Although clinical advancements have significantly reduced mortality rates, they have also led to an increase in the number of patients with disorders of consciousness (DoC) (1). Patients with DoC require prolonged and intensive clinical management, imposing a significant burden on families and society. DoC encompasses conditions characterized by diminished awareness or impaired cognition of oneself and the surrounding environment, including coma, vegetative state (VS)/ unresponsive wakefulness syndrome (UWS), and minimally conscious state (MCS). Patients in a VS/UWS exhibit intermittent wakefulness without any signs of self-awareness or environmental recognition (2). In contrast, patients in an MCS demonstrate fluctuating but reproducible signs of conscious awareness through clinical motor responses, although their level of awareness is unstable (3). MCS is further subdivided into MCS+ and MCS−, reflecting higher and lower levels of behavioral responsiveness, respectively (4, 5). The Coma Recovery Scale-Revised (CRS-R) is widely regarded as the most reliable clinical tool for diagnosing patients with prolonged DoC (6). However, clinical consensus-based assessments are associated with a misdiagnosis rate of approximately 40% (7).
Patients with DoC require prolonged and intensive clinical management, imposing a significant burden on families and society. In the past few years, the advancement of neuroimaging techniques such as functional magnetic resonance imaging (fMRI), positron emission tomography (PET), and electroencephalography (EEG) has provided objective diagnostic tools for assessing cognitive function, predicting neurological recovery, and determining prognosis in patients with disorders of consciousness (8, 9). EEG offers advantages in its superior temporal resolution and real-time responsiveness, however, its low spatial resolution and susceptibility to artifacts limit the diagnostic accuracy of DoC. In contrast, fMRI and PET provide high spatial resolution but are hindered by high costs, low portability, and limited real-time responsiveness (10, 11). Functional near-infrared spectroscopy (fNIRS) emerges as a promising alternative technique, offering a non-invasive, portable, and high temporal resolution for monitoring brain activity. Unlike fMRI and PET, fNIRS enables long-term bedside monitoring, making it particularly suitable for DoC patients. Additionally, fNIRS is less sensitive to motion artifacts than fMRI, enhancing its feasibility in clinical settings (12, 13). By facilitating early and accurate diagnosis, fNIRS provide valuable insights in guiding targeted interventions, reducing unnecessary treatments, shortening hospitalization durations, and alleviating the financial and logistical burden on healthcare systems. Ultimately, it optimizes healthcare resource allocation for DoC patients.
fNIRS is a non-invasive optical neuroimaging technique that utilizes near-infrared light (700–900 nm) to measure changes in tissue concentrations of oxy-hemoglobin (HbO), deoxy-hemoglobin (HbR), and total-oxyhemoglobin (HbT), providing (14–16). The selection of near-infrared light is based on its unique optical properties rather than its penetration depth. Within the near-infrared spectrum, light exhibits minimal absorption by water and significant absorption differences between HbO and HbR, enabling fNIRS to differentiate chromophores effectively. This “optical window” allows light to penetrate several centimeters into cortical tissue, facilitating reliable measurements of hemodynamic changes in highly scattering biological tissues (17–19). Advancements in fNIRS instrumentation, including continuous wave, frequency domain, and time domain systems, have further improved its sensitivity and broadened its clinical and research applications. These advancements have enabled the integration of fNIRS with other modalities, such as EEG, for comprehensive assessments of brain function (20, 21) fNIRS offers several strengths, including its non-invasive nature, portability, real-time measurement capabilities, and continuous repeatability (22–24). Compared to fMRI, fNIRS is less sensitive to interference from devices such as hearing aids, pacemakers, or cochlear implants, making it valuable for monitoring scalp oxygenation in patients with such medical implants (25). fNIRS has demonstrated its utility in identifying functional differences in key brain regions, including the occipital lobe, temporal lobe, and prefrontal lobe, in patients with various types of DoCs (26, 27). Moreover, it can detect neural responses to active stimuli in MCS and assess brain activity during verbal and motor imagery (MI) tasks, providing critical insights into residual cognitive and functional capacities (28–30).
As a non-invasive neuroimaging technique, fNIRS has gained significant attention in the field of DoC. It has demonstrated high sensitivity in diagnosing varying degrees of DoC, evaluating and predicting changes in brain function, and monitoring therapeutic outcomes (31). Notably, fNIRS offers unique advantages in assessing the effects of neuromodulator therapies on DoC patients (32, 33). By providing real-time insights into neural responses and treatment efficacy, fNIRS serves as an essential tool for guiding and optimizing treatment strategies. This review provides an overview of the clinical applications of fNIRS from 1993 to 2024, with a focus on the latest advances in fNIRS technology for DoC patient assessment, functional neuromodulation therapies, and brain-computer interfaces (BCIs) (Table 1). The flowchart in Figure 1 illustrates the search strategy, including keyword retrieval and paper screening processes. Additionally, recent clinical studies are summarized in Table 2, with data sourced from the World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP). This review also explores emerging trends in clinical applications while addressing the current challenges and limitations of using fNIRS to inform DoC treatment strategies (Figure 2).
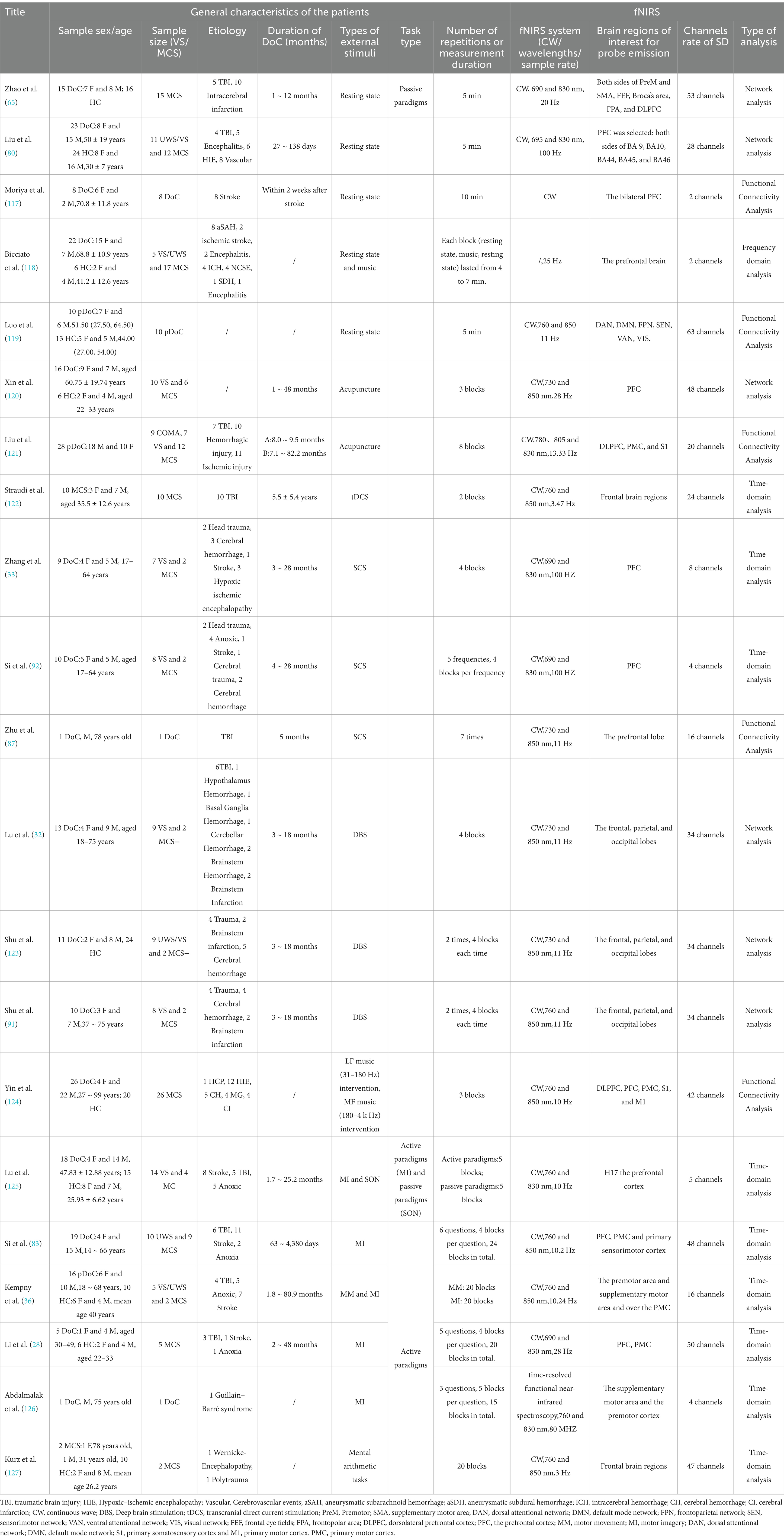
Table 1. Summary of fNIRS studies conducted on DoC patients to date.
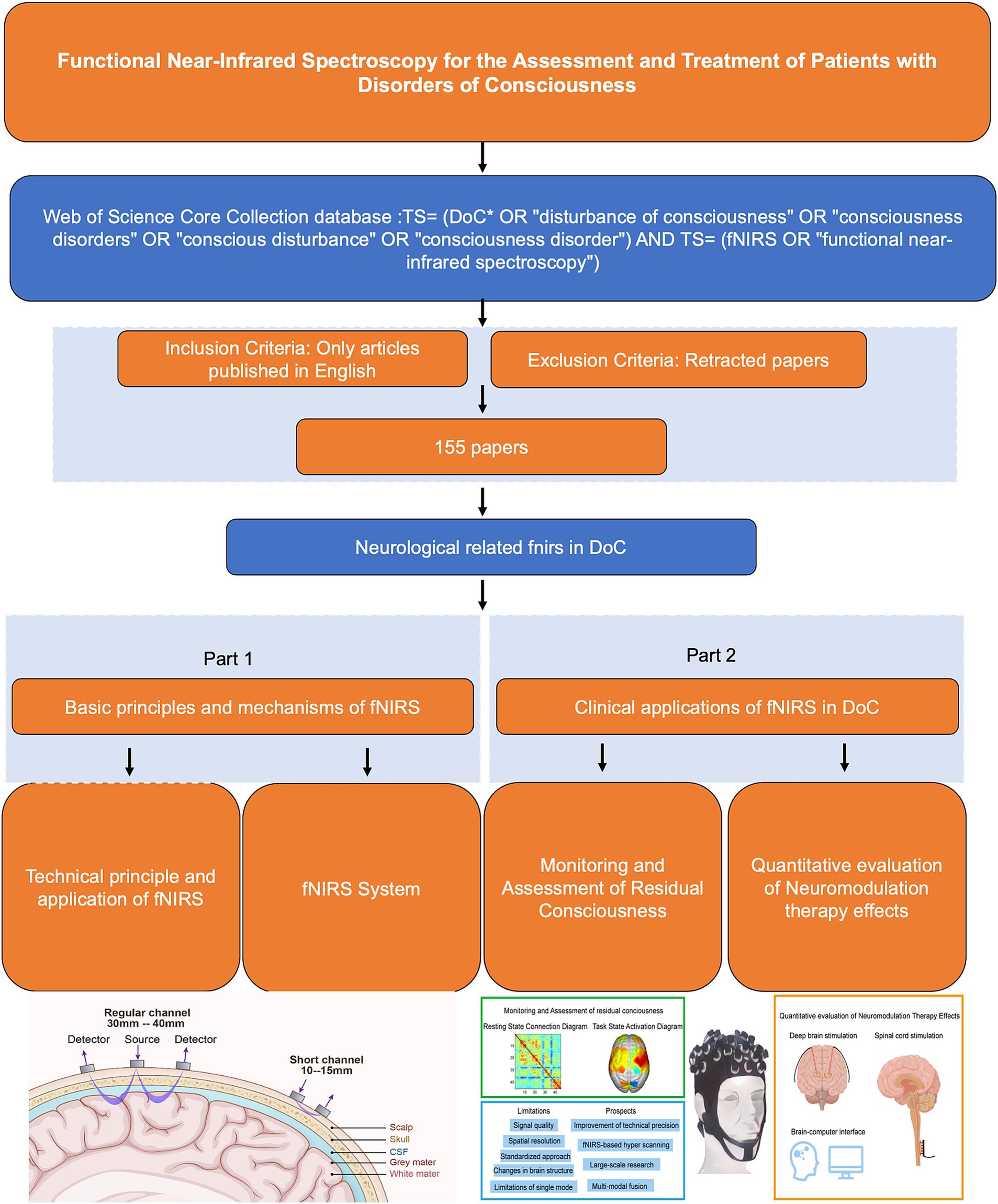
Figure 1. Flow chart.
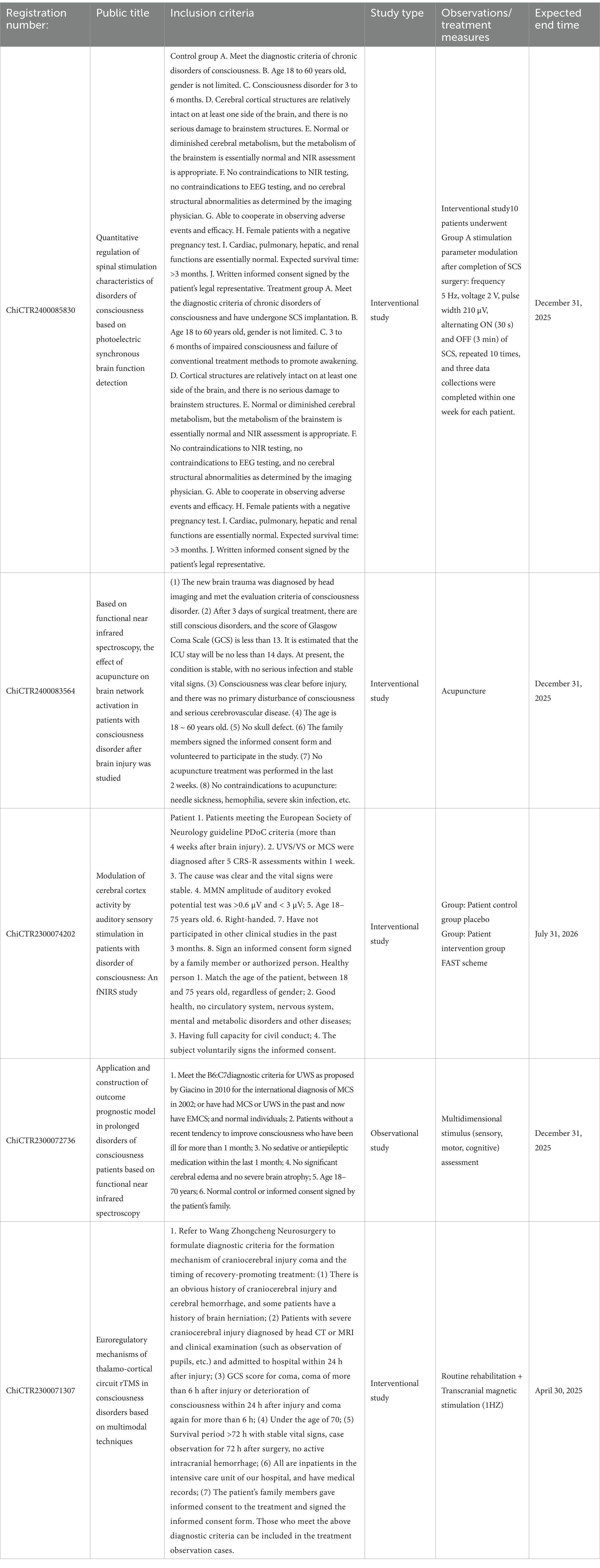
Table 2. Summary of ongoing clinical trials of fNIRS registered by WHO at home and abroad in the last 10 years.
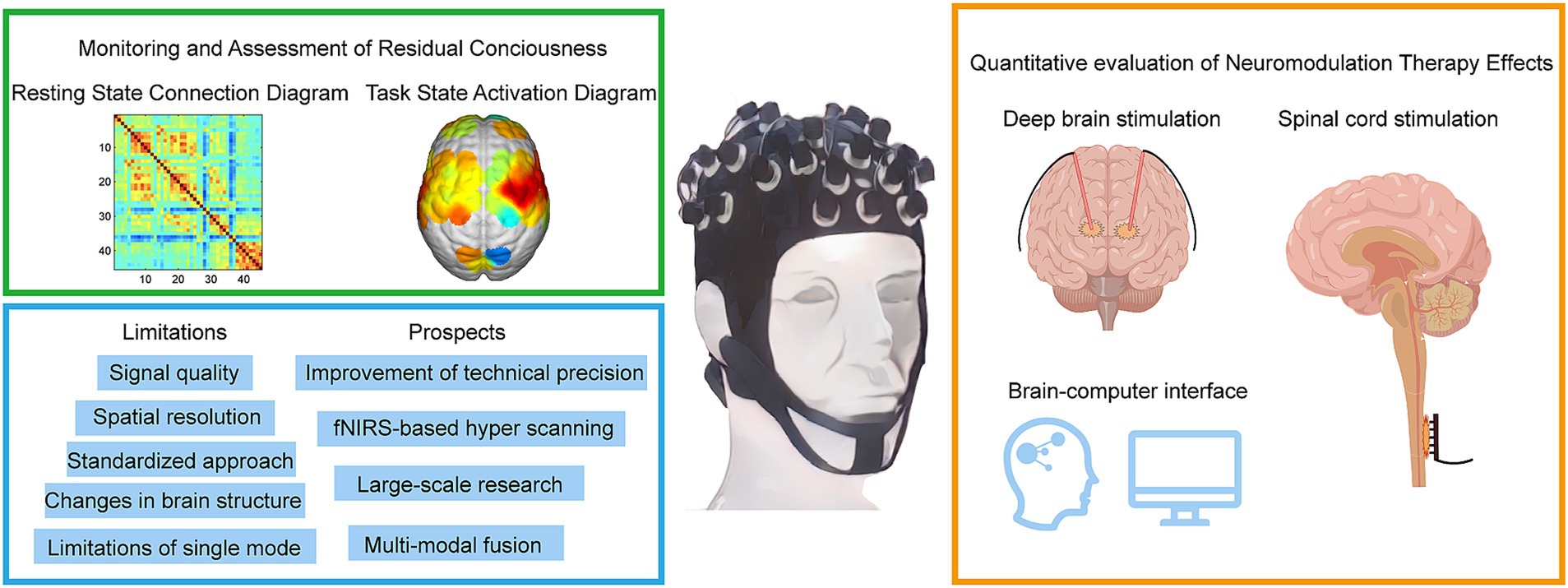
Figure 2. Methods for diagnosing and assessing different levels of DoC by fNIRS. fNIRS technology has made recent advances in the direction of deep brain electrical stimulation, spinal cord electrical stimulation, and brain-computer interfaces. fNIRS has problems and challenges, as well as trends in future clinical applications.
2 Basic principles of fNIRS 2.1 Technical principle fNIRSThe light within the 700–900 nm range, referred to as the ‘optical window’, possesses several distinct characteristics: firstly, it can penetrate tissues such as the scalp and skull to reach the intracranial space; secondly, the overall absorption coefficients of chromophores (water, hemoglobin, etc.) are relatively low, ensuring that the light is not entirely absorbed before it exits the scalp and reaches the detector; and lastly, there is a significant difference in the absorption coefficients of HbO and HbR within this range. Consequently, it is possible to select two or more wavelengths and, by leveraging the differences in absorption coefficients between HbO and HbR at these wavelengths, separately determine the concentration changes of HbO and HbR. After near-infrared light is emitted from the source, it passes through the scalp, skull, and cerebrospinal fluid, entering the cerebral cortex where it undergoes absorption, scattering, and refraction before reaching the detector, exhibiting a typical “banana” shape, as shown in Figure 3. The typical fNIRS system consisted of several sources and detectors with a 3 cm source-detector separation (34). During the data analysis process, the modified Beer–Lambert law (MBLL) (35) is applied to infer backward from changes in light intensity to calculate changes in hemodynamic responses, which allows for the deduction of neural activity in the brain, and subsequently, the analysis of the synergistic actions between various brain regions during task execution.
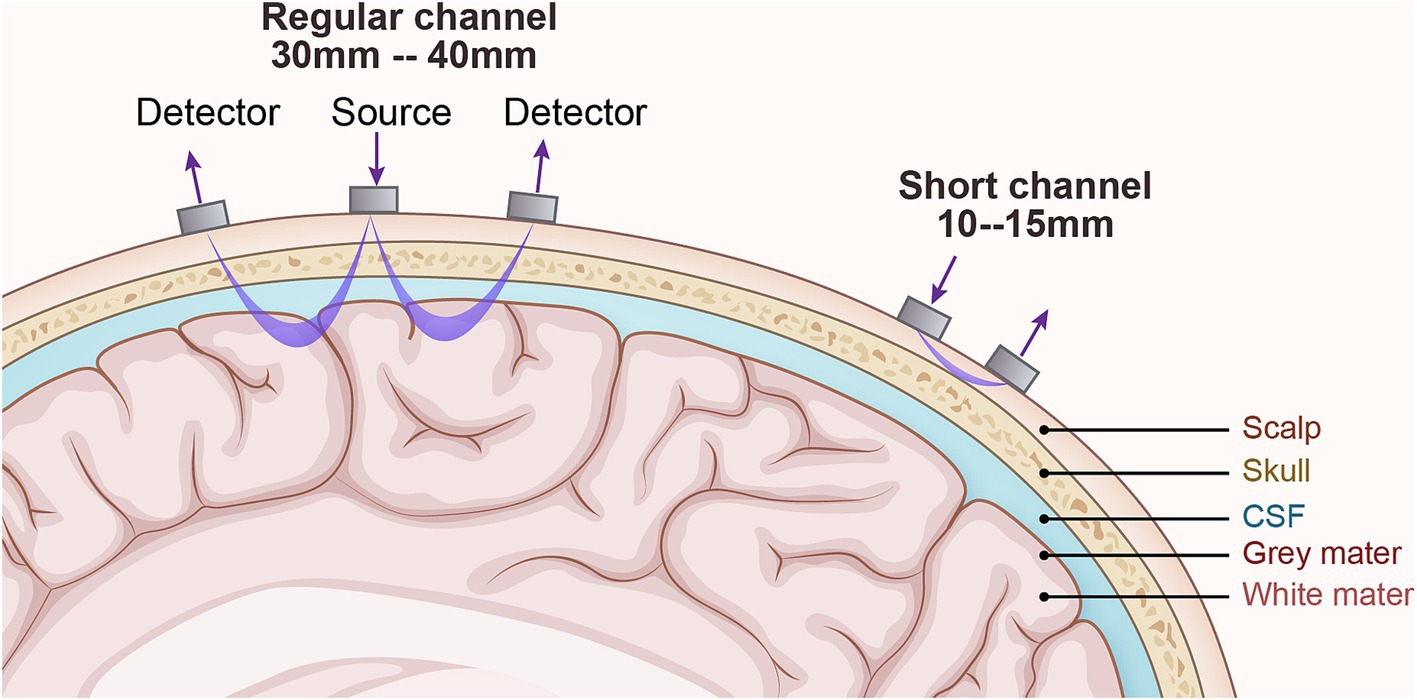
Figure 3. The technical principles of fNIRS. The detector is the receiving light source, and the source is the emitting light source. Generally, the detector and source are 3-4cm for adults and 1–1.5 cm for children. The changes in light intensity of the emitting and receiving light sources respond to changes in blood oxygen concentration in the cerebral cortex and then speculate on neuronal activity.
In the field of DoC, fNIRS plays a pivotal role in assessing residual consciousness by measuring changes in hemodynamics and evaluating brain functional connectivity (31). It can also assess brain activity in DoC patients using MI task paradigms (36). Additionally, fNIRS is effective in evaluating treatment efficacy by monitoring oxygenation changes during various therapeutic interventions (32).
2.2 fNIRS systemfNIRS is a non-invasive imaging technology that measures blood oxygenation changes in brain tissue, offering significant potential in assessing patients with DoC (37). Key aspects of fNIRS use in DoC include system types, sensor configuration, and data analysis methods, each of which has a profound impact on measurement accuracy, data complexity, and clinical applicability. Table 1 presents a comprehensive overview of the application of fNIRS in patients with DoC. The table is organized into two main sections: Patient Information and Equipment Details. In the Patient Information section, key demographic and clinical details are provided, including age, gender, etiology, and time since onset. Additionally, the paradigms employed in the studies are summarized, encompassing resting-state assessments, active paradigms (e.g., MI tasks), and passive paradigms (e.g., subject’s name). The Equipment Details section outlines the technical aspects of the studies, such as the specific fNIRS systems used, the number of channels employed, and the analytical methods applied. It also includes information on the targeted brain regions selected based on the experimental design and clinical objectives, highlighting the tailored approach for evaluating DoC patients.
2.3 fNIRS instrumentationfNIRS systems can be classified into three main categories: continuous wave (CW), frequency domain (FD), and time domain (TD) systems. CW systems are the most used fNIRS systems. They utilize a constant-intensity near-infrared light source to measure absorption differences across various wavelengths, thus estimating relative concentration changes in HbO and HbR (19). CW systems are relatively simple, low-cost, and allow for rapid data acquisition, making them ideal for bedside monitoring and high-throughput research. However, CW systems measure only relative concentration changes, lacking the capacity to provide absolute concentrations of HbO and HbR. Without information on photon travel time or phase shift, CW systems have inherent limitations in accuracy (38). FD technology employs intensity-modulated near-infrared light, where the incident light intensity is modulated with a sinusoidal wave at a frequency of 100–200 MHz on top of a steady-state value (39). As the incident light propagates through tissue, it experiences attenuation and scattering, leading to a reduction in the amplitude of the high-frequency sinusoidal wave and a phase delay. By detecting the emitted light and demodulating it, amplitude attenuation and phase shift can be utilized to obtain the tissue’s absorption and scattering coefficients. From these optical parameters, the absolute concentrations of oxygenated and deoxygenated hemoglobin in the tissue can be determined (40). FD systems use modulated light sources to measure phase shifts and amplitude variations, enabling the estimation of absolute HbO and HbR concentrations (40). FD systems provide high accuracy and absolute concentration measurements, which are critical for precise hemodynamic assessments in DoC patients. Compared to CW systems, FD systems are more complex and expensive, limiting their widespread application in clinical settings (25). TD systems emit ultrashort light pulses and measure the distribution of photon travel times within tissue, capturing detailed absorption and scattering properties (41). TD systems offer the most precise measurements of absolute concentrations and tissue scattering, making them suitable for in-depth research. TD systems are the most complex and costly, with high data processing demands, making them less feasible for routine clinical use. TD systems and FD systems can provide more detailed information, but their technology is more complex, and the equipment is significantly more expensive than that of CW systems, with lower signal-to-noise ratios, thus limiting their applications (42). The vast majority of near-infrared imaging instruments utilize CW systems. In cases where absolute concentrations of HbO and HbR are required, FD systems offer a higher signal-to-noise ratio and faster acquisition speed compared to TD systems, but it is slightly inferior to TD systems in distinguishing between scattering and absorption effects and in terms of depth resolution (16, 42). A comparative analysis of three technologies is presented in Table 3.
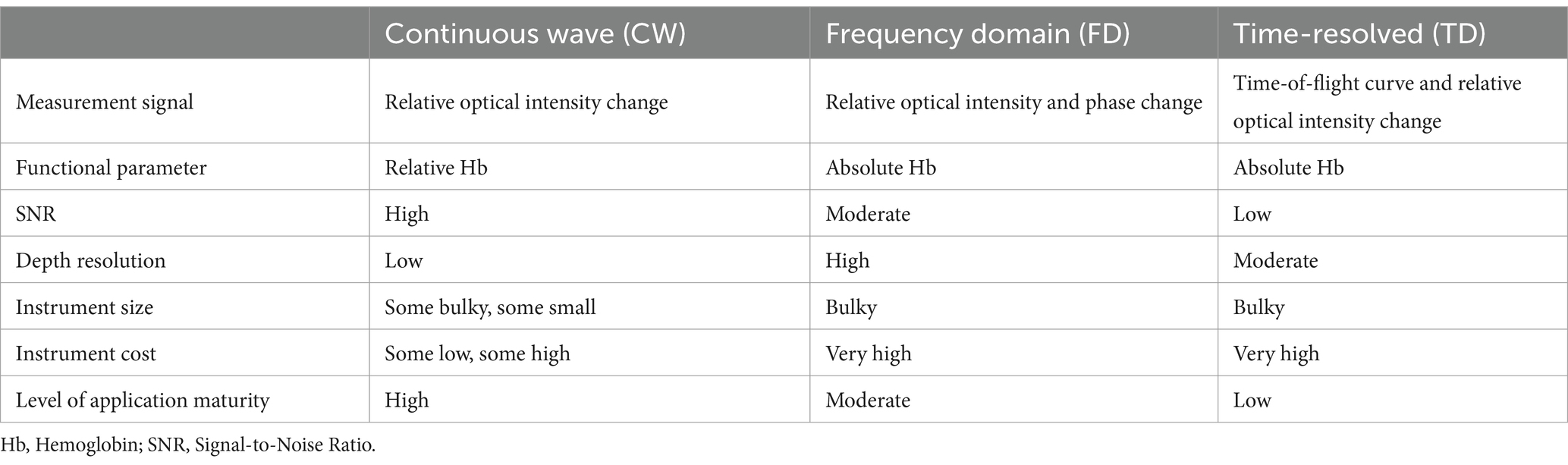
Table 3. Comparison of fNIRS systems with three different modulation techniques.
2.4 Arrangement of fNIRS optodesThe number and placement of fNIRS sensors (light sources and detectors) significantly impact measurement accuracy and spatial resolution. A higher number of fNIRS optodes enables greater cortical coverage and precision, but it also increases data volume and complexity. Configurations typically range from a few to dozens of optodes, depending on the areas of interest (20).
The International 10–20 system is widely adopted in fNIRS studies for its standardized and consistent approach to optode placement, ensuring optimal coverage of critical cortical regions such as the prefrontal, parietal, and temporal lobes. Approximately 70% of fNIRS studies in DoC research utilize montages based on the 10–20 system due to its reliability and compatibility with existing neuroimaging methods (41, 43). This system facilitates cross-study comparisons and provides a foundation for interpreting functional brain activity in DoC patients. However, alternative optode configurations have also been employed to meet specific research and clinical needs. High-density optode grids, for instance, offer improved spatial resolution, allowing for more detailed mapping of cortical activation patterns. Despite their advantages, these configurations often require more complex data processing and are less portable, making them challenging to implement in bedside settings (16). Conversely, sparse optode arrangements prioritize portability and ease of application, particularly in clinical environments. While these setups are advantageous for rapid assessments, they may compromise spatial resolution and limit the ability to detect activity in deeper cortical regions (44). Hybrid approaches, which combine high-density grids for key cortical areas with sparse arrangements for peripheral regions, aim to balance these trade-offs (45). These variations in optode placement underscore the importance of selecting appropriate configurations based on study objectives and patient conditions. The flexibility of fNIRS in accommodating different sensor montages highlights its adaptability and potential for diverse clinical and research applications in the DoC field.
2.5 Data analysis methods in fNIRSTheoretically, fNIRS data would solely encompass the blood oxygenation signal components induced by experimental tasks. However, in practical data acquisition processes, various types of noise are inevitably superimposed on the data. The sources of noise in fNIRS data are diverse: the measurement system, physiological origin, and head/body motion (46). Below are several common types of fNIRS noise and their removal methods. Firstly, systemic physiological changes affect cerebral hemodynamics. The primary physiological factors that can confound fNIRS measurements include fluctuations in the partial pressure of carbon dioxide (PaCO2) (around 0.2–0.6 Hz), systemic blood pressure (around 0.1 Hz), and alterations in heart rate (around 0.8–1.6 Hz) (47, 48). Secondly, signal drift is a pervasive type of noise in fNIRS brain imaging signals, typically manifesting as slow fluctuations over extended periods. Signal drift can alter the baseline of fNIRS signals. The sources of fNIRS brain imaging signal drift are complex and include interference noise during the signal acquisition process of the imaging system (such as the effects of the machine gradually warming up during operation), as well as the slow head movements of the subject that are imperceptible to the naked eye during the experiment. Thirdly, head motion and motion artifacts (MA) are a common type of noise in fNIRS signals; subject head movements or limb activities may lead to poor contact between the optodes and the scalp, often reflected as large jumps in the signal. Generally, fNIRS has a low signal-to-noise ratio, and without “denoising,” it will directly affect the subsequent calculation of individual hemodynamic response indicators and may even lead to false group activations. Bandpass filtering can be used to remove high-frequency cardiac signals and low-frequency drifts in the optical signal (49). It is crucial to pay special attention to not disrupt the frequencies related to the experimental tasks during the filtering process. Otherwise, the expected activation results cannot be obtained. Nevertheless, the frequency range of respiratory and Mayer wave oscillations coincides with that of the hemodynamic response, thus precluding their simple elimination via bandpass filtering (50). Due to the abundance of capillaries in superficial tissues such as the scalp, skull, and meninges, physiological fluctuations such as respiration and heartbeat, as well as task-related autonomic nervous activities, can cause changes in the concentration of hemoglobin in these capillaries. When near-infrared light passes through these superficial tissues, changes in the concentration of hemoglobin in the superficial vessels also lead to variations in the optical attenuation of fNIRS. Superficial physiological noise has a significant impact on fNIRS signals (51). Currently, the short-channel subtraction has been considered the ‘gold standard’ to reduce the responses from the extracerebral tissue in fNIRS data (52). The optical path of short interoptode channels is relatively shallow, generally passing only through superficial tissues without reaching the cerebral cortex. Therefore, it is generally believed in the academic community that short interoptode channels primarily record superficial physiological noise. This method employs additional short-distance fNIRS channels to record the superficial physiological noise and subsequently removes it from the signal (53). Alternative approaches are data-driven signal processing methods that decompose the fNIRS signals into its brain and systemic components, e.g., principal component analysis (PCA) (46). In previous publications, PCA has been widely used to remove head motion and motion artifacts in fNIRS data (54–56). In DoC patients, sensor placement strategies that reduce susceptibility to movement artifacts are particularly valuable given the limited ability of these patients to remain still (57, 58).
Time-domain analysis evaluates changes in light intensity over time to correlate hemodynamic responses with specific tasks or stimuli. In DoC research, this method is widely used to assess brain responses to external stimuli, helping to detect residual cognitive activity. For instance, Pipeline 1 involves preprocessing steps such as motion artifact removal and signal detrending, followed by task-based averaging to identify significant activation. Pipeline 2 incorporates additional steps like deconvolution modeling to enhance temporal resolution, allowing for the differentiation of overlapping hemodynamic responses. A recent meta-analysis revealed that 70% of studies employed Pipeline 1 due to its simplicity, while 30% utilized Pipeline 2 for more complex paradigms. Pipeline 1 is advantageous for rapid analysis but is limited in detecting subtle temporal changes, whereas Pipeline 2 provides greater accuracy at the cost of computational complexity (16, 44). Frequency Domain Analysis examines the power spectral density of fNIRS signals, capturing brain wave activities such as alpha and theta waves, as well as low-frequency oscillations (0.01–0.1 Hz) (45). Frequency domain analysis is useful for understanding neural networks and functional connectivity changes in DoC patients. By combining multi-channel data, spatial analysis generates maps of brain activity, allowing for the visualization of functional distribution across different cortical areas (59). Spatial analysis generates brain activity maps by combining multi-channel data to visualize functional distribution across cortical areas. Techniques such as t-maps, derived from statistical comparisons of task versus baseline HbO levels, are commonly employed to identify active regions. Additionally, functional nodes and network hubs are identified through clustering algorithms, offering insights into the spatial organization of neural networks. This approach has been used to map activity in regions like the PFC, SMA, and FPA, providing a detailed understanding of localized brain functions (45, 60). This approach provides valuable insights into the regional brain activity patterns in DoC patients. Functional connectivity analysis assesses the synchronized activity across brain regions, revealing the organization of functional networks (16). Changes in functional connectivity offer essential information on the integrity of neural networks, making this approach particularly useful in DoC assessments.
In summary, fNIRS is a promising tool for DoC assessment, with unique advantages such as non-invasiveness, portability, and real-time monitoring capabilities. This discussion highlights several gaps in current research on fNIRS for DoC applications and potential directions for future studies: Current DoC studies predominantly utilize CW systems, with limited exploration of FD and TD systems in clinical settings. Future studies should investigate FD and TD systems to improve accuracy and enable absolute concentration measurements. Furthermore, increasing sensor density and optimizing placement strategies for critical cortical regions, such as the PFC and SMA, could enhance spatial resolution and reduce MA. Finally, adopting multidimensional analyses, including spatial and connectivity measures, may uncover complex network dynamics in DoC patients, advancing diagnostic and therapeutic strategies.
3 Clinical applications of fNIRS in DoCFigure 2 provides a comprehensive summary of the application of fNIRS in the field of DoC, highlighting its current and future roles in clinical and therapeutic contexts. The figure is divided into four sections, including a schematic representation of fNIRS applications in DoC patients. In the upper-left green frame, the focus is on the diagnostic and assessment capabilities of fNIRS for DoC patients. This includes detecting residual consciousness during resting states and evaluating brain activation during task states. These applications underscore the potential of fNIRS as a non-invasive tool for identifying preserved cognitive and motor functions in patients with DoC. The lower-left blue frame addresses the main limitations and prospects of fNIRS. Key challenges include limited spatial resolution, signal interference from extracerebral tissues, and the need for advanced signal processing techniques. Future advancements, such as improved sensor designs and multimodal integration with other imaging modalities, are anticipated to enhance the accuracy and clinical utility of fNIRS. In the yellow frame on the right, the therapeutic applications and future potential of fNIRS are explored. Emphasis is placed on its integration with neuromodulation techniques, such as DBS and SCS, to monitor and optimize therapeutic outcomes. Additionally, the development of BCIs presents a promising direction for combining fNIRS with advanced neurotechnology to refine treatment strategies and improve patient prognoses.
From 1993 to 2024, 243 institutions have participated in fNIRS research (Figure 4). Research on fNIRS spans 35 countries, with the United States leading in publication output: the earliest U.S. publications date back to 2002, with 40 articles. The U.S. also holds the strongest intermediary position in collaborative networks. China ranks second, with 35 publications. Although China’s engagement in fNIRS research began later than the U.S., the minimal difference in publication volume underscores China’s growing interest and active involvement in fNIRS research (Figure 5). In China, fNIRS research is mainly conducted at leading cognitive psychology institutions such as Beijing Normal University. fNIRS research involves psychology, sociology, aerospace, and other related cognitive-behavioral research (61, 62). In the medical field, fNIRS is increasingly used in psychiatry, pain management, rehabilitation, and neurosurgery. Its applications range from disease diagnosis to supporting the development of brain-computer interface technologies, demonstrating its growing importance as an auxiliary clinical diagnostic tool (63–65). These statistical diversities of the literature represent the current research hotspots of fNIRS in the field of DoC, highlighting the expanding role of fNIRS in bridging cognitive science and clinical medicine, paving the way for future research and practice advances.
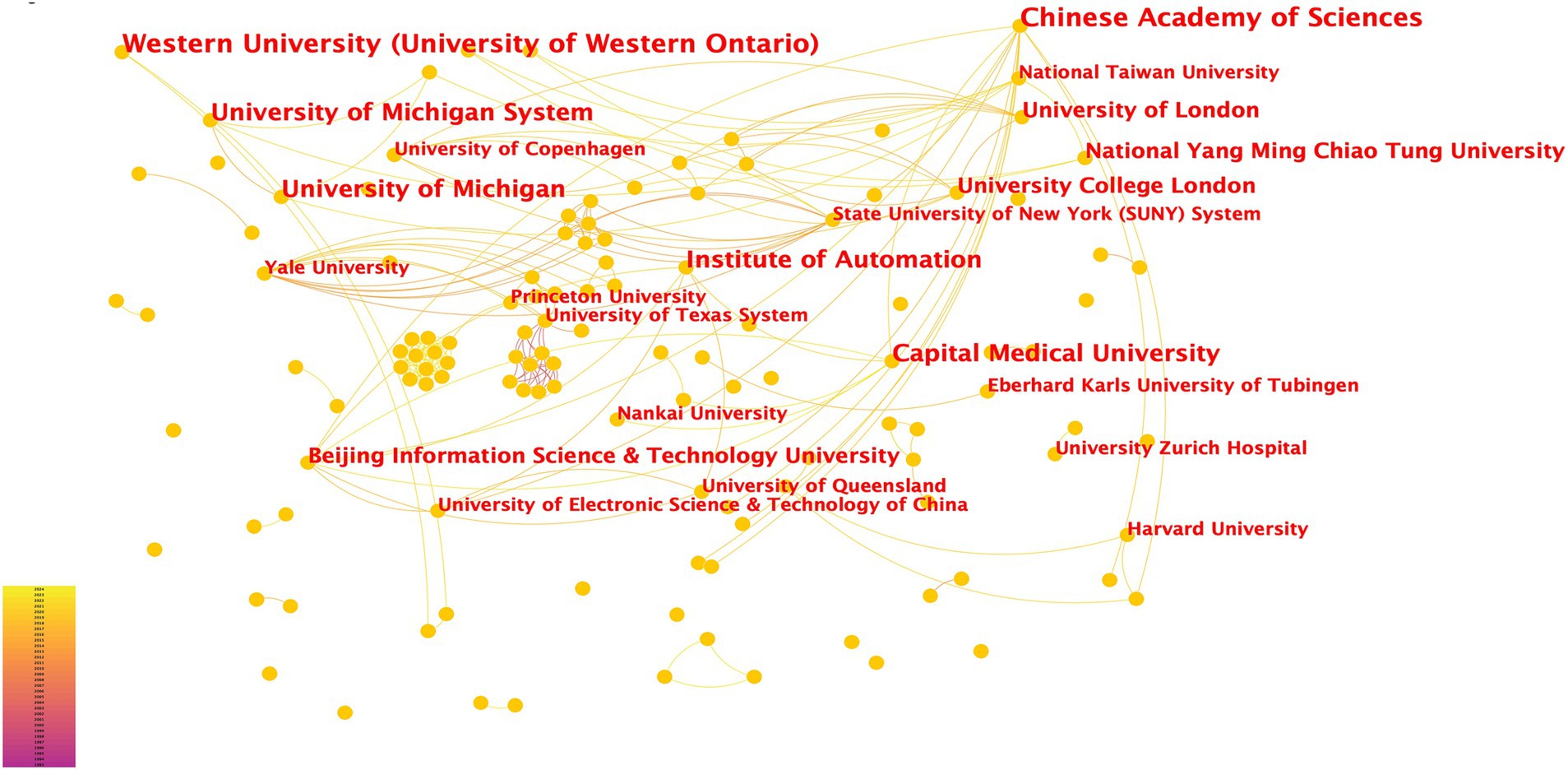
Figure 4. Institutional Contributions to fNIRS Research. Each node in the figure represents a research institution. The font size of the institution name corresponds to the number of articles published, with larger font sizes indicating higher publication output. The density of connections between nodes reflects the level of collaboration between institutions, with thicker or denser connections signifying stronger cooperative relationships.
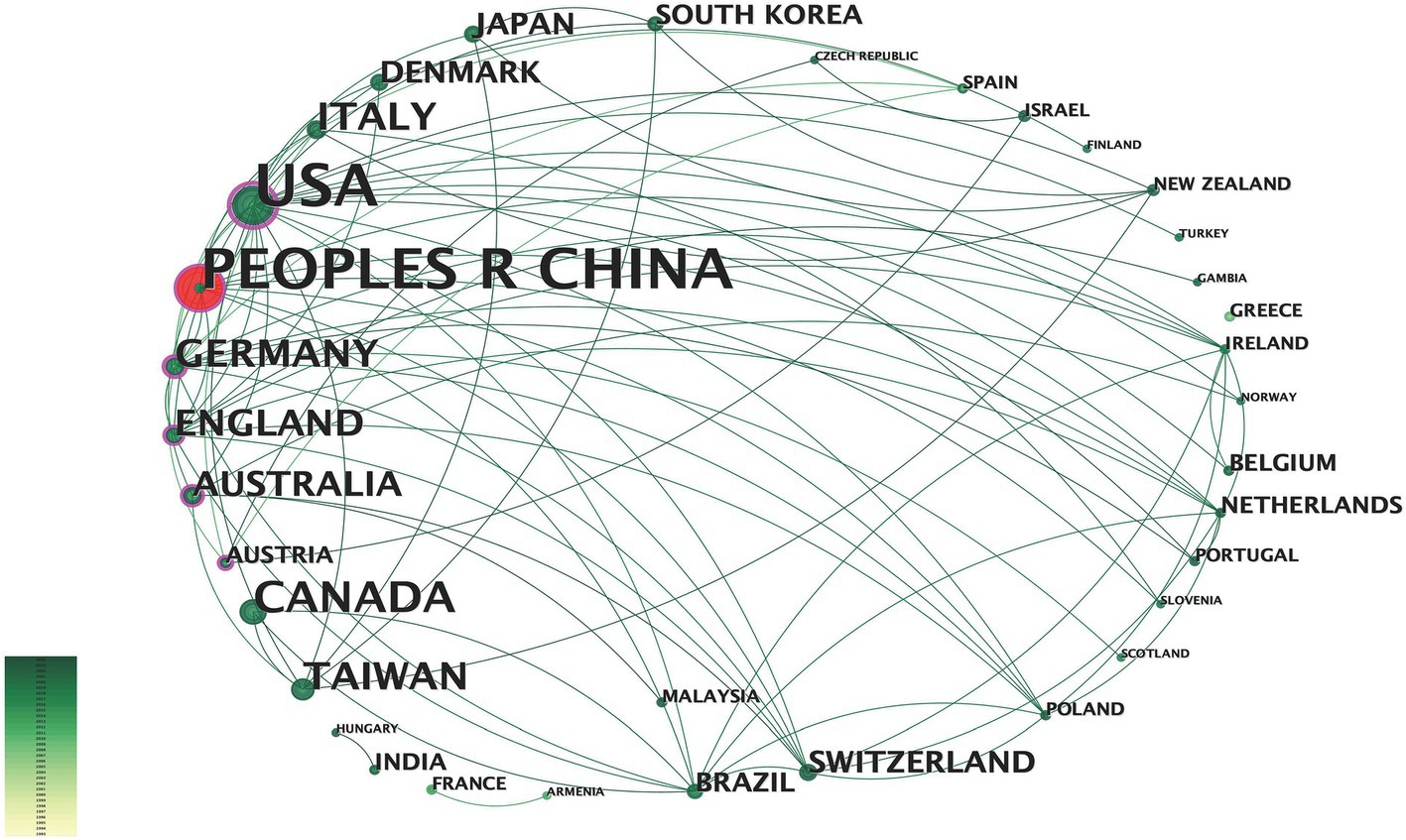
Figure 5. Countries or regions contributing to fNIRS research. The larger the text in the node, the greater the number of articles sent by the country. The node center red indicates a dramatic increase in the number of posts from that country or region.
3.1 Monitoring and assessment of residual consciousnessNumerous studies suggest that the human brain operates within a sophisticated network for functional connectivity and integration. This network exhibits a delicate balance between spontaneous segregation and integration during neuronal activity, which is essential for cognitive, perceptual, or motor signaling (66, 67). Its strength is typically assessed using correlation coefficients and quantified using graph-theoretic topological analysis of resting-state data (68, 69).
In patients with DoC, the functional connectivity of the resting-state brain network is significantly disrupted, with diminished strength compared to that of healthy individuals (70). Specifically, the functional connectivity in the frontal lobes, particularly the frontal pole region and the right dorsolateral prefrontal cortex, is severely impaired in patients with MCS (71–73). CRS-R remains the clinical gold standard for distinguishing between VS and MCS (74). However, behavioral assessments based on the CRS-R are prone to subjective, influenced by the clinical assessor’s understanding of DoC criteria and their application of quantitative scales. Such variability may result in biased or erroneous diagnoses (6, 75, 76) To address these limitations, objective assessment tools are essential for accurately evaluating the sensory, motor, and cognitive functions of DoC patients and for predicting neurological recovery (76, 77). These tools provide a crucial complement to subjective assessments, enabling more reliable and standardized evaluation methods. Experimental paradigms for detecting and assessing levels of consciousness typically fall into two categories: resting-state brain functional connectivity analyses and task-based brain response analyses, the latter of which includes both passive and active paradigms.
3.1.1 Resting-state paradigmThe fNIRS resting-state paradigm is primarily utilized to evaluate the functional brain state of patients with DoC. It allows clinicians to more accurately assess patients’ level of consciousness, thereby supporting informed clinical management and decision-making. After mitigating noise from physiological artifacts, such as respiration and heartbeat, resting-state fNIRS data analysis can produce robust correlation maps and theoretical indicators. These indicators represent functional connectivity between brain regions, offering valuable insights into spontaneous brain activity in the absence of specific tasks and its relationship with disease (78, 79).
Liu et al. demonstrated the efficacy of resting-state fNIRS in detecting residual neurofunctional connectivity, confirming its effectiveness in identifying the status of DoC patients (80). Similarly, Urquhart et al. highlighted the feasibility of using fNIRS in various functional connectivity metrics, showing how physical activity influences cortical networks across different frequency bands (81). Furthermore, He et al. assessed residual consciousness in 18 DoC patients using resting-state fNIRS and applied graph-theoretic analyses to improve classification accuracy. Their findings provided valuable insights into the diagnosis of DoC patients and enhanced understanding of the pathological mechanisms underlying DoC, thereby advancing prospects for neurological recovery (31).
3.1.2 Active paradigmUsing the fNIRS, patients with DoC can be assessed for residual consciousness through passive tasks and active commands-based paradigms. In clinical practice, fNIRS has demonstrated its value in identifying patients with locked-in syndrome or impaired consciousness, thereby expanding its clinical applicability (12). “One approach involves measuring changes in prefrontal blood oxygenation in response to auditory stimuli. For instance, patients are asked to listen to two simple questions and imagine answering ‘yes’ or ‘no.’”. Results showed that some of the MCS patients and one patient with UWS exhibited blood oxygenation changes consistent with patterns observed in healthy controls, indicating residual verbal comprehension and volitional expression (42). Another study demonstrated that three out of five MCS patients produced with one patient answering all questions accurately using this method. This study confirms the feasibility of the fNIRS in detecting residual cognitive abilities in DoC patients through active command-driven MI (28). fNIRS technology provides a non-invasive and reliable means to measure neuronal activity and assess stimulus–response relationships in DoC patients, offering critical insights into their potential for neurological recovery. This capability can significantly influence their future treatment strategies and clinical management.
Patients with different types of docs are instructed to imagine performing simple or complex movements, such as speaking or shaking a fist, to assess their motor function and recovery potential using MI tasks under active command, which can be divided into visual and motor tasks. These tasks, categorized as visual or motor, are closely linked to the corticomotor system, as demonstrated by visual and kinesthetic imagery (82). One study found that some MCS could follow commands to imagine shaking a fist with either their left or right hand. The cortical hemodynamic changes measured by fNIRS in these patients were consistent with those observed in healthy controls (36). Another study compared the hemodynamic responses of the MCS and UWS patients during imagery tasks, revealing that the MCS group exhibited higher hemodynamic responses and better CRS-R scores, whereas the UWS group showed no significant changes (83). These findings suggest that MCS patients have greater potential for neurological recovery. Furthermore, fNIRS has proven to be a convenient and effective tool for detecting residual communication and awareness in DoC patients.
3.1.3 Passive paradigmfNIRS can assess different degrees and types of Docs by detecting cognitive or perceptual responses to stimuli that do not require active participation or response. These stimuli may include touch, images, and sounds. For instance, one study used fNIRS to measure prefrontal blood oxygenation changes in patients with prefrontal blood oxygen changes in patients with VS, MIS, and MCS as they listened to recordings of their names and unfamiliar names (84).
3.2 Quantitative evaluation of neuromodulation therapy effectsNeuromodulation therapy plays a pivotal role in treating refractory neurological disorders such as epilepsy, Parkinson’s disease, and depression (85, 86). This technology encompasses both non-invasive and implantable approaches, delivering electromagnetic stimulation or chemical agents to targeted regions of the management of DoC, SCS and DBS are the most commonly employed neuromodulation techniques (87).
3.2.1 Deep brain stimulationThe precise mechanism of action of DBS remains unclear, but it is widely believed to regulate neurotransmitter release, inhibit abnormal neural activity, stimulate synapse activity and neural remodeling, and alleviate symptoms of brain dysfunction (88). The first documented use of DBS in treating DoC was reported in 1969, demonstrating its potential to restore conscious arousal and pathways following loss of consciousness (89, 90).
DBS is a critical tool in neuromodulation and monitoring cognitive function during stimulus on and off periods is essential to evaluate its modulatory effects. A recent study utilized fNIRS to measure blood flow changes in the frontal, parietal, and occipital lobes. The findings revealed that global communication efficiency correlated significantly with the CRS-R index (slope = 57.384, p < 0.05, r = 0.483), while no significant relationship was observed between changes in communication strength across six brain lobes and the CRS-R index (91). The integration of fNIRS into DBS therapy provides valuable insights into its impact on neurological restoration and recovery in DoC patients. This combination underscores the potential of fNIRS-based functional connectivity analysis as a powerful quantitative assessment tool in neuromodulation research and clinical applications.
3.2.2 Spinal cord stimulationSCS has been studied using the fNIRS passive paradigm. Unlike fMRI, fNIRS is unaffected by metallic implants, such as deep or spinal cord stimulators, making it suitable for assessing real-time activity changes during neuromodulation therapy in patients with DoC. This capability provides crucial clinical guidance for regulating the frequency and duration of stimulation during SCS therapy. Zhang et al. reported a significant increase in cerebral blood volume in the prefrontal and occipital cortex when SCS was applied at a stimulation interval of 30 s. Their findings suggest that optimizing stimulation intervals can promote neurological function recovery in DoC patients, offering robust evidence and a feasible research paradigm for further quantitative assessment of treatment effects (33). Si et al. measured hemodynamic changes in the prefrontal and occipital lobes of DoC patients at varying frequencies using SCS. They observed that high-frequency SCS at 70 and 100 Hz significantly enhanced the cerebral hemodynamic response in the prefrontal region, with SCS at 70 Hz notably improving functional connectivity between the prefrontal and occipital lobes (92). In conclusion, fNIRS-based visualization enhanced the standardization of SCS therapy and supports neurological recovery in DoC patients.
Additionally, fNIRS-based visualization enhances the standardization of SCS therapy and supports neurological recovery in DoC patients. Additionally, closed-loop neuromodulation techniques such as repetitive transcranial magnetic stimulation (rTMS) and vagus nerve stimulation (VNS) are widely employed in DoC treatment. However, research combining these techniques with fNIRS remains limited. Future studies exploring the use of fNIRS to evaluate the therapeutic effects of rTMS and VNS in DoC may provide valuable insights into the advancement of neuromodulation strategies.
3.3 BCIBCI technology enables direct interaction between the brain and external devices by deciphering neural activity, serving as a means of communication and neurological function recovery. It has been applied in various fields, including communication, artificial intelligence, and biomedicine. In recent years, fNIRS-based BCI has shown significant potential in facilitating communication for patients with DoC. Natio et al. demonstrated the feasibility of using fNIRS to analyze cerebral blood flow, enabling communication with amyotrophic lateral sclerosis patients in complete locked-in syndrome. This study highlights the potential of fNIRS in establishing effective BCI systems (93). Notably, fast and efficient fNIRS-BCI communication has been achieved in as little as 2 s (94).
MI and mental arithmetic are the two most common paradigms in fNIRS-based BCI applications. The MI paradigm is particularly suitable for patients with severe physical impairments, as it does not require intact thalamocortical tracts (95). Linguistic imagery tasks, where patients can imagine speaking sentences, words, or numbers, are also employed to evaluate language-related brain activity and assess nerve conduction, linguistic function, and prognosis (84, 96, 97). fNIRS has been used to measure blood oxygenation changes in the frontotemporal lobes of doC patients imagining themselves uttering a sequence of numbers. Some MCS patients exhibited blood oxygenation patterns consistent with those of healthy controls, suggesting residual memory and language production abilities, as well as potential for rehabilitation (98). Integrating fNIRS with EEG enhances BCI performance, creating a weighted assessment system capable of stable long-term monitoring (99). Mental arithmetic, involving simple calculations without external aids, is another widely used paradigm. Since the prefrontal cortex is less prone to data noise due to minimal hair interference, it is often the preferred region for fNIRS studies (100, 101). Mental arithmetic has been successfully applied in BCI research with both brain-injured patients and healthy controls (100).
The fNIRS-based BCI classification approach currently lacks the consistency needed to meet the performance standards required for practical, real-world applications. A “real BCI” refers to a system designed for dynamic, real-world environments that demands high classification accuracy (typically >80%), robustness across users and settings, sufficient information transfer rates, and ease of use. These criteria are critical for reliable applications in clinical and assistive technologies. Recent research has demonstrated promising advancements in this area. Zhang et al. developed a convolution-based conditional generative adversarial network (CGAN) with an improved Inception-ResNet architecture for fNIRS-BCI classification. Their method, tested on MA and mental singing (MS) tasks, achieved a maximum accuracy of 92%, showcasing its potential for high-precision applications (102) Similarly, Jinuk et al. utilized a deep convolutional neural network to distinguish between idle-state and mental arithmetic tasks, achieving classification accuracies exceeding 85%. This approach reduced the need for extensive calibration sessions, thereby improving the practicality of subject-independent fNIRS-BCI systems (103). In addition to widely used machine learning algorithms such as support vector machines (SVM) and linear discriminant analysis (LDA), channel selection methods based on positive Z-scores have been employed to enhance fNIRS-BCI performance. The Z-score method significantly improved classification accuracies, achieving 87.2 ± 7.0%, 88.4 ± 6.2%, and 88.1 ± 6.9% (p < 0.0167) for left MI vs. rest, right MI vs. rest, and mental arithmetic vs. rest, respectively. This approach was further validated using an open-access dataset of 17 subjects (104).
BCI systems have been extensively applied to detect cognitive functioning and consciousness in patients with DoC. For example, Guger et al. reported that BCI systems enable efficient and effective communication in patients with locked-in syndrome (105). Additionally, EEG-BCI systems have been used to detect mood alterations and command-following abilities in one-third of DoC patients (106). While the utility of BCI systems in neurological applications is well-documented, research specifically on fNIRS-based BCI systems for DoC patients remains limited. Preliminary findings from other populations suggest that fNIRS-BCI technology could provide valuable insights into brain function. However, its feasibility and effectiveness in supporting prognostic judgment and clinical decision-making for DoC patients require further validation. Nevertheless, the non-invasive nature, portability, and cortical activity assessment capabilities of fNIRS-BCI systems position them as promising tools for aiding functional and cognitive recovery in DoC patients. Continued research in this field could establish a robust foundation for integrating fNIRS-BCI into clinical practice.
4 Limitations and prospects 4.1 LimitationsfNIRS is an innovative neuroimaging technique capable of assessing residual consciousness and potential neural functions in patients with DoC. By utilizing various near-infrared brain imaging paradigms or tasks, fNIRS can explore cortical structures and neural network restoration, providing valuable guidance for diagnosis, evaluation, and treatment (107, 108). However, several limitations and challenges remain. These include issues with signal quality, spatial resolution, and the lack of standardized protocols, all of which require further investigation and refinement for clinical use. Technical challenges in fNIRS data collection often arise from craniocerebral injuries, such as the effects of intracranial hemorrhages on imaging and poor signal quality due to suboptimal contact between cranial surgical probes and the scalp. Additionally, structural changes in the brain may further degrade signal quality in functional regions (109, 110). Furthermore, fluctuations in alertness, awareness, and concentration in DoC patients present another challenge, complicating the reliability of fNIRS measurements.
4.2 ProspectsAmong neuroimaging modalities for DoC detection, EEG and fMRI are more commonly used. EEG offers high temporal resolution and has advanced task-based paradigms for assessing and classifying DoC patients, while fMRI provides high spatial resolution and unique insights into functional brain networks. However, fNIRS has emerged as a promising tool for DoC assessment, offering moderate spatial and temporal resolution, superior portability, and cost-effectiveness. Future research in the following areas could enhance its utility.
4.2.1 Improvement of technical precisionMost fNIRS research has focused on detecting brain activity through sensorimotor activity in MI paradigms. However, its sensitivity for detecting task-related activation in single-subject data remains inadequate for clinical applications. Many studies rely on simpler, less sensitive continuous-wave devices. Advancing the use of high-channel-density frequency-domain and time-domain fNIRS systems could enable the capture of clinically meaningful brain responses at higher resolutions. Developing sensitive fNIRS markers for MI, particularly in the sensorimotor cortex, which is easily accessible via scalp-based sensors, would be invaluable for DoC patients.
4.2.2 Multi-modal fusionMultimodal brain imaging can address the limitations of single modalities by extracting complementary features. Integrating fNIRS with other neuroimaging methods, such as EEG, could provide a more comprehensive and accurate assessment of DoC. Advanced machine learning techniques, including artificial neural networks, could further enhance the sensitivity of fNIRS (111). Recent studies combining fNIRS and EEG for BCI applications have shown that these techniques can meet clinical standards for DoC assessment. The consistent and complementary nature of EEG and fNIRS signals—EEG offering high temporal resolution and fNIRS providing insights into neurovascular coupling—has proven effective. For instance, coupling between fNIRS oxygenated hemoglobin (0.07–0.13 Hz) and EEG band power (1–12 Hz) in the frontal region has been shown to predict consciousness recovery following acute brain injury. Such findings highlight the potential of fNIRS-EEG as a valuable addition to multimodal neuromonitoring (112, 113). Some studies use fMRI to improve the modeling of fNIRS-collected brain data to provide an initial roadmap and for fMRI researchers to add fNIRS experimental assays to their study subjects (114). Mixed-modality imaging may become the future standard for assessing disorders of consciousness.
4.2.3 Large-scale researchUnderstanding the relationship between fNIRS signals and patients’ consciousness states requires larger datasets. Different types of DoC may exhibit distinct fNIRS signal patterns, necessitating extensive clinical studies to classify and validate these differences. Large-scale research will be crucial for verifying the validity and feasibility of fNIRS as a reliable tool for detecting and evaluating consciousness in DoC patients.
4.2.4 fNIRS-based hyperscanningHyperscanning, which allows simultaneous recording of neural signals from two or more participants, was first applied to fNIRS in 2012 to study neural activation during social interactions (115). For example, eye contact between individuals activated relevant neural systems that could not be captured under static conditions. The central subcentral gyrus (BA43) was selectively activated during eye contact, demonstrating connections to facial, linguistic, sensorimotor, and executive functions. This study provided insights into the continuous, bidirectional flow of social interactions. Future fNIRS research could focus on sensory processes underlying coherent neural activity during real-time, spontaneous human interactions, offering new avenues for understanding social and neural dynamics (116).
5 ConclusionThis review highlights recent advances in the application of fNIRS for DoC while addressing its current challenges and limitations. As an emerging neuroimaging technique, fNIRS provides a unique capability to explore residual consciousness and potential neural functions in DoC patients, offering valuable guidance for diagnosis, prognostic assessment, and therapeutic management. However, significant challenges remain, including issues with signal quality, spatial resolution, standardized data analysis, and its limited ability to elucidate specific mechanisms of neural recovery. Addressing these challenges will require continued research and technological development.
The integration of multiple imaging modalities to jointly assess consciousness states presents a promising avenue for improving clinical diagnosis and prognosis. fNIRS stands out as a non-invasive, portable, and reliable method for assessing neural activity and evaluating therapeutic outcomes in DoC patients. Its ability to capture real-time brain function changes positions it as a critical tool for guiding treatment strategies and monitoring the efficacy of neuromodulation therapies. Nevertheless, further research is essential to enhance its accuracy, spatial resolution, and integration with other neuroimaging techniques, thereby unlocking its full clinical potential.
Author contributionsNW: Conceptualization, Formal analysis, Writing – original draft. YH: Conceptualization, Formal analysis, Writing – original draft. SZ: Writing – review & editing. DL: Writing – review & editing. XC: Investigation, Writing – review & editing. QH: Investigation, Writing – review & editing. TC: Investigation, Writing – review & editing. JH: Methodology, Writing – review & editing. JL: Methodology, Writing – review & editing. JS: Supervision, Validation, Writing – review & editing. YY: Supervision, Validation, Writing – review & editing. JZ: Supervision, Validation, Writing – re
留言 (0)