Remnant cholesterol (RC) refers to the cholesterol content of all triglyceride-rich lipoproteins, including very low-density lipoprotein (VLDL), synthesized by the liver; chylomicrons, derived from the intestinal tract; and intermediate-density lipoproteins (1). RC is increasingly recognized as a key factor in residual cardiovascular risk (2–4), helping to explain cardiovascular risks and mortality that persist even after achieving target low-density lipoprotein cholesterol (LDL-C) levels (5, 6). Dyslipidemia is not only strongly linked to atherosclerosis and stroke (7, 8) but also affects the smooth muscle and endothelial function of cerebral arteries (9). Research has also highlighted the pro-inflammatory and anti-inflammatory effects of different lipid components (10). In addition, lipids have been found to correlate with brain-derived neurotrophic factor (BDNF), which is essential for the development, maintenance, and recovery of the central nervous system (11, 12). These findings suggest a potential link between RC and stroke outcomes. However, the impact of RC on ischemic stroke outcomes remains unclear.
Non-alcoholic fatty liver disease (NAFLD) is the most common chronic liver disease, affecting approximately 40% of acute stroke patients (13). Although existing studies suggest that patients with NAFLD may have worse stroke prognoses (14), research on the underlying mechanisms remains limited. In particular, the relationship between NAFLD and lipid metabolism-related factors has not been fully elucidated. Patients with NAFLD showed increased expression levels of ANGPTL3, which regulates residual cholesterol synthesis, leading to elevated remnant cholesterol (15). Conversely, RC levels were positively associated with NAFLD risk (16). In addition, higher serum levels of remnant cholesterol were linked to more severe hepatic steatosis, independent of conventional lipid parameters (17). A previous study found that NAFLD was independently associated with higher circulating remnant cholesterol, and elevated remnant cholesterol levels were shown to predict major adverse cardiovascular and cerebrovascular events in NAFLD patients (18).
In this analysis of the CNSR-III trial, we aimed to examine the association between residual cholesterol and stroke outcomes in a large, prospective, multicenter cohort. Furthermore, we investigated the interaction between residual cholesterol and NAFLD in patients with ischemic stroke.
2 Methods 2.1 Study design and participantsThis analysis utilized data from the Third China National Stroke Registry (CNSR-III), which is a comprehensive, prospective, multicenter cohort registry dedicated to patients with acute ischemic cerebrovascular events (19). The cohort included 15,166 patients from 201 sites across eastern, western, and central China. The inclusion criteria were as follows: (1) individuals aged 18 years or older; (2) individuals diagnosed with ischemic stroke or transient ischemic attack; and (3) individuals enrolled within 7 days of symptom onset. The exclusion criteria were as follows: (1) individuals with transient ischemic attack; (2) individuals with viral hepatitis; (3) individuals with a history of substantial alcohol consumption; (4) individuals lacking essential measurements, such as height, weight, and waist circumference, or critical laboratory data required for NAFLD and residual cholesterol assessment; and (5) individuals with no follow-up modified Rankin Scale (mRS) scores recorded. Ethical approval for CNSR-III was granted by the ethics committees of the participating institutions, and written informed consent was obtained from the patients or their legal representatives.
2.2 Baseline data collectionExperienced research coordinators collected baseline data from the enrolled patients through face-to-face interviews or electronic medical records, following a standardized protocol for data collection. The collected data included information regarding patient demographics, body mass index (BMI, calculated as weight in kilograms divided by the square of height in meters), smoking status, alcohol consumption, the National Institutes of Health Stroke Scale (NIHSS) score at admission, and medical history. Medical history included hypertension, diabetes, and coronary artery disease. Ischemic stroke etiology was classified according to the Trial of ORG 10172 in Acute Stroke Treatment (TOAST) criteria. The laboratory parameters, including total cholesterol (TC), triglycerides (TG), low-density lipoprotein cholesterol (LDL-C), and high-density lipoprotein cholesterol (HDL-C), were recorded. In addition, details regarding medications administered during hospitalization and the utilization of reperfusion therapy were documented.
2.3 Measurements of the RCThe fasting blood samples collected in EDTA tubes were obtained within 24 h of admission from 171 study sites. The samples were transported to the central laboratory at Beijing Tiantan Hospital under a maintained cold chain and stored at −80°C until analysis. The remnant cholesterol (RC) was calculated as total cholesterol minus the measured low-density lipoprotein cholesterol (LDL-C) and high-density lipoprotein cholesterol (HDL-C) (3).
2.4 NAFLD screening indexThe fatty liver index (FLI) (20) was calculated using the measurements of triglycerides (mmol/L), γ-glutamyl transferase (GGT) levels (U/L), and waist circumference (WC) (cm), with the formula as follows:
FLI=e0.953×lntriglycerides+0.139×BMI+0.718×lnGGT+0.053×WC−15.745/1+e0.953×lntriglycerides+0.139×BMI+0.718×lnGGT+0.053×WC−15.745×100
where GGT = γ-glutamyl transferase, WC = waist circumference, and BMI = body mass index. Based on previously reported standards for Asian populations, the patients were categorized into three groups: (I) no NAFLD: FLI < 25 for the male participants and FLI < 10 for the female participants; (II) possible NAFLD: 25 ≤ FLI < 35 for the male participants and 10 ≤ FLI < 20 for the female participants; (III) NAFLD: FLI ≥ 35 for the male participants and FLI ≥ 20 for the female participants (21).
2.5 Outcomes and follow-upThe participants were followed up with face-to-face interviews at 3 months by trained research coordinators using a standardized interview protocol (19). The poor functional outcomes were defined as a modified Rankin Scale score of 3 to 6 at 3 months (22).
2.6 Statistical analysisAll participants were classified into four groups based on the quartiles of the fasting remnant cholesterol levels, and the baseline characteristics were compared across these quartiles. Continuous variables were presented as mean (SD) or median (interquartile range), while categorical variables were expressed as frequency (%). The trend across the quartiles was analyzed using the Cochran–Armitage test for the categorical variables, and ANOVA or the Kruskal–Wallis test was used for the continuous variables, as appropriate.
To identify significant predictors of the poor outcomes at the 3-month follow-up, both univariable and multivariable analyses were performed using logistic regression models to estimate odds ratios (ORs) and 95% confidence intervals (CIs). Multivariable regression models were employed to adjust for potential confounding factors, including age, sex, smoking status, alcohol consumption, BMI, hypertension, diabetes, coronary heart disease (CHD), NIHSS score at admission, TOAST classification, antihypertensive drug use, and use of antiplatelet agents.
A restricted cubic spline (RCS) model was used to explore the potential non-linear relationship between the remnant cholesterol (RC) levels and poor functional outcomes. The RCS method allows for flexible modeling of non-linear associations by fitting cubic spline functions at predefined knots. The knots were placed at the 5th, 50th, and 95th percentiles of the RC levels. The significance of non-linearity was assessed using the likelihood ratio test, comparing the model with linear terms only to the model with non-linear spline terms. The resulting RCS plot was constructed with the RC values on the x-axis and the odds ratios (ORs) with 95% confidence intervals (CIs) on the y-axis to visually depict the association.
Interaction between the remnant cholesterol groups and non-alcoholic fatty liver disease (NAFLD) categories was assessed by including interaction terms in the models. All statistical analyses were conducted using SAS, version 9.4 (SAS Institute). Two-sided tests were employed, and p-values less than 0.05 were considered statistically significant.
3 Results 3.1 Baseline characteristicsThe Third China National Stroke Registry initially enrolled 15,166 participants. After excluding patients with diagnoses of transient ischemic attack (1,020) or viral hepatitis (235), as well as those with incomplete laboratory or clinical data (4,620), heavy alcohol consumption (1,978), and missing 3-month follow-up Modified Rankin Scale (mRS) records (79), a total of 7,234 patients were included in the final analysis (Figure 1). These patients had a mean age of 63.00 years (SD = 11.44), and the male participants accounted for 63.2% of the cohort. The initial median NIHSS score was 3 (interquartile range: 2–6). The patients with higher remnant cholesterol (RC) levels were more likely to be women, had a higher body mass index, had a history of diabetes and hypertension, had elevated levels of triglycerides and total cholesterol, and had lower levels of high-density lipoproteins (HDL) and low-density lipoproteins (LDL) at admission (Table 1).
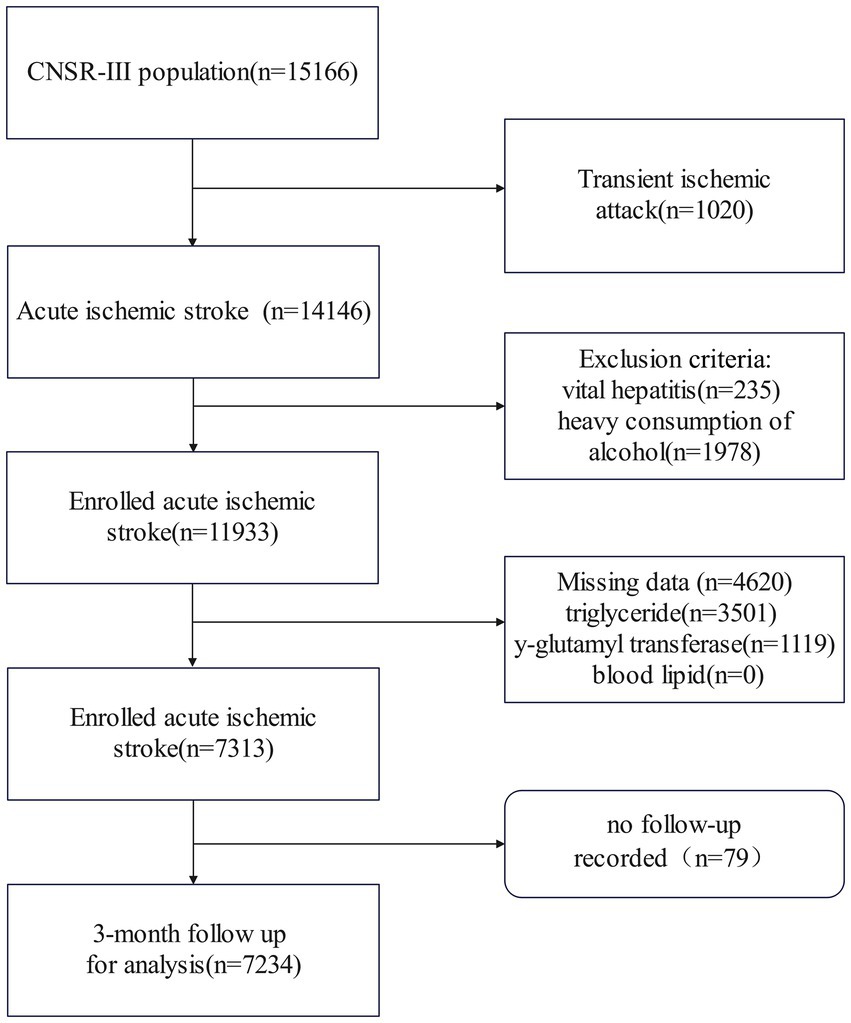
Figure 1. Flowchart of patient selection. CNSR-III stands for the Third China National Stroke Registry.

Table 1. Clinical characteristics of participants stratified by quartiles of remnant cholesterol.
According to the FLI, 1,350 (18.7%) patients were classified as having possible NAFLD and 3,780 (52.3%) were diagnosed with NAFLD. The crude prevalence of NAFLD among the acute ischemic stroke (AIS) patients is shown in Table 1.
3.2 Association between the RC and 3-month poor functional outcomeAmong the 7,234 patients, 98.9% completed the 3-month follow-up or were deceased, while 1.1% were lost to follow-up. In the univariate analysis, the rates of the poor functional outcomes (mRS 3–6) at 3 months were 12.1 and 18.5% for the patients in the highest quartile of the remnant cholesterol (RC) levels (Q4), compared to those in the lowest quartile of the RC levels (Q1). The odds ratio (OR) for the poor functional outcomes was 0.98(95% CI: 0.96–1) for the highest quartile (Q4) compared to the lowest quartile (Q1). A significant trend was observed across the RC quartiles and poor functional outcomes (p for trend <0.001). This association persisted after adjusting for age, sex, smoking status, alcohol consumption, BMI, hypertension, diabetes, coronary heart disease (CHD), NIHSS score at admission, TOAST classification, antihypertensive medication, and antiplatelet therapy (p for trend = 0.039). Details are provided in Table 2.
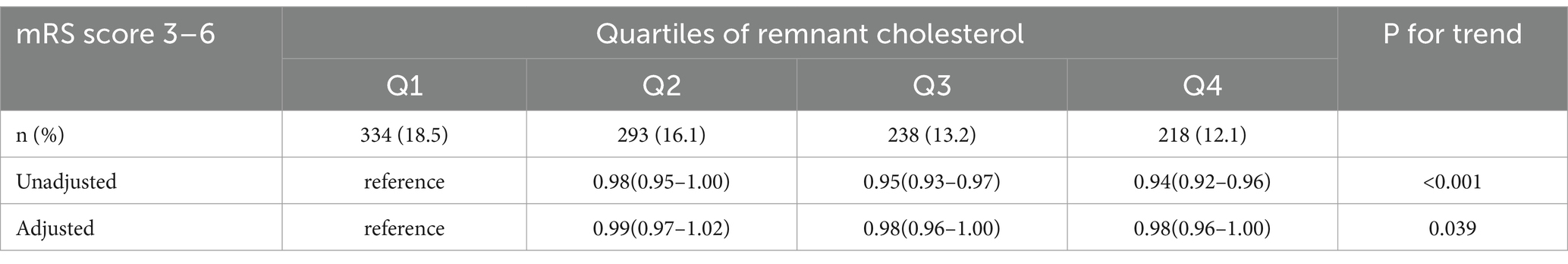
Table 2. Adjusted ORs of the outcomes at 3 months according to RC quartile categories.
3.3 Subgroup analysisFigure 2 presents the unadjusted and adjusted odds ratios (ORs) and forest plots from the logistic regression analyses examining the association between the remnant cholesterol (RC) percentile groupings (Q1 as the reference group) and the risk of the poor functional outcomes, stratified by NAFLD status. Compared to the individuals in Q1, those in Q2, Q3, and Q4 of the RC had adjusted ORs of 0.98 (95% CI: 0.95–1.02), 0.94 (95% CI: 0.91–0.97), and 0.96 (95% CI: 0.93–0.99), respectively. The ORs were lower in the individuals with NAFLD compared to those without NAFLD or with possible NAFLD (P for interaction = 0.038).
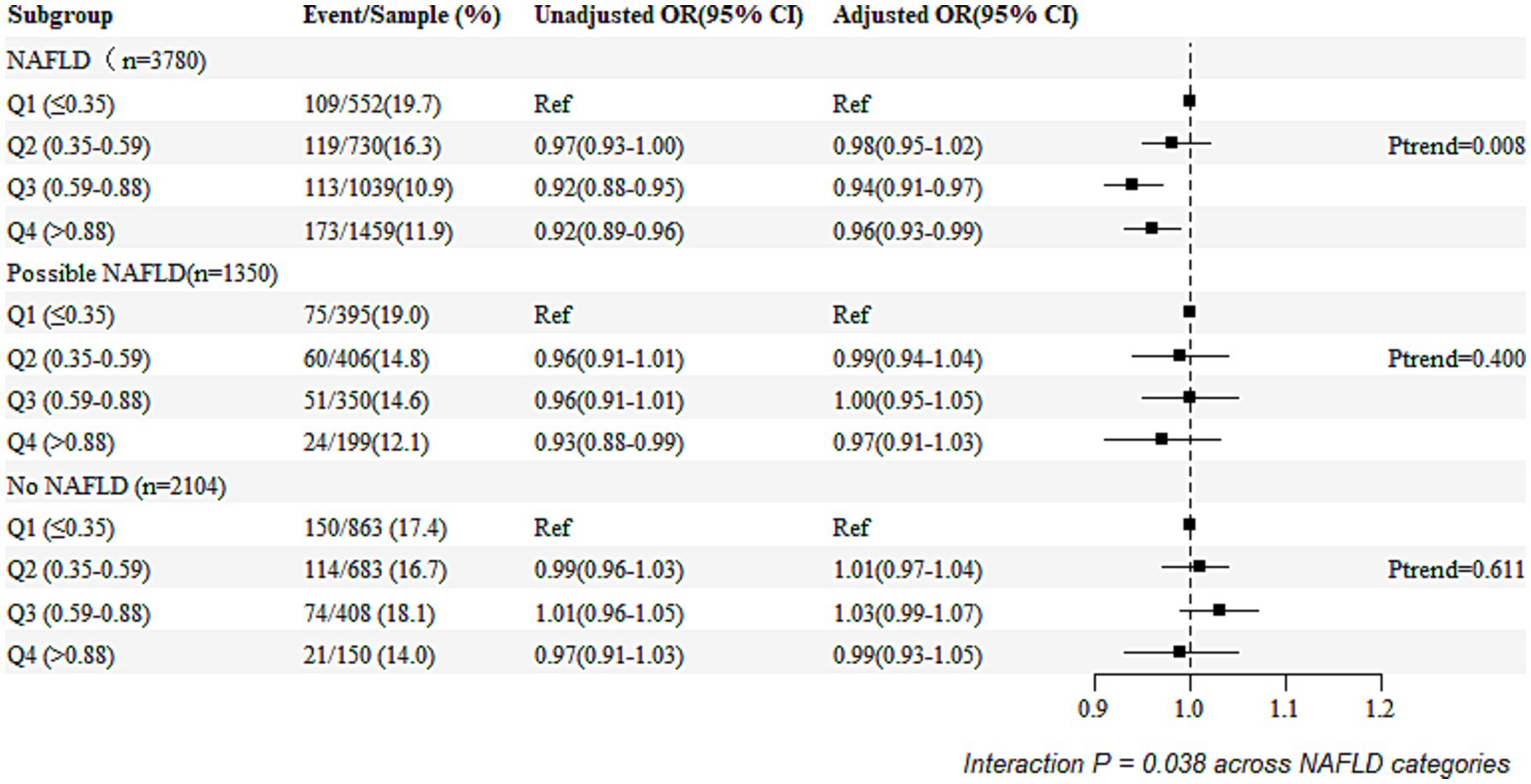
Figure 2. Relationship between NAFLD and poor functional outcomes based on RC quartile categories.
3.4 Association between the remnant cholesterol and poor functional outcomesThe restricted cubic spline (RCS) analysis showed no evidence of a non-linear relationship between remnant cholesterol (RC) levels and poor functional outcomes (p-value for non-linearity = 0.9327). A linear trend was observed, with an OR of 1 at an RC value of 0.56. The RCS plot is shown in Figure 3.
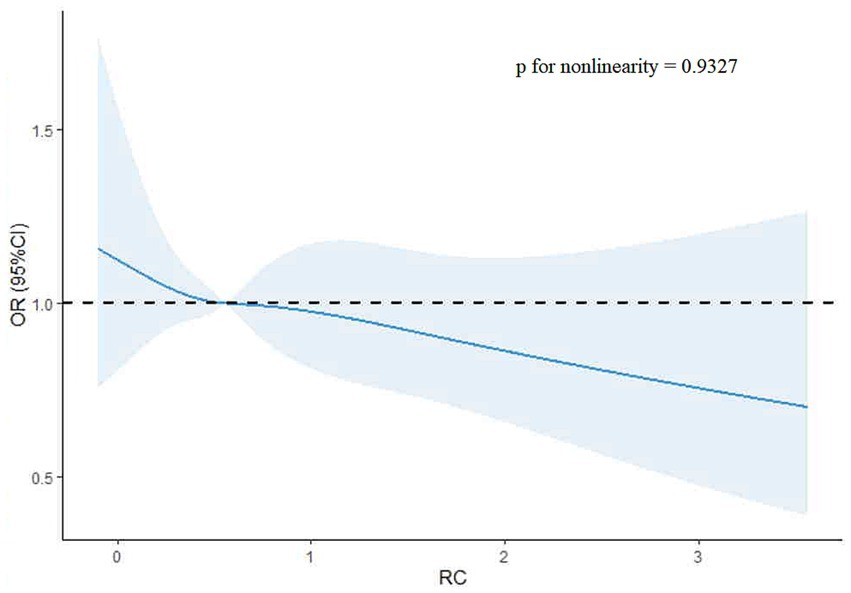
Figure 3. Restricted cubic spline plot of remnant cholesterol and poor functional outcomes.
4 DiscussionIn this prospective registry study, we found that higher levels of the remnant cholesterol (RC) were associated with a reduced likelihood of the poor functional outcomes in the patients with ischemic stroke. Specifically, a linear relationship between the RC and poor outcomes was observed, with an RC value of 0.56 serving as the threshold where the odds ratio crossed 1. In addition, non-alcoholic fatty liver disease (NAFLD) was found to modify the relationship between the RC and poor functional outcomes, as indicated by a significant interaction (p-value for interaction = 0.038). These findings suggest that RC may serve as a potential predictor of functional recovery in ischemic stroke patients, with NAFLD playing a role in modifying this association.
To date, evidence on the relationship between blood lipids and stroke prognosis is limited, with existing results being controversial. A recent retrospective study involving 165 patients who underwent mechanical thrombectomy found that elevated remnant cholesterol was associated with a less favorable prognosis (mRS ≤ 2) in patients with non-large artery atherosclerosis strokes (adjusted OR = 0.31, 95% CI: 0.11–0.85, p = 0.023) (23). Conversely, LDL-C has been reported to exhibit a U-shaped relationship with infarct volume in strokes caused by large artery occlusion, with higher early LDL-C concentrations being associated with better clinical outcomes (mRS 0–2) at 3 months (24). Similarly, cholesterol levels were found to be negatively associated with poor outcomes at 3 months (β = −0.17, p = 0.031) (25). However, these studies were small-sample observational studies and lacked evidence from larger, multicenter studies. The results of the current study are inconsistent with previous findings but align with Hutanu’s research. Interestingly, in our multicenter, nationwide, prospective registry cohort, high levels of the remnant cholesterol were associated with a decreased risk of poor functional outcomes at the 3-month follow-up in patients with NAFLD. However, this relationship was not observed in the stroke patients without NAFLD.
Potential mechanisms may explain why elevated RC levels in the presence of NAFLD could be associated with better stroke outcomes. One possibility is the “obesity paradox,” where a higher body mass index (BMI) is sometimes linked to better outcomes after ischemic stroke (26–28). Our study found that the patients with higher RC levels had higher BMI values at admission, and NAFLD is associated with increased obesity (29). This could suggest that obese patients, due to chronic low-grade inflammation, might develop tolerance to inflammatory responses and hypercatabolic states, which may confer some protection against stroke (30). In addition, cholesterol might serve as a buffer against free radicals released during ischemic injury, potentially limiting infarct expansion (31). Furthermore, brain-derived neurotrophic factor (BDNF), which is critical for the central nervous system’s development and recovery, has been associated with blood lipid levels. Higher BDNF levels have been reported to be correlated with lower HDL and higher triglyceride concentrations (21), which might influence stroke recovery and outcomes.
To the best of our knowledge, this study is the first to explore the association between residual cholesterol and poor prognosis following acute ischemic stroke and its interaction with NAFLD. However, our study has several limitations. First, NAFLD was defined using the fatty liver index because liver biopsy and ultrasonography are not suitable for large sample sizes. Nevertheless, the fatty liver index is a well-established, non-invasive biomarker for predicting hepatic steatosis and has been validated in both Chinese (32) and global populations (20). In addition, as a cohort study, we were unable to explore the detailed pathophysiological mechanisms underlying the effects of RC abnormalities on stroke outcomes in the NAFLD patients. Further research with specifically designed studies is needed to validate and gain a better understanding of these findings. Baseline differences between the included and excluded patients were also observed, but we believe that these differences did not likely substantially affect the results, given our adjustments for potential confounders. However, residual confounding and limited generalizability must be considered when interpreting the findings.
5 ConclusionWe found an inverse relationship between RC levels and poor functional outcomes in patients with ischemic stroke, which was influenced by NAFLD. Future studies on optimal target RC levels in patients with NAFLD are needed.
Data availability statementThe raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statementThe studies involving humans were approved by ethics committee of Beijing Tiantan Hospital (IRB approval number: KY2023-247-02). The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributionsSJ: Writing – original draft. AJ: Data curation, Writing – original draft. WX: Writing – review & editing. JJ: Writing – review & editing.
FundingThe author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This research received support from the National Natural Science Foundation of China (grant no. 82271329).
AcknowledgmentsWe would like to thank the staff and participants of the Third China National Stroke Registry (CNSR-III) for their valuable contributions.
Conflict of interestThe reviewer KK declared a shared affiliation with the authors AJ and JJ at the time of review.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s noteAll claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary materialThe Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2025.1472871/full#supplementary-material
References1. Stürzebecher, PE, Katzmann, JL, and Laufs, U. What is 'remnant cholesterol'? Eur Heart J. (2023) 44:1446–8. doi: 10.1093/eurheartj/ehac783
Crossref Full Text | Google Scholar
2. Sandesara, PB, Virani, SS, Fazio, S, and Shapiro, MD. The forgotten lipids: triglycerides, remnant cholesterol, and atherosclerotic cardiovascular disease risk. Endocr Rev. (2019) 40:537–57. doi: 10.1210/er.2018-00184
PubMed Abstract | Crossref Full Text | Google Scholar
3. Castañer, O, Pintó, X, Subirana, I, Amor, AJ, Ros, E, Hernáez, Á, et al. Remnant cholesterol, not LDL cholesterol, is associated with incident cardiovascular disease. J Am Coll Cardiol. (2020) 76:2712–24. doi: 10.1016/j.jacc.2020.10.008
Crossref Full Text | Google Scholar
4. Wadström, BN, Pedersen, KM, Wulff, AB, and Nordestgaard, BG. Elevated remnant cholesterol, plasma triglycerides, and cardiovascular and non-cardiovascular mortality. Eur Heart J. (2023) 44:1432–45. doi: 10.1093/eurheartj/ehac822
PubMed Abstract | Crossref Full Text | Google Scholar
6. Wadström, BN, Pedersen, KM, Wulff, AB, and Nordestgaard, BG. Elevated remnant cholesterol and atherosclerotic cardiovascular disease in diabetes: a population-based prospective cohort study. Diabetologia. (2023) 66:2238–49. doi: 10.1007/s00125-023-06016-0
PubMed Abstract | Crossref Full Text | Google Scholar
7. Hong, KS, Bang, OY, Kang, DW, Yu, KH, Bae, HJ, Lee, JS, et al. Stroke statistics in Korea: part I. Epidemiology and risk factors: a report from the korean stroke society and clinical research center for stroke. J stroke. (2013) 15:2–20. doi: 10.5853/jos.2013.15.1.2
PubMed Abstract | Crossref Full Text | Google Scholar
8. Amarenco, P, and Labreuche, J. Lipid management in the prevention of stroke: review and updated meta-analysis of statins for stroke prevention. Lancet Neurol. (2009) 8:453–63. doi: 10.1016/S1474-4422(09)70058-4
Crossref Full Text | Google Scholar
9. Kitayama, J, Faraci, FM, Lentz, SR, and Heistad, DD. Cerebral vascular dysfunction during hypercholesterolemia. Stroke. (2007) 38:2136–41. doi: 10.1161/STROKEAHA.107.481879
Crossref Full Text | Google Scholar
10. Barter, PJ, Nicholls, S, Rye, KA, Anantharamaiah, GM, Navab, M, and Fogelman, AM. Antiinflammatory properties of HDL. Circ Res. (2004) 95:764–72. doi: 10.1161/01.RES.0000146094.59640.13
PubMed Abstract | Crossref Full Text | Google Scholar
11. Lima Giacobbo, B, Doorduin, J, Klein, HC, Dierckx, RAJO, Bromberg, E, and de Vries, EFJ. Brain-derived neurotrophic factor in brain disorders: focus on Neuroinflammation. Mol Neurobiol. (2019) 56:3295–312. doi: 10.1007/s12035-018-1283-6
PubMed Abstract | Crossref Full Text | Google Scholar
12. Tuwar, MN, Chen, WH, Yeh, HL, and Bai, CH. Association between brain-derived neurotrophic factor and lipid profiles in acute ischemic stroke patients. Int J Mol Sci. (2024) 25:2380. doi: 10.3390/ijms25042380
PubMed Abstract | Crossref Full Text | Google Scholar
13. Mori, T, Yoshioka, K, and Tanno, Y. Non-alcoholic fatty liver disease frequency and associated factors at admission of acute stroke. Hepatol Int. (2022) 16:81–8. doi: 10.1007/s12072-021-10253-z
PubMed Abstract | Crossref Full Text | Google Scholar
14. Kim, KS, Hong, S, Han, K, and Park, CY. Association of non-alcoholic fatty liver disease with cardiovascular disease and all cause death in patients with type 2 diabetes mellitus: nationwide population based study. BMJ. (2024) 384:e076388. doi: 10.1136/bmj-2023-076388
PubMed Abstract | Crossref Full Text | Google Scholar
15. Barchetta, I, Cimini, FA, Chiappetta, C, Bertoccini, L, Ceccarelli, V, Capoccia, D, et al. Relationship between hepatic and systemic angiopoietin-like 3, hepatic vitamin D receptor expression and NAFLD in obesity. Liver Int: Official J Int Assoc Study of the Liver. (2020) 40:2139–47. doi: 10.1111/liv.14554
PubMed Abstract | Crossref Full Text | Google Scholar
16. Huang, H, Xie, J, Zeng, Y, Liu, Z, Miao, M, Xu, L, et al. Remnant cholesterol independently predicts the development of nonalcoholic fatty liver disease. J Clin Endocrinol Metab. (2023) 108:2907–15. doi: 10.1210/clinem/dgad272
PubMed Abstract | Crossref Full Text | Google Scholar
17. Huang, H, Wang, J, Wu, L, Ruan, J, Hou, L, Shen, C, et al. Remnant cholesterol and severity of nonalcoholic fatty liver disease. Diabetol Metab Syndr. (2023) 15:238. doi: 10.1186/s13098-023-01220-9
PubMed Abstract | Crossref Full Text | Google Scholar
18. Pastori, D, Baratta, F, Novo, M, Cocomello, N, Violi, F, Angelico, F, et al. Remnant lipoprotein cholesterol and cardiovascular and cerebrovascular events in patients with non-alcoholic fatty liver disease. J Clin Med. (2018) 7:378. doi: 10.3390/jcm7110378
PubMed Abstract | Crossref Full Text | Google Scholar
19. Wang, Y, Jing, J, Meng, X, Pan, Y, Wang, Y, Zhao, X, et al. The third China National Stroke Registry (CNSR-III) for patients with acute ischaemic stroke or transient ischaemic attack: design, rationale and baseline patient characteristics. Stroke Vascular Neurol. (2019) 4:158–64. doi: 10.1136/svn-2019-000242
PubMed Abstract | Crossref Full Text | Google Scholar
20. Bedogni, G, Bellentani, S, Miglioli, L, Masutti, F, Passalacqua, M, Castiglione, A, et al. The fatty liver index: a simple and accurate predictor of hepatic steatosis in the general population. BMC Gastroenterol. (2006) 6:33. doi: 10.1186/1471-230X-6-33
PubMed Abstract | Crossref Full Text | Google Scholar
21. Yang, BL, Wu, WC, Fang, KC, Wang, YC, Huo, TI, Huang, YH, et al. External validation of fatty liver index for identifying ultrasonographic fatty liver in a large-scale cross-sectional study in Taiwan. PLoS One. (2015) 10:e0120443. doi: 10.1371/journal.pone.0120443
PubMed Abstract | Crossref Full Text | Google Scholar
23. Li, M, Wang, M, Zhao, Y, Zhong, R, Chen, W, Lei, X, et al. Effects of elevated remnant cholesterol on outcomes of acute ischemic stroke patients receiving mechanical thrombectomy. J Thromb Thrombolysis. (2024) 57:390–401. doi: 10.1007/s11239-023-02939-y
PubMed Abstract | Crossref Full Text | Google Scholar
24. Pikija, S, Sztriha, LK, Killer-Oberpfalzer, M, Weymayr, F, Hecker, C, Ramesmayer, C, et al. Contribution of serum lipid profiles to outcome after endovascular Thrombectomy for anterior circulation ischemic stroke. Mol Neurobiol. (2019) 56:4582–8. doi: 10.1007/s12035-018-1391-3
PubMed Abstract | Crossref Full Text | Google Scholar
25. Hutanu, A, Iancu, M, Dobreanu, M, Oprea, O, Barbu, S, Maier, S, et al. Extended lipid profile in Romanian ischemic stroke patients in relation to stroke severity and outcome: a path analysis model. Archives of Medical Sci: AMS. (2021) 17:864–73. doi: 10.5114/aoms.2019.89302
PubMed Abstract | Crossref Full Text | Google Scholar
26. Lee, SH, Jung, JM, and Park, MH. Obesity paradox and stroke outcomes according to stroke subtype: a propensity score-matched analysis. Int J Obes. (2005) 47:669–76. doi: 10.1038/s41366-023-01318-0
Crossref Full Text | Google Scholar
27. Kim, BJ, Lee, SH, Jung, KH, Yu, KH, Lee, BC, Roh, JK, et al. Dynamics of obesity paradox after stroke, related to time from onset, age, and causes of death. Neurology. (2012) 79:856–63. doi: 10.1212/WNL.0b013e318266fad1
PubMed Abstract | Crossref Full Text | Google Scholar
28. Pirson, FAV, Hinsenveld, WH, Staals, J, de Greef, BTA, van Zwam, WH, Dippel, DWJ, et al. The effect of body mass index on outcome after endovascular treatment in acute ischemic stroke patients: a post hoc analysis of the MR CLEAN trial. Cerebrovascular Dis (Basel, Switzerland). (2019) 48:200–6. doi: 10.1159/000504744
PubMed Abstract | Crossref Full Text | Google Scholar
29. Friedman, SL, Neuschwander-Tetri, BA, Rinella, M, and Sanyal, AJ. Mechanisms of NAFLD development and therapeutic strategies. Nat Med. (2018) 24:908–22. doi: 10.1038/s41591-018-0104-9
PubMed Abstract | Crossref Full Text | Google Scholar
30. Rodríguez-Castro, E, Rodríguez-Yáñez, M, Arias-Rivas, S, Santamaría-Cadavid, M, López-Dequidt, I, Hervella, P, et al. Obesity paradox in ischemic stroke: clinical and molecular insights. Transl Stroke Res. (2019) 10:639–49. doi: 10.1007/s12975-019-00695-x
PubMed Abstract | Crossref Full Text | Google Scholar
31. Joseph, JA, Villalobos-Molinas, R, Denisova, NA, Erat, S, and Strain, J. Cholesterol: a two-edged sword in brain aging. Free Radic Biol Med. (1997) 22:455–62. doi: 10.1016/S0891-5849(96)00376-0
PubMed Abstract | Crossref Full Text | Google Scholar
32. Huang, X, Xu, M, Chen, Y, Peng, K, Huang, Y, Wang, P, et al. Validation of the fatty liver index for nonalcoholic fatty liver disease in middle-aged and elderly Chinese. Medicine. (2015) 94:e1682. doi: 10.1097/MD.0000000000001682
留言 (0)