Among hospitalized patients with stroke in China, about 83.3% suffer from acute ischemic stroke (AIS) (1), with an annual recurrence rate of about 9.6%−17.7% (2, 3). Chronic kidney disease (CKD) is prevalent among stroke patients, with decreased renal function observed in 20%−35% of AIS cases (4). The presence of abnormal renal function significantly increases the risk of stroke recurrence in patients with AIS (5, 6). Accurate assessment of the risk factors for stroke recurrence in patients with AIS and abnormal renal function is of great significance for the secondary prevention of ischemic stroke and for taking measures to reduce the risk of recurrence.
Serum uric acid (SUA) is the final metabolite of purine catabolism (7). Previous studies have produced conflicting results regarding the relationship between SUA levels at admission and acute stroke outcomes, with findings varying from positive outcomes (8–10) to insignificant association (11, 12) and negative outcomes (13, 14). In addition, some studies have found a U-shaped association between excessively low or high SUA levels and worse stroke outcomes (15). As SUA is mainly excreted by the kidneys, variations in renal clearance may contribute to the inconsistent results of previous studies (16). Studies have demonstrated that elevated SUA levels are associated with a higher risk of in-hospital mortality in patients with ischemic stroke and kidney disease, whereas this association is not observed in patients with normal renal function. The results of these studies suggest that the impact of kidney function should be considered when evaluating the association of SUA with stroke outcomes (17).
Serum creatinine (SCr) is an important marker for identifying minor alterations in glomerular filtration rate (GFR) and is widely recognized as a dependable biomarker for early detection of chronic kidney disease (CKD) (18). Given that renal function typically influences the renal clearance of SUA, the SUA to SCr ratio (SUA/SCr) has emerged as a novel marker that normalizes SUA to renal function, thereby providing a more accurate reflection of the net SUA level (19–21). Prior research has identified a correlation between the SUA/SCr ratio and conditions such as non-alcoholic fatty liver disease, metabolic syndrome, and impaired islet function (19–21). Nonetheless, the association between the SUA/SCr ratio and stroke recurrence remains underexplored, with limited studies addressing this, particularly in AIS with abnormal renal function, for whom no reports have been published to date. This study sought to explore the association between the SUA/SCr ratio and 1-year stroke recurrence in patients with AIS and abnormal renal function, utilizing data from the Xi'an Stroke Registry Study database. The goal is to offer a scientific foundation for the prevention and treatment of 1-year stroke recurrence in this patient population.
2 Materials and methods 2.1 Study design and study populationThis was a prospective, multicenter, observational cohort study. The study population consisted of patients with stroke admitted to four grade A tertiary hospitals in Xi'an, China, between January and December 2015. At the initial stage of the study, 3,117 patients with stroke underwent a comprehensive medical examination, with follow-up conducted at 1, 3, 6, and 12 months after onset of symptoms. The inclusion criteria were as follows: a clinical diagnosis of AIS, with imaging findings in line with the diagnostic criteria outlined by the American Heart Association/American Stroke Association and confirmed through brain computed tomography or cranial magnetic resonance imaging (22). The patients were between the ages of 18 and 97 years and had experienced symptom onset within 7 days. The exclusion criteria were as follows: patients with cerebral or subarachnoid hemorrhage (n = 416), patients with loss of SUA/SCr value (n = 139), patients with eGFR ≥ 90 ml/min/1.73 m2 (n = 414), and patients lost to follow-up (n = 216). Ultimately, 1,932 patients with AIS were included in the study. The detailed screening process and study flowchart are presented in Figure 1. Consistent diagnostic criteria were applied across all participating hospitals.
Figure 1. Screening flow charts for enrolled patients. AIS, acute ischemic stroke; SUA, Serum Uric Acid; SCr, Serum Creatinine; eGFR; estimated glomerular filtration rate.
2.2 Standard protocol approval and patient consentThis study adhered to the principles outlined in the Declaration of Helsinki. Ethical approval was obtained from the Academic Committee of Xi'an No. 1 Hospital and the ethics committees of all participating hospitals (Approval No. 2014 (5); Registration Number: ChiCTR-EOC-17012190). Written or verbal informed consent was obtained from each patient prior to participation.
2.3 Baseline data collectionThis multicenter observational cohort study utilized data from the Xi'an Stroke Registry. Baseline information, including sociodemographic details, medical history, admission assessments, and key laboratory results, was collected (Supplementary Table 1). The criteria for medical history, risk factors, and definitions were aligned with those used in the Chinese National Stroke Registry (CNSR) Study (23). SUA/SCr was evaluated as both a continuous and categorical variable (quartiles, Q1–Q4). The quartile ranges for SUA/SCr were as follows, from lowest to highest: Q1: < 3.091, Q2: 3.091–3.895, Q3: 3.896–4.773, Q4: ≥4.774.
2.4 Measurements and outcomesIn this study, AIS refers to a type of disease caused by impaired blood supply to brain tissue due to various reasons, leading to ischemic and hypoxic necrosis of brain tissue and ultimately resulting in brain dysfunction. This primarily includes acute cerebral infarction and transient ischemic attack, with diagnostic criteria based on the World Health Organization's standards (24). The SUA/SCr measurements were performed by experienced nurses using blood samples collected on the morning following patient admission. These samples were subsequently sent to the laboratory for analysis. All technicians involved in testing across the hospitals received specialized training, and the instruments used were uniformly calibrated and of identical models. Fasting venous blood samples were obtained the day after admission following overnight fasting. Smoking was defined as the consumption of at least one cigarette per day for a minimum of 6 months prior to stroke onset, while smoking cessation was classified as meeting the smoking criteria previously but refraining from smoking for at least 6 months before the stroke. Alcohol consumption was defined as consuming at least one drink per week, with a standard drink equating to 45 ml of liquor, 360 ml of beer, or 120 ml of wine. Pneumonia was identified within the 1st week post-stroke in patients not requiring mechanical ventilation, diagnosed based on a chest radiograph, clinical symptoms like fever, purulent sputum, and cough, alongside abnormal laboratory findings such as altered white blood cell counts (25). Abnormal Renal function was defined by the estimated glomerular filtration rate (eGFR) < 90 mL/min/1.73 m2 (26). The eGFR was determined using the CKD-EPI creatinine equation with a correction factor of 1.1 for the Asian population (27, 28). In this study, the the endpoint event of stroke recurrence refers to the occurrence of a new stroke event (including cerebral infarction, cerebral hemorrhage, and subarachnoid hemorrhage) during the 1-year follow-up period. A committee of four to five stroke experts from each hospital confirmed the endpoint events.
2.5 Follow-upAll patients underwent routine follow-up at 1, 3, 6, and 12 months following the AIS diagnosis. Trained study coordinators carried out follow-ups with all enrolled patients either through phone interviews or in-person visits, ensuring that the deviation in follow-up timing did not exceed 5 days and precisely documenting the dates of any stroke recurrence. Patients who withdrew from the study or could not be reached after three daily attempts over five consecutive working days were classified as lost to follow-up.
2.6 Statistical analysesContinuous variables with normal distribution were expressed as mean ± standard deviation, while categorical variables were reported as frequencies and percentages. Non-normally distributed variables were presented as medians with interquartile ranges. Group comparisons were performed using one-way ANOVA, Chi-square test, or non-parametric tests depending on data distribution. Fisher's exact test was applied when expected frequencies were < 10. Multivariable Cox proportional hazards models were used to assess the association between SUA/SCr and 1-year stroke recurrence. The Kaplan-Meier (K-M) method and log-rank test were used to analyze the 1-year cumulative stroke recurrence rate stratified by SUA/SCr quartile groups. Covariates were included in the adjusted model if they altered the SUA/SCr estimates for 1-year stroke recurrence by more than 10% or were significantly related to stroke recurrence at 1 year. Subgroup interactions were evaluated using a likelihood ratio test. Statistical significance was defined as a two-tailed P-value < 0.05. All analyses were conducted using Free Statistics software version 1.7 (http://www.clinicalscientists.cn, Free Clinical Medical Technology, Inc., Beijing, China) and R software version 3.3.2 (http://www.R-project.org, The R Foundation).
3 Results 3.1 Baseline characteristicsAfter 1 year of follow-up, 216 patients were lost to follow-up, resulting in a final cohort of 1,932 patients. A comparison of clinical characteristics between the followed and lost-to-follow-up groups revealed statistically significant differences only in education level, smoking history, systolic blood pressure (SBP) on admission, and atrial fibrillation, with no significant differences observed in other variables (Supplementary Table 1). This suggests that the key characteristics of patients lost to follow-up were similar to those retained, indicating that the analyzed cohort is representative of the initially enrolled population.
The study included 1,932 patients, comprising 1,261 men and 671 women, with a mean age of 66.7 ± 11.3 years. The average SUA/SCr ratio was 4.0 ± 1.5. Baseline demographic, clinical, and biochemical characteristics across SUA/SCr quartiles (Q1–Q4) were compared (Table 1). There were significant differences in age, presence of co-morbidities such as diabetes mellitus and pneumonia, National Institutes of Health Stroke Scale (NIHSS) score, levels of blood parameters such as triglycerides, fasting plasma glucose (FPG), alanine aminotransferase, homocysteine, aspartate aminotransferase, blood urea nitrogen, serum uric acid, serum creatinine, and white blood cell count, eGFR, and international normalized ratio (INR) among different SUA/SCr values (P < 0.05). No statistically significant differences were observed across SUA/SCr quartiles in terms of sex, type of medical insurance, education level, smoking history, alcohol consumption, co-morbidities such as hypertension, atrial fibrillation, and previous stroke, body mass index (BMI), SBP and diastolic blood pressure on admission (DBP), heart rate, or blood parameters including total cholesterol, high-density lipoprotein (HDL) cholesterol, low-density lipoprotein (LDL) cholesterol, glycated hemoglobin, alkaline phosphatase, and platelet count.
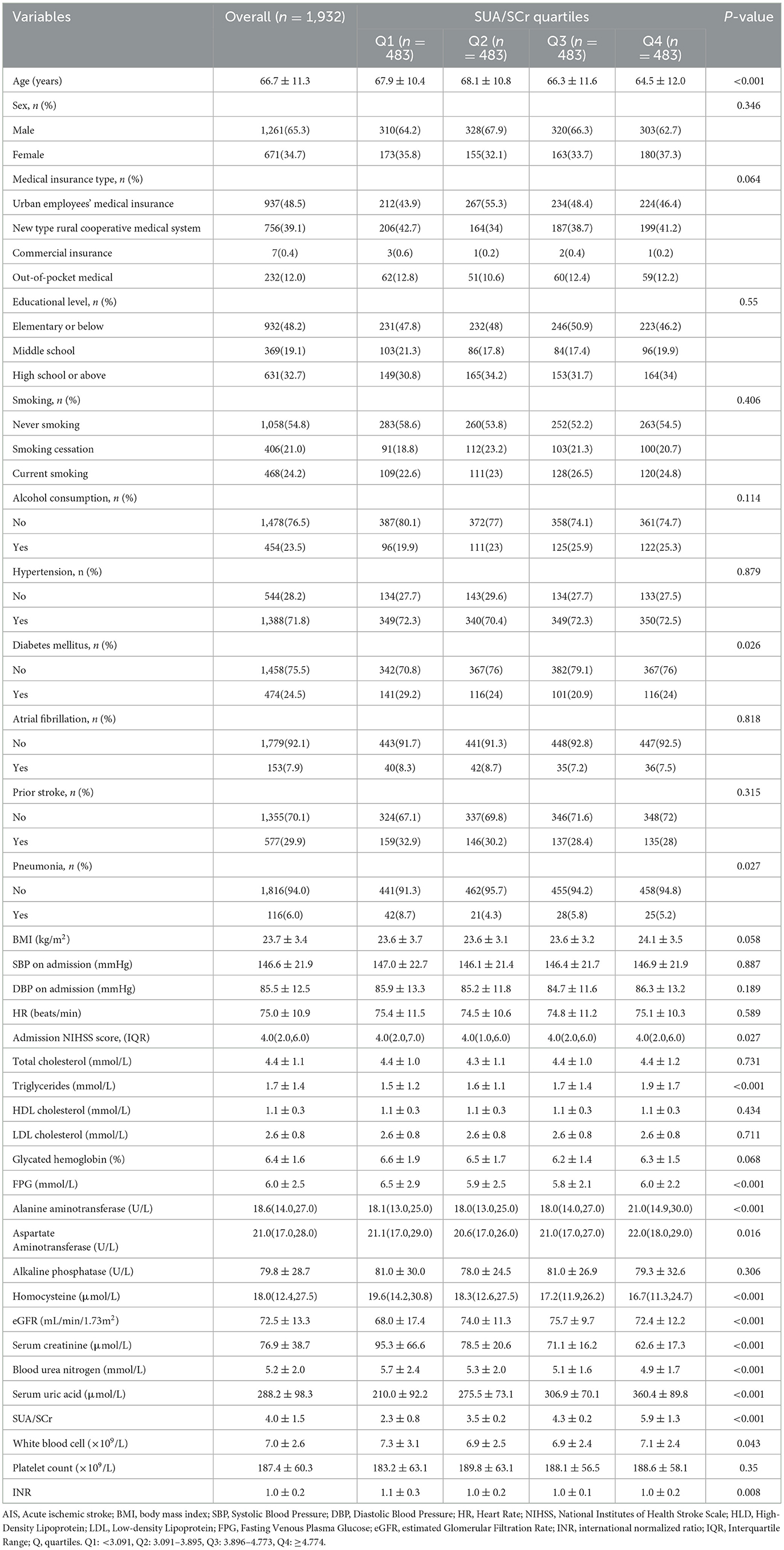
Table 1. Baseline and biochemical characteristics by SUA/SCr quartiles (Q1-Q4) in patients with AIS and abnormal renal function.
3.2 Association between SUA/SCr and 1-year stroke recurrence in patients with AIS and abnormal renal functionConsidering SUA/SCr as a continuous variable, multivariable Cox regression analysis, adjusted for confounders, revealed a 17% decrease in 1-year stroke recurrence risk for each 1-unit increase in SUA/SCr (HR = 0.83, 95% CI 0.73 to 0.96, P = 0.009) (Table 2). When SUA/SCr was categorized into quartiles, with Q1 as the reference, the risk of 1-year stroke recurrence was 54% lower in Q2 (HR = 0.46, 95% CI 0.27 to 0.79, P = 0.005) and 53% lower in Q4 (HR = 0.47, 95% CI 0.27 to 0.81, P = 0.007) compared to Q1, with no significant difference observed between Q3 and Q1. Trend analysis indicated a significant decreasing trend in stroke recurrence risk across the quartiles from Q1 to Q4 (P = 0.01) (Table 2). Further curve-fitting analysis suggested a negative but non-linear association between SUA/SCr and 1-year stroke recurrence in patients withAIS and abnormal renal function (Figure 2). KM curve analysis also indicated that, compared to the Q1 group, the 1-year stroke recurrence risk in patients with AISand abnormal renal function was significantly lower in the Q2 and Q4 (Figure 3).
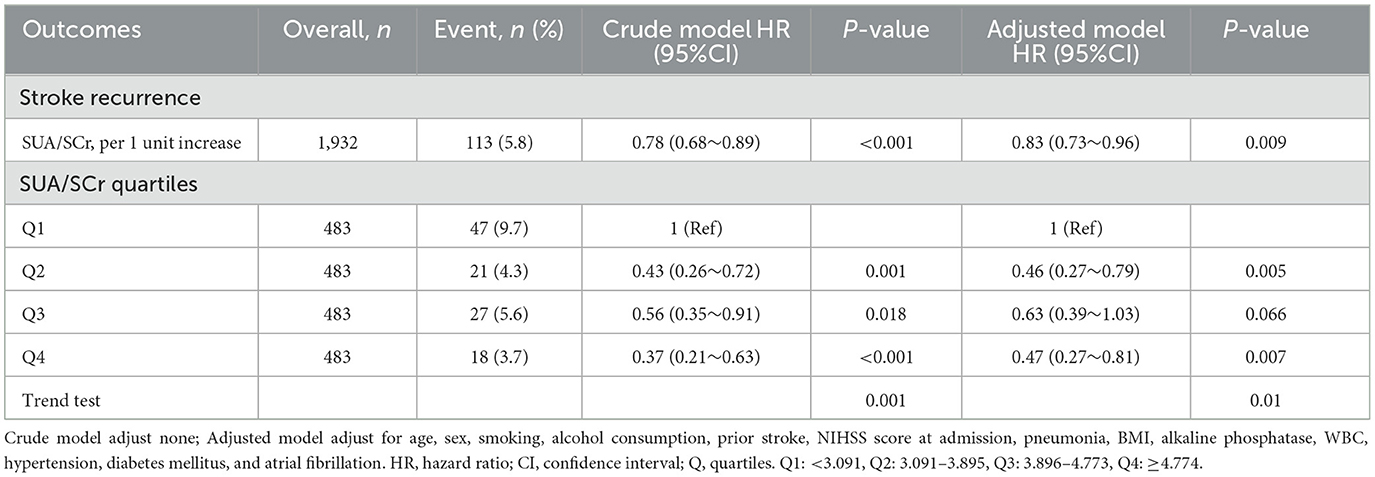
Table 2. Multivariable Cox analyses of the SUA/SCr and 1-year stroke recurrence in patients with AIS and abnormal renal function.
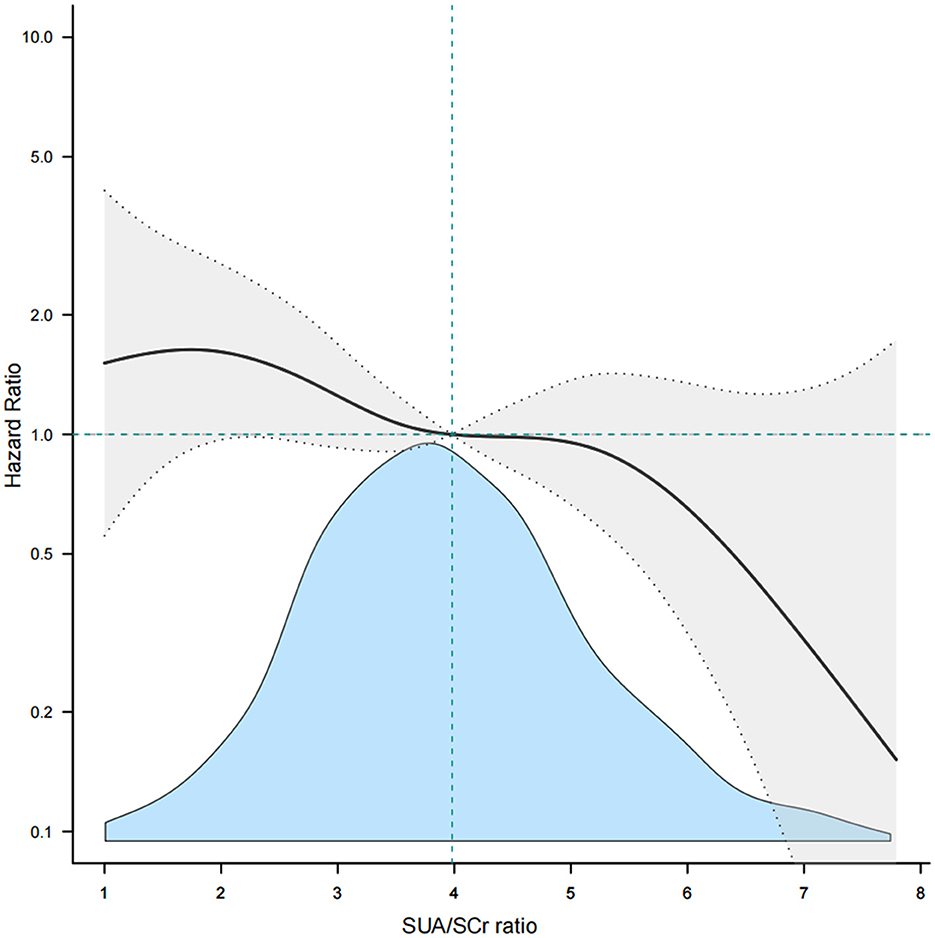
Figure 2. Smooth curve fitting of SUA/SCr to 1-year stroke recurrence. SUA, Serum Uric Acid; SCr, Serum Creatinine.
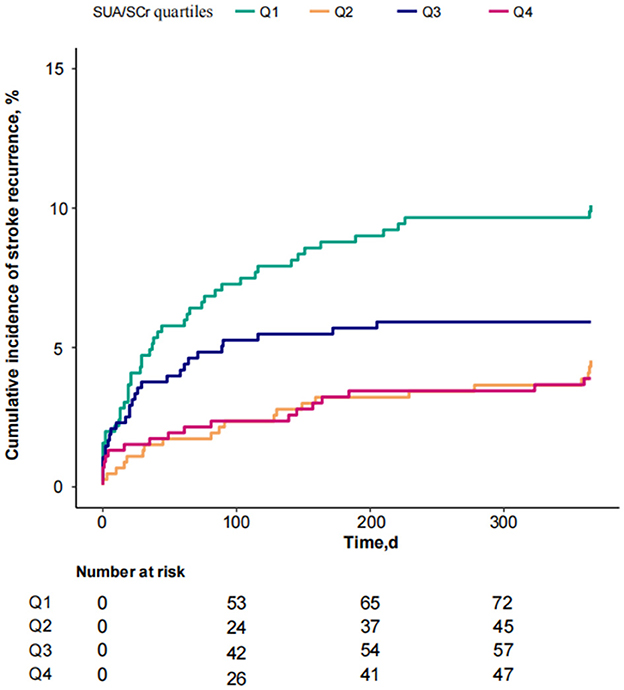
Figure 3. KM curve analysis of 1-year stroke recurrence in patients with AIS and abnormal renal function stratified by SUA/SCr quartiles.
3.3 Association of SUA/SCr with 1-year stroke recurrence stratified according to preservation of renal functionBased on previous reports on renal dysfunction (29, 30), eGFR ≥ 60 ml/min/1.73 m2 was defined as preserved renal function. Therefore, patients with AIS and abnormal renal function were stratified into two groups: eGFR < 60 ml/min/1.73 m2 and ≥60 ml/min/1.73 m2. In patients with eGFR < 60 ml/min/1.73 m2, the risk of 1-year stroke recurrence in the Q4 group significantly decreased compared with the Q1 group after adjusting for potential confounders (HR = 0.19, 95% CI 0.04 to 0.86, P = 0.031). The trend of stroke recurrence significantly differed between groups Q1 and Q4 both in the crude and adjusted models (P = 0.034 and P = 0.013 for trend tests, respectively) (Table 3). Among patients with eGFR ≥ 60 ml/min/1.73 m2, the risk of 1-year stroke recurrence was reduced significantly in both the Q2 and Q4 groups compared with the Q1 group in a crude model (Q2: HR = 0.43, 95% CI 0.24 to 0.79, P = 0.007; Q4: HR = 0.44, 95% CI 0.24 to 0.81, P = 0.008). The trend of stroke recurrence was significantly different between Q1 and Q4 (P = 0.023). After adjustment for potential confounding factors, compared with the Q1 group, the risk of 1-year stroke recurrence in group Q2 was significantly reduced (HR = 0.48, 95% CI 0.25 to 0.9, P = 0.023), while the risk of 1-year stroke recurrence in group Q4 was not significantly different (HR = 0.67, 95% CI 0.36 to 1.26, P = 0.216). There was also no statistically significant difference in the trend of recurrence between Q1 and Q4 (P = 0.345).
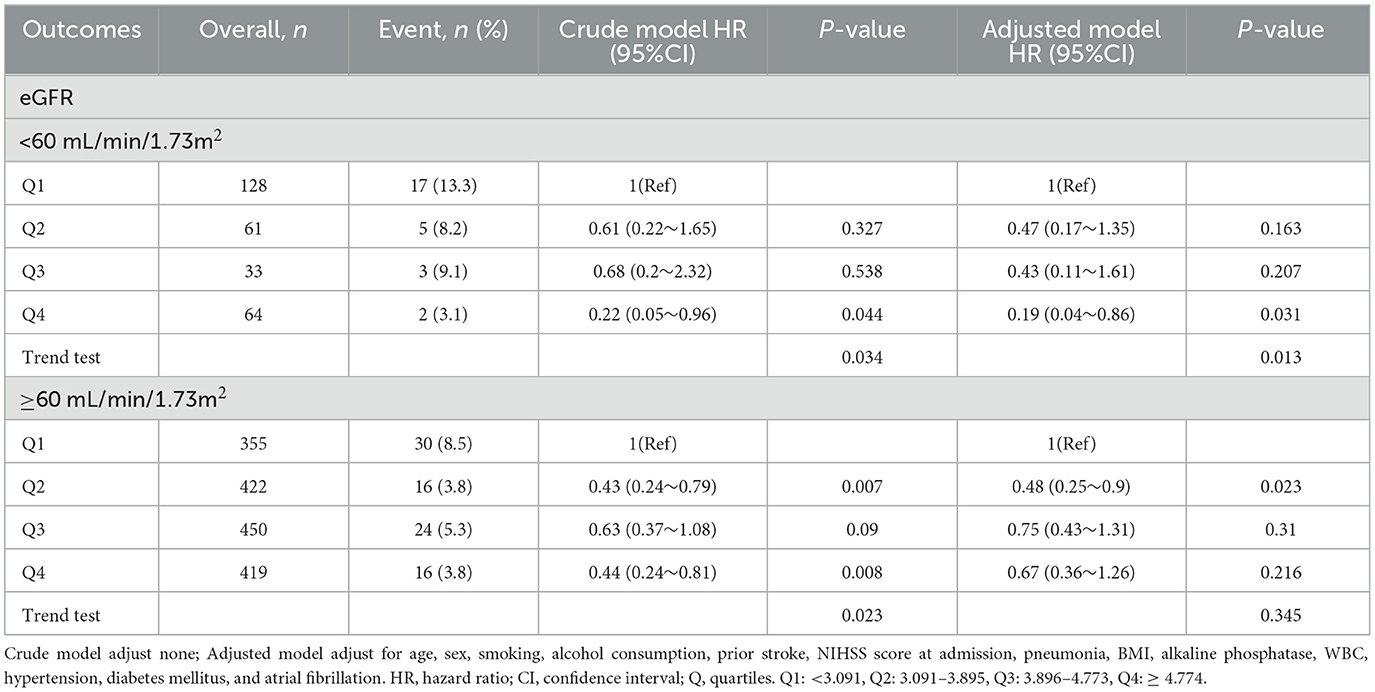
Table 3. SUA/SCr quartiles and 1-year stroke recurrence were analyzed separately stratified according to eGFR value.
3.4 Subgroup analysesStratified and interaction analyses were conducted to assess the consistency of the association between SUA/SCr and 1-year stroke recurrence across different subgroups (Figure 4). The results revealed that there was no significant interaction between age, sex, and the presence of co-morbidities such as pneumonia, hypertension, diabetes mellitus, atrial fibrillation, and prior stroke,. However, stratified analysis still found a lower risk of 1-year stroke recurrence in patients with age ≥65 years (HR = 0.83, 95% CI 0.7 to 0.97, P = 0.023), male patients (HR = 0.79, 95% CI 0.66 to 0.95, P = 0.012), those without pneumonia (HR = 0.78, 95% CI 0.67 to 0.92, P = 0.003), those with hypertension (HR = 0.85, 95% CI 0.73 to 0.98, P = 0.025), those without atrial fibrillation (HR = 0.83, 95% CI 0.72 to 0.96, P = 0.013), and those without prior stroke (HR = 0.8, 95% CI 0.68 to 0.95, P = 0.012).
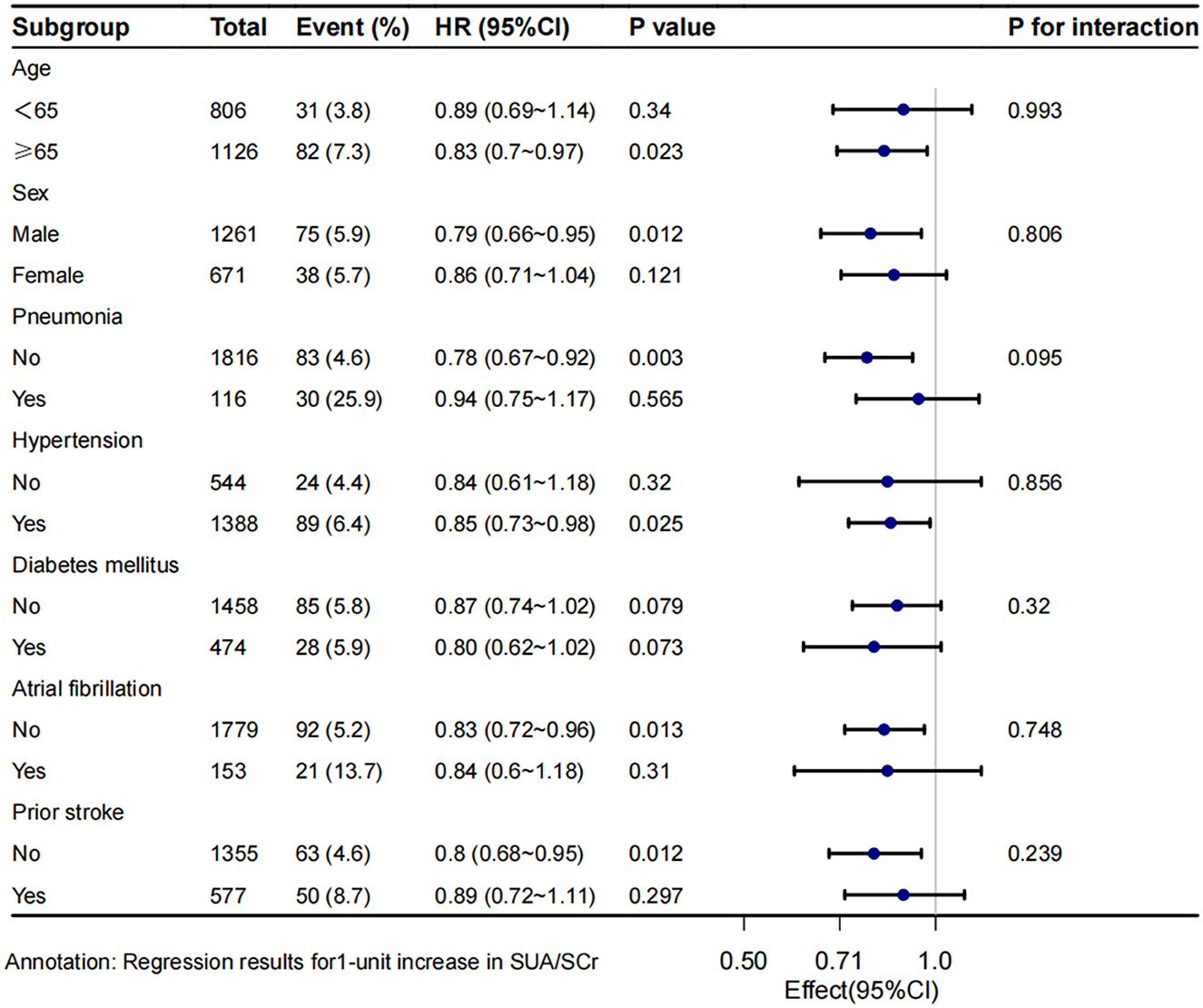
Figure 4. Subgroup analysis of SUA/SCr and 1-year stroke recurrence in patients with AIS and abnormal renal function. Crude model adjust none; Adjusted model adjust for sex, smoking, alcohol consumption, prior stroke, NIHSS score at admission, BMI, alkaline phosphatase, WBC. HR, hazard ratio; CI, confidence interval.
4 DiscussionIn this multicenter prospective cohort study, we identified low SUA/SCr as an independent risk factor for 1-year stroke recurrence, especially in patients with eGFR < 60 mL/min/1.73 m2, revealing a non-linear negative relationship between the two variables. Subgroup analyses showed that in patients with eGFR < 60 ml/min/1.73 m2, Q4 had significantly lower 1-year stroke recurrence risk than Q1. These results underscores the need for clinicians to closely monitor SUA/SCr levels in AIS patients with renal impairment.
Previous studies have demonstrated that a lower SUA/SCr ratio has been linked to poorer functional outcomes at 3 months and 1 year in AIS patients, and SUA/SCr is also positively correlated with the risk of metabolically unhealthy phenotypes (31, 32). Notably, recent studies have shown that SUA/SCr is positively associated with the risk of stroke recurrence in young ischemic stroke patients (33). This is not consistent with our findings from this multicenter study, where we found that lower SUA/SCr levels were associated with 1-year stroke recurrence. Variances in research outcomes can be ascribed to discrepancies in patient populations, number of patients, research approaches, and regions. These findings, all related to stroke, suggest that abnormal SUA/SCr levels are associated with the occurrence and progression of stroke. However, no relevant studies have been conducted in AIS patients with impaired renal function. Our study conducted a prospective analysis of 1,932 patients (mean age: 66.7 ± 11.3 years) with AIS and abnormal renal function to investigate the causal relationship between SUA/SCr and stroke recurrence in patients with abnormal renal function.
Gong et al. utilized data from the Third China National Stroke Registry III and found that lower SUA/SCr values were associated with poor functional outcomes at 3 months and 1 year (31). The association between SUA/SCr and poor functional outcomes was non-linear, showing somewhat L-shaped associations. They also observed a trend suggesting a protective effect of SUA/SCr in patients with abnormal renal function (eGFR < 90 ml/min/1.73 m2), although the association did not reach statistical significance (31). Similarly, our results indicate a protective effect of SUA/SCr in patients with AIS and abnormal renal function. However, we found that lower values of SUA/SCr were associated with 1-year stroke recurrence, and this association was more significant in patients with eGFR < 60 ml/min/1.73 m2. SUA/SCr ratio and 1-year stroke recurrence were negatively and non-linearly associated. In addition to differences in research methodologies and outcomes, their study was based on national average data, whereas ours was based on regional data; consequently, our findings hold implications for regional prevention. Most previous studies have explored and analyzed the association between SUA levels and stroke outcomes, including poor prognosis and mortality. However, the association between SUA levels and stroke recurrence has been poorly reported. A meta-analysis of 23 articles, including 15,733 patients with AIS, showed that SUA has a linear dose-response relationship with outcomes in patients with AIS within a certain limit, with higher baseline SUA indicating better outcomes after AIS (9). Our observations are in line with this conclusion, indicating a protective effect of SUA/SCr on stroke outcomes.
Our study results suggest that among patients with impaired renal function (eGFR < 60 mL/min/1.73 m2), there may be a more pronounced non-linear negative correlation between SUA/SCr levels and the risk of stroke recurrence. The trend of changes across the Q1–Q4 groups showed significant statistical significance, which may indicate a threshold effect of SUA/SCr levels in this population. Specifically, when SUA/SCr levels are higher (Q4 group), the 1-year risk of stroke recurrence may be significantly lower compared to when SUA/SCr levels remain low (Q1–Q3 groups).
Relevant prior studies are limited. A review by Lee et al. (34) reported that baseline eGFR < 60 mL/min/1.73 m2 was independently associated with stroke events across various populations and study designs. Additionally, a study by Cui et al. (35) showed that lower eGFR levels were associated with the highest risk of cardiovascular disease, suggesting that renal function may act as a mediating factor in the relationship between cardiovascular risk factors. Both previous studies and our findings suggest that impaired renal function may also mediate the relationship between SUA/SCr levels and stroke recurrence in AIS patients.
Furthermore, by comparing the clinical characteristics of AIS patients with eGFR < 60 mL/min/1.73 m2 across SUA/SCr quartiles, we found that, compared to the Q1 group, patients in the Q4 group were younger, had lower blood urea nitrogen levels, and higher serum uric acid levels. Other clinical characteristics showed no statistically significant differences among the groups (Supplementary Table 2). This indicates that the relationship between SUA/SCr levels and stroke recurrence in patients with eGFR < 60 is likely influenced by these clinical characteristics. However, further validation in larger and more diverse populations is needed in future studies.
Previous studies have referred to patients with eGFR > 60 mL/min/1.73 m2 as having preserved renal function (29). Among these AIS patients, stroke risk decreased in the Q2 group compared to the Q1 group as SUA/SCr levels increased; however, the trend of changes across groups did not reach statistical significance. This finding suggests that variations in SUA/SCr levels may have a relatively minor impact on stroke recurrence risk in patients with preserved renal function. Although the results we obtained reflect an independent effect after adjusting for relevant confounding factors, the differences in clinical characteristics among the groups may also play a role. By comparing the clinical characteristics of AIS patients with eGFR > 60 across SUA/SCr quartiles, we observed that, compared to the Q1 group, patients in the Q2 group had lower FP and aspartate aminotransferase levels, as well as higher triglyceride and serum uric acid levels (Supplementary Table 3). These differences in clinical characteristics merit attention and warrant further investigation in subsequent studies.
Subgroup analysis revealed no significant interactions in patients with pneumonia, atrial fibrillation, or prior stroke. However, in patients without these conditions, the risk of 1-year stroke recurrence decreased significantly as SUA/SCr levels increased. Previous studies have indicated a positive correlation between SUA/SCr and the severity of dyspnea in patients with chronic obstructive pulmonary disease (COPD), suggesting its predictive value for COPD exacerbation and the severity of the disease (36). In addition, pneumonia has been linked to a higher risk of stroke recurrence (37), and these results support our findings. Interestingly, our subgroup analyses found no significant correlation between increased SUA/SCr values in patients with pneumonia and reduced stroke recurrence risk within 1 year. Similarly, previous studies have shown that AIS related to atrial fibrillation is associated with a high risk of recurrence (38). Prior stroke is also associated with an increased risk of recurrence (39). The results of these studies support our research findings. The association of SUA/SCr with the risk of stroke recurrence was less significant in patients with atrial fibrillation or prior stroke. In summary, for patients without pneumonia, atrial fibrillation, or prior stroke, it is essential to monitor SUA/SCr. Maintaining higher SUA/SCr values may potentially reduce the 1-year risk of stroke recurrence.
The mechanism linking high SUA/SCr with reduced stroke recurrence remains unclear. Previous studies suggest that one possible explanation is the direct antioxidant effect of SUA. As a powerful antioxidant, SUA contributes to approximately two-thirds of the antioxidant capacity in human plasma (40). In ischemic stroke, brain damage is primarily driven by the production of peroxynitrite and free radicals, which are highly reactive oxidant molecules (41). Peroxynitrite is mainly generated in the ischemic penumbra. Free radical levels gradually rise during cerebral ischemia, with a more pronounced increase occurring during reperfusion (42). SUA specifically inhibits peroxynitrite formation and acts as a scavenger of peroxynitrite (43). SUA effectively neutralizes free radicals generated from peroxynitrite decomposition (42). These findings suggest that SUA serves as an endogenous neuroprotective molecule, which may partly explain our results. In addition, our data analysis revealed that higher SUA/SCr levels in patients with AIS and abnormal renal function might be associated with younger age, less of diabetes mellitus and pneumonia, and lower initial stroke severity scores. This characteristic aligns with findings from previous studies, which indicate that stroke recurrence is more likely to occur in elderly patients, as well as in those with diabetes, pneumonia, or severe neurological impairment (37, 44–46). These findings highlight the importance of early attention to SUA/SCr levels, beyond routine interventions targeting comorbidities. Early recognition and timely intervention could significantly reduce the risk of 1-year stroke recurrence in patients with AIS and abnormal renal function. Furthermore, our study showed that higher SUA/SCr levels in these patients might also be associated with elevated triglyceride and aspartate aminotransferase levels. These characteristics suggest that higher SUA/SCr levels may be linked to increased dyslipidemia and liver dysfunction. This finding implies that abnormalities in lipid metabolism and liver function could influence the interaction between SUA/SCr levels and stroke recurrence in patients with AIS and abnormal renal function, warranting further in-depth research and analysis in the future. Our study has some limitations. Firstly, we did not record dynamic changes in the SUA/SCr, which may have affected the stroke outcomes during the follow-up period. Future studies should address this by monitoring these changes over time. Secondly, owing to the lack of data on patients undergoing mechanical thrombectomy, intravenous thrombolysis, or both, we could not analyze this subgroup. Finally, the selected hospitals were all tertiary hospitals in the urban area of Xi'an, and data from surrounding community hospitals were not collected, possibly introducing selection bias. In the future, we plan to expand the scope and amount of data for more in-depth research.
5 ConclusionLow SUA/SCr is an independent predictor of 1-year stroke recurrence in AIS patients with abnormal renal function, particularly in those with eGFR < 60 mL/min/1.73 m2. This underscores the need for clinicians to closely monitor SUA/SCr levels in AIS patients with renal impairment.
Data availability statementThe original contributions presented in the study are included in the article/Supplementary material. Further inquiries can be directed to the corresponding author. Requests to access these datasets should be directed to Songdi Wu, d3Vzb25nZGlAZ21haWwuY29t.
Ethics statementThe studies involving humans were approved by the Academic Committee of Xi'an No. 1 Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributionsZLi: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Software, Writing – original draft. DZ: Conceptualization, Data curation, Writing – original draft. LZ: Conceptualization, Formal analysis, Investigation, Methodology, Project administration, Supervision, Visualization, Writing – review & editing. WG: Data curation, Resources, Writing – review & editing. QL: Data curation, Funding acquisition, Investigation, Resources, Writing – review & editing. ZLe: Data curation, Resources, Writing – review & editing. YH: Data curation, Resources, Writing – review & editing. PL: Conceptualization, Data curation, Investigation, Resources, Writing – review & editing. TL: Data curation, Investigation, Resources, Writing – review & editing. LP: Data curation, Investigation, Resources, Writing – review & editing. QC: Data curation, Investigation, Resources, Writing – review & editing. MZ: Data curation, Investigation, Resources, Writing – review & editing. XL: Conceptualization, Data curation, Funding acquisition, Investigation, Supervision, Validation, Visualization, Writing – review & editing. FW: Data curation, Investigation, Resources, Writing – review & editing. SW: Conceptualization, Funding acquisition, Investigation, Project administration, Supervision, Visualization, Writing – review & editing.
FundingThe author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was supported by the Scientific Research Project of the Xi'an Health Commission (Grant Nos. 2023qn03, 2023qn04, and 2024yb15), the Science and Technology Program of Xi'an city (Grant Nos. 22YXYJ0061, 22YXYJ0074, 23YXYJ0055, 23YXYJ0060, 24YXYJ0081, 24YXYJ0097, and 24YXYJ0089), the Science and Technology Program of Shaanxi Province (Grant Nos. 2023-YBSF-041, 2023-YBSF-048, and 2023-YBSF-052), the Project of Shaanxi Administration of Traditional Chinese Medicine (Grant No. 2022-SLRH-LJ-013) and the Scientific Research Project of the Shaanxi Health Commission (Grant No. 2022C005). The design and analyses of the trial were done independently from the funders.
AcknowledgmentsWe appreciated the contributions of all participating hospitals and the cooperation of all participants in this study.
Conflict of interestThe authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's noteAll claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary materialThe Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2025.1496791/full#supplementary-material
References1. Gu HQ, Yang X, Wang CJ, Zhao XQ, Wang YL, Liu LP, et al. Clinical characteristics, management, and in-hospital outcomes in patients with stroke or transient ischemic attack in China. JAMA Netw Open. (2021) 4:e2120745. doi: 10.1001/jamanetworkopen.2021.20745
PubMed Abstract | Crossref Full Text | Google Scholar
2. Wang Y, Xu J, Zhao X, Wang D, Wang C, Liu L, et al. Association of hypertension with stroke recurrence depends on ischemic stroke subtype. Stroke. (2013) 44:1232–7. doi: 10.1161/STROKEAHA.111.000302
PubMed Abstract | Crossref Full Text | Google Scholar
3. Pan Y, Li Z, Li J, Jin A, Lin J, Jing J, et al. Residual risk and its risk factors for ischemic stroke with adherence to guideline-based secondary stroke prevention. J Stroke. (2021) 23:51–60. doi: 10.5853/jos.2020.03391
PubMed Abstract | Crossref Full Text | Google Scholar
5. Agarwal A, Cheung AK, Ma J, Cho M, Li M. Effect of baseline kidney function on the risk of recurrent stroke and on effects of intensive blood pressure control in patients with previous lacunar stroke: a post hoc analysis of the SPS3 trial (secondary prevention of small subcortical strokes). J Am Heart Assoc. (2019) 8:e013098. doi: 10.1161/JAHA.119.013098
PubMed Abstract | Crossref Full Text | Google Scholar
6. Ovbiagele B, Bath PM, Cotton D, Sha N, Diener H-C, Investigators P. Low glomerular filtration rate, recurrent stroke risk, and effect of renin-angiotensin system modulation. Stroke. (2013) 44:3223–5. doi: 10.1161/STROKEAHA.113.002463
PubMed Abstract | Crossref Full Text | Google Scholar
8. Wang C, Cui T, Wang L, Zhu Q, Wang A, Yuan Y, et al. Prognostic significance of uric acid change in acute ischemic stroke patients with reperfusion therapy. Eur J Neurol. (2021) 28:1218–24. doi: 10.1111/ene.14643
PubMed Abstract | Crossref Full Text | Google Scholar
9. Zhang P, Wang R, Qu Y, Guo ZN, Zhen Q, Yang Y. Serum uric acid levels and outcome of acute ischemic stroke: a dose-response meta-analysis. Mol Neurobiol. (2024) 61:1704–13. doi: 10.1007/s12035-023-03634-y
PubMed Abstract | Crossref Full Text | Google Scholar
10. Amaro S, Urra X, Gómez-Choco M, Obach V, Cervera A, Vargas M, et al. Uric acid levels are relevant in patients with stroke treated with thrombolysis. Stroke. (2011) 42:S28–32. doi: 10.1161/STROKEAHA.110.596528
PubMed Abstract | Crossref Full Text | Google Scholar
11. Zhong J, Cai H, Zhang Z, Wang J, Xiao L, Zhang P, et al. Serum uric acid and prognosis of ischemic stroke: cohort study, meta-analysis and Mendelian randomization study. Eur Stroke J. (2023) 2023:23969873231209620. doi: 10.1177/23969873231209620
PubMed Abstract | Crossref Full Text | Google Scholar
12. Miedema I, Uyttenboogaart M, Koch M, Kremer B, de Keyser J, Luijckx GJ. Lack of association between serum uric acid levels and outcome in acute ischemic stroke. J Neurol Sci. (2012) 319:51–5. doi: 10.1016/j.jns.2012.05.019
PubMed Abstract | Crossref Full Text | Google Scholar
13. Newman EJ, Rahman FS, Lees KR, Weir CJ, Walters MR. Elevated serum urate concentration independently predicts poor outcome following stroke in patients with diabetes. Diabetes Metab Res Rev. (2006) 22:79–82. doi: 10.1002/dmrr.585
PubMed Abstract | Crossref Full Text | Google Scholar
14. Zhu H-Y, Zhao S-Z, Zhang M-L, Wang Y, Pan Z-M, Cheng H-R, et al. Elevated serum uric acid increases the risk of ischemic stroke recurrence and its inflammatory mechanism in older adults. Front Aging Neurosci. (2022) 14:822350. doi: 10.3389/fnagi.2022.822350
PubMed Abstract | Crossref Full Text | Google Scholar
15. Seet RC, Kasiman K, Gruber J, Tang SY, Wong MC, Chang HM, et al. Is uric acid protective or deleterious in acute ischemic stroke? A prospective cohort study. Atherosclerosis. (2010) 209:215–9. doi: 10.1016/j.atherosclerosis.2009.08.012
PubMed Abstract | Crossref Full Text | Google Scholar
16. Johnson RJ, Bakris GL, Borghi C, Chonchol MB, Feldman D, Lanaspa MA, et al. Hyperuricemia, acute and chronic kidney disease, hypertension, and cardiovascular disease: report of a scientific workshop organized by the national kidney foundation. Am J Kidney Dis. (2018) 71:851–65. doi: 10.1053/j.ajkd.2017.12.009
PubMed Abstract | Crossref Full Text | Google Scholar
17. Falsetti L, Capeci W, Tarquinio N, Viticchi G, Silvestrini M, Catozzo V, et al. Serum uric acid, kidney function and acute ischemic stroke outcomes in elderly patients: a single-cohort, perspective study. Neurol Int. (2017) 9:6920. doi: 10.4081/ni.2017.6920
PubMed Abstract | Crossref Full Text | Google Scholar
19. Li M, Gu L, Yang J, Lou Q. Serum uric acid to creatinine ratio correlates with beta-cell function in type 2 diabetes. Diabetes Metab Res Rev. (2018) 34:e3001. doi: 10.1002/dmrr.3001
PubMed Abstract | Crossref Full Text | Google Scholar
20. Ma C, Liu Y, He S, Zeng J, Li P, Ma C, et al. C-Peptide: a mediator of the association between serum uric acid to creatinine ratio and non-alcoholic fatty liver disease in a Chinese population with normal serum uric acid levels. Front Endocrinol. (2020) 11:600472. doi: 10.3389/fendo.2020.600472
PubMed Abstract | Crossref Full Text | Google Scholar
21. Zhong D, Liu D, Guo Y, Huang H, Li L, Wu F, et al. Association of the serum uric acid to creatinine ratio with metabolic syndrome in the middle age and older population in China. Front Endocrinol. (2022) 13:1060442. doi: 10.3389/fendo.2022.1060442
PubMed Abstract | Crossref Full Text | Google Scholar
22. Kleindorfer DO, Towfighi A, Chaturvedi S, Cockroft KM, Gutierrez J, Lombardi-Hill D, et al. 2021 guideline for the prevention of stroke in patients with stroke and transient ischemic attack: a guideline from the American Heart Association/American Stroke Association. Stroke. (2021) 52:e364–467. doi: 10.1161/STR.0000000000000375
PubMed Abstract | Crossref Full Text | Google Scholar
23. Wang Y, Cui L, Ji X, Dong Q, Zeng J, Wang Y, et al. The China National Stroke Registry for patients with acute cerebrovascular events: design, rationale, and baseline patient characteristics. Int J Stroke. (2011) 6:355–61. doi: 10.1111/j.1747-4949.2011.00584.x
PubMed Abstract | Crossref Full Text | Google Scholar
24. Asplund K, Tuomilehto J, Stegmayr B, Wester PO, Tunstall-Pedoe H. Diagnostic criteria and quality control of the registration of stroke events in the MONICA project. Acta Med Scand Suppl. (1988) 728:26
留言 (0)