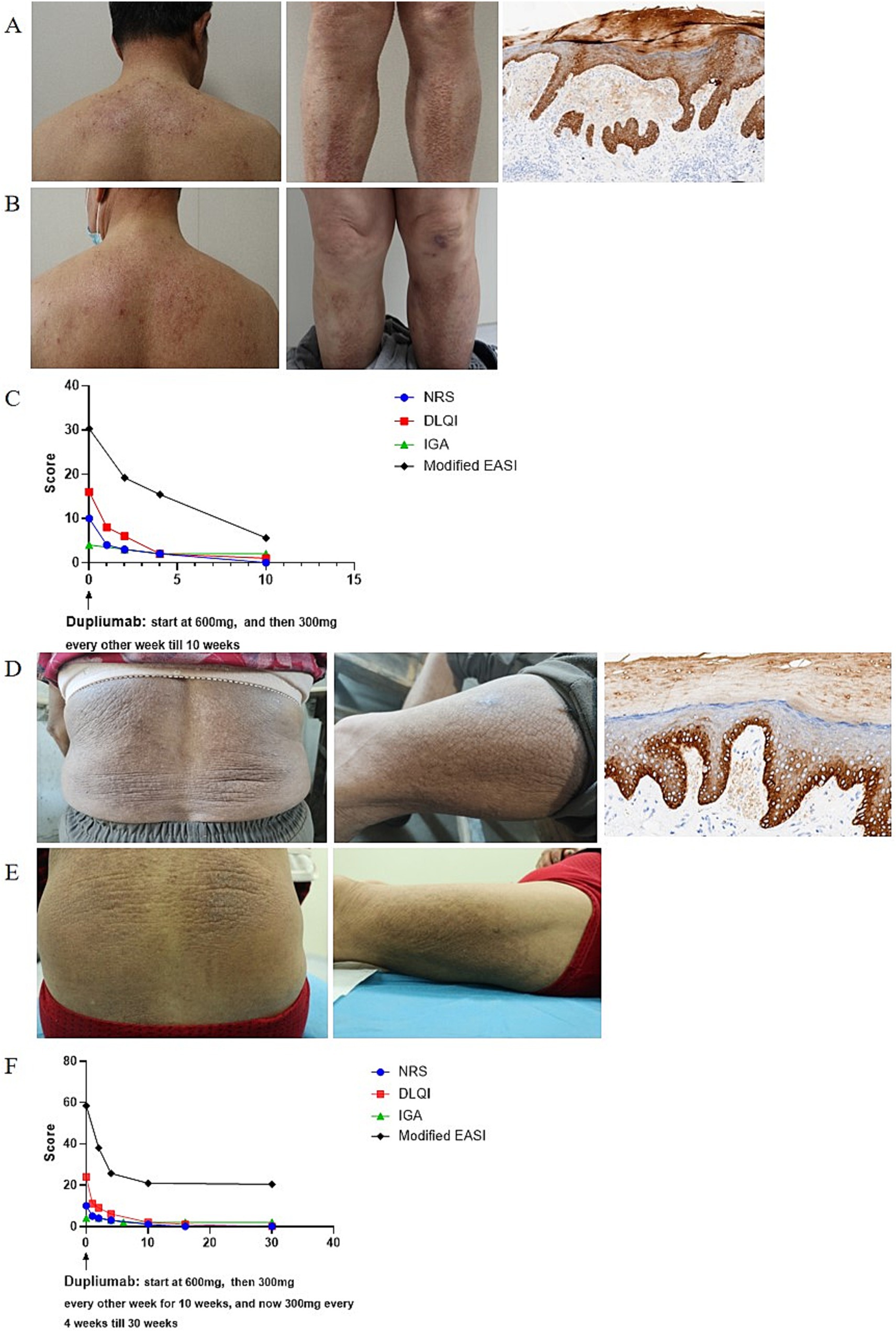
Figure 1. (A,D) Clinical and histopathological photographs at initial presentation. (B,E) Clinical photographs at last visit in July 2024. (C,F) Dupilumab treatment significantly alleviated skin lesions and pruritus. m-EASI is identical to EASI but included an additional assessment of pruritus (6).
Case 2A 69-year-old women presented to dermatology clinic with a 3-year history of generalized rashes with severe itching (NRS: 10; IGA: 4; DLQI: 24; m-EASI: 58.4). Physical examination revealed red papules, patches, and plaques distributed on the trunk and limbs (Figure 1D). Her personal and family history were unremarkable. Laboratory examinations were all within normal range. A skin biopsy taken from the leg showed hyperkeratosis, dyskeratosis, acanthosis, and eosinophilic material deposition on the dermal papilla; eosinophilic deposition immunohistochemistry was positive for CK5/6 (Figure 1D), supporting a diagnosis of LA. After the failure of phototherapy, topical corticosteroids, and oral anti-histamines, a course of dupilumab therapy was initiated on December 27th, 2023, which started with 600 mg as a loading dose followed by 300 mg biweekly injections for 10 weeks and then 300 mg monthly injections thereafter. The pruritus was relieved after 1 week and the skin lesions improved after 2 weeks (Figure 1E). During the treatment, all scores continued to decline (NRS: 0; IGA: 2; DLQI:0; m-EASI: 20.4 at 30 weeks) (Figure 1F), without adverse reactions.
DiscussionPCA is a frequently encountered skin disease characterized by extracellular deposition of heterogenic amyloid protein in previous normal skin in the absence of visceral organs involvement (1). Traditionally, it can be divided into macular amyloidosis, LA, and nodular amyloidosis (7). LA is the most common form of PCA, typically presenting as multiple localized or rarely generalized, hyper-pigmented grouped papules with a predilection for the calves, shins, ankles, and thighs. These lesions not only bring cosmetic concerns to patients, but also negatively impair patients’ quality of life since they are mostly associated with severe pruritus (1, 8). PCA lesions are currently considered difficult to treat, since no consistently effective therapy has been reported despite many therapeutic modalities have been tried in PCA treatment (3).
Dupilumab, a fully human anti-IL-4 receptor-α monoclonal antibody, blocks IL-4 and IL-13 signaling to downregulate itching-associated cytokines, chemokines, and IgE levels, which has been applied in allergic diseases treatment, i.e., AD and asthma treatment (4, 9, 10). Studies have shown that serum and cutaneous levels of type 2 cytokines (IL-4, IL-13, IL-31) and their receptors were elevated in patients with PCA, and their expression were decreased when symptoms were alleviated, indicating that type 2 inflammation may involve in LA pathogenesis (11, 12). Therefore, we speculated that dupilumab might be an alternative treatment for LA.
In this article, we successfully treated 2 refractory LA patients with dupilumab and achieved satisfactory response. The itching was relieved within 1 week after dupilumab treatment and rashes improved since 2 weeks. During the treatment, no adverse events were reported. Due to economic reasons, our patient 2 received monthly injection of dupilumab since 10 weeks after treatment onset. Though rashes showed no sustained clinical improvement since then, pruritus score and quality-of-life score maintained improvement, making the patients satisfied with therapy. We also searched the published literature reporting dupilumab treatment for PCA and summarized them in Table 1.
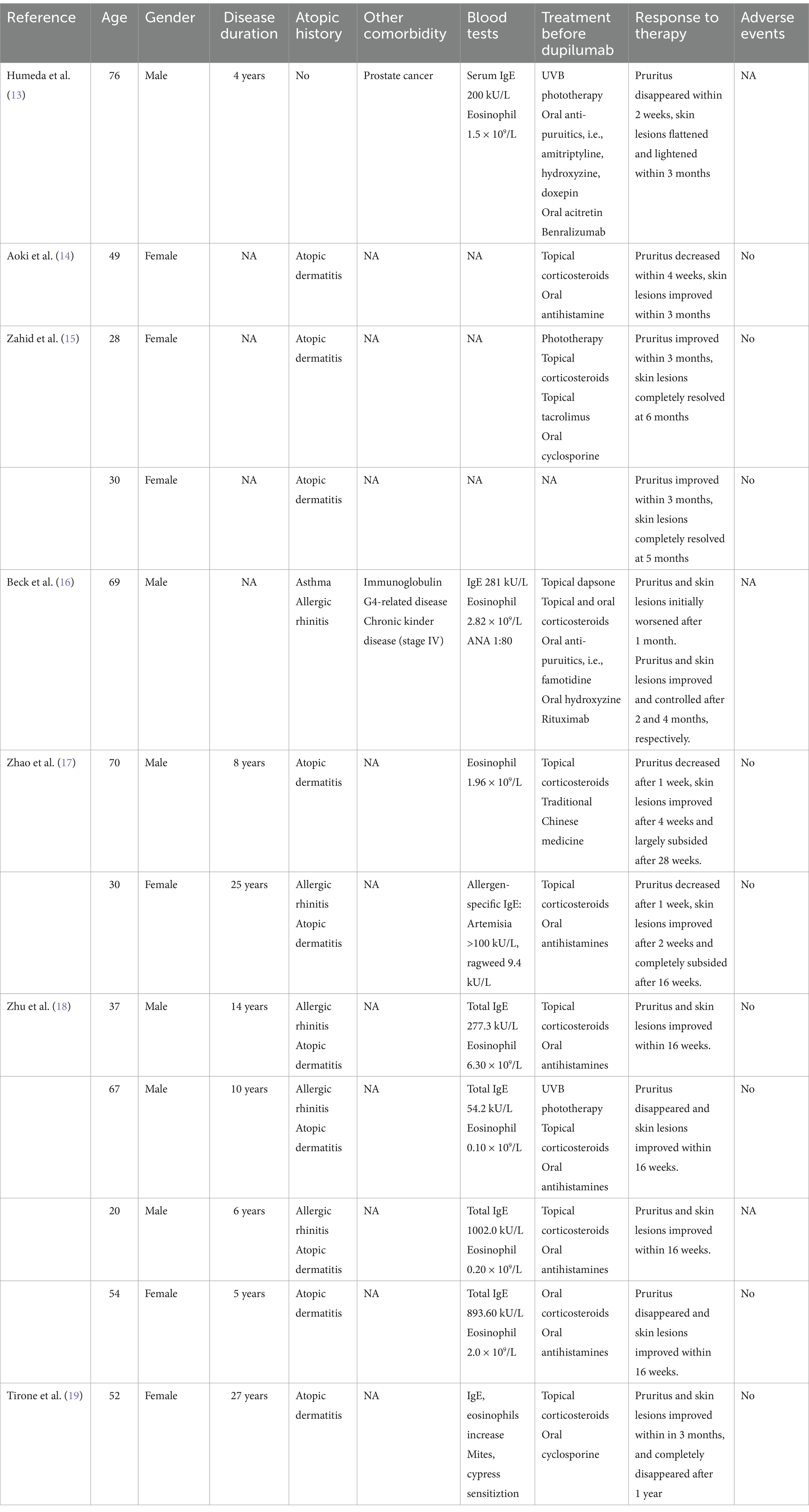
Table 1. Publications of patients with primary cutaneous amyloidosis treated with dupilumab.
As of October 2024, 14 patients with PCA (including our 2 patients) tried dupilumab treatment, with female to male ratio of 7:7. These patients aged 20–76 years old, and their medical history of PCA ranged from 3–27 years. All of them resisted to traditional therapy for PCA, and achieved disease relief on dupilumab treatment. Itching usually alleviated firstly, with a reported remission time of 1–12 weeks after treatment. Skin lesions improved later, which began and largely resolved after 4 weeks and 28 weeks, respectively. 4 patient patients got complete skin lesions remission, and most patients achieved significant alleviation and improvement. Notably, unlike most reported PCA patients using dupilumab, our patients denied personal and family history of atopic diseases, indicating that dupilumab may be an appropriate off-label indication for PCA treatment. Dupilumab was also successfully tried in 1 PCA patient with prostate cancer, suggesting that tumor is not a contraindication for dupilumab. However, due to the limited experience, we propose that more observation and trial of PCA treated with dupilumab are needed to confirm its efficacy and safety.
In summary, we speculate that dupilumab may be a promising therapy for PCA, with excellent effectiveness and safety. However, further studies are necessary to clarify the complex pathogenesis of PCA and the role of dupilumab in its treatment.
ConclusionDupilumab may be a promising therapy for PCA, which alleviate pruritus and rashes without apparent adverse effects.
Data availability statementThe raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statementWritten informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributionsFG: Writing – original draft, Data curation, Supervision. HZ: Writing – original draft, Supervision. YW: Conceptualization, Writing – original draft. XX: Investigation, Writing – original draft. JT: Methodology, Writing – original draft. QZ: Writing – review & editing, Visualization. ST: Writing – review & editing, Visualization.
FundingThe author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This work was supported by the Huzhou City Medical Innovation Discipline (CXXK-HT-202302A) and Huzhou Science and Tech-nology Bureau Public Welfare Application Research Project (2024GY10).
Conflict of interestThe authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statementThe author(s) declare that no Gen AI was used in the creation of this manuscript.
Publisher’s noteAll claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References1. Hamie, L, Haddad, I, Nasser, N, Kurban, M, and Abbas, O. Primary localized cutaneous amyloidosis of keratinocyte origin: an update with emphasis on atypical clinical variants. Am J Clin Dermatol. (2021) 22:667–80. doi: 10.1007/s40257-021-00620-9
PubMed Abstract | Crossref Full Text | Google Scholar
2. Sinha, A, Manjunath, GV, and Basavaraj, V. Primary cutaneous amyloidosis: a clinicopathological, histochemical, and immunohistochemical study. Indian J Pathol Microbiol. (2021) 64:323–8. doi: 10.4103/IJPM.IJPM_32_20
PubMed Abstract | Crossref Full Text | Google Scholar
3. Weidner, T, Illing, T, and Elsner, P. Primary localized cutaneous amyloidosis: a systematic treatment review. Am J Clin Dermatol. (2017) 18:629–42. doi: 10.1007/s40257-017-0278-9
PubMed Abstract | Crossref Full Text | Google Scholar
5. Chiricozzi, A, Maurelli, M, Gori, N, Argenziano, G, de Simone, C, Calabrese, G, et al. Dupilumab improves clinical manifestations, symptoms, and quality of life in adult patients with chronic nodular prurigo. J Am Acad Dermatol. (2020) 83:39–45. doi: 10.1016/j.jaad.2020.03.049
PubMed Abstract | Crossref Full Text | Google Scholar
6. Reitamo, S, Ortonne, JP, Sand, C, Cambazard, F, Bieber, T, Folster-Holst, R, et al. A multicentre, randomized, double-blind, controlled study of long-term treatment with 0.1% tacrolimus ointment in adults with moderate to severe atopic dermatitis. Br J Dermatol. (2005) 152:1282–9. doi: 10.1111/j.1365-2133.2005.06592.x
PubMed Abstract | Crossref Full Text | Google Scholar
8. Ahramiyanpour, N, Akbari, Z, Sarasyabi, MS, Aflatoonian, M, Saki, N, and Shafie’ei, M. The therapeutic role of lasers in primary localized cutaneous amyloidosis: a systematic review. Lasers Med Sci. (2021) 37:799–813. doi: 10.1007/s10103-021-03429-4
PubMed Abstract | Crossref Full Text | Google Scholar
9. Thibodeaux, Q, Smith, MP, Ly, K, Beck, K, Liao, W, and Bhutani, T. A review of dupilumab in the treatment of atopic diseases. Hum Vaccin Immunother. (2019) 15:2129–39. doi: 10.1080/21645515.2019.1582403
PubMed Abstract | Crossref Full Text | Google Scholar
10. Maurer, M, Casale, TB, Saini, SS, Ben-Shoshan, M, Laws, E, Maloney, J, et al. Dupilumab reduces Urticaria activity, itch, and hives in patients with chronic spontaneous Urticaria regardless of baseline serum immunoglobulin E levels. Dermatol Ther. (2024) 14:2427–41. doi: 10.1007/s13555-024-01231-y
PubMed Abstract | Crossref Full Text | Google Scholar
11. Adams, R, Colmont, C, Mukhtar, A, Morgan, H, and Patel, GK. A novel oncostatin M/interleukin-31 receptor mutation in familial primary localized cutaneous amyloidosis. Clin Exp Dermatol. (2019) 45:254–6. doi: 10.1111/ced.14059
PubMed Abstract | Crossref Full Text | Google Scholar
12. Tey, HL, Cao, T, Nattkemper, LA, Tan, VWD, Pramono, ZAD, and Yosipovitch, G. Pathophysiology of pruritus in primary localized cutaneous amyloidosis. Br J Dermatol. (2016) 174:1345–50. doi: 10.1111/bjd.14391
PubMed Abstract | Crossref Full Text | Google Scholar
13. Humeda, Y, Beasley, J, and Calder, K. Clinical resolution of generalized lichen amyloidosis with dupilumab: a new alternative therapy. Dermatol Online J. (2020) 26. doi: 10.5070/D32612051364
PubMed Abstract | Crossref Full Text | Google Scholar
14. Aoki, K, Ohyama, M, and Mizukawa, Y. A case of lichen amyloidosis associated with atopic dermatitis successfully treated with dupilumab: a case report and literature review. Dermatol Ther. (2021) 34:e15005. doi: 10.1111/dth.15005
PubMed Abstract | Crossref Full Text | Google Scholar
15. Zahid, S, Saussine, A, Calugareanu, A, Jachiet, M, Vignon-Pennamen, MD, Rybojad, M, et al. Dramatic response to dupilumab in papular amyloidosis. J Eur Acad Dermatol Venereol. (2022) 36:e1071–2. doi: 10.1111/jdv.18482
PubMed Abstract | Crossref Full Text | Google Scholar
16. Beck, TC, Plante, J, Robinson, I, Khatskevich, K, Forcucci, J, and Valdebran, M. Immunoglobulin G4-related disease-associated dermatitis with pruritus: a positive response to Dupilumab. Life (Basel). (2023) 13:833. doi: 10.3390/life13030833
PubMed Abstract | Crossref Full Text | Google Scholar
17. Zhao, XQ, Zhu, WJ, Mou, Y, Xu, M, and Xia, JX. Dupilumab for treatment of severe atopic dermatitis accompanied by lichenoid amyloidosis in adults: two case reports. World J Clin Cases. (2023) 11:2301–7. doi: 10.12998/wjcc.v11.i10.2301
PubMed Abstract | Crossref Full Text | Google Scholar
18. Zhu, Q, Gao, BQ, Zhang, JF, Shi, LP, and Zhang, GQ. Successful treatment of lichen amyloidosis coexisting with atopic dermatitis by dupilumab: four case reports. World J Clin Cases. (2023) 11:2549–58. doi: 10.12998/wjcc.v11.i11.2549
PubMed Abstract | Crossref Full Text | Google Scholar
19. Tirone, B, Cazzato, G, Ambrogio, F, Foti, C, and Bellino, M. Lichen amyloidosis in an atopic patient treated with Dupilumab: a new therapeutic option. Diseases. (2024) 12:94. doi: 10.3390/diseases12050094
留言 (0)