Mental disorders are common and burdensome. Among people with severe mental illness, deaths from unnatural causes have increased significantly, occurring 13 times more frequently compared to the general population, with suicide being the leading cause (20 times higher) (Revier et al., 2015). Antidepressants and antipsychotics remain the primary strategies for treating psychiatric symptoms. However, the long-term efficacy of drug treatment has been questioned. For many individuals with mental illness, antipsychotics do not result in clinically meaningful long-term improvements and often cause significant side effects (Malhi and Mann, 2018), such as weight gain, elevated blood sugar levels, elevated blood lipids, and loss of sexual interest. These side effects frequently lead to withdrawal and discomfort (De Hert et al., 2011; Vancampfort et al., 2015). Over the past few decades, the use of traditional Chinese medicine (TCM) to treat various mental disorders, including depression, has grown significantly. Studies suggest that TCM is a safer alternative to drug therapy, with a lower risk of side effects (Yeung K. S. et al., 2018).
Traditional Chinese medicine operates under the guidance of traditional Chinese medicine theory. It exhibits characteristics such as multi-metabolite, multi-target, multi-approach, and holistic concept (Ma et al., 2023; Wang and Zhang, 2017; Zheng et al., 2024; Song et al., 2023). While traditional Chinese medicine demonstrates remarkable clinical efficacy, its modern development faces constraints due to unclear efficacy substances and mechanisms of action. In recent years, there has been rapid development in metabolomics technology (Misra, 2018; Bingol and Brüschweiler, 2017). Its research strategy, which is based on detecting the dynamic changes of global metabolites, aligns with traditional Chinese medicine theory (Zhang et al., 2010; Wei et al., 2024). This alignment presents new opportunities to address the developmental challenges faced by traditional Chinese medicine. Metabolomics technology has been extensively employed in researching the material basis and pharmacodynamic mechanisms of traditional Chinese medicine, yielding promising results (Wang et al., 2017).
However, the metabolism and distribution of traditional Chinese medicine metabolites in organisms often exhibit precise spatial positioning (Bai et al., 2022). The efficacy of the medication is closely linked to the spatial distribution of biological tissue or micro-regions. Nevertheless, traditional metabolomics methods have limitations in sample pre-processing, resulting in the absence of spatial distribution information of metabolites in tissues (Liu G. X. et al., 2023). This absence poses challenges in fully and objectively interpreting the sites of action and pharmacodynamic mechanisms of traditional Chinese medicine. Spatial metabolomics enables the correlation of metabolites and their biological functions with the anatomical characteristics of biological tissues (Sun et al., 2019; Nakabayashi et al., 2021). This approach allows for a more accurate and scientific analysis of the pharmacodynamic metabolites of traditional Chinese medicines and the regulatory mechanisms of diseases within organisms.
Indeed, these methods also facilitate the comprehensive characterization of metabolic functions at physiological and pathological time scales with high spatial resolution. Mass spectrometry imaging (MSI) is a powerful method to perform in situ analysis of the molecular composition of biological tissues while retaining spatial information (Parrot et al., 2018). Furthermore, spatial metabolic characterization holds significant relevance to our comprehension of normal physiological processes and the neuropathological manifestations of neurological disorders (Wang et al., 2022).
Hence, spatial metabolomics technology was utilized to establish the relationship of “molecular structure-spatial distribution-content change-metabolic pathway,” offering novel insights into the search for medicinal metabolites, therapeutic targets, and mechanisms of action of traditional Chinese medicine (Zhao et al., 2023a).
This paper provides a comprehensive overview of the research progress in spatial metabolomics technology concerning the quality control, metabolic distribution, pharmacodynamic mechanisms, and toxicity mechanisms of Chinese medicine. Additionally, it critically examines the limitations and future development directions of spatial metabolomics in the study of Chinese medicine for treating mental diseases. These insights aim to furnish a theoretical basis for advancing the modernization and internationalization of Chinese medicine in the treatment of mental diseases.
2 Traditional metabolomics and spatial metabolomicsMetabolomics involves the systematic study of small and medium molecules in biological fluids. The term “metabolomics” was first coined by Dr. Nicholson of Imperial College London in 1999 (Yu et al., 2017). While metabolomic analysis shares similarities with other high-throughput methods like genome sequencing, its rapid response to both exogenous and endogenous stimuli renders it particularly sensitive to changes in health status (Dona et al., 2016). Spatial metabolomics has been developed based on mass spectrometry imaging technology, characterized by its lack of labeling, matrix, and short analysis cycle (McDonnell and Heeren, 2007; Zang et al., 2021). Serving as a novel molecular imaging technology, spatial metabolomics can directly provide spatial distribution information of numerous known or unknown endogenous metabolites and exogenous drugs from biological tissues (Wang Z. et al., 2021). By employing mass spectrometry imaging technology, spatial metabolomics enables the analysis of metabolites in different tissues and organs in three dimensions, including qualitative, quantitative, and localization aspects. This breakthrough overcomes the limitations of traditional metabolomics research, which often loses spatial information. The comparative analysis of traditional and spatial metabolomics platforms and their respective application conditions are summarized in Table 1.
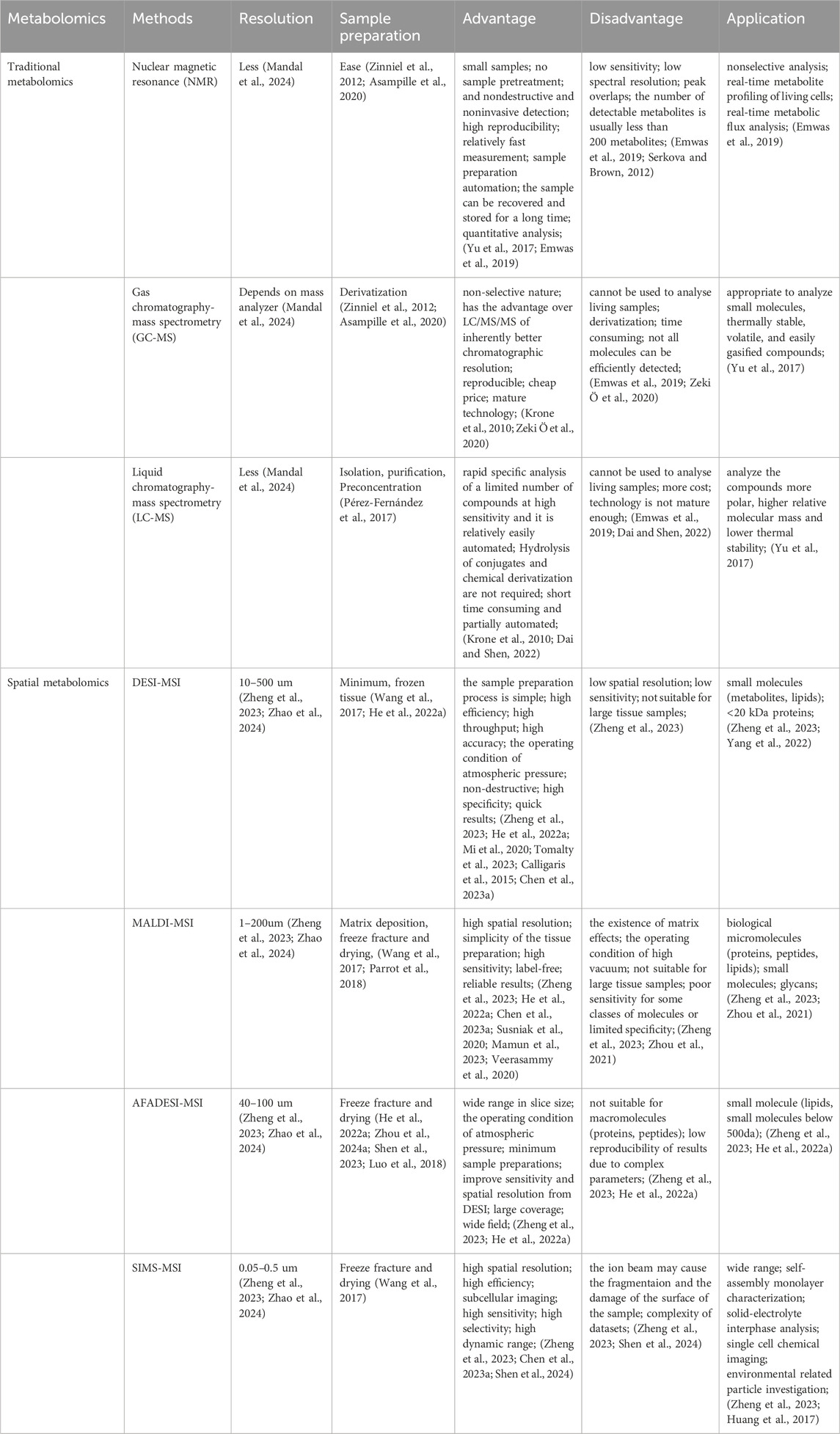
Table 1. Traditional metabolomics and spatial metabolomics.
2.1 Desorption electrospray ionization mass spectrometry (DESI-MSI)DESI-MS, introduced in 2004, is an atmospheric pressure environmental ionization method that directly ionizes solid-phase samples (Takáts et al., 2004). DESI-MSI employs the fundamental principle of electrospray ionization, wherein solvent droplets are rasterized and desorbed directly onto the sample surface (Parrot et al., 2018; Eberlin et al., 2011). DESI operates at room temperature, eliminating the need for freeze-drying prior to analysis. This method of tissue imaging minimizes sample damage through environmental ionization mass spectrometry, enabling repeated measurements of samples from diverse biological sources (Soudah et al., 2023). Ambient MSI offers a user-friendly interface and facilitates the rapid analysis of larger samples, thereby facilitating real-time diagnostic capabilities (Luo et al., 2013; Keller et al., 2018).
However, enhancing the sensitivity of DESI-MSI presents substantial challenges (Wang et al., 2017). Recent studies have demonstrated that the sensitivity and specificity of DESI-MSI nanoparticles can be enhanced by incorporating silver ions into the solvents (Lillja and Lanekoff, 2022). Researchers have developed a compact post-photoionization module integrated with DESI, enabling the detection of enhanced signal strength for non-polar compounds. This advancement significantly enhances the sensitivity of DESI-MSI to non-polar compounds (Liu C. et al., 2019). Furthermore, there are emerging indications that the spatial resolution of DESI will pose a substantial impediment in numerous other applications where it could be potentially beneficial (Qi et al., 2021). Consequently, scientists are currently engaged in a concerted effort to significantly enhance the spatial resolution of DESI. Subsequent research has demonstrated that nano DESI-MSI possesses the potential to attain even finer spatial resolution, potentially reaching a resolution of 10 microns (Yin et al., 2018; Yin et al., 2019; Yang et al., 2023).
2.2 Matrix-assisted laser desorption/ionization mass spectrometry imaging (MALDI-MSI)The concept of MALDI-MSI was initially introduced in the early 2000s (Morisasa et al., 2019). MALDI is a soft ionization technique that involves the co-crystallization of a sample molecule or analyte with a matrix to form a sample matrix crystal. The matrix functions as a proton donor or acceptor, ionizing the analyte (Yalcin and de la Monte, 2015). MALDI-MSI operates by directing a laser beam at the surface of a specimen, typically a frozen section of tissue. This laser action induces the desorption of ions from the tissue, which are subsequently analyzed through a mass spectrometer (Basu and Agar, 2021; Kuik et al., 2024). Despite ongoing technological advancements, the low detection sensitivity of certain compounds poses a significant challenge that requires effective solutions. Research has indicated that poor sensitivity is often associated with reduced ionization efficiency, low analyte and matrix ion abundance, or endogenous interference. Histochemical derivatization has emerged as a crucial strategy to address these challenges, preserve tissue integrity, and mitigate potential dislocations (Merdas et al., 2021).
2.3 Airflow-assisted desorption and electrospray ionization mass spectrometry imaging (AFADESI-MSI)AFADESI-MSI employs DESI technology to directly ionize the sample using an electrospray plume. Subsequently, a gas stream propels the ions over extended distances, enabling mass spectrometry imaging (Luo et al., 2013). In addition to inheriting the advantages of DESI-MSI, AFADESI-MSI can also attain exceptionally high metabolite coverage. It is an environmental molecular imaging technology characterized by its high sensitivity, extensive coverage, and exceptional chemical specificity (He M. J. et al., 2022). A significant advantage of this approach is its direct predictive applicability to a substantial number of candidate metabolites and metabolic enzymes, eliminating the necessity to define a specific target of interest beforehand (Sun et al., 2019). Although this novel technique yields drug signal strength, it cannot objectively reflect the absolute drug content in various tissues due to sample heterogeneity, ion inhibition, analyte extraction efficiency, and ionization efficiency (Zhang et al., 2020).
2.4 Secondary ion mass spectrometry (SIMS-MSI)The SIMS instrument bombards the sample surface with a finely focused primary ion beam (an analysis gun) to generate characteristic secondary ions from the sample surface. These secondary ions are subsequently detected using a mass analyzer. By rasterizing the primary ion beam on the surface of a solid sample, mass-resolved secondary ion images can be obtained, thereby providing chemical mapping of each component of the surface (Huang et al., 2017). The primary advantage of SIMS lies in its capability to measure the spatial localization of molecules with exceptional spatial resolution. It is particularly effective in targeting inorganic compounds or biomolecules with relatively low molecular weights (Wu et al., 2013). Although samples for SIMS do not necessitate any special surface treatment, it is important to note that SIMS can be a destructive analysis technique, which may lead to sample loss. Furthermore, quantifying the composition of SIMS samples can be challenging due to matrix effects and fragmentation processes that occur during SIMS analysis (Huang et al., 2017).
In summary, mass spectrometry imaging (MSI), a tool capable of in situ quantitative qualitative and two-dimensional imaging, is characterized by high stability, high throughput, and label-free. The above MSI techniques have their characteristics. MALDI is suitable for detecting various small and large molecules, and it is the most used technique in multiple fields, but the preparation process is relatively complicated. DESI is more accurate for the in situ localization of small molecules in tissue slices, but the spatial resolution is relatively low compared with the other techniques. DESI has a broader range of application scenarios than the different techniques, and it can be used for detection at room temperature. SIMS can measure the spatial localization of molecules with high spatial resolution, but the sample components used for SIMS may be lost, generating fragment ions that can severely interfere with the detection signals of small chemical molecules. AFADESI directly inherits the advantages of DESI but also optimizes the technology based on it.
3 Spatial metabolomics and Chinese medicine3.1 Quality controlChinese medicine quality control plays a vital role in ensuring the clinical efficacy of Chinese medicine (Li X. R. et al., 2021). The medicinal parts, metabolites, and distribution of traditional Chinese medicine directly reflect its quality, but traditional analysis methods often face challenges in achieving comprehensive assessments. MSI emerges as a novel analytical method that overcomes the technical limitations of traditional approaches. MSI technology encompasses secondary ionization (SI), matrix-assisted laser desorption ionization (MALDI), and desorption electrospray ionization (DESI) methods based on ionization techniques (Ganesana et al., 2017). Notably, MSI eliminates the need for intricate sample pretreatment steps and offers the capability to detect known or unknown metabolites with high throughput, sensitivity, and resolution (Zheng et al., 2023). It serves as a straightforward and swift approach for identifying quality markers in Chinese medicine, enabling the direct characterization of chemical features and spatial distribution across various samples. Consequently, MSI holds promising applications in Chinese medicine quality control (Jiang H. et al., 2022; Dong and Aharoni, 2022). Table 2 presents an overview of spatial metabolomics studies in Chinese medicine quality control.
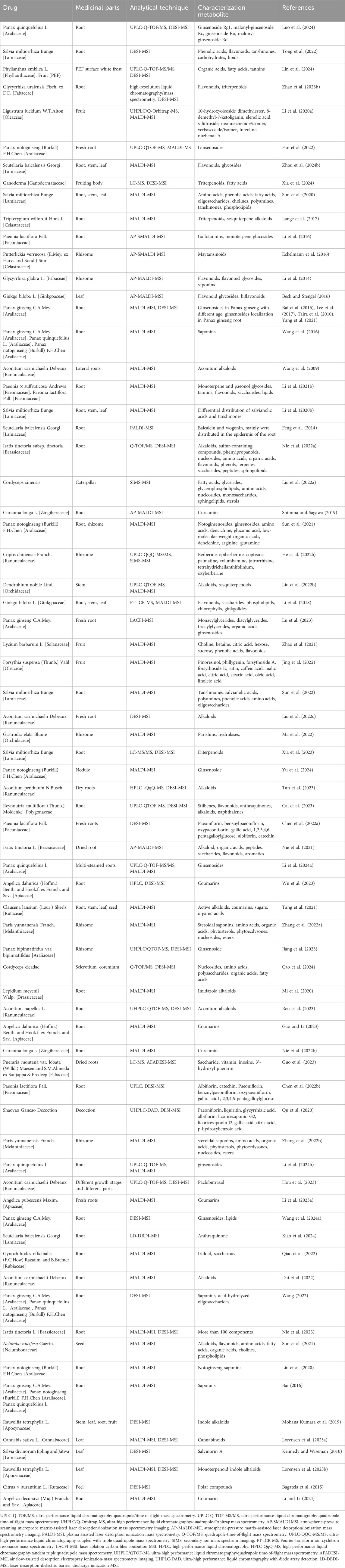
Table 2. Spatial metabolomics and quality control.
3.2 Spatial distribution and pharmacodynamic mechanismThe distribution and metabolism of TCM active metabolites in tissues are crucial for identifying target organs, understanding the pharmacodynamic material basis, and evaluating potential adverse reactions of TCM. However, traditional analysis techniques often destroy tissue structure during sample preparation, making it difficult to clearly characterize the regional distribution of active ingredients and metabolites of TCM. MSI can extract extensive data and provide information about the spatial distribution of these data by analyzing tissue slices (Xu et al., 2022). Spatial metabolomics can simultaneously characterize the spatial metabolic distribution of TCM active metabolites and their metabolites in the whole or micro-regions of different tissues and organs. This approach presents a more complete metabolic process and is a significant analytical technique in neuroscience research (Liang et al., 2022). Table 3 shows studies on the metabolic distribution of TCM in organisms.
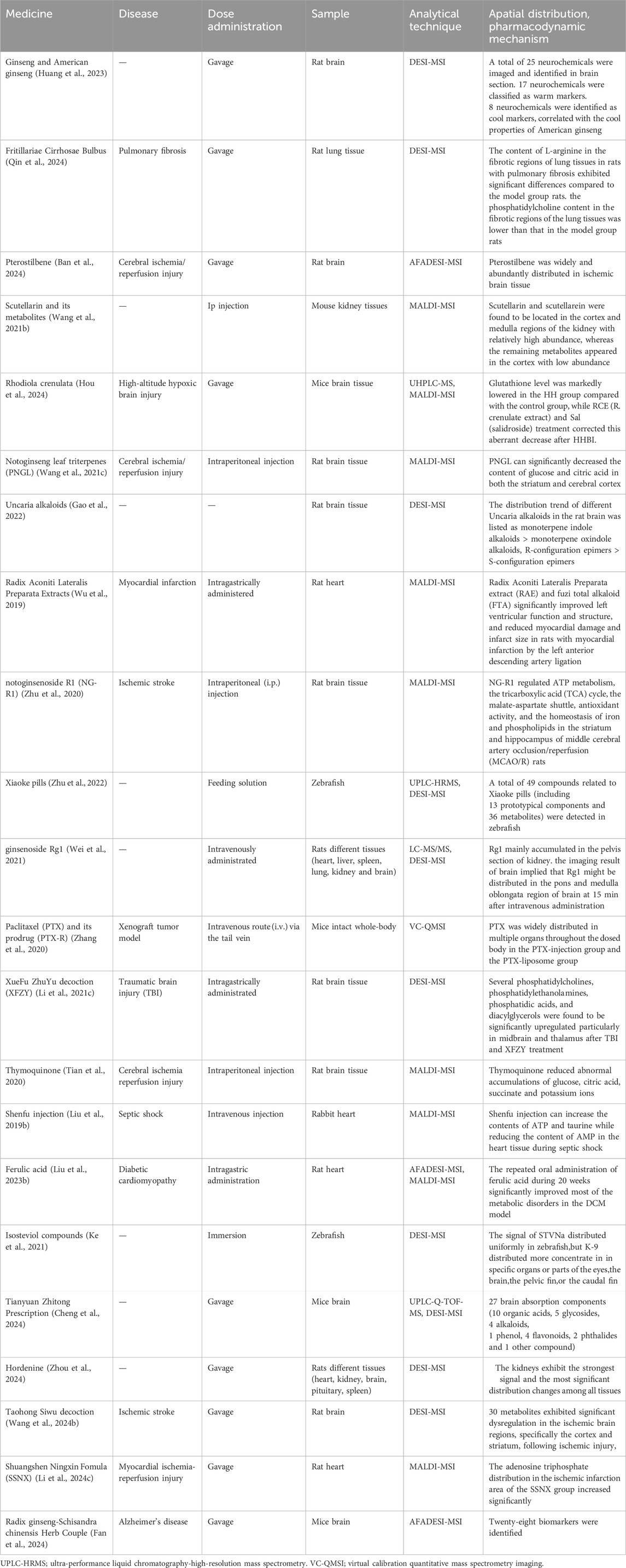
Table 3. Spatial metabolome, spatial distribution and pharmacodynamic mechanism.
3.3 Toxicity mechanismDrug safety poses a significant threat to human health. Toxicological analysis and safety evaluation are crucial aspects of drug development. Conventional analysis methods cannot provide spatial distribution information. However, spatial dimension analysis can supplement safety evaluations, enabling better prediction and assessment of drug toxicity (Chen et al., 2023b). Spatial metabolomics allows us to study the distribution of toxic Chinese medicine components and their metabolites in tissues and organs. This technique provides a scientific basis for identifying toxic target organs and revealing toxic molecular mechanisms (Table 4).
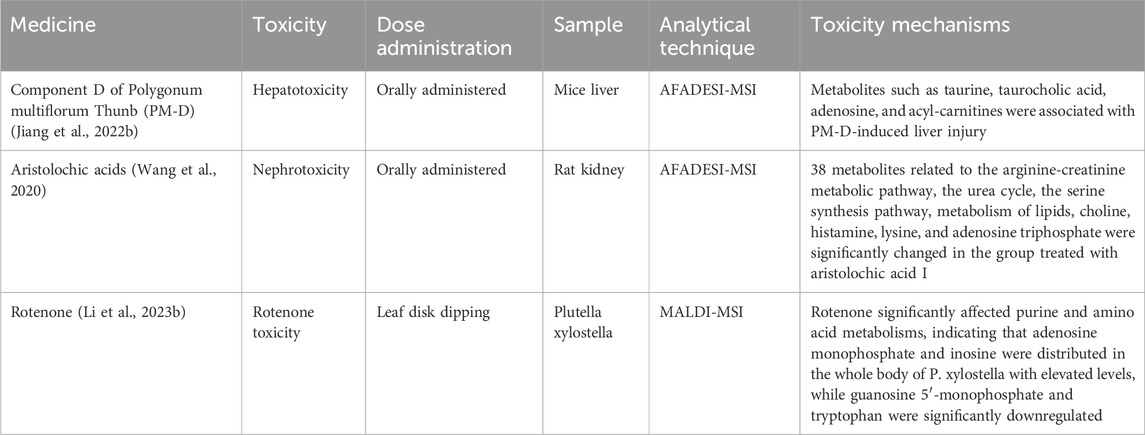
Table 4. Spatial metabolome and toxicity mechanisms.
4 Spatial metabolomics and mental disorders4.1 Spatial metabolomics studies on schizophreniaSchizophrenia, a major mental illness, involves lipids playing a crucial role. The authors (Matsumoto et al., 2011) have demonstrated the association between lipid analysis and brain functional mapping in postmortem human brains. They identified the types of lipids in normal human brains using LC/ESI-MS/MS. Subsequently, MALDI-MSI analysis of brain tissue was conducted to screen for differentially expressed lipid types between the control group and two schizophrenia patients. In this study, the authors report the abnormal distribution of a molecular species of phosphatidylcholine (PC), specifically in the cortical layer of the frontal cortex region, postmortem in patients with schizophrenia. Additionally, PC (diacyl-16:0/20:4) containing arachidonic acid showed an increase in the frontal cortex of patients with schizophrenia. MALDI-MSI holds a specific advantage in revealing abnormalities in local lipid metabolism in the human brain after death. Moreover, it complements previous findings indicating abnormal brain lipid composition in schizophrenia patients (McNamara et al., 2007; Taha et al., 2013).
The corpus callosum (CC) serves to connect the brain’s hemispheres, yet individuals with schizophrenia exhibit impaired interhemispheric communication, potentially contributing to brain disconnection (Guo et al., 2013). Researchers (Vendramini et al., 2016) utilized DESI-MSI to compare lipid content in postmortem CC samples from two schizophrenia patients and two controls in a label-free manner. The findings reveal a noteworthy reduction in the distribution of phosphatidylcholine in patients with schizophrenia. Interestingly, the 760 Da ions show a much lower abundance of phosphatidylcholine compared to the 788 Da ions. This study marks the first investigation into CC white matter in schizophrenia patients and strongly supports the hypothesis that phospholipid dysfunction is prevalent in schizophrenia (Ross et al., 1997). Despite limitations in sample size, these studies contribute to the molecular understanding of the disease, as well as the identification of biomarkers and drug targets. Phospholipids are bioactive substances crucial for brain function. To analyze differences in the amount and type of phospholipids present in the brain tissue of schizophrenic patients, the authors (Matsumoto et al., 2017) conducted a comprehensive analysis of phospholipids in the postmortem brains of elderly schizophrenic patients. In LC-ESI/MS/MS, the authors found significantly reduced levels of 16:0/20:4-phosphatidylinositol (PI) in the prefrontal cortex of the brain in patients with schizophrenia, while 16:0/20:4-PI was most notably reduced in the gray matter in MALDI-MSI.
4.2 Spatial metabolomics studies on depressionStress represents a risk factor for the development and exacerbation of various diseases, including neuropsychiatric disorders and depression (Sanacora et al., 2022; Park et al., 2019). The endocannabinoid 2-arachidonoylglycerol (2AG) serves as a vital regulator of stress response, with its brain levels increasing in response to heightened stress. Researchers (Islam et al., 2022) investigated the impact of stress on 2AG levels in specific brain regions of senescence-accelerated mouse prone (SAMP8). Utilizing DESI-MSI, they observed a significant increase in 2AG levels in the hypothalamus, midbrain, and hindbrain of SAMP8 mice following 3 days of water immersion stress. Previous reports (Zhai et al., 2023) utilizing DESI-MSI analysis of coronal brain sections from stressed mice indicated that 2-AG levels were highest in the hypothalamus region and lowest in the hippocampus, spanning from forebrain to cerebellum. Furthermore, this study demonstrated elevated levels of endocannabinoid 2-AG in the Anterior Cingulate Cortex, Caudate Putamen, Nucleus Accumbens, and Piriform Cortex in individuals experiencing chronic stress. postpartum depression (PPD) presents a severe mental disorder with significant adverse effects on maternal health. Researchers (Sheng et al., 2024) employed MSI and targeted metabolomics analysis to investigate metabolic changes in the brains of postpartum mice with GABAAR Delta-subunit defects (Gabrd−/−), serving as a specific preclinical model of PPD. This study identified the downregulation of prostaglandin D2 (PGD2) in the central amygdala (CeA) as the most notable change in PPD.
4.3 Spatial metabolomics studies on drug addictionDrug addiction remains a significant global health concern. Researchers (Uys et al., 2010) employed a combination of MALDI-MSI tissue mapping, MALDI-MSI tissue imaging, and bioinformatics analysis to discern differences in protein expression and localization in the nucleus accumbens (NAc) of cocaine-sensitized rats. Through additional sequencing experiments via MALDI tandem mass spectrometry and a database search of measurement quality, they identified an increase in expression of secretoneurin (m/z 3653). Moreover, the distribution of secretoneurin in the NAc was determined through MALDI tissue imaging, and the heightened expression of its precursor protein, secreted granuloprotein II, was verified via Western blotting. This spatial localization aligns with previous immunolocalization studies of secreted neurotin (Marksteiner et al., 1993). Prolonged exposure to morphine can lead to the development of addictive behaviors, and early diagnosis may mitigate the adverse effects of these behaviors on individuals and society. The authors (Bodzon-Kulakowska et al., 2016) utilized the brains of morphine-addicted rats for DESI analysis. Following morphine administration, the substance exhibited marked overexpression in the medial forebrain bundle, hypothalamic nuclei, and fornix region. Furthermore, two systems (BioMap, Datacube) were utilized to analyze images of rat brain tissue under morphine and compare their ease of use and the quality of results obtained. The ST (22:0) ratio of morphine to control rat brain peak intensity was 3.44 for BioMap and 3.55 for Datacube. Although the results were similar, the authors posit that BioMap proves more beneficial for DESI IMS analysis. The application of spatial metabolomics to mental disorders is summarized in Figure 1.
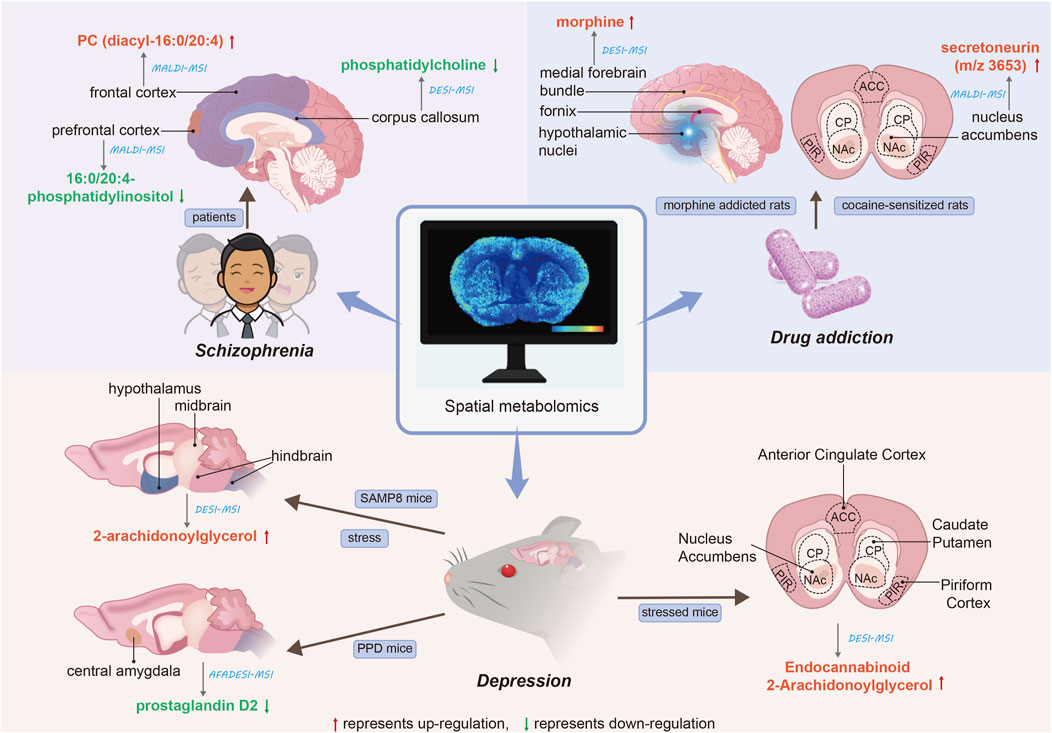
Figure 1. Applications of spatial metabolomics to mental disorders. The red arrows denote the upregulation of the corresponding metabolite, while the green arrows indicate the downregulation of the corresponding metabolite. The straight lines illustrate the spatial distribution of metabolites within brain tissue. The blue italics represent the analytical techniques employed, and the blue text boxes indicate the research subjects. The black italics denote the type of mental disorder.
5 Spatial metabolomics, Chinese medicine and mental disordersChinese medicine has unique advantages in psychiatric disorders and the adverse effects of antipsychotic drugs. Therefore, Chinese medicine’s efficacy in treating psychiatric disorders has been gradually emphasized in clinical practice. The application of antipsychotic drugs is still the primary treatment for mental disorders at this stage. However, poor patient compliance, a sense of discrimination, adverse drug reactions, and complex interactions between different drugs often adversely affect clinical efficacy during treatment. Moreover, almost all the essential principles of drug action established in Western psychopharmacology in the 20th century were discovered empirically in TCM during the 2000 years of evolution (Shorter and Segesser, 2013). In recent years, researchers have made significant progress in basic research and clinical treatment of mental disorders based on TCM characteristics. In today’s clinical therapeutic practice, TCM therapy combined with Western medicine is mainly used for treatment, which TCM treatment is diverse, including but not limited to decoction, Chinese patent drug, acupuncture, TCM gongfu (Baduanjin, Qigong, and Tai-Chi) and Five-Element Music (Xu et al., 2011; Lin et al., 2012; Chan et al., 2015; Zhao et al., 2019; Li et al., 2022; Lam et al., 2024; Zhou, 2020; Wu et al., 2024; Chen et al., 2015; Yeung A. et al., 2018; Zou et al., 2018).
TCM has a complex and diffuse composition, and its formulation is a complex combination of several natural medicines. The study of TCM on the etiology and pathogenesis of mental disorders is still at an exploratory stage. In recent years, accumulated studies have revealed the application of spatial metabolomics approaches to study the etiology and pathophysiology of complex systemic disorders, including depression and other psychiatric disorders, as well as the mechanisms of TCM effects. However, a single “metabonomics” technique may not fully reflect the mechanisms by which TCM treats mental disorders. Therefore, in the study of mental disorders, data from spatial metabolomics, spatial proteomics, and spatial transcriptomics should be integrated to decipher the biological significance and spatial correlation from differential metabolites, proteins, and genes further to explore the mechanism of TCM for mental disorders. So far, most of the studies on TCM for mental disorders first started with untargeted metabolomics. Then, a series of different endogenous metabolites were obtained from standard controls to infer disease-related metabolic pathways, which provided clues for further mechanistic studies but, at the same time, lacked specificity (Gu et al., 2021). Based on this phenomenon, we propose that future studies should not be limited to full-spectrum metabolites but should also focus on targeted metabolomics for further validation. In addition, each metabolomics platform has its advantages and limitations, and multiple platforms should be clustered to apply for targeted metabolomics studies to obtain different spatial metabolomics data to discover and characterize common biomarkers when conditions allow, which in turn will collectively provide new ideas for the development of antidepressant natural products for psychiatric disorders. Despite the rapid growth in the application of metabolomics for treating psychiatric disorders under TCM interventions (Liu, 2020; Zhu et al., 2024; Lv et al., 2022; Zhou et al., 2020), the application of spatial metabolomics is still in the preliminary research stage. We can foresee that shortly, researchers will vigorously carry out corresponding animal models and even clinical studies in spatial metabolomics research of TCM for the treatment of mental disorders. Meanwhile, under the extensive guidance of spatial metabolomics, TCM is expected to become a more acceptable therapeutic option for treating mental disorders.
6 Challenges and perspectivesToday, the unique position of spatial metabolomics in the field of nervous system research is widely acknowledged, and it has begun to find application in studying the metabolic mechanisms of human mental diseases and in the development of new drugs. Serving as a breakthrough technology, spatial metabolomics has opened up numerous new opportunities for the molecular diagnosis of mental diseases treated with traditional Chinese medicine. Nevertheless, it also encounters various challenges, such as metabolite identification and chromatographic separation, as well as issues related to mass spectrometry databases and data sharing (Collins et al., 2021).
Fortunately, advancements in instrumentation, experimental techniques, and analytical software have helped alleviate many of these challenges. For instance, researchers can overlay MS images with optical or HE scans and focus on tissue microregions or lesions of interest to accurately extract mass spectrometry data for the target region in metabolic studies. This approach mitigates the challenges associated with the difficult isolation of study specimens (Jiang H. et al., 2022). In future studies, we can explore three-dimensional MSI, construct multiple slices of two-dimensional MSI data, and visualize another dimension of drug distribution. Furthermore, the biological computing challenges associated with increased spatial resolution also necessitate the development of more efficient data mining tools (Angel and Caprioli, 2013).
It can be predicted that multi-omics joint analysis will become a key research strategy in the future. This approach not only mitigates the data deficiencies stemming from data noise and missingness in single omics analysis, but also reduces the false positive outcomes generated by single omics analysis through the mutual verification of multiple omics data resources. Consequently, multi-omics joint analysis is more conducive to systematically analyzing the multifaceted mechanisms or phenotypic connections of biological models at various levels and perspectives. Moreover, it facilitates the collaborative exploration of potential regulatory network mechanisms within organisms (Zheng et al., 2023).
Specifically, in-depth studies on the spatial distribution of active/toxic ingredients and their metabolites about different metabolites in vivo will be carried out to clarify the active/poisonous ingredients and their target areas and to elucidate the mechanisms of the efficacy or toxicity of traditional Chinese medicines more accurately. We can combine spatial metabolomics with spatial proteomics and spatial transcriptomics to realize multi-dimensional studies on quality control, metabolic distribution, and pharmacodynamic or toxicity mechanisms of TCM at metabolic, protein, and gene levels. The blood-brain barrier maintains the relative stability of the intracerebral environment and blocks drug molecules outside the barrier. The combination of MSI and 3D imaging is also strategically important in studying the intracerebral distribution of drugs and neurological side effects.
Currently, spatial metabolomics has shown vigorous development in exploring the metabolic mechanisms of the nervous system. However, the nascent application of traditional Chinese medicine in the treatment of mental diseases remains underdeveloped. It is worthwhile to expect that MSI technology will provide a new vision for treating mental disorders in Chinese medicine, and the application of spatial metabolomics in treating mental disorders in Chinese medicine will become a key research direction.
Therefore, further research is imperative, as it holds significant guiding implications for studying the metabolic mechanisms underlying TCM treatment of mental diseases. In summary, there exists substantial room for the development of spatial metabolomics in the realm of traditional Chinese medicine and mental illness. Through continual refinement and innovation, it can significantly contribute to the modernization of traditional Chinese medicine.
Author contributionsCL: Writing–original draft, Writing–review and editing, Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Project administration, Software, Supervision, Validation, Visualization. JC: Conceptualization, Methodology, Writing–review and editing. ZC: Conceptualization, Methodology, Writing–review and editing. CM: Data curation, Investigation, Writing–review and editing, Methodology. XC: Investigation, Methodology, Writing–review and editing. XT: Investigation, Writing–review and editing. XS: Investigation, Writing–review and editing. JD: Investigation, Software, Writing–review and editing. SW: Data curation, Investigation, Writing–review and editing. JJ: Data curation, Software, Writing–review and editing. LX: Methodology, Supervision, Writing–review and editing. DW: Supervision, Writing–review and editing.
FundingThe author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This work was supported by National Natural Science Foundation of China (No. 82104831), Furong Laboratory Science and Technology Project (No. 2023SK2113-2), Hunan Science and Technology Innovation Project (No. 2021SK51005), Hunan Science and Technology Innovation Project (No. 2023RC3215).
Conflict of interestThe authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s noteAll claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
ReferencesAngel, P. M., and Caprioli, R. M. (2013). Matrix-assisted laser desorption ionization imaging mass spectrometry: in situ molecular mapping. Biochemistry 52 (22), 3818–3828. doi:10.1021/bi301519p
PubMed Abstract | CrossRef Full Text | Google Scholar
Asampille, G., Cheredath, A., Joseph, D., Adiga, S. K., and Atreya, H. S. (2020). The utility of nuclear magnetic resonance spectroscopy in assisted reproduction. Open Biol. 10 (11), 200092. doi:10.1098/rsob.200092
PubMed Abstract | CrossRef Full Text | Google Scholar
Bagatela, B. S., Lopes, A. P., Cabral, E. C., Perazzo, F. F., and Ifa, D. R. (2015). High-performance thin-layer chromatography/desorption electrospray ionization mass spectrometry imaging of the crude extract from the peels of Citrus aurantium L. (Rutaceae). Rapid Commun. Mass Spectrom. 29 (16), 1530–1534. doi:10.1002/rcm.7246
PubMed Abstract | CrossRef Full Text | Google Scholar
Bai, H., Wang, S., Liu, J., Gao, D., Jiang, Y., Liu, H., et al. (2016). Localization of ginsenosides in Panax ginseng with different age by matrix-assisted laser-desorption/ionization time-of-flight mass spectrometry imaging. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 1026, 263–271. doi:10.1016/j.jchromb.2015.09.024
PubMed Abstract | CrossRef Full Text | Google Scholar
Bai, R. H. (2016). Analysis of saponins in panax species using MALDI-TOF mass spectrometry imaging. Tsinghua University.
Bai, X., Zhu, C., Chen, J., Jiang, X., Jin, Y., Shen, R., et al. (2022). Recent progress on mass spectrum based approaches for absorption, distribution, metabolism, and excretion characterization of traditional Chinese medicine. Curr. Drug Metab. 23 (2), 99–112. doi:10.2174/1389200223666220211093548
PubMed Abstract | CrossRef Full Text | Google Scholar
Ban, W., Jiang, X., Lv, L., Jiao, Y., Huang, J., Yang, Z., et al. (2024). Illustrate the distribution and metabolic regulatory effects of pterostilbene in cerebral ischemia-reperfusion rat brain by mass spectrometry imaging and spatial metabolomics. Talanta 266 (Pt 2), 125060. doi:10.1016/j.talanta.2023.125060
PubMed Abstract | CrossRef Full Text | Google Scholar
Basu, S. S., and Agar, N. Y. R. (2021). Bringing matrix-assisted laser desorption/ionization mass spectrometry imaging to the clinics. Clin. Lab. Med. 41 (2), 309–324. doi:10.1016/j.cll.2021.03.009
PubMed Abstract | CrossRef Full Text | Google Scholar
Beck, S., and Stengel, J. (2016). Mass spectrometric imaging of flavonoid glycosides and biflavonoids in Ginkgo biloba L. Phytochemistry 130, 201–206. doi:10.1016/j.phytochem.2016.05.005
PubMed Abstract | CrossRef Full Text | Google Scholar
Bingol, K., and Brüschweiler, R. (2017). Knowns and unknowns in metabolomics identified by multidimensional NMR and hybrid MS/NMR methods. Curr. Opin. Biotechnol. 43, 17–24. doi:10.1016/j.copbio.2016.07.006
PubMed Abstract | CrossRef Full Text | Google Scholar
Bodzon-Kulakowska, A., Marszalek-Grabska, M., Antolak, A., Drabik, A., Kotlinska, J. H., and Suder, P. (2016). “Comparison of two freely available software packages for mass spectrometry imaging data analysis using brains from morphine addicted rats,”Eur. J. Mass Spectrom., 22. 229–233. doi:10.1255/ejms.1445
PubMed Abstract | CrossRef Full Text | Google Scholar
Cai, M. T., Zhou, Y., Ding, W. L., Huang, Y. H., Ren, Y. S., Yang, Z. Y., et al. (2023). Identification and localization of morphological feature-specific metabolites in Reynoutria multiflora roots. Phytochemistry 206, 113527. doi:10.1016/j.phytochem.2022.113527
PubMed Abstract | CrossRef Full Text | Google Scholar
Calligaris, D., Feldman, D. R., Norton, I., Brastianos, P. K., Dunn, I. F., Santagata, S., et al. (2015). Molecular typing of meningiomas by desorption electrospray ionization mass spectrometry imaging for surgical decision-making. Int. J. Mass Spectrom. 377, 690–698. doi:10.1016/j.ijms.2014.06.024
PubMed Abstract | CrossRef Full Text | Google Scholar
Cao, M., Wu, J., Zhu, X., Jia, Z., Zhou, Y., Yu, L., et al. (2024). Tissue distribution of metabolites in Cordyceps cicadae determined by DESI-MSI analysis. Anal. Bioanal. Chem. 416 (8), 1883–1906. doi:10.1007/s00216-024-05188-x
PubMed Abstract | CrossRef Full Text | Google Scholar
Chan, Y. Y., Lo, W. Y., Yang, S. N., Chen, Y. H., and Lin, J. G. (2015). The benefit of combined acupuncture and antidepressant medication for depression: a systematic review and meta-analysis. J. Affect Disord. 176, 106–117. doi:10.1016/j.jad.2015.01.048
PubMed Abstract | CrossRef Full Text | Google Scholar
Chen, C. J., Sung, H. C., Lee, M. S., and Chang, C. Y. (2015). The effects of Chinese five-element music therapy on nursing students with depressed mood. Int. J. Nurs. Pract. 21 (2), 192–199. doi:10.1111/ijn.12236
PubMed Abstract | CrossRef Full Text | Google Scholar
Chen, W. J., Zheng, Y. N., Zhao, L., Song, S. H., Long, F., Pei, Z. Q., et al. (2022a). Distribution of bioactive compounds in different tissues of Paeonia lactiflora roots by DESI-MSI and UPLC. 47(16): p. 4333–4340. doi:10.19540/j.cnki.cjcmm.20220514.105
PubMed Abstract | CrossRef Full Text | Google Scholar
Chen, W. J., Zheng, Y. N., Zhao, L., Song, S. H., Long, F., Pei, Z. Q., et al. (2022b). Distribution of bioactive compounds in different tissues of Paeonia lactiflora roots by DESI-MSI and UPLC. China J. Chin. Materia Medica. 47 (16): p. 4333–4340. doi:10.19540/j.cnki.cjcmm.20220514.105
PubMed Abstract | CrossRef Full Text | Google Scholar
Chen, Y., Liu, Y., Li, X., He, Y., Li, W., Peng, Y., et al. (2023a). Recent advances in mass spectrometry-based spatially resolved molecular imaging of drug disposition and metabolomics. Drug Metab. Dispos. 51 (10), 1273–1283. doi:10.1124/dmd.122.001069
PubMed Abstract | CrossRef Full Text | Google Scholar
Chen, Y., Xie, Y., Li, L., Wang, Z., and Yang, L. (2023b). Advances in mass spectrometry imaging for toxicological analysis and safety evaluation of pharmaceuticals. Mass Spectrom. Rev. 42 (5), 2207–2233. doi:10.1002/mas.21807
PubMed Abstract | CrossRef Full Text | Google Scholar
Cheng, Y., Lan, Q., Wu, B. Y., Wang, J. Y., Liu, D. W., and Tong, Y. (2024). Analysis of brain absorption components and their distribution of tianyuan zhitong prescription based on UPLC-Q-TOF-MS and DESI-MSI. Chin. J. Exp. Traditional Med. Formulae. 30(12): p. 166–172. doi:10.13422/j.cnki.syfjx.20240661
CrossRef Full Text | Google Scholar
Collins, S. L., Koo, I., Peters, J. M., Smith, P. B., and Patterson, A. D. (2021). Current challenges and recent developments in mass spectrometry-based metabolomics, Annu. Rev. Anal. Chem. 14(1): p. 467–487. doi:10.1146/annurev-anchem-091620-015205
PubMed Abstract | CrossRef Full Text | Google Scholar
Dai, S. Y., Jiang, S. H., Dong, J., Lian, C. J., Qiao, F., Zheng, J., et al. (2022). Visualization analysis of spatial distribution of alkaloid s in aconiti radix cocta during processing process by matrix assisted laser desorption ionization mass spectrometry imaging. Chin. Pharm. J. 57 (10), 834–839.
De Hert, M., Detraux, J., van Winkel, R., Yu, W., and Correll, C. U. (2011). Metabolic and cardiovascular adverse effects associated with antipsychotic drugs. Nat. Rev. Endocrinol. 8 (2), 114–126. doi:10.1038/nrendo.2011.156
PubMed Abstract | CrossRef Full Text | Google Scholar
Dona, A. C., Coffey, S., and Figtree, G. (2016). Translational and emerging clinical applications of metabolomics in cardiovascular disease diagnosis and treatment. Eur. J. Prev. Cardiol. 23 (15), 1578–1589. doi:10.1177/2047487316645469
PubMed Abstract | CrossRef Full Text | Google Scholar
Eberlin, L. S., Ferreira, C. R., Dill, A. L., Ifa, D. R., and Cooks, R. G. (2011). Desorption electrospray ionization mass spectrometry for lipid characterization and biological tissue imaging. Biochim. Biophys. Acta 1811 (11), 946–960. doi:10.1016/j.bbalip.2011.05.006
PubMed Abstract | CrossRef Full Text | Google Scholar
Eckelmann, D., Kusari, S., and Spiteller, M. (2016). Occurrence and spatial distribution of maytansinoids in Putterlickia pyracantha, an unexplored resource of anticancer compounds. Fitoterapia 113, 175–181. doi:10.1016/j.fitote.2016.08.006
PubMed Abstract | CrossRef Full Text | Google Scholar
Emwas, A. H., Roy, R., McKay, R. T., Tenori, L., Saccenti, E., Gowda, G. N., et al. (2019). NMR Spectroscopy for metabolomics research. Metabolites. 9(7). doi:10.3390/metabo9070123
PubMed Abstract | CrossRef Full Text | Google Scholar
Fan, W., Yang, Y., Li, L., Fan, L., Wang, Z., and Yang, L. (2022). Mass spectrometry-based profiling and imaging strategy, a fit-for-purpose tool for unveiling the transformations of g
留言 (0)