Preeclampsia, a hypertensive condition unique to pregnancy, is characterized by high blood pressure and either end-organ dysfunction or proteinuria, typically developing after the 20th week of gestation (1). Preeclampsia serves as a primary contributor to maternal and neonatal mortality and morbidity worldwide, leading to serious complications in both the short term and long term (2), including placental abruption, damage to the maternal liver, kidneys, or brain, premature birth, fetal growth restriction, and an increased risk of chronic hypertension and cardiovascular diseases. Additionally, children born to mothers with preeclampsia may face neurodevelopmental issues and chronic health problems. Preeclampsia with severe complications, including hemolysis, elevated liver enzyme levels, and low platelet levels (HELLP syndrome), along with other serious maternal and fetal health issues, can be further classified as severe preeclampsia (3).
Body mass index (BMI) serves as an important indicator for assessing the nutritional status of women. The effect of pre-pregnancy BMI on adverse obstetric outcomes has been well-documented, such as stillbirth, large for gestational age, gestational diabetes, neonatal mortality, and preterm birth (4–7). In addition, increasing amounts of research have indicated that pre-pregnancy BMI is closely related to the risk of preeclampsia, particularly in women classified as obese or overweight (8–10), with this association potentially being even more significant. The pathophysiological mechanisms underlying obesity and preeclampsia share commonalities, such as heightened oxidative stress, an inflammatory environment, vasoconstriction, and endothelial dysfunction (11).
Despite the well-documented connection between pre-pregnancy BMI and preeclampsia, relatively few studies have specifically addressed the effect of pre-pregnancy overweight or obesity on the risk of severe preeclampsia. The majority of these studies are outdated (12–14), highlighting the ongoing need for updated research. Early reports suggested that women with a pre-pregnancy BMI value ranging from 25.0 to 29.9 or ≥ 30.0 exhibited a higher risk of severe preeclampsia (15). However, with the evolution of society, the constitution and nutritional status of the female population have changed, accompanied by increased awareness and shifting perspectives on maternal health during pregnancy (16, 17). Moreover, there are differences in obesity diagnostic criteria across countries (18, 19), further underscoring the urgent need for a contemporary study with a large sample size, specifically focusing on the domestic female population in China.
Given the evidence and potential clinical implications, our study aimed to explore the relationship between BMI before pregnancy and preeclampsia risk, with a specific focus on severe preeclampsia, based on patient data obtained from Shanghai First Maternity and Infant Hospital in China. This research highlights the significance of weight management before conception in preventing preeclampsia, which can help mitigate severe adverse pregnancy outcomes and related complications. Our research aimed to provide precise estimates on the relationship between preeclampsia and pre-pregnancy BMI, particularly in high-risk populations with extreme BMI values, ultimately enhancing maternal and fetal health outcomes.
Materials and methods Study population and data sourceThe investigation was initiated at Shanghai First Maternity and Infant Hospital in China. The inclusion criteria were as follows: (1) Participants had a live birth of a single infant; (2) participants must have delivered between 22nd and 42nd week of gestation; (3) participants must have given birth at Shanghai First Maternity and Infant Hospital in China; (4) delivery records must have been documented between the years 2016 and 2020; and (5) delivery records must be accessible in the hospital’s electronic medical system. Participants with records meeting the following criteria were excluded: (1) Twin or multiple pregnancies; (2) foreign nationality; (3) incomplete clinical information; and (4) underlying diseases such as pre-pregnancy hypertension and diabetes. Among the recruited participants, 73,988 individuals did not have preeclampsia, while 1,785 were diagnosed with preeclampsia, including 778 cases classified as severe preeclampsia. (Figure 1). The research was approved by the Ethics Committee of Shanghai First Maternity and Infant Hospital, School of Medicine, Tongji University.
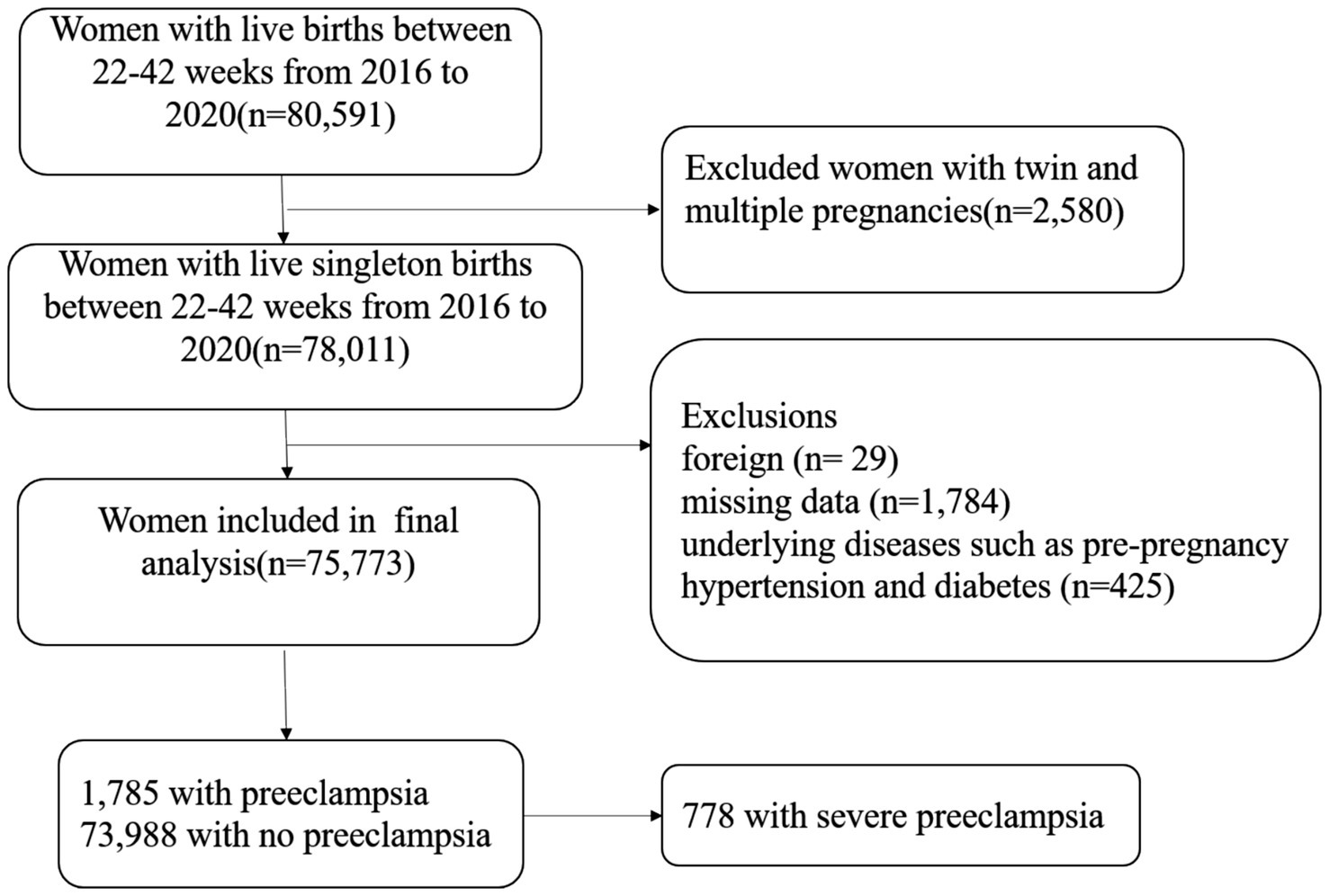
Figure 1. Illustration of the participant recruitment process.
Personal health and sociodemographic information was collected during outpatient consultation, including maternal age, parity status (nulliparous/multiparous), whether conception was through assisted reproductive technology (ART), native place, nationality, employment status, maternal height, and maternal weight before pregnancy. The value of BMI was computed as weight (in kilograms)/height (in meters squared) and classified as underweight (<18.5 kg/m2), optimal weight (18.5–23 kg/m2), overweight (23–27.5 kg/m2), and obesity (≥27.5 kg/m2), based on the criteria suitable for the Asian population. Gestational age was determined based on the date of the last menstrual period. Information about the delivery mode and newborn status was extracted from the electronic system.
Diagnosis of preeclampsia and severe preeclampsiaDiagnosis of (severe) preeclampsia was based on the patient information documented in discharge records. The diagnosis criteria followed the guidelines by the Société Française d’Anesthésie et de Réanimation (3). Preeclampsia is defined as the onset of systolic blood pressure (SBP) ≥ 140 and/or diastolic blood pressure (DBP) ≥ 90 mmHg after the 20th week of gestation, along with proteinuria ≥0.3 g/24 h. Severe preeclampsia is diagnosed based on the presence of more than one of the following criteria: (1) Thrombocytopenia with a platelet count <100,000/mm3; (2) proteinuria >3 g/24 h; (3) neurological symptoms; (4) shortness of breath, acute pulmonary edema, or chest pain; (5) oliguria ≤500 mL/24 h or ≤ 25 mL/h; (6) uncontrolled hypertension with continuing DBP ≥ 110 mmHg or SBP ≥ 160 mmHg; (7) cytolysis in hepatic cells with AST/ALT levels greater than twice the normal limit, persistently; (8) persistent or severe upper abdominal pain, particularly in the right upper quadrant; and (9) serum creatinine ≥90 μmol/L.
Statistical analysesThe χ2 test was applied to evaluate the associations between categorical variables with sufficient expected frequencies. To assess non-linear relationships between the pre-pregnancy BMI levels and (severe) preeclampsia outcomes while controlling for confounding variables, a logistic regression model was applied. The categorical confounders were defined based on prior references. The only factors found to be clinically significant in the univariate logistic regression analysis were adjusted for in the resulting model. All data processing and analyses were conducted using IBM SPSS Statistics, Version 26.0 (Armonk, NY: IBM Corp.). A significance level of p < 0.05 was applied for all statistical tests.
ResultsAs illustrated in Table 1, the enrolled pregnant women were categorized by the BMI levels before pregnancy. The underweight cohort comprised 11,360 individuals with BMI values <18.5 kg/m2, the normal weight cohort comprised 53,912 individuals with BMI values ranging from 18.5 to <24 kg/m2, the overweight group comprised 8,756 individuals with BMI values ranging from 24 to <28 kg/m2, and the obese group comprised 1,745 individuals with BMI values ≥28 kg/m2.

Table 1. Information about the enrolled participants, divided based on pre-pregnancy BMI values.
Compared to the normal BMI cohort, the overweight and obese cohorts exhibited a higher proportion of individuals aged 35 years and older (14.73% vs. 21.29 and 18.34%). In contrast, the underweight individuals were less likely to be of advanced maternal age (14.73% vs. 7.68%). The comparisons mentioned above regarding age showed statistically significant differences (p < 0.05). In addition, the overweight and obese populations showed a higher likelihood of being multiparous (24.97% vs. 30.74 and 30.09%), undergoing a cesarean delivery (31.74% vs. 44.77 and 50.32%), conceiving through assisted reproductive technology (ART) (6.05% vs. 9.94 and 13.52%), having a residence in Shanghai (29.53% vs. 36.38% and. 45.33%), and experiencing gestational diabetes mellitus (10.55% vs. 19.85% and. 27.62%), all of which were statistically significant. On the other hand, the underweight population was more likely to be primiparous (24.97% vs. 17.29%), received a cesarean delivery (31.74% vs. 24.67%), and conceived through ART (6.05% vs. 3.82%), all of which were also statistically significant.
Compared to the women with normal weight, the underweight pregnant women showed a significantly lower incidence of preeclampsia (1.27% vs. 2.08%), while the overweight and obese women exhibited notably higher preeclampsia rates, at 4.49 and 7.22%, respectively. Similarly, for severe preeclampsia, the incidence was significantly lower in the underweight women (0.67% vs. 0.96%), while the overweight and obese women exhibited significantly higher rates, at 1.59 and 2.58%, respectively, compared to the normal BMI group (Table 2).
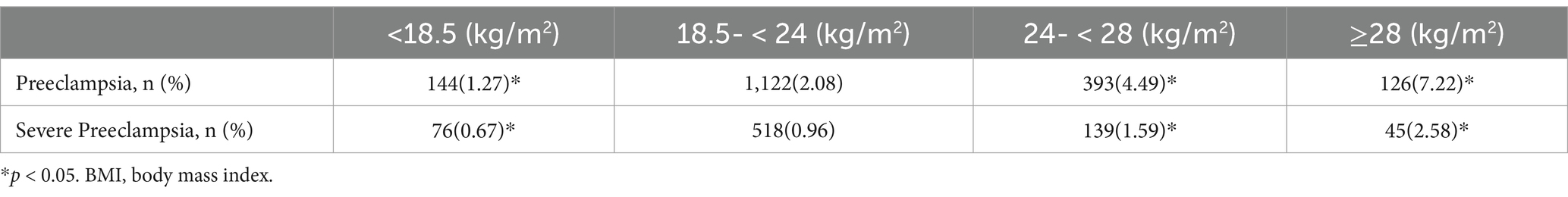
Table 2. Relationship between the BMI values before pregnancy and (severe) preeclampsia in the singleton pregnant women.
Logistic regression was applied to explore the relationship between BMI before pregnancy and the risks of preeclampsia and severe preeclampsia. Overweight and obese populations exhibited a higher risk of preeclampsia, with ORs (95% CI) of 2.211 (1.967–2.486) and 3.662 (3.026–4.431), respectively. In contrast, the individuals with a BMI value <18.5 kg/m2 exhibited a lower risk of preeclampsia, with an OR (95% CI) of 0.604 (0.507–0.719). After adjusting for confounders, including maternal age, gestational diabetes mellitus, parity, assisted reproductive technology, and employment status, these risks remained significant, with ORs (95% CI) of 2.152(1.911–2.425) for overweight women, 3.493(2.874–4.245) for obese women, and 0.609(0.511–0.727) for underweight women. All these differences were statistically significant. For severe preeclampsia, after adjusting for confounders, overweight and obese women still exhibited higher risks, with ORs (95% CI) of 1.652(1.364–2.001) and 2.762(2.014–3.788), respectively, while the underweight population exhibited a lower risk, with an OR (95% CI) of 0.720(0.565–0.919) (Table 3).
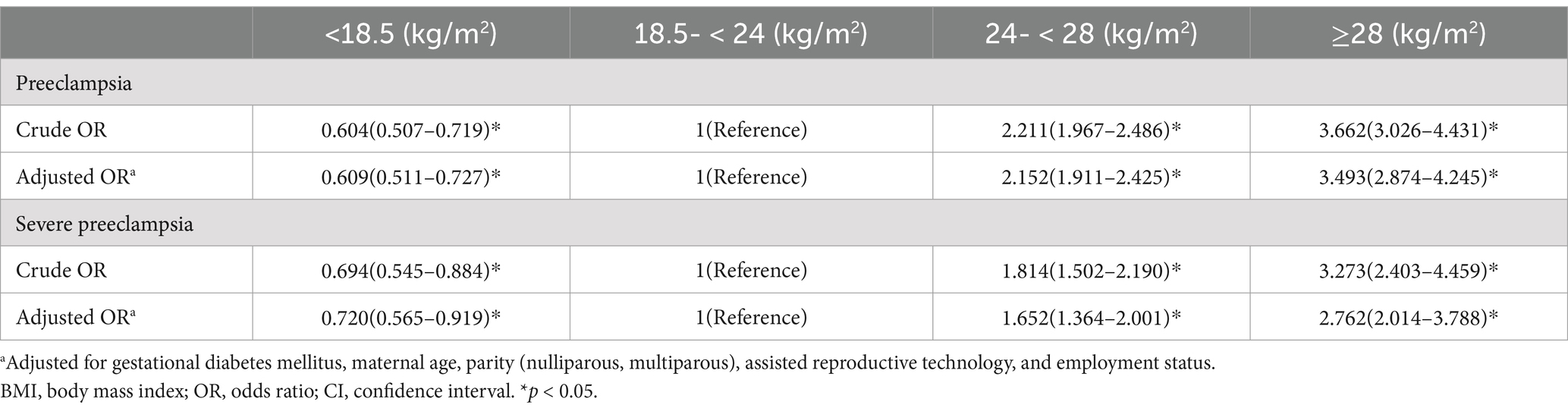
Table 3. Crude and adjusted ORs (95% CI) for the relationship between BMI before pregnancy and (severe) preeclampsia.
The women were stratified by age to explore whether the relationship between BMI before pregnancy and the risks of preeclampsia and severe preeclampsia varied across different age groups. Among the women younger than 35 years, after adjusting for confounders, those with a BMI value <18.5 kg/m2 exhibited a lower risk of preeclampsia (OR = 0.588, 95% CI =0.487–0.708), while the overweight (OR = 2.119, 95% CI =1.852–2.424) and obese (OR = 3.751, 95% CI = 3.035–4.635) women exhibited higher risks. For severe preeclampsia, the underweight population showed a lower risk (OR = 0.739, 95% CI = 0.573–0.954), while the overweight (OR = 1.605, 95% CI = 1.285–2.003) and obese (OR = 2.890, 95% CI = 2.039–4.095) women still showed an elevated risk. Among women aged 35 years or older, there was no reduction in the risk of preeclampsia or severe preeclampsia in underweight women compared to those with a normal BMI. However, the risk was higher for overweight and obese women (Table 4). In addition, these risks were not significantly associated with maternal age.
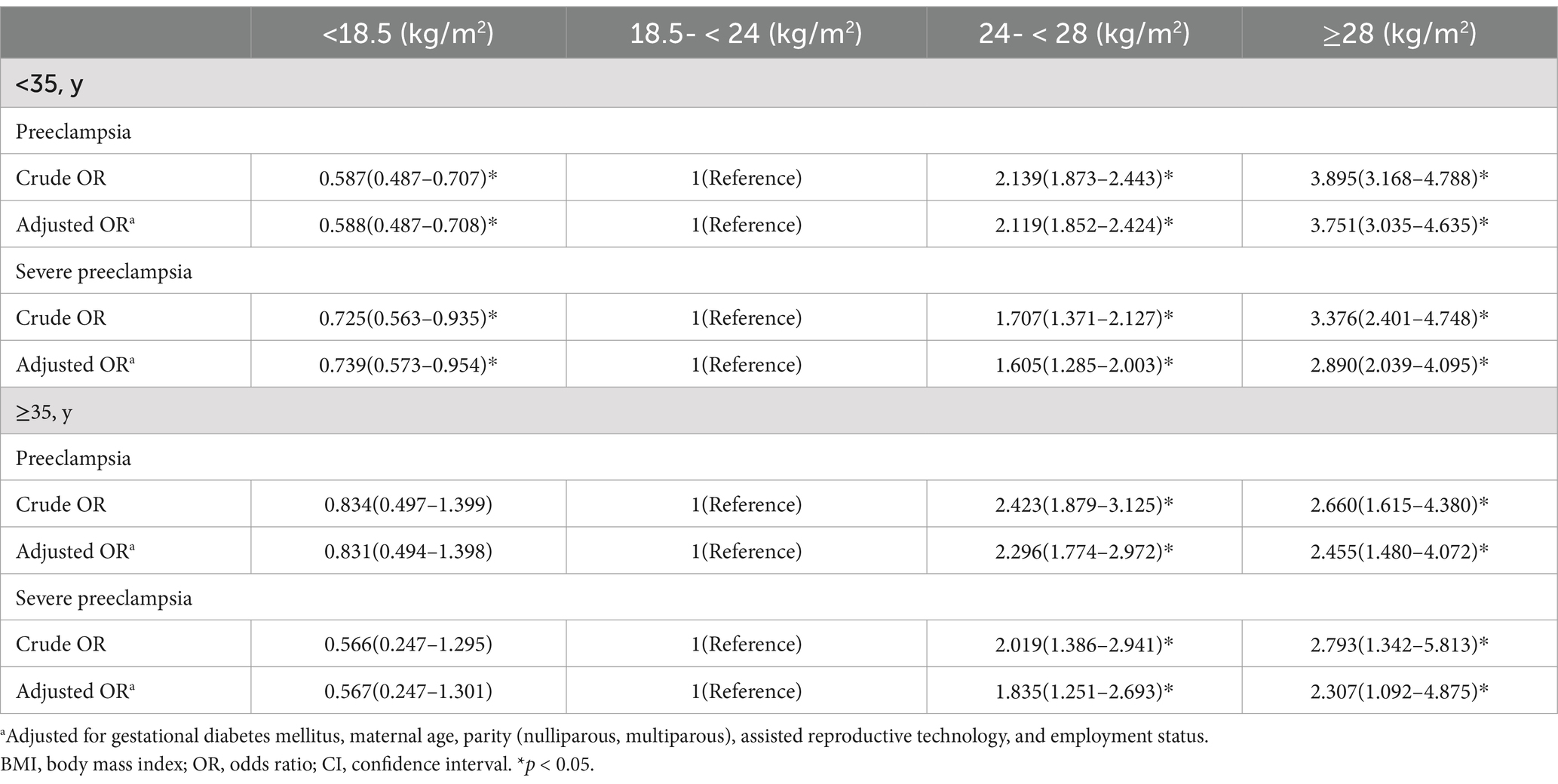
Table 4. Crude and adjusted ORs (95% CI) for the relationship between BMI before pregnancy and (severe) preeclampsia categorized by age.
DiscussionAs we know, this is currently the largest retrospective study in China investigating the relationship between pre-pregnancy BMI and preeclampsia, as well as severe preeclampsia. In this study, the pregnant women who were overweight or obese before pregnancy were observed to have a higher risk of (severe) preeclampsia, while those who were underweight had a lower risk of developing preeclampsia. In addition, these risks were not significantly associated with maternal age.
Maternal obesity has been identified as an established risk factor for preeclampsia (15). The aforementioned positive connection between overweight/obesity before pregnancy and preeclampsia has been well-established (20–23). This research further confirmed that this correlation persists regardless of age or parity. The significance of the relationship became even more significant after excluding the effects of the factors mentioned above, as maternal overweight highly correlates with advanced age and multiparity (24–26). The risk of preeclampsia with serious complications was also increased in the overweight and obese populations and decreased in the underweight population. After adjusting for confounding factors, these correlations became even more significant. The underlying mechanisms still need further investigation, and it is hypothesized that both the initiation and progression of preeclampsia are influenced by similar adverse environmental factors in the overweight population.
There is evidence supporting the relationship between overweight and obesity and an increased risk of preeclampsia, especially its severe form. The onset of preeclampsia is characterized by the activation of endothelial cells, intravascular inflammation, and stress in the syncytiotrophoblast. These conditions are often triggered by a physiological environment in individuals with metabolic abnormalities, which are marked by systemic inflammation, increased oxidative stress, and endothelial dysfunction. Another possibility is that preeclampsia and metabolic abnormalities (including overweight and obese) share multiple co-activation pathways (27). This association is particularly pronounced in late-onset severe preeclampsia (28) as many manifestations of severe preeclampsia are associated with a highly inflammatory and hyper-reactive internal environment (29). As a clinically complex syndrome with multifactorial causes, the initiation and progression of preeclampsia involve various etiological factors.
Among multi-organ severe damage caused by preeclampsia across various systemic functions, conditions such as renal impairment, thrombocytopenia, and cardiopulmonary symptoms show a typical relationship with obesity-induced endothelial dysfunction. Excessive release of adipokines and inflammatory cytokines from the adipose tissue contributes to the chronic inflammatory state, damaging endothelial cells and affecting coagulation mechanisms. This is associated with vascular lesions and impairment in the regulation of blood pressure. Hypertension and systemic endothelial injury ultimately lead to end-organ dysfunction. This condition is also linked to short-term symptoms of preeclampsia, such as headache, blurred vision, and photophobia (30). Moreover, abnormal fatty acid metabolism is another issue commonly seen in the overweight population as it contributes to placental oxidative stress (31) and modulates the balance between thromboxane and prostacyclin (32). Therefore, the disruption of long-chain polyunsaturated fatty acids, which is commonly observed in the overweight or obese population, may contribute to the onset of preeclampsia (33). However, since this was a retrospective study and lacked sufficiently detailed information on lipid metabolism, we could not be certain if it is related to this factor. Furthermore, obesity increases the overall fluid load and renal burden, leading to altered renal blood perfusion. This ultimately causes or enhances kidney injury, which is manifested as elevated serum creatinine levels. Concurrently, renal dysfunction is a critical hallmark of severe preeclampsia. Obesity not only amplifies cardiac load, leading to cardiopulmonary symptoms, but also poses a risk of inducing structural and functional heart alterations over the long term, which, in turn, can elevate the risk of heart failure. Overall, the complex pathogenic pathways in pre-pregnancy overweight or obese population create a conducive foundation for the onset and progression of preeclampsia, thereby strengthening the theoretical basis for our analysis and conclusions.
Therefore, it can be concluded that optimal weight management before conception is crucial in mitigating the risks associated with preeclampsia. Such practices would not only help lower the incidence of preeclampsia but also reduce the likelihood of developing its severe forms, which are characterized by life-threatening complications. Preeclampsia is a prevalent obstetric complication, with an incidence rate as high as 2.2–9.4% in China (21, 34, 35). It is the leading cause of maternal death globally, second only to postpartum hemorrhage, and impacts the health of both the fetus and mother in the short term and long term, making it a critical issue in obstetrics. By managing weight, the pathophysiological factors that contribute to the development of preeclampsia can be more effectively moderated. Consequently, this approach leads to a reduction in the severity and incidence of preeclampsia, thereby enhancing the safety and viability of pregnancies. This study still has several limitations. As a retrospective analysis, all the data used were sourced only from the electronic register system. The lack of records regarding prior pregnancies with preeclampsia, placental abnormalities, weight gain during pregnancy, fetal gender, autoimmune and kidney diseases, socioeconomic status, weight loss treatments or relevant medication use, and the pregnancy stage at which preeclampsia was diagnosed makes it difficult to rule out these confounding factors or perform a detailed stratification of preeclampsia into early- or late-onset categories. Moreover, the absence of information on the specific complication of each patient diagnosed with severe preeclampsia limited the discussion on the role that pre-pregnancy BMI plays in different situations, such as dysfunction occurring in various organs, including hepatic, renal, and blood pressure regulation disorders.
ConclusionOur study demonstrated that pre-pregnancy underweight individuals exhibited a significantly lower chance of developing preeclampsia, irrespective of confounding factors, while pre-pregnancy overweight and obese individuals exhibited an elevated risk for both preeclampsia and severe preeclampsia. The results showed no correlation with maternal age, suggesting that BMI before pregnancy is a critical determinant in assessing the risk of preeclampsia. The findings reveal the significance of weight management before pregnancy in reducing the incidence of preeclampsia and its severe forms.
Data availability statementThe raw data supporting the conclusions of this article will be made available by the authors, upon reasonable request. Requests to access these datasets should be directed to MjU2NTQ4NDg0NkBxcS5jb20=.
Ethics statementThe studies involving humans were approved by Ethics Committee of the Shanghai First Maternal and Infant Hospital, affiliated with Tongji University School of Medicine (KS1998). The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and institutional requirements.
Author contributionsJM: Writing – original draft. HS: Conceptualization, Writing – original draft. QS: Data curation, Writing – original draft. CZ: Methodology, Writing – original draft. YY: Investigation, Writing – original draft. QD: Funding acquisition, Writing – review & editing.
FundingThe author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This work was supported by National Natural Science Foundation (grant No.82371693), Shanghai Municipal Health Commission (grant No. 202340113), Pudong New Area Health Commission (grant No. PW2023E-04), and Open Project of Shanghai Key Laboratory of Maternal and Fetal Medicine (grant No. mfmkf202202).
Conflict of interestThe authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statementThe author(s) declare that no Gen AI was used in the creation of this manuscript.
Publisher’s noteAll claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References2. GBD 2015 Child Mortality Collaborators. Global, regional, national, and selected subnational levels of stillbirths, neonatal, infant, and under-5 mortality, 1980-2015: a systematic analysis for the global burden of disease study 2015. Lancet. (2016) 388:1725–74. doi: 10.1016/S0140-6736(16)31575-6
Crossref Full Text | Google Scholar
3. Bonnet, MP, Garnier, M, Keita, H, Compère, V, Arthuis, C, Raia-Barjat, T, et al. Guidelines for the management of women with severe pre-eclampsia. Anaesthesia Crit Care Pain Med. (2021) 40:100901. doi: 10.1016/j.accpm.2021.100901
PubMed Abstract | Crossref Full Text | Google Scholar
4. Bartsch, E, Medcalf, KE, Park, AL, and Ray, JG. Clinical risk factors for pre-eclampsia determined in early pregnancy: systematic review and meta-analysis of large cohort studies. BMJ. (2016) 353:i1753. doi: 10.1136/bmj.i1753
PubMed Abstract | Crossref Full Text | Google Scholar
5. Xiong, Y, Wang, J, Huang, S, Liu, C, Liu, Y, Qi, Y, et al. Association between maternal prepregnancy body mass index and pregnancy outcomes following assisted reproductive technology: a systematic review and dose-response meta-analysis. Obes Rev. (2021) 22:e13219. doi: 10.1111/obr.13219
PubMed Abstract | Crossref Full Text | Google Scholar
6. Flenady, V, Koopmans, L, Middleton, P, Frøen, JF, Smith, GC, Gibbons, K, et al. Major risk factors for stillbirth in high-income countries: a systematic review and meta-analysis. Lancet. (2011) 377:1331–40. doi: 10.1016/S0140-6736(10)62233-7
PubMed Abstract | Crossref Full Text | Google Scholar
7. Najafi, F, Hasani, J, Izadi, N, Hashemi-Nazari, SS, Namvar, Z, Mohammadi, S, et al. The effect of prepregnancy body mass index on the risk of gestational diabetes mellitus: a systematic review and dose-response meta-analysis. Obes Rev. (2019) 20:472–86. doi: 10.1111/obr.12803
PubMed Abstract | Crossref Full Text | Google Scholar
8. Vats, H, Saxena, R, Sachdeva, MP, Walia, GK, and Gupta, V. Impact of maternal pre-pregnancy body mass index on maternal, fetal and neonatal adverse outcomes in the worldwide populations: a systematic review and meta-analysis. Obes Res Clin Pract. (2021) 15:536–45. doi: 10.1016/j.orcp.2021.10.005
PubMed Abstract | Crossref Full Text | Google Scholar
9. Nagpal, TS, Souza, SCS, Moffat, M, Hayes, L, Nuyts, T, Liu, RH, et al. Does prepregnancy weight change have an effect on subsequent pregnancy health outcomes? A systematic review and meta-analysis. Obes Rev. (2022) 23:e13324. doi: 10.1111/obr.13324
PubMed Abstract | Crossref Full Text | Google Scholar
10. Rahman, MM, Abe, SK, Kanda, M, Narita, S, Rahman, MS, Bilano, V, et al. Maternal body mass index and risk of birth and maternal health outcomes in low- and middle-income countries: a systematic review and meta-analysis. Obes Rev. (2015) 16:758–70. doi: 10.1111/obr.12293
PubMed Abstract | Crossref Full Text | Google Scholar
11. Teefey, CP, Durnwald, CP, Srinivas, SK, and Levine, LD. Adverse maternal outcomes differ between obese and nonobese women with severe preeclampsia. Am J Perinatol. (2019) 36:74–8. doi: 10.1055/s-0038-1661403
PubMed Abstract | Crossref Full Text | Google Scholar
13. Lu, J, Zhao, YY, Qiao, J, Zhang, HJ, Ge, L, and Wei, Y. A follow-up study of women with a history of severe preeclampsia: relationship between metabolic syndrome and preeclampsia. Chin Med J. (2011) 124:775–9.
PubMed Abstract | Google Scholar
15. Sudjai, D. Association of pre-pregnancy body mass index with early- and late-onset severe preeclampsia. Eur J Obstetr Gynecol Reprod Biol. (2023) 19:100223. doi: 10.1016/j.eurox.2023.100223
PubMed Abstract | Crossref Full Text | Google Scholar
16. Shirvanifar, M, Ahlqvist, VH, Lundberg, M, Kosidou, K, Herraiz-Adillo, Á, Berglind, D, et al. Adverse pregnancy outcomes attributable to overweight and obesity across maternal birth regions: a Swedish population-based cohort study. Lancet Public Health. (2024) 9:e776–86. doi: 10.1016/S2468-2667(24)00188-9
PubMed Abstract | Crossref Full Text | Google Scholar
18. Fan, Y, Li, W, Liu, H, Wang, L, Zhang, S, Li, W, et al. Effects of obesity and a history of gestational diabetes on the risk of postpartum diabetes and hyperglycemia in Chinese women: obesity, GDM and diabetes risk. Diabetes Res Clin Pract. (2019) 156:107828. doi: 10.1016/j.diabres.2019.107828
PubMed Abstract | Crossref Full Text | Google Scholar
19. Gu, ZJ, Song, QJ, Gu, WQ, Zhang, GP, Su, Y, Tang, Y, et al. New approaches in the diagnosis and prognosis of gestational diabetes mellitus. Eur Rev Med Pharmacol Sci. (2023) 27:10583–94. doi: 10.26355/eurrev_202311_34338
PubMed Abstract | Crossref Full Text | Google Scholar
20. Catov, JM, Ness, RB, Kip, KE, and Olsen, J. Risk of early or severe pre-eclampsia related to pre-existing conditions. Int J Epidemiol. (2007) 36:412–9. doi: 10.1093/ije/dyl271
PubMed Abstract | Crossref Full Text | Google Scholar
21. Yang, Y, Le Ray, I, Zhu, J, Zhang, J, Hua, J, and Reilly, M. Preeclampsia prevalence, risk factors, and pregnancy outcomes in Sweden and China. JAMA Netw Open. (2021) 4:e218401. doi: 10.1001/jamanetworkopen.2021.8401
PubMed Abstract | Crossref Full Text | Google Scholar
22. Phipps, EA, Thadhani, R, Benzing, T, and Karumanchi, SA. Pre-eclampsia: pathogenesis, novel diagnostics and therapies. Nat Rev Nephrol. (2019) 15:275–89. doi: 10.1038/s41581-019-0119-6
PubMed Abstract | Crossref Full Text | Google Scholar
23. Chang, KJ, Seow, KM, and Chen, KH. Preeclampsia: recent advances in predicting, preventing, and managing the maternal and fetal life-threatening condition. Int J Environ Res Public Health. (2023) 20:994. doi: 10.3390/ijerph20042994
PubMed Abstract | Crossref Full Text | Google Scholar
24. Onubi, OJ, Marais, D, Aucott, L, Okonofua, F, and Poobalan, AS. Maternal obesity in Africa: a systematic review and meta-analysis. J Public Health. (2016) 38:e218–31. doi: 10.1093/pubmed/fdv138
PubMed Abstract | Crossref Full Text | Google Scholar
25. Voerman, E, Santos, S, Inskip, H, Amiano, P, Barros, H, Charles, MA, et al. Association of Gestational Weight Gain with Adverse Maternal and Infant Outcomes. JAMA. (2019) 321:1702–15. doi: 10.1001/jama.2019.3820
PubMed Abstract | Crossref Full Text | Google Scholar
26. Jang, HC, Cho, NH, Min, YK, Han, IK, Jung, KB, and Metzger, BE. Increased macrosomia and perinatal morbidity independent of maternal obesity and advanced age in Korean women with GDM. Diabetes Care. (1997) 20:1582–8. doi: 10.2337/diacare.20.10.1582
PubMed Abstract | Crossref Full Text | Google Scholar
29. Zhu, L, Zhang, Z, Zhang, L, Shi, Y, Qi, J, Chang, A, et al. HMGB1-RAGE signaling pathway in severe preeclampsia. Placenta. (2015) 36:1148–52. doi: 10.1016/j.placenta.2015.08.006
PubMed Abstract | Crossref Full Text | Google Scholar
30. Ives, CW, Sinkey, R, Rajapreyar, I, Tita, ATN, and Oparil, S. Preeclampsia-pathophysiology and clinical presentations: JACC state-of-the-art review. J Am Coll Cardiol. (2020) 76:1690–702. doi: 10.1016/j.jacc.2020.08.014
PubMed Abstract | Crossref Full Text | Google Scholar
31. Jadhav, A, Khaire, A, and Joshi, S. Exploring the role of oxidative stress, fatty acids and neurotrophins in gestational diabetes mellitus. Growth Fact. (2020) 38:226–34. doi: 10.1080/08977194.2021.1895143
PubMed Abstract | Crossref Full Text | Google Scholar
32. Kain, V, Ingle, KA, Kachman, M, Baum, H, Shanmugam, G, Rajasekaran, NS, et al. Excess ω-6 fatty acids influx in aging drives metabolic dysregulation, electrocardiographic alterations, and low-grade chronic inflammation. Am J Physiol Heart Circ Physiol. (2018) 314:H160–9. doi: 10.1152/ajpheart.00297.2017
PubMed Abstract | Crossref Full Text | Google Scholar
34. Wu, J, Lu, AD, Zhang, LP, Zuo, YX, and Jia, YP. [study of clinical outcome and prognosis in pediatric core binding factor-acute myeloid leukemia]. Zhonghua Xueyexue Zazhi. (2019) 40:52–7. doi: 10.3760/cma.j.issn.0253-2727.2019.01.010
PubMed Abstract | Crossref Full Text | Google Scholar
35. Zeng, S, Liu, H, Li, B, Guo, X, Chen, S, Li, X, et al. Association of air temperature exposure during pregnancy with risk of preeclampsia in Guangzhou. China Environ Int. (2024) 186:108646. doi: 10.1016/j.envint.2024.108646
留言 (0)