Tomato is the second-most produced and valuable/consumed vegetable after potato, accounting for up to 20.1% of the total vegetables production, and belongs to Solanaceae family (Horticultural Crops Directorate, 2019). According to epidemiological studies, there is lower risk of cancer and cardiovascular disease when tomato is consumed raw (Clinton, 1998; Giovannucci et al., 2002). On the other hand, tomato bioactive components have antioxidant properties and have been credited with protective functions (Borguini and Torres, 2009). However, tomato production is constrained by pests and diseases that cause high yield loss. In Africa, significant yield loss ranged from 11 to 43% on average, but could reach 100% in some areas due to both direct and indirect effects of insect pests and diseases on tomato (Rwomushana et al., 2019). Among the insect pests that threatened tomato production and productivity, whitefly is ranked as one of the major well-known sucking pests which causes significant crop damage and yield losses as result of nymphs and adults feeding and which also transmits viruses (Abubakar et al., 2022).
The greenhouse whitefly, Trialeurodes vaporariorum Westwood (Hemiptera: Aleyrodidae), is one of the major pests of horticultural crops and causes significant damage to both open-field and protected crops (Wang et al., 2017). Trialeurodes vaporariorum has massive spread on tomato and is the most common and dominant species in various agroecological zones of Kenya (Khamis et al., 2021). Both adults and nymphs of T. vaporariorum damage the plant by feeding on the phloem, which causes significant nutrients lost or deficiency, and consequently lower plant productivity (Arnó i Pujol et al., 2009; Gao et al., 2017). In crops like tomato, Solanum lycopersicum, the honey-dew excreted by whiteflies during feeding also promotes the growth of sooty mould, which discolors and reduces the quality of harvestable/marketable fruits (Anderson et al., 2005). Trialeurodes vaporariorum not only infest the crops directly but also indirectly cause significant economic damage by spreading a number of plant viruses such as,the tomato infectious chlorosis virus (TICV) and the tomato chlorosis virus (ToCV) (Navas-Castillo et al., 2011). The pest transmitted viruses losses hinge on the viral type, disease’s prevalence, and developmental phase of the crop (Lapidot et al., 2014). Tomato infectious chlorosis virus symptoms include interveinal chlorosis, interveinal leaves portions turn brilliant yellow as symptoms worsen but the veins are ever green (Hartono et al., 2003); whereas in tomato chlorosis virus, older leaves have light green polygonal spots that lead to interveinal yellowing, which resembles a magnesium deficiency. Within the yellowed sections, bronzing and red patches are common (Louro et al., 2000).
To manage the greenhouse whitefly, T. vaporariorum transmitted plant viruses, growers rely on vector management mostly through chemical pesticide applications (Whitfield et al., 2015). However, frequent use of pesticides has negative impacts on the dynamics of whitefly vector populations and lead to quick development of resistance as well as resurgence of pesticide resistant whitefly populations (Denholm et al., 2003). Furthermore, thick whitefly’s cuticular layer prevents active ingredients from penetrating the insect’s cuticle, which leads to the difficulty of controlling them in the field using pesticides (Patel et al., 2001; James et al., 2003). Moreover, this difficulty in managing the pest is also aggravated by whitefly’s cryptic behavior (Wang et al., 2017). It is established that alternative hosts diversity provides safety for insects evading pesticide applications, and some insects receiving sub-lethal doses. These insects may therefore carry resistance genes and viruses from different taxonomic groups, thus, new disease complexes developed (Gilbertson et al., 2015).
In addition, synthetic pesticides application in controlling the whitefly has negative effects on the environment, biodiversity, public/human health, and toxic effect to the pollinators as well as chemical residues in the harvested products (Abubakar et al., 2022). Therefore, exploring some agroecologically safe and sustainable approaches as alternatives could overcome the synthetic pesticides drawbacks (Kim et al., 2014). As a result, biological control approaches using microorganism-based products (microbial-based biopesticides) and parasitoids or predators become more sustainable alternatives to be used and promoted in different cropping systems (Naranjo, 2001; Jaber et al., 2018; Sani et al., 2020; Akutse et al., 2020). In addition, endophytes, key microscopic organisms that live inside their host plants without causing any disease symptoms, are also among biological agents that induce protection of their hosts against herbivorous and diseases (Vega et al., 2008; Poveda, 2021b). They have been demonstrated to cause improved protection against herbivorous insects through the induction of systemic resistance, most likely as a result of changes in biochemistry and physiology of the associated host plants (Rostás et al., 2006; Pineda et al., 2012). Biocontrol agent such as Encarsia formosa (Hymenoptera: Aphelinidae) is a well known host-specific parasitoid of T. vaporariorum but its application at a larger scale was significantly hampered by the abusive use of synthetic chemicals. In addition, it was also established that the efficacy of entomopathogenic fungus Beauveria bassiana in controlling insect pests like the greenhouse whitefly highly depends on the environmental factors such as temperature and relative humidity, and its pathogenicity/virulence level is pests specific. Paradza et al. (2021b) previously demonstrated that the fungal endophytes Hypocrea lixii F3ST1 and Trichoderma asperellum M2RT4 endophytically colonized S. lycopersicum and suppress T. vaporariorum nymph development and adult emergence. Therefore, this study explored the validation of these findings under field conditions. In addition, the authors also hypothesized that the suppression of T. vaporariorum vector could also reduce the viral diseases transmitted by the pest. The current study was therefore designed to explore the efficacy of these two fungal endophytes in controlling the T. vaporariorum vector and their potency in reducing tomato infectious chlorosis virus and tomato chlorosis virus incidence and severity under field conditions.
2 Materials and methods2.1 Study sites and field preparation for plantingField trials were conducted at Ngoliba (Kiambu county) and Mwea (Kirinyaga county), Kenya with geographical references of 1° 04’ 22.8”S 37°18”19.0”E, 1399 m a.s.l and 0° 37’7.35”S 37° 22’.973 “E, 1159 m a.s.l, respectively, for two consecutive seasons: season one (October 2022 – February 2023) and season two (April – August 2023). The field site in Mwea had deep and moderately fertile volcanic soils while Ngoliba has Pellic vertisol soil type. The experimental fields were prepared by slashing, tilling and harrowing to obtain fine tilth. Each of the experimental field was set at 21 cm × 29 m plots and was sub-divided into 28 experimental units of 4 m × 3 m each; with a path of 1 m between them.
2.2 Fungal cultures and insecticideThe fungal endophytes, Trichoderma asperellum M2RT4 and Hypocrea lixii F3ST1 which were previously reported to colonize tomatoes (Agbessenou et al., 2020; Paradza et al., 2021a) were obtained from International Centre of Insect Physiology and Ecology (icipe)’s germplasm at Arthropod Pathology Unit. The two endophytes were also reported to suppress T. vaporariorum nymph’s population and adult emergence. Trichoderma asperellum M2RT4 and H. lixii F3ST1 were cultured on Potato Dextrose Agar (PDA), while Sabouraud Dextrose Agar (SDA) was used to culture Metarhizium anisopliae ICIPE 18 which was also proved to be effective against T. vaporariorum adults and nymphs through inundative application (Paradza et al., 2021b). The culture plates were incubated at 25 ± 2°C in complete darkness.
In addition, Buprofezin insecticide, recommended insecticide according to the Pest Control Products Board (PCPB-Kenya) used in this study was obtained from an Agrovet (Nairobi, Kenya). Buprofezin insecticide was selected because of its effectiveness against the whiteflies and commonly used by farmers (Sain et al., 2021).
2.3 Mass production of fungal isolates and viability assessmentFor the field trials against the T. vaporariorum vector and targeted diseases, conidia of T. asperellum M2RT4, H. lixii F3ST1 and M. anisopliae ICIPE 18 were mass produced using Pishori grain rice on a long-rice substrate in Milner bags (60 × 35 cm). Rice substrate was autoclaved for 1 hour at 121°C, allowed to cool and inoculated with a 3-day-old culture of blastospores T. asperellum M2RT4, H. lixii F3ST1, and M. anisopliae ICIPE 18 in separate Milner bags (Jenkins et al., 1998). The inoculated bags were then incubated for 21 days at 20 – 26 ° C and 40 – 70% relative humidity RH). The rice substrate containing the sporulated fungal spores was then allowed to dry for five days at room temperature while in the Milner bags. Conidia were harvested by sifting the substrate through a 295-μm mesh sieve and then stored in plastic bags in a refrigerator for less than three weeks at 4 - 6°C using Opisa et al. (2018) approach.
The viability of the fungal isolates was determined before being used for field trials. The harvested conidia suspended in 10 ml distilled water with 0.05% Triton X-100 in universal bottles containing glass beads (Φ = 3 mm). The suspension was vortexed for 5 min at 700 rpm to break the conidial clumps and ensure a homogeneous suspension. Conidial concentrations were quantified using a haemocytometer under a light microscope. The conidial suspensions were adjusted to 1 × 108 conidia/ml through dilution prior to field applications. For viability test, a concentration of 3 × 106 conidia/ml was prepared, and 0.1 ml of the suspension was evenly spread on SDA or PDA plate and three sterile microscope cover slips were placed randomly on the surface of each inoculated plate. The plates were sealed with Parafilm and incubated under complete darkness at 25 ± 2°C. Conidia germination was assessed after 18 hours by counting 100 randomly selected conidia beneath each coverslip under a light microscope at 400× magnification. Conidia were considered to have germinated when the length of the germ tube was at least twice the diameter of the conidium (Goettel and Inglis, 1997; Inglis et al., 2012). Four replicate plates were used per isolate, and viability of each fungal isolate was determined, where approximately 96% of conidial germination was observed in all the fungal isolates.
2.4 Seed inoculation, seedlings maintenance and transplantationTomato (Solanum lycopersicum L. cv. “Moneymaker”) seeds were obtained from Simlaw Seeds Company Ltd., Nairobi, Kenya and were surface sterilized by washing them up successively in 70% ethanol for 2 min followed by 1.5% sodium hypochlorite for three 3 min, and finally rinsed three times in sterile distilled water. The surface sterilized seeds were placed on sterile filter paper on a clean working surface in a cabinet until the residual water evaporated. Effectiveness of the surface sterilization technique was confirmed by plating out 0.1 ml of the last rinse water onto potato dextrose agar and also imprinting of surface sterilized seeds onto PDA (tissue imprint) supplemented with 100 mg/l Streptomycin and plates were incubated at 25°C for 14 days (Schultz et al., 1998; Akutse et al., 2013). Seeds were then soaked overnight for 12 hours in conidial suspensions titrated at 1 × 108 conidia ml-1. For the controls, sterilized seeds were soaked overnight for 12 hours in sterile distilled titrated (0.05% Triton X-100) water (Akutse et al., 2013; Agbessenou et al., 2020; Paradza et al., 2021a).
Seeds were then sown in each planting trays containing the planting substrate (mixture of manure and soil 1:5) in screen house as per the defined treatments. The substrate was sterilized in an autoclave for 2 h at 121°C and allowed to cool for 72 h prior to planting. Seeds were sowed and maintained at room temperature (25 ± 2°C, 60% RH and 12:12 L:D photoperiod). The planting trays were transferred immediately after germination to the screen house (2.8 m length × 1.8 m width × 2.2 m height) at 25 ± 2°C, 55% RH and 12:12 L:D photoperiod. Seedlings were watered twice per day (morning and evening) until when they developed 4-6 leaves. No additional fertilizer was added to the planting substrate prior to the seedlings’ transplantation.
Three weeks after germination, seedlings were transplanted at both prepared sites (Ngoliba and Mwea) on planting bed in holes which were amended with 20 g each of well decomposed goat manure mixed well with soil. Transplanting was done at a spacing of 0.6 × 0.75 m in 4 × 3 m plot giving the total number of 16 plants per plot.
2.5 Endophytic colonization of Solanum lycopersicum assessmentEndophytic colonization was determined when seedlings were three-week-old after transplanting. Seedlings (both inoculated and uninoculated) were randomly selected from each treatment, uprooted and washed under running tap water to remove any soil attached to the plants after which they were separated into different parts each (root, stem, and leaf) and cut into 1 × 1 cm pieces, and surface sterilized under a hood as described above (Jaber et al., 2018). Five fragments of each section of the plant parts were plated 4 cm from one another on PDA plates amended with antibiotics (0.25 g/l w/v chloramphenicol) (Akello et al., 2007a; Batta, 2013; Gathage et al., 2016). The plates were incubated for 10 days at 25 ± 2 °C, after which the presence of endophytes was determined. The last rinse water was also plated to assess the effectiveness of the surface sterilization procedure as described earlier. Plate imprinting was also conducted to assess effective surface sterilization of the plant materials (Schultz et al., 1998; Akutse et al., 2013). The colonization of the different plant parts was recorded by counting the number of pieces of the different plant parts that showed the presence of inoculated fungal growth/mycelia according to Koch’s postulates (Petrini and Fisher, 1986). Only the presence of endophytes that were inoculated was scored. The colonized and reisolated fungal isolates were identified morphologically using slides which were prepared from the mother plates. Treatments were arranged in a randomized complete block design (RCBD) with four replicates per experiment (Akutse et al., 2013; Agbessenou et al., 2020). The proportion of the plant parts colonized by the inoculated fungal isolate was calculated for each treatment as the number of plant pieces showing fungal outgrowth divided by the total number of plant pieces plated (Paradza et al., 2021a).
2.6 Experimental design, treatments and trial managementField experimental trial was laid out in a Randomized Complete Block Design (RCBD) with seven treatments, replicated four times per season at each site. The fungal inoculated and uninoculated seedlings were transplanted as per the defined treatments. The following treatments were applied: H. lixii F3ST1-inoculated tomato seedlings (T1), T. asperellum M2RT4-inoculated tomato seedlings (T2), F3ST1- inoculated tomato seedlings + M. anisopliae ICIPE 18 (T3), T. asperellum M2RT4-inoculated tomato seedlings + M. anisopliae ICIPE 18 (T4), uninoculated tomato seedlings + M. anisopliae ICIPE 18 (T5), uninoculated seedlings with Buprofezin insecticide (T6), and uninoculated seedlings alone (Control) (T7). 0.75 L of Buprofezin insecticide was formulated in 15 L of water and sprayed using Knapsack twice at third and fifth weeks after transplanting. In addition to the endophytes, the M. anisopliae ICIPE 18 which was initially identified very effective against T. vaporariorum adults and nymphs, was applied in inundative application, where the fungus was formulated in olive oil (obtained from Bidco Africa Limited, Thika, Kenya) (Paradza et al., 2021b). The oil formulation was done using dry conidia that were formulated with the oil by emulsifying 2% (v/v) in 0.05% Triton X-100 water containing 1 × 108 conidia/ml fungal suspension (Kirubakaran et al., 2014; Paradza et al., 2021b). In addition, the conidia were suspended in olive oil with 0.05% Integra (sticker, Greenlife Crop Protection Africa Ltd, Nairobi, Kenya) with 0.1% nutrient agar, 0.1% glycerine and 0.5% molasses added as protectants and attractants, respectively. The application was done using Knapsack sprayer at the rate of 0.15 l suspension (where 10 ml of oil-formulated product in 20 l of water) per experimental unit. Control treatment was sprayed with water containing 0.05% Triton X-100, 0.05% Integra, 0.1% nutrient agar, 0.05% molasses and 0.1% glycerine without any fungal conidia and any insecticide solution (Kabaale et al., 2022; Mweke et al., 2019). Two applications were performed at three and five weeks, respectively after transplanting. The applications were done early in the morning for two times per season.
The field experimental trial was repeated for two consecutive seasons at the two sites (Mwea and Ngoliba plots). After transplantation, watering was done using hose pipe as required and weeding was done whenever necessary using hand hoe. At the vegetative stage, booster foliar fertilizer was applied at the rate of 0.5 L in 50 L of water to help in vegetative growth of the tomato crop following the tomato farmers’ practices as per the experimental sites. Staking was done using supporting sticks and there were no fungicides or other pesticides applications throughout the experimental trials.
2.7 Sampling and molecular identification of the whitefly collected from the experimental sitesWhiteflies were randomly sampled from the experimental field sites. During the sampling whiteflies were collected from the underside of the leaves using camel brush. Twenty (20) whiteflies were randomly selected and placed in universal bottles containing 95% ethanol and transported to the laboratory where they were stored at -20 °C. Prior to DNA extraction, each of the insect was surface sterilized in 3% Sodium hypochlorite and rinsed three times with sterile distilled water. The genomic DNA was extracted using isolate II genomic DNA Kit (Bioline, London, UK), following the manufacturer’s instructions (Khamis et al., 2021). The purity and concentration of the resultant extracted DNA was determined using Nanodrop 2000/2000c Spectrophotometer (Thermo Fischer Scientific, Wilmington, USA) then stored at − 20°C for used in downstream processes. Polymerase chain reaction (PCR) was done to amplify a portion of the 16S ribosomal RNA (rRNA) gene region using WF-F (50 -CGCCTGTTTAACAAAAACAT-30) and WF-R (50 - CCGGTCTGAACTCAGATCACGT-30) primers (Alhudaib et al, 2014; Frohlich et al., 1999). The PCR was carried out in a total reaction volume of 20 μL containing 5X My Taq Reaction Buffer (5 mM dNTPs, 15 mM MgCl2, stabilizers and enhancers) (Bioline, London, UK), 0.5 pmol μl−1 of each primer, 0.5 mM MgCl2, 0.0625 U μl−1 My Taq DNA polymerase and 15 ng μl−1 of DNA template. This reaction was set up in the Mastercycler gradient Nexus thermal cycler (Eppendorf, Hamburg, Germany). The following cycling conditions were used: initial denaturation for 2 min at 95°C, followed by 40 cycles of 30 s at 95°C, 40 s annealing and 1 min at 72°C, then a final elongation step of 10 min at 72°C. The target gene region was 650–700 base pairs. The amplified PCR products were resolved through a 1.2% agarose gel. The DNA bands on the gel were analyzed and documented using KETA GL imaging system trans-illuminator (Wealtec Corp, Meadowvale Way Sparks, Nevada, USA). Successively amplified products were excised and purified using Isolate II PCR and Gel Kit (Bioline) following the manufacturer’s instructions. The purified samples were shipped to Macrogen Inc. Europe Laboratory, Amsterdam, the Netherlands, for bi-directional sequencing. The target species from both study sites were identified to be T. vaporariorum and the sequences have been submitted/deposited to GenBank, a database maintained by the National Center for Biotechnology Information (NCBI) under accession numbers OK500119.1, MW60307.1, NC_006280.1.
2.8 Trialeurodes vaporariorum population densitiesWeekly samplings were conducted to determine T. vaporariorum population densities. Five plants were randomly selected and tagged using masking tape in each plot/unit. Using a magnifying lens, T. vaporariorum nymphs were counted from two lower, two middle, and two upper leaves of each plant by turning over the leaves because nymphs cluster on the undersides. Leaves were marked with mark pen to avoid sampling the same leaves many times, and a different marker was used in each sampling point. The total number of nymphs was recorded and averaged for five weeks during the vegetative stage of the crop.
2.9 Assessment of tomato infectious chlorosis and tomato chlorosis disease incidence and severityThe disease incidence and severity symptoms of both viruses were determined visually in all the experimental plots/units. The percentage of each disease incidence was determined by counting the total number of diseased plants in each plot divided by the total number of plants in the plot/unit, multiplied by hundred. On the other hand, disease severity was determined using a ranking scale of 0 – 5 as described by Nelson et al. (1999)with 0- there are no signs or symptoms of the disease; 1- mild mottling; 2- mottling on the leaf area/light downward cupping; 3- pronounced downward or upward leaf chlorosis leaf mottling; 4- severe mosaic/leaf distortion/crinkled leaf/plant stunting/leaf bunching; 5- severe leaf distortion/necrosis/narrowed or shoes-string leaf.
The incidence and severity were recorded at weekly intervals and averaged during the vegetative stage of the crop time over time.
2.10 Identification of the tomato infectious chlorosis and tomato chlorosis virusesTo confirm the viruses causing each targeted and assessed disease, symptomatic leaves of tomato infectious chlorosis and tomato chlorosis viruses were collected and put in paper bags and brought to the library in cool boxes and were stored in – 80°C incubators. They were later ground in cold extraction buffer (0.5 M trisodium citrate 0.1% thioglycolic acid). RNA was extracted from 200 µL homogenate in line with manufacturer’s instructions. Homogenate was heated at 90°C for 5 min and RT- PCR, TOCV specific primer was designed based on end region sequence of TOCV RNA, and synthesis was done by DNA synthesis. RT-PCR was then performed using specific primers created in the coat protein (CP) gene or the HSP70 homologue gene (Jacquemond et al., 2009). Each sample was lysed using a BioSpec Mini-Beadbeater-16 high-energy cell disruptor (USA) in a sterile 1.5 ml microcentrifuge tube containing plastic beads and 500 l of lysis buffer. In the final step before elution, the column was spun dried to get rid of any unwanted leftover ethanol in the wash buffer. Thermo Fisher Scientific’s nanodrop 1000 spectrophotometer was used to measure the final elution. Nested RT-PCR assays were run under the following conditions: a first denaturizing step at 95°C for 2 min, 40 cycles divided into 30 s at 95°C,1 min at 72°C, annealing at 40 s and finally one final extension step at 72°C for 10 min. The target DNA was detected by 1.5% agarose gel electrophoresis in 0.1X TBE buffer and ethidium bromide staining of the final RT-PCR products were visualized under UV light. The cleaned samples were sent to the Amsterdam, Netherlands-based Microgen Inc. Europe Laboratory for sequencing, and the sequences were subsequently uploaded into GenBank.
2.11 Solanum lycopersicum yieldTomato fruits were harvested after they have reached maturation stage, at weekly interval and the fruits were sorted into damaged, cracked, diseased and marketable categories. The average weight of both damaged, cracked, diseased and marketable fruits was taken and recorded as per each experimental unit. In addition, three yield parameters such as number of fruits, fruit weight, and yield were collected in the manner outlined by Liu et al. (2019), where the total number of fruits per experimental unit and the individual fruit weight were obtained by counting and weighing each marketable fruit. The yield (kg per experimental unit) was obtained by totaling the weight of all fruits harvested from each plant over time. The yield estimation in tons per ha was calculated using the below formula described by Ali et al. (2016):
Yield (T ha−1)= Yield per experimental unit (kg) x 10,000Experimental unit area (m2) x 1,0002.12 Data analysisData on fungal endophytic colonization, whitefly nymph population densities, disease severity were analyzed using Generalized Linear Model (GLM) with binomial distribution and logit link function to determine treatments and study sites effects. Where there was over-dispersion in the data a quassi- Poisson distribution was used. Means were separated using Tukey’s Honesty significance difference (Tukey’s HSD) at a significance level of P < 0.05. Data for each of the site were analyzed separately per season, however comparisons were done among the treatments. The approximate χ2 distribution of the deviance, which reflects the treatment effects in GLM, is presented as test statistics. The data on disease incidence and yield were analyzed using analysis of variance (ANOVA) and the means were separated using HSD and Student–Newman–Keuls (SNK) test, respectively. All the data were analyzed using R version 4.2.2 statistical software (R Core Team, 2022).
3 Results3.1 Endophytic colonization of Solanum lycopersicumThe two endophytes T. asperellum M2RT4 and H. lixii F3ST1 successfully colonized the roots, stems and leaves of the tomato plants in both sites and seasons. The colonization of the two endophytes was confirmed not only by colonization rates, but also by the presence of mycelia growth of the same inoculated fugus in comparison with the mother plates. However, no colonization was observed in the uninoculated plant treatments at the two experimental sites (Figure 1). In season 1, the colonization ranged between 90 - 100%, 65 - 95% and 35 - 85% for the root, stem and leaf respectively in Mwea (Figure 1A); while in Ngoliba 100%, 75 - 95% and 45 - 85% colonization were recorded for root, stem and leaf respectively (Figure 1B). At Mwea in season 1, there were significant differences in the colonization rates of the roots (χ2 = 8.46, df= 6, P = 0.04), stems (χ2 = 9.11, df = 6, P = 0.02) and leaves (χ2 = 13.02, df = 6, P = 0.005) among the treatments (Figure 1A). High colonization rates of the roots were recorded for T. asperellum M2RT4 (90 – 100%) and H. lixii F3ST1 (100%). However, T. asperellum M2RT4 colonized 85 – 95% of the stems compared to 65 – 70% for H. lixii F3ST1; while 60 – 70% and 35 – 85% colonization rates of the leaves were recorded for T. asperellum M2RT4 and H. lixii F3ST1, respectively (Figure 1A). Similar trends were also observed at Ngoliba in season 1 where no significant differences were observed in roots colonization among both endophyte treatments (P = 1), where the root colonization rate was 100% for both H. lixii F3ST1 and T. asperellum M2RT4 (Figure 1B). Similarly, no significant difference (P = 0.21) was observed in the colonization of the stems by H. lixii F3ST1 (75 – 80%) and T. asperellum M2RT4 (87.5 – 95%). However, there was significant difference (χ2 = 9.73, df = 6, P = 0.02) in the colonization of the leaves by H. lixii F3ST1 (45 – 85%) and T. asperellum M2RT4 (55 – 70%). No colonization was recorded for uninoculated treatment plants (Figure 1B).
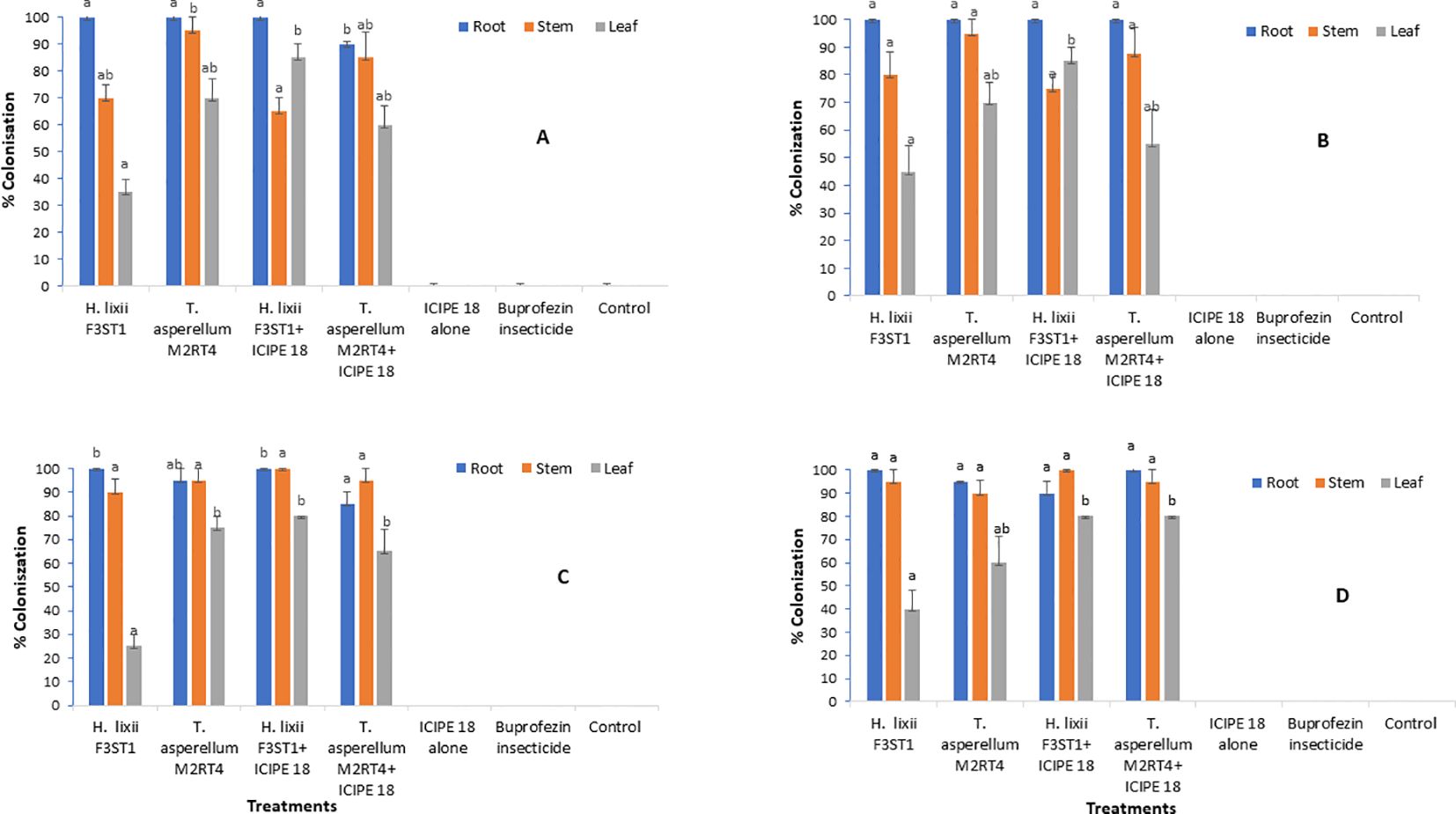
Figure 1. Endophytic colonization rates of the different parts of Solanum lycopersicum plants at Mwea (A, C) and Ngoliba (B, D) during season 1 (A, B) and season 2 (C, D). Means followed by the same letters are not significantly different from each other at P < 0.05.
In season 2 at Mwea, significant difference (χ2 = 11.54, df = 6, P = 0.009) was observed in root colonization rates where H. lixii F3ST1 treatments recorded 100% compared to 85 – 95% in T. asperellum M2RT4 treatments (Figure 1C). Similarly, there was significant difference (χ2 = 52.53, df = 6, P < 0.0001) in the colonization rates of the leaf by H. lixii F3ST1 treatments (25 – 80%) as compared to 65 – 75% for T. asperellum M2RT4 treatments (Figure 1C). However, there was no significant difference (χ2 = 2.34, df = 6, P = 0.50) in the colonization rates of the stem where H. lixii F3ST1 treatments recorded 90 – 100% compared to 95% for T. asperellum M2RT4 treatments (Figure 1C). Similar trends were also observed in season 2 at Ngoliba with no significant difference (P = 0.21) in root colonization rates by H. lixii F3ST1 treatments (90 – 100%) compared to 95 – 100% for T. asperellum M2RT4 treatments (Figure 1D). Similarly, no significant difference (P = 0.50) was observed in the colonization of the stem with H. lixii F3ST1 treatments recording 95 – 100% compared to T. asperellum M2RT4 treatment which recorded 90 – 95%. However, significant difference (χ2 = 18.51, df = 6, P = 0.0003) was observed in the colonization rates of the leaves where H. lixii F3ST1 treatments colonized 40 – 80% compared to 60 – 80% for T. asperellum M2RT4 treatments (Figure 1D). The colonization rates of the leaves observed which were lower compared to the other parts of the plant in both seasons and sites could be attributed to endophytic fungus tissue-specificity and the physiological conditions of the plants.
3.2 Effect of fungal endophytes on Trialeurodes vaporariorum nymphs’ population densitiesThe two fungal endophytes H. lixii F3ST1 and T. asperellum M2RT4 suppressed the T. vaporariorum nymph’s population. However, there was no significant interaction between sites and treatments in season 1 (χ2 = 1.72, df = 6, P = 0.94). In addition, no significant difference was observed between both (Mwea and Ngoliba) sites (χ2 = 0.05, df = 6, P = 0.82). However, there were significant differences between the treatments at Mwea (χ2 = 39.62, df = 6, P < 0.0001) and Ngoliba (χ2 = 27.87, df = 6, P < 0.0001) regarding the T. vaporariorum nymphs’ population densities in season 1 (Figures 2A, B). The overall mean number of T. vaporariorum nymphs ranged between 24.70 and 58.75 and between 25.80 and 56.35 per plant in each treatment plot at Mwea (Figure 2A) and Ngoliba (Figure 2B) respectively. In season 1, fewer mean numbers of nymphs were recorded in plants inoculated with H. lixii F3ST1 (24.70 ± 3.8) and T. asperellum M2RT4 (30.75 ± 6.5) compared to the control plots (58.75 ± 6.6) at Mwea (Figure 2A) versus H. lixii F3ST1 (25.80 ± 3.6) and T. asperellum M2RT4 (28.85 ± 2.6) compared to control (56.35 ± 4.2) at Ngoliba (Figure 2B). In addition, the endophytes treated plots recorded the fewer number of nymphs in season 1 compared to buprofezin insecticide treated plots (Figures 2A, B).
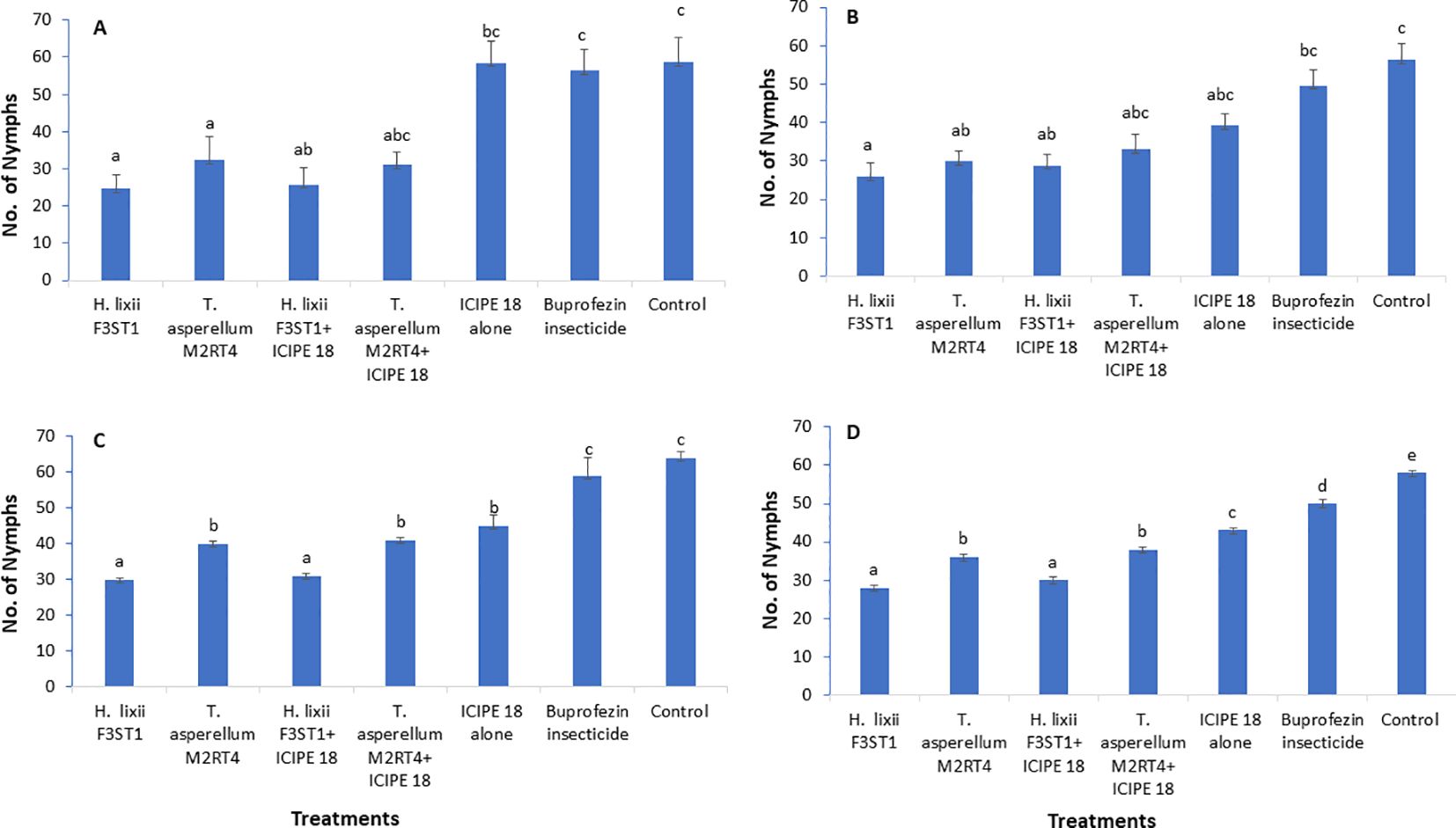
Figure 2. Effect of the different treatments on mean number of Trialeurodes vaporarium nymphs per plant within a plot/experimental unit at Mwea (A, C) and Ngoliba (B, D) during season 1 (A, B) and season 2 (C, D). Bars represent means ± SE. Means followed by the same letters are not significantly different from each other at P < 0.05.
In season 2, there was a significant difference between both (Mwea and Ngoliba) sites (χ2 = 18.12, df = 6, P < 0.0002). However, no significant interaction was observed between the sites and treatments (χ2 = 7.8, df = 6, P = 0.25). There were highly significant variations between the treatments with more T. vaporariorum nymphs recorded in the controls than in the endophyte treatments at Mwea (χ2 = 208.6, df = 6, P < 0. 0001; Figure 2C) and Ngoliba (χ2 = 1124.3, df = 6, P < 0.0001; Figure 2D). Moreover, the endophytes treated plots recorded the fewer number of nymphs in season 2 compared to buprofezin insecticide treated plots (Figures 2C, D). The number of nymphs varied between 30 and 41 and between 45 and 64 in endophyte-inoculated treatments compared to uninoculated plots, respectively at Mwea (Figure 2C); while at Ngoliba, the number of nymphs varied between 28 and 38 and between 43 and 58 respectively (Figure 2D). Fewer numbers of nymphs were recorded in H. lixii F3ST1 (30 ± 0.51) and T. asperellum M2RT4 (40 ± 0.6) compared to the control (64 ± 1.7) plots at Mwea (Figure 2C), versus in H. lixii F3ST1 (28 ± 0.7) and T. asperellum M2RT4 (36 ± 0.8) compared to the control (58 ± 0.5) plots at Ngoliba (Figure 2D).
3.3 Effect of fungal endophytes on incidence of tomato infectious chlorosis diseaseThere was no significant interaction between sites and treatments in season 1 (F6, 42 = 0.2, P = 0.97) and no significant variation was also observed between the experimental sites (F1, 6 = 3.18; P = 0.08). Significant differences were observed among the treatments at Mwea (F6, 21 = 5.09, P = 0.002) and Ngoliba (F6, 21 = 7.24, P = 0.0002) in disease incidence. The disease incidence rates varied between 20.73 ± 0.51 and 38.16 ± 1.82% and between 18.09 ± 1.77 and 29.30 ± 2.45% in endophytically colonized treatments at Mwea and Ngoliba respectively (Table 1). However, the lowest disease incidence rates were recorded in H. lixii F3ST1 endophytically colonized plants at Mwea (20.73 ± 1.51%) and Ngoliba (18.09 ± 1.77%); while the incidence rates of 38.16 ± 3.65 and 25.2 ± 1.55% were obtained in T. asperellum M2RT4 endophytically colonized treatments at Mwea and Ngoliba, respectively. The highest disease incidence rates were recorded in the controls at Mwea (55.55 ± 5.83%) and Ngoliba (52.34 ± 5.18%) in season 1 (Table 1). Furthermore, the endophytes treated plots significantly reduced the incidence of tomato infectious chlorosis virus compared to the treatments where buprofezin insecticide was applied (Table 1).
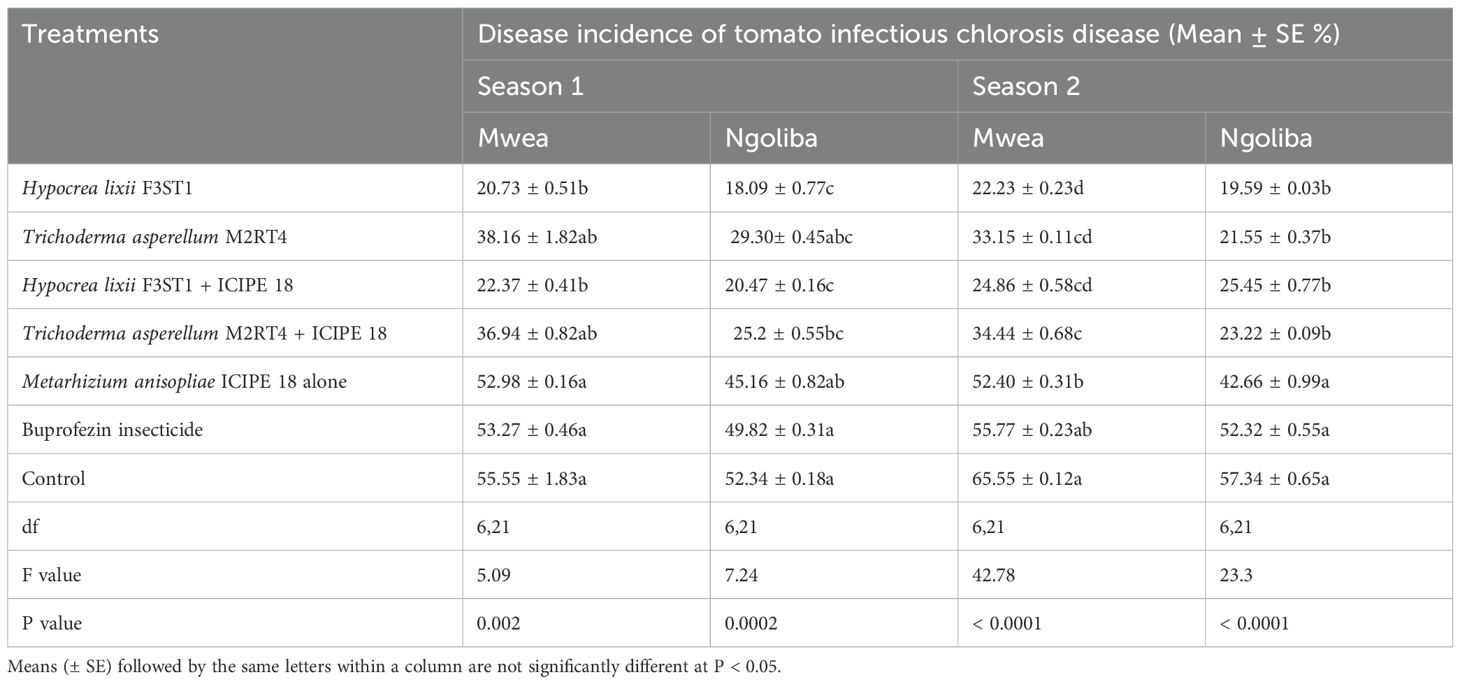
Table 1. Effect of the various treatments on the mean percentage of tomato infectious chlorosis disease incidence during the two seasons at Mwea and Ngoliba.
In season 2, similar trends were also observed in both experimental sites with significance differences in disease incidence among treatments at Mwea (F6, 21 = 42. 78, P = 0.0001) and Ngoliba (F6, 21 = 23.3, P = 0.0001). Regarding the site effects, significant difference was observed between the two sites (F1, 6 = 17.72; P = 0.0001), while no significant interaction was observed between the sites and treatments (F6, 42 = 1.26, P = 0.29). The lowest disease incidence rates were recorded in H. lixii F3ST1 endophytically colonized treatments at Mwea (22.23 ± 1.23%) and Ngoliba (19.59 ± 1.03%); while the incidence rates of 33.15 ± 1.11 and 21.55 ± 0.37% were obtained in T. asperellum M2RT4 endophytically colonized tomato crops compared to the control treatments at Mwea (65.55 ± 1.12%) and Ngoliba (57.34 ± 3.65%) respectively (Table 1). On the other hand, the endophytes treated plots reduced the incidence of tomato infectious chlorosis virus compared to the treatments where buprofezin insecticide was applied (Table 1). In general, the fungal endophytes significantly reduced the incidence of tomato infectious chlorosis viral disease and could be developed as fungal-based biopesticide to manage the incidence of the viral disease in tomato.
3.4 Effect of fungal endophytes on severity of tomato infectious chlorosis diseaseThe fungal endophytes reduced the severity of tomato infectious chlorosis disease. In season 1, significant differences were observed in disease severity among the various treatments at Mwea (χ2 = 85.43, df = 6, P <0.0001; Figure 3A) and Ngoliba (χ2 = 30.49, df = 6, P < 0.0001; Figure 3B). However, there was no significant interaction between the sites and treatments (P = 0.89), and no significant variation was also observed between the two experimental sites (P = 0.930). The endophytically colonized S. lycopersicon crops had lower disease severity than the control treatments at both Mwea and Ngoliba (Figures 3A, B). In season 2, there was no significant interaction between both sites and the treatments (χ2 = 8.6, df = 6, P = 0.19). However, there were significant differences among the treatments at Mwea (χ2 = 129.4, df = 6, P < 0.0001; Figure 3C) and Ngoliba (χ2 = 77.1, df =6, P < 0.0001; Figure 3D) regarding the disease severity. For instance, the highest disease severity was recorded in the control treatments at Mwea (24.51 ± 1.04%) and Ngoliba (22.87 ± 2.39%) as compared to the endophytically colonized treatments which recorded the lowest disease severity at both sites (Figures 3C, D). The endophytes treated tomato crops recorded the lowest disease severity compared to the insecticide treatments which recorded the highest disease severity (Figures 3A–D).
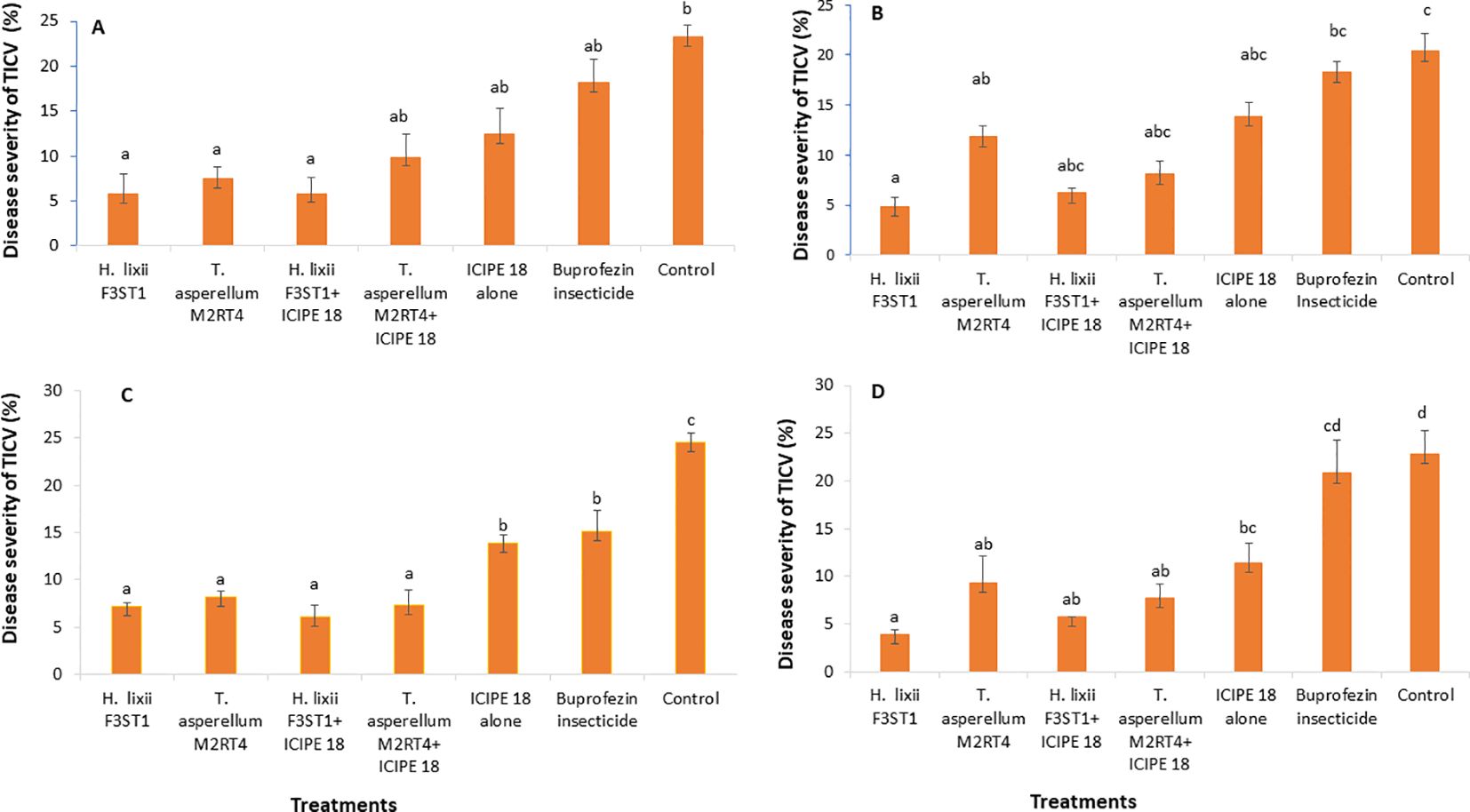
Figure 3. Effect of the various treatments on the mean percentage of tomato infectious chlorosis disease severity at both Mwea (A, C) and Ngoliba (B, D) during season 1 (A, B) and season 2 (C, D). Bars denote means ± SE and means followed by the same letters are not significantly different at P < 0.05.
3.5 Effect of fungal endophytes on incidence of tomato chlorosis diseaseThere was high reduction in the incidence of tomato chlorosis disease in the fungal endophyte plots which could be attributed to a tendency toward delay in symptoms development in inoculated plants compared to the controls. In addition, there was significant difference between the experimental sites (F1, 6 = 0.54; P < 0.0008) in season 1, but no significant interaction was observed between the sites and the treatments (F6, 42 = 1.07; P= 0.39). However, there were significant differences in tomato chlorosis disease incidence among the treatments at Mwea (F6, 21 = 3.41; P= 0.02) and Ngoliba (F6, 21 = 4.73; P= 0.003). The lowest disease incidence rates were recorded in fungal inoculated treatments compared to controls at both Mwea and Ngoliba (Table 2). Similarly, in season 2, there was significant difference between both sites (F6, 42 = 24.38; P < 0.0001) and significant interactions were also observed between the sites and treatments (F6, 21 = 24.38; P = 0.04). The disease incidence rates varied among the treatments with the lowest incidence recorded in H. lixii F3ST1 and T. asperellum M2RT4 endophytically colonized plots. For instance, in season 2, significant differences were observed between endophyte inoculated and uninoculated treatments at Mwea (F6, 21 = 21.68; P < 0.0001) and Ngoliba (F6, 21 = 19.06; P < 0.0001). The disease incidence rates also varied among the treatments with the highest rates recorded in the controls at Mwea (65.58 ± 0.52%) and Ngoliba (52.21 ± 0.32%) compared to the endophyte treated plots; where the lowest disease incidence rates were recorded in H. lixii F3ST1 (18.88 ± 0.90 and 16.49 ± 025%) and T. asperellum M2RT4 (34.04 ± 0.14 and 27.57 ± 0.94%) endophytically colonized tomato crops at Mwea and Ngoliba, respectively (Table 2). The endophytes treated tomato crops recorded the lowest tomato chlorosis virus disease incidence compared to the insecticide treated plots which recorded the highest disease severity (Table 2).
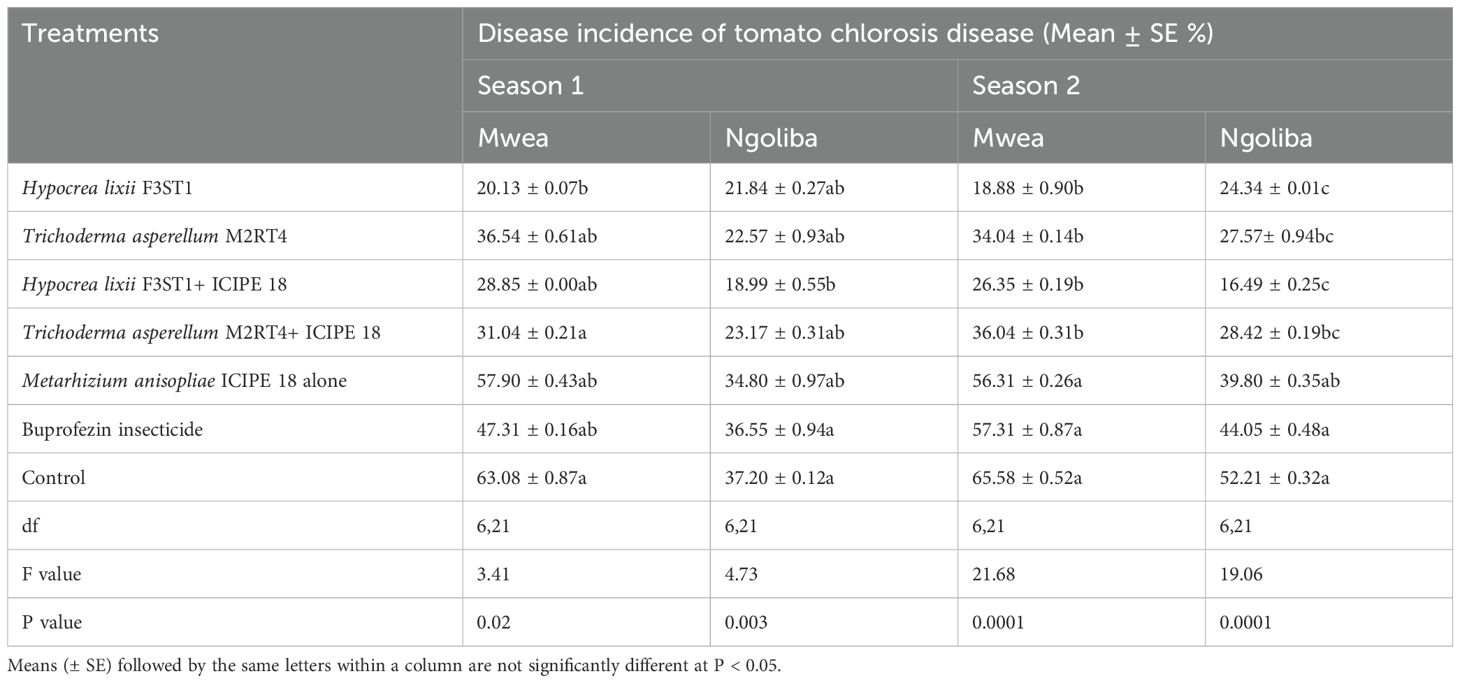
Table 2. Effect of the various treatments on the mean percentage of tomato chlorosis disease incidence during the two seasons at Mwea and Ngoliba.
3.6 Effect of fungal endophytes on severity of tomato chlorosis diseaseIt was observed that the fungal endophytes reduced the severity of tomato chlorosis viral disease. This observed result was associated with reduced expression of viral symptoms such as leaf area reduction, leaf chlorosis, leaf mottling, severe mosaic, leaf distortion, crinkling, plant stunting and severe necrosis. There was no significant interaction between the sites and the treatments (P = 0.83) and no significant site/location effects between the two experimental sites (P = 0.41). The highest disease severity was recorded in the controls than in the endophyte’s treatments. There were significant differences between the treatments in both Mwea (χ2 = 37.24, df = 6, P < 0.0001) and Ngoliba (χ2 = 20.9, df = 6, P < 0.000) in season 1, where the percentage disease severity rates varied between 8.9 and 10.7% in endophyte treatments compared to 24.2 and 29% in the control treatments in both Ngoliba and Mwea, respectively (Figures 4A, B). In season 2, there was significant difference between the sites and treatments (χ2 = 41.62, df = 6, P < 0.0001), while no significant variation was observed between the experimental sites (χ2 = 0.70, df = 1, P = 0.40). In addition, there were significant differences among the treatments at Mwea (χ2 = 41.83, df = 6, P < 0.0001) and Ngoliba (χ2 = 17.28, df = 6, P = 0.008) as regard to the disease severity in season 2 (Figures 4C, D). The lowest disease severity was recorded in H. lixii F3ST1 treatment alone at Mwea (10.72%) and Ngoliba (8.90%) in season 1 (Figures 4A, B). Similarly, higher variability was observed in the disease severity among the treatments in season 2 with H. lixii F3ST1 (10.71 ± 0.18 and 9.9 ± 1.93%) and T. asperellum M2RT4 (19.45 ± 5.22, and 16.75 ± 1.08%) endophytically colonized treatments recording the lowest disease severity rates compared the controls (30.00 ± 2.28 and 29.22 ± 0.91%) at both Mwea and Ngoliba, respectively (Figures 4C, D). The lowest disease severity was recorded in endophytes treated tomato plots compared to buprofezin insecticide treated tomato plots which recorded the highest disease severity (Figures 4A–D).
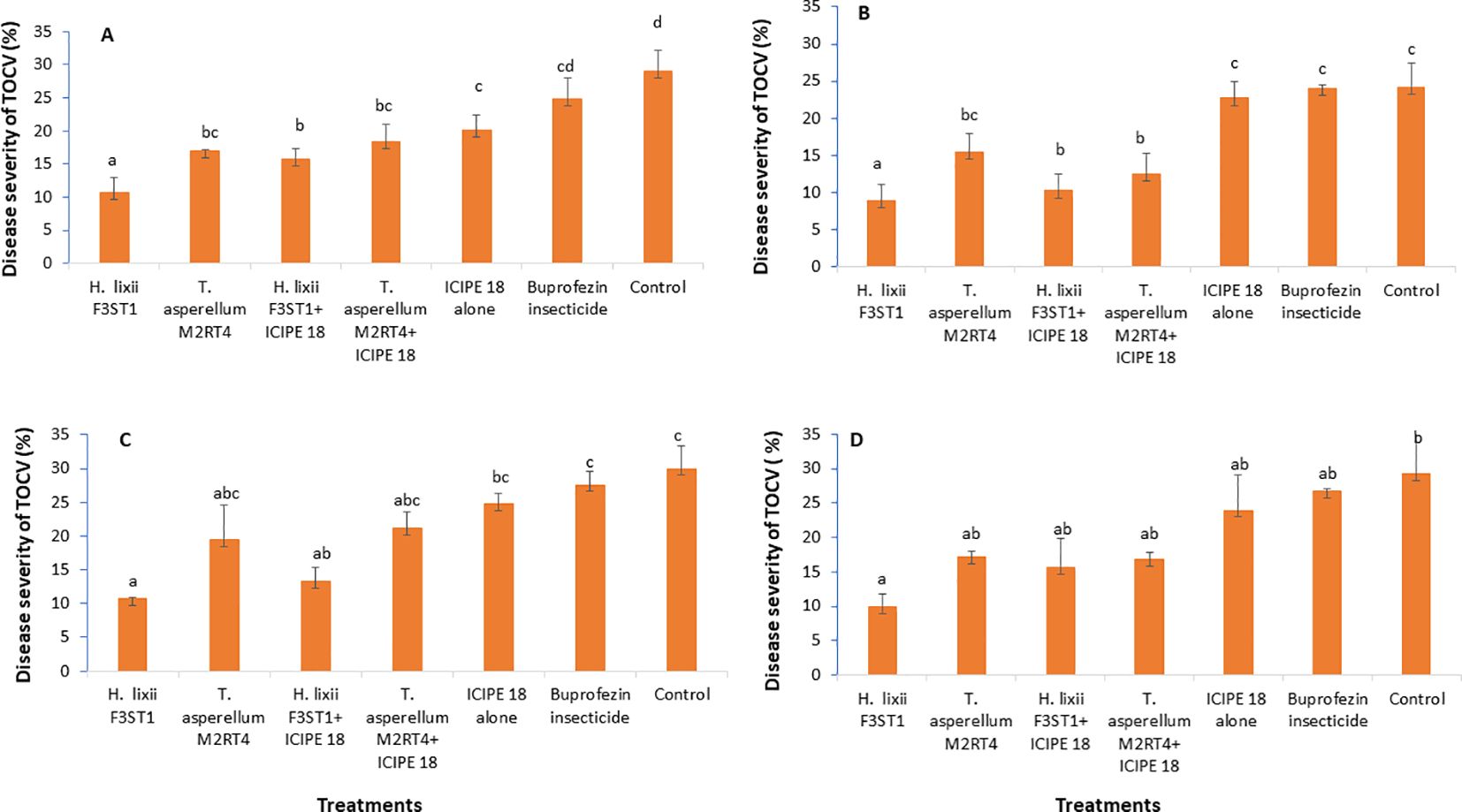
Figure 4. Effect of the various treatments on the mean percentage of tomato chlorosis disease severity at both Mwea (A, C) and Ngoliba (B, D) during season 1 (A, B) and season 2 (C, D). Bars denote means ± SE, and means followed by the same letters are not significantly different at P < 0.05.
3.7 Confirmation of the tomato infectious chlorosis and tomato chlorosis virusesIn additions to the morphological identification through disease symptoms of the pathogens, their identity was also confirmed via molecular tools. For the tomato chlorosis virus, all identity levels percentage similarities ranged from 95.45 – 100% with accession number of LC437668.1 according to BLAST GenBank queries. This high identity rate confirmed test for the virus. However, although the tomato infectious chlorosis virus/disease symptoms were observed in the field, the molecular confirmatory result for the virus based on the protocols described above did not show the virus, which could be due to low titers/status, thus the RNA was not enough for sequencing. It is therefore important that further analysis using stronger and more advanced tools like transcriptomics could also be employed in order to confirm tomato infectious chlorosis virus.
3.8 Effect of fungal isolates on the tomato fruits yieldHigher yield was recorded in endophytes treated tomato plants than the controls, and this could be attributed to the vector pest population suppression that resulted to reduced incidence and severity of viral diseases. In season 1, significant differences were observed in the marketable tomato fruits yield among the treatments at Mwea (F6, 21 = 7.95; P = 0.0001) and Ngoliba (F6, 21 = 6.5; P = 0.0005). On the other hand, there were also significant differences in non-marketable fruits yield among the various treatments at Mwea (F6, 21 = 71.06; P < 0.0001) and Ngoliba (F6, 21 = 10.53; P < 0.0001) in season 1. The marketable yield was higher in the combination of both endophytes and M. anisopliae ICIPE 18 compared to the controls in both sites (Table 3). The highest marketable fruits yield was recorded in H. lixii F3ST1 + ICIPE 18 treatment in both sites compared to the controls (Table 3). Similarly, in season 2, there were also significance differences in the marketable fruits yield (F6, 21 = 52.27; P < 0.0001) and non-marketable fruits yield (F6, 21 = 480.9; P < 0.0001) among the treatments at Mwea. However, the highest marketable fruits yield was recorded in H. lixii F3ST1 + ICIPE 18 treatment compared to the control (Table 3). Similar to the trends observed in Mwea, there were also significant variations among the treatments as regard to the marketable fruits yield (F6, 21 = 164; P < 0.0001) and non-marketable fruits yield (F6, 21 = 198.2; P < 0.0001) at Ngoliba. In addition, the highest marketable tomato fruits yields were recorded in H. lixii F3ST1 + ICIPE 18 and T. asperellum M2RT4 + ICIPE 18 plots compared to the controls (Table 3). There were significant differences in the number of fruits per plant in both at Mwea (F6, 21 = 19.2; P < 0.0001) and Ngoliba (F6, 21 = 15.2; P < 0.0001) in season 1. Similar trends were also observed in season 2 at Mwea (F6, 21 = 39.7.9; P < 0.0001) and Ngoliba (F6, 21 = 33.6; P < 0.0001) respectively (Table 4).
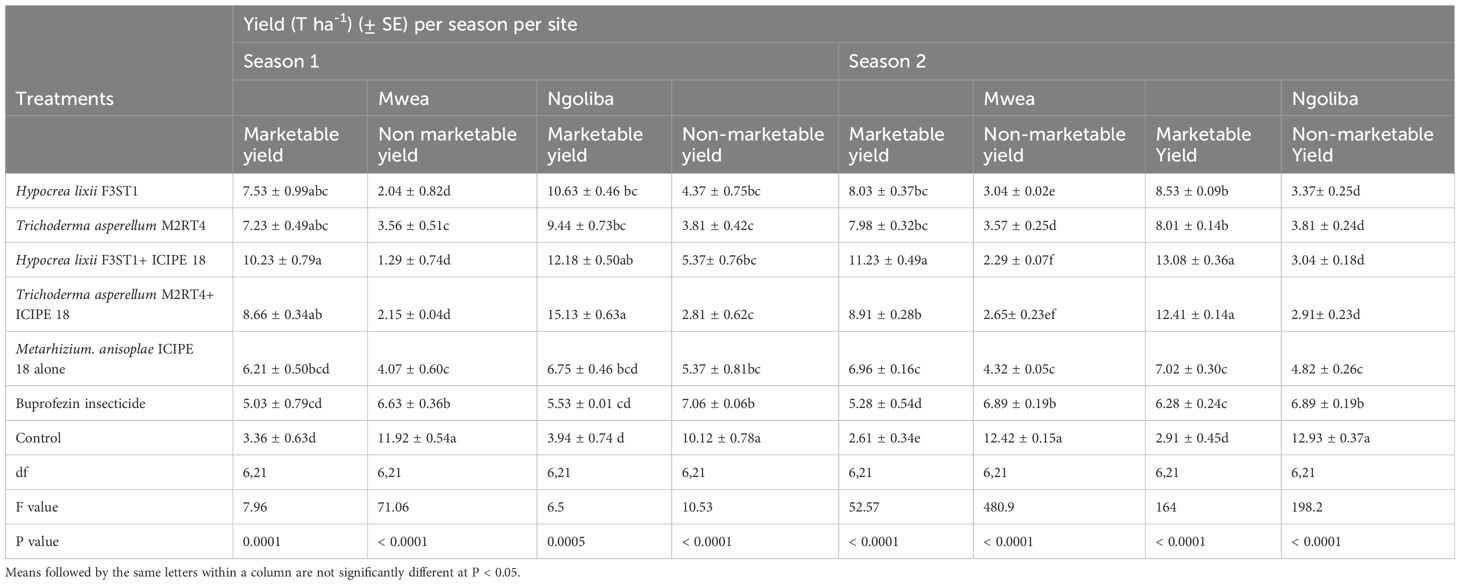
Table 3. Calculated mean yield of Solanum lycopersicon fruits in both sites and during the two seasons.
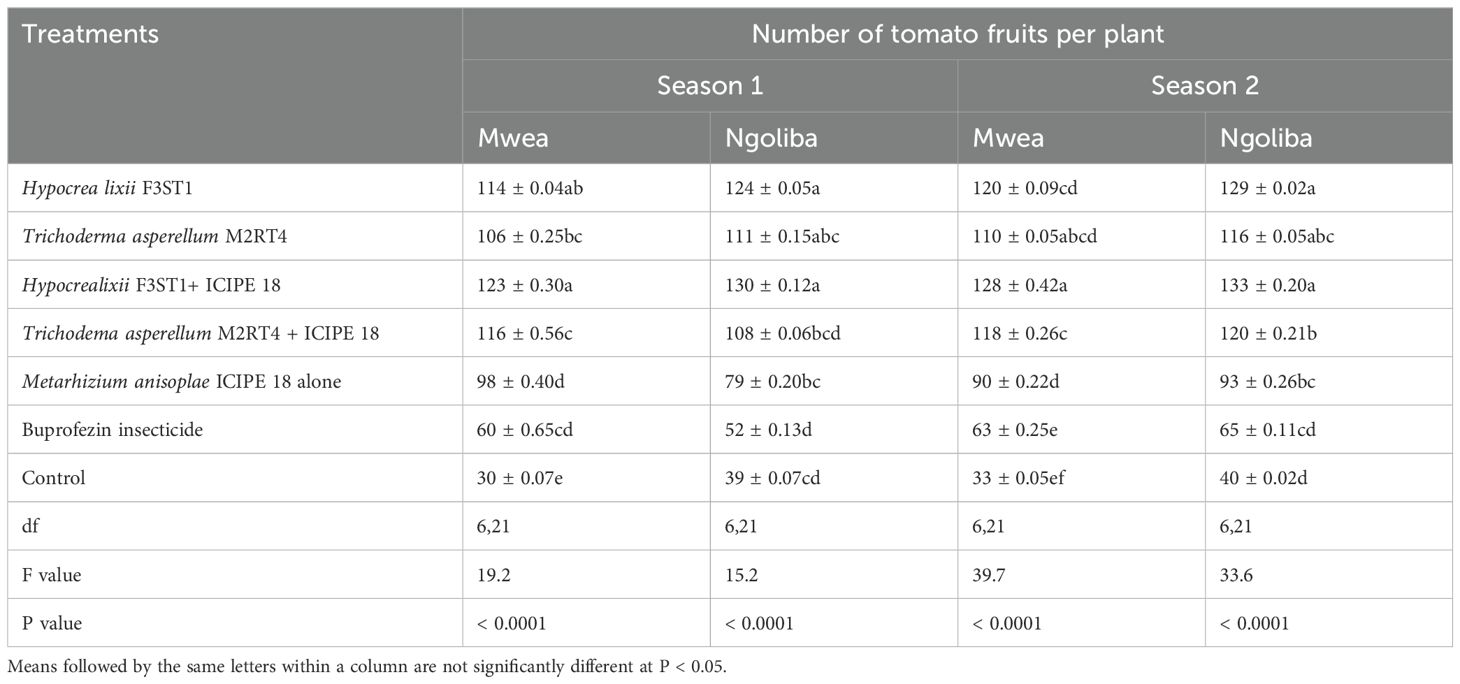
Table 4. Mean number of fruits per plant in both sites and during the two seasons.
4 DiscussionThe results of this study showed that the two fungal endophytes, T. asperellum M2RT4 and H. lixii F3ST1 successfully colonized all the tomato plant parts (roots, stems and leaves) at both sites as previously reported by Paradza et al. (2021a) and Agbessenou et al. (2020) in the screenhouse. Similar results were also reported by Mutune et al. (2016) where T. asperellum M2RT4, T. atroviride F2S21, and H. lixii F3ST1 were found to endophytically colonize the various parts of the common bean (Phaseolus vulgaris) after artificial seeds inoculation. In addition, Gathage et al. (2016) demonstrated that H. lixii F3ST1 successfully colonized all the various parts of P. vulgaris under field conditions. Following seed inoculation, T. harzianum F2R41, T. atroviride F2S21, T. asperellum M2RT4 and H. lixii F3ST1 were also able to endophytically colonize the various tissues of maize plants (Kiarie et al., 2020; Kinyungu et al., 2023). However, the colonization rates varied significantly as per the plant species and the host plant parts. The ability of these fungal isolates to endophytically colonize these different plant species and with varied colonization rates through artificial inoculation might be due to that the plant species’ physiology and chemistry that may have an impact on the effectiveness of colonization (Akello et al., 2007b; Gathage et al., 2016; Gange et al., 2019). In addition, our results showed that even under field conditions, T. asperellum M2RT4 and H. lixii F3ST1 were able to outcompete other possible endophytes and systemically colonized S. lycopersicon host plants in the different treatments. The ability of these artificially inoculated endophytes to outcompete th
留言 (0)