Traumatic brain injury (TBI) is a form of externally acquired injury to the brain that can produce cognitive, emotional, social, and physical deficits (McDonald, 2013). One of the most common complications is headache, which has been shown to contribute to anxiety and depression in patients (Mofatteh, 2021). Additionally, a general deficit that can be seen in patients suffering from mild-to-moderate TBI (mmTBI) is balance injury (Li Y. et al., 2013). Physical therapy, a vestibular-based therapeutic exercise focusing on gait and balance training, is the typical standard of care for treating these functional deficits and promoting neurological health in mmTBI (Gottshall, 2011). However, this vestibular rehabilitation therapy also has a limited effect on its persistent functional recovery (Han et al., 2011).
Translingual neural stimulation (TLNS) is a novel therapeutic intervention that combines superficial electrical stimulation of the facial and trigeminal nerves with physical therapy focused on reducing balance and gait deficits (Danilov et al., 2015). TLNS is provided via the Portable Neuromodulation Stimulator (PoNS®, Helius Medical Technologies, Newtown, Pennsylvania, USA), a compact electrical apparatus that delivers comfortable electrical stimulation to the surface of the tongue (Tyler et al., 2019). The stimuli induce action potentials in the facial and trigeminal nerves that subsequently propagate to the cerebellum and brainstem, which may ultimately lead to functional changes in brain structures (Wildenberg et al., 2011).
A previous study showed that TLNS combined with physical therapy can affect the rehabilitation outcomes (Bolognini et al., 2009; Motamed Vaziri et al., 2014), and non-invasive brain stimulation can affect neural excitability and facilitate motor skill learning (Li et al., 2015). A study by our group showed that TLNS combined with PT can significantly improve outcomes in patients with degenerative neurologic disease, spinal cord injury or stroke (Tyler et al., 2014).
Studies on TLNS treatment with mmTBI patients have shown that targeted physical rehabilitation combined with neurostimulation can reduce symptoms and benefit neuronal recovery (e.g., the cerebellum, brainstem, and pons) (Danilov et al., 2015; Wildenberg et al., 2011). A previous voxel-based morphometry study found that the gray matter volume (GMV) significantly increased in the temporal and cerebellar regions, which are responsible for the automatic processing of visual motion, motor control, balance, and gait. However, significant decreases in the frontal and occipital regions, involved with effortful processing of vision, motor plan or control, were also seen. Our recent resting-state functional magnetic resonance imaging (fMRI) study further revealed increased resting state functional connectivity (RSFC) in specific regions-of-interest (ROIs), namely the left postcentral gyrus, left inferior parietal lobule, and between the right culmen and right declive (Hou et al., 2022), indicating positive effects of TLNS treatment on brain plasticity of somatosensory and visual inputs, visual-vestibular interactions, and balance control in mmTBI patients.
However, seed-based RSFC only provides information regarding functional interactions between specific brain regions; its analysis usually requires a priori region of interest (ROI) definition, and the results strongly depend upon and are limited by the ROI chosen (Smith et al., 2011; Tian et al., 2012). Moreover, the human brain is organized as a network, where the local architecture (e.g., short-range connections) is integrated with the large architecture (e.g., long-range connections) in order to support high-level cognitive function (Li et al., 2018; Park and Friston, 2013). Thus, network-based approaches offer a broader perspective on brain activity by capturing the interactions across the entire brain, without the need to restrict the analysis to predefined regions. Some clinical practices have employed the resting-state functional network approach to stroke (Falcon et al., 2016), schizophrenia (Bassett et al., 2012) and intracranial space-occupying lesions (Guan et al., 2022).
The present study specifically examines the intra- and inter-network functional connectivity changes from before (pre-) and after (post-) TLNS intervention in mmTBI patients. The subsequent functional network changes were correlated to changes in behavioral testing of gait and balance before and after TLNS intervention. We hypothesized that TLNS treatment in mmTBI patients would lead to increased RSFC measures between motor and sensory networks with a corresponding observable increase in balance and gait.
2 Methods 2.1 ParticipantsThis study was performed between June and October, 2016, and the participants were recruited through print and radio advertising. The behavioral testers and participants were blinded to the participants' intervention status. Nine participants with mmTBI were involved (3 males, 6 females; 43–62 years old, mean age = 53.11 ± 6.60 years (see Table 1). Their mmTBI occurred at least 1 year before enrollment. Participants had previously participated in physical therapy, had reached a plateau in their functional recovery, and still scored at least 16-points below normal (after age adjustment) on the Sensory Organization Test (SOT), a quantitative dynamic posturographic analysis system (NeuroCom®). Their mmTBI diagnoses were made according to the guidelines established by the Veterans Affairs/Department of Defense (Management of Concussion/mTBI Working Group, 2009). This study was approved by the Institutional Review Board at School of Medicine and Public Health, University of Wisconsin–Madison. All participants in this study provided informed consent before the experiments.
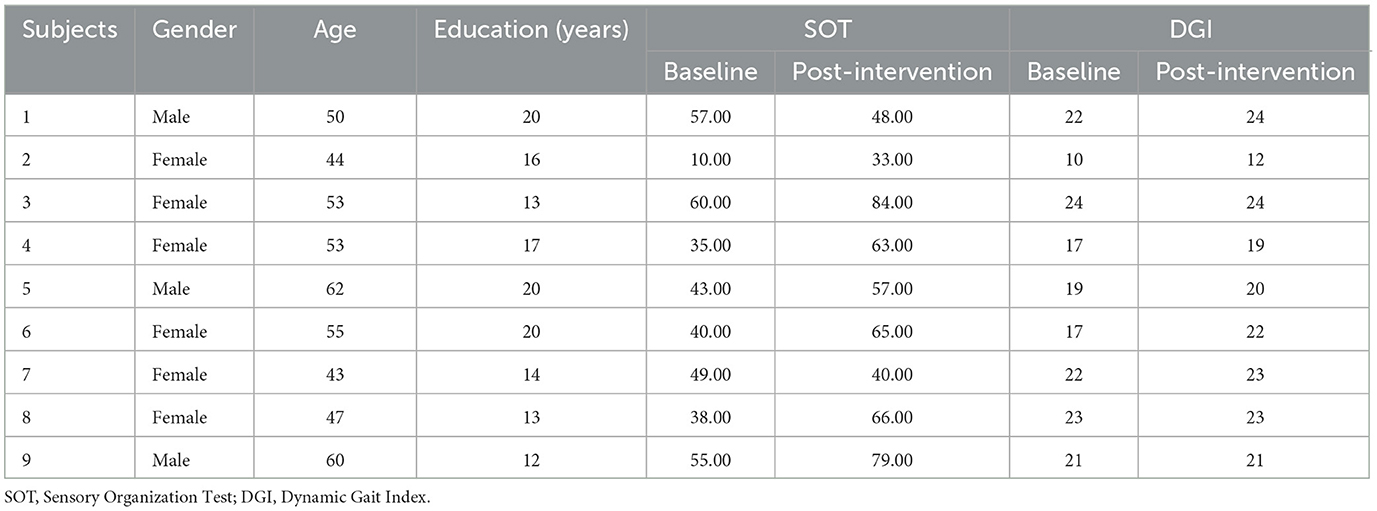
Table 1. The characteristics of mmTBI patients.
All nine participants received HFP or LFP stimulation. Inclusion criteria were: Participants were able to walk independently for at least 20 min, had access to a treadmill while not in the clinic, and had no medication changes for at least 3 months before the study. Exclusion criteria were: No additional medical problems such as oral health problems, unmanaged hypertension, diabetes, chronic infectious disease, a penetrating head injury, craniotomy, or refractory subdural hematoma, history of treatment for cancer other than basal cell carcinoma within the past year, neurological disorders other than those attributed to their primary diagnosis, non-removable metal orthodontic devices, or oral cavity piercings that could interfere with TLNS use. Additionally, long-term use of psychoactive or psychostimulant medications could compromise participants' ability to comprehend and perform study activities was also grounds for exclusion, as was the presence of a pacemaker or elevated risk for cardiovascular events. Furthermore, participants with a history of substance use disorder were not included. In the end, individuals with a lower extremity biomechanical prosthetic, history of seizures, or a “severe” score in any of the attention, memory, or executive functions categories on the Cognitive Linguistic Quick Test (CLQT) were also excluded (Tyler et al., 2019).
2.2 InterventionAn experimental PoNS device (version 2.5) was utilized to deliver the TLNS, using 143 electrodes on the tongue array to administer 19-volt amplitude-controlled, pulse-width modulated, unbalanced biphasic pulses to the anterior and superior surface of the tongue. The waveform delivers a zero net direct current to minimize the potential for tissue irritation (Tyler et al., 2019). This experimental PoNS device produced the same electrical stimulation as a commercially available PoNS device (Helius Medical Technologies), which received regulatory clearance for treating balance and gait disorders arising from mmTBI and multiple sclerosis (MS) in Canada, MS in the USA, and all neurologically-based balance and gait disorders in Australia. The 2-week TLNS intervention program, specifically stimulation during focused physical therapy focused on recovery of gait and balance, included twice-daily treatment in the laboratory and the same program at home during the intervening weekend. The participants also received physical exercise training focusing on motor coordination and mobility as part of the TLNS training. All participants worked with a physical therapist twice daily for a total of 1-h (each session being 30 min in length) to perform the different training modules (two balance, two gait, one warm-up, one movement control exercise, one breathing and awareness training [BAT]), followed by a BAT session completed independently at home (Ptito et al., 2021).
2.3 Behavioral testingAll participants received both SOT and the Dynamic Gait Index (DGI) testing at baseline (before, or pre-intervention), and after 2 weeks of twice-daily intervention (post-intervention) as part of the behavioral assessment battery. The SOT is an objective and automated testing of sensory-motor integration that assesses the levels of somatosensory, visual, and vestibular balance. The DGI is a clinician-scored examination of eight facets of gait and is scored from 0 (worst) to 24 (normal). A score change of 3 points is generally considered clinically significant (Tyler et al., 2019).
2.4 MRI acquisitionBoth resting-state fMRI and T1 structural MRI data (3T MRI GE750 scanner, GE Healthcare, Waukesha, Wisconsin, USA) were acquired at baseline (pre-) and immediately after a 2-week (post-) TLNS intervention. Ten minutes of eyes-closed resting-state fMRI was acquired with the following parameters: repetition time (TR) = 2,000 ms, echo time (TE) = 22 ms, flip angle (θ) = 60°, field of view (FOV) = 100 mm and matrix size = 100 × 100, voxel size = 3.5 mm3 isotropic. The anatomical data scan was acquired using a T1-weighted, three-dimensional, gradient-echo pulse-sequence (MPRAGE) with TR = 8,132 ms, TE = 3.18 ms, TI = 450 ms, flip angle θ = 12°, FOV = 100 mm and matrix size = 100 × 100, and in-plane resolution = 1 mm2 isotropic. Participants' head motion was minimized by MRI compatible foam pads.
2.5 Data preprocessingPreprocessing of RS-fMRI data was performed using the Data Processing and Analysis of Brain Imaging (DPABI) toolbox version 6.0 (http://rfmri.org/dpabi), which includes the sub-toolbox of Data Processing Assistant for Resting-state fMRI Advanced Edition toolbox (DPARSF V5.3) (Chang and Glover, 2010; Yan et al., 2016). DPARSF is an easy plug-in software tool that works with Statistical Parametric Mapping (SPM, version 12) (https://www.fil.ion.ucl.ac.uk/spm/software/spm12/) integrated in Matlab (Chao-Gan and Yu-Feng, 2010). The first five volumes were discarded to allow the magnetization to approach a dynamic equilibrium so the participants could become accustomed to the scanner noise. The preprocessing steps, in order, included slice timing correction, realignment, regressing out head motion parameters (scrubbing with Friston 24-parameter model regression; bad time points were identified using a threshold of frame-wise displacement >0.2 mm, and 1 volume before and 2 volumes after at the individual-subject level as well as accounting for head motion at the group-level (i.e., covariate analysis) (Power et al., 2012; Yan et al., 2013, 2016), normalization (spatial normalization to the MNI template, resampling voxel size of 3.5 × 3.5 × 3.5 mm3), and smoothing (a spatial Gaussian filter of 4 mm full-width at half maximum was used) (Chao-Gan and Yu-Feng, 2010; Kuhn et al., 2012). The temporal correlations as spontaneous neural connectivity were calculated to quantify RSFC. Additionally, the symmetric correlation matrices for a 160 × 160 network with the Dosenbach atlas, which defines 160 regions or nodes distributed across the brain (Dosenbach et al., 2010), were generated for each participant for pre- and post-interventions. Based on the matrix, each participant had a total of 25,600 unique pairwise functional connections at each pre- and post-intervention stage. Only half of the pairwise functional connections within the network were used for further brain network construction because the top right half and bottom left half were the same in the matrix.
2.6 Network functional connectivity constructionNetwork functional connectivity (FC) was constructed for each participant with the DPABINet V1.1 that was integrated in DPABI V6.0. The 160 nodes in the Dosenbach atlas were classified into eight subnetworks using the Yeo et al. classification: visual network (VN), somatosensory network (SMN), ventral attention network (VAN), dorsal attention network (DAN), default mode network (DMN), subcortical network (SC), frontoparietal network (FPN), and cerebellum network (CN) (Yeo et al., 2011). Based on the 160 × 160 matrix produced at the preprocessing step, the network FC for any pair of nodes was calculated as a Pearson's linear correlation coefficient, which was then Fisher-z transformed to derive a symmetric z score matrix that represented the functional connectivity network.
2.7 Statistical analysisA paired t-test between pre- vs. post-intervention was performed to compare the changes exhibited in the network FC, and two-tailed permutation testing in DPABINet was used with 5,000 permutations (Winkler et al., 2016). The head motion (mean framewise displacement [FD]) was included in the model as a covariate. False discovery rate (FDR) corrected p < 0.05 was used for multiple comparisons correction, and the results were visualized using DPABINet Viewer. The correlation analysis between SOT (or DGI; post- minus pre-) and RSFC (post- minus pre-) was corrected at p < 0.05 with IBM SPSS version 23.
3 Results 3.1 Behavioral scoresTLNS intervention led to significant increases in both SOT scores (t(8) = 2.742, p = 0.028) and DGI scores (t(8) = 2.855, p = 0.024) from pre-intervention to post-intervention.
3.2 Intra-network functional connectivityTable 2 and Figures 1, 2 present group average results and demonstrate significant intra-network FC increases in multiple networks following TLNS intervention. Specifically, within the somatosensory network (SMN), significantly increased FC was observed between the right frontal gyrus and right parietal lobule (t = 8.904, p = 0.002), as well as between the right precentral gyrus and right parietal lobule (t = 7.817, p = 0.002). The default mode network (DMN) also showed significantly increased connectivity between the right posterior cingulate cortex and left precuneus (t = 7.762, p = 0.002). Additionally, intra-network FC within the frontoparietal network (FPN) significantly increased between the left and right inferior parietal regions (t = 10.767, p = 0.001) and between the right dorsal prefrontal cortex and left occipital gyrus (t = 8.261, p = 0.002). The visual network (VN) also exhibited significantly increased connectivity, particularly between bilateral regions of the occipital gyrus (t = 10.247, p = 0.002).
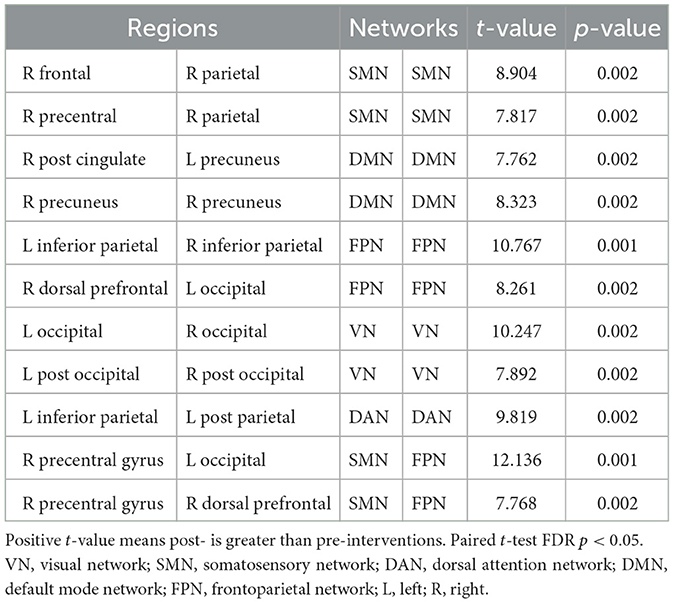
Table 2. The significant network functional connectivity differences between post- vs. pre-intervention.
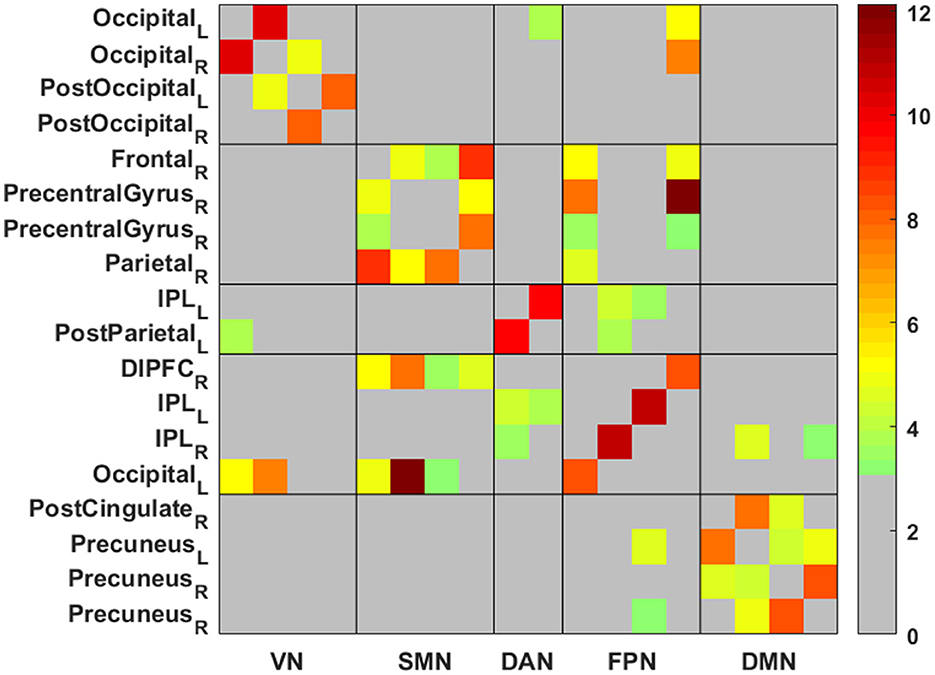
Figure 1. The matrix of network functional connectivity (FC) differences between post- vs. pre-intervention. Color bar represents Fisher's z-transformed Pearson correlation coefficient. VN, visual network; SMN, somatosensory network; DAN, dorsal attention network; DMN, default mode network; FPN, frontoparietal network; L, left; R, right. Shown are 18 × 18 regions matrix (all are within the five networks) with color shading indicating statistically significant results. The network FC for the pair of regions with significant results were calculated as a Pearson's linear correlation coefficient, which was then Fisher-z transformed to derive a symmetric z score matrix that represented the functional connectivity network.
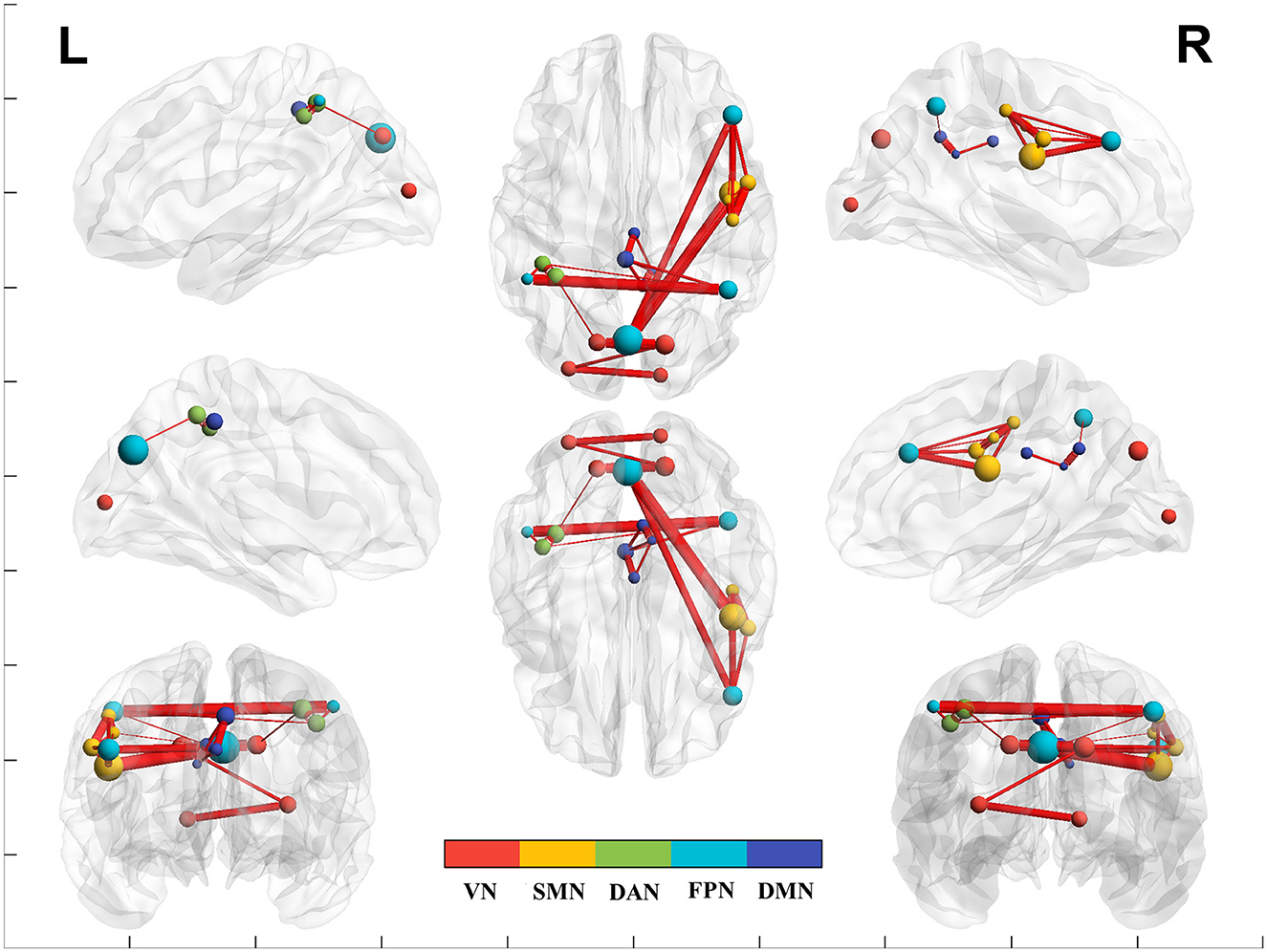
Figure 2. The brain network functional connectivity (FC) between post- vs. pre-intervention. Red line indicates increased network FC post- than pre-intervention. VN, visual network; SMN, somatosensory network; DAN, dorsal attention network; DMN, default mode network; FPN, frontoparietal network.
3.3 Inter-network functional connectivityTable 2 and Figures 1, 2 also present group average results and demonstrate significant inter-network FC increases from pre-intervention to post-intervention in several key networks. Notably, the inter-network FC between the SMN and FPN was significantly increased, particularly between the right precentral gyrus and left occipital gyrus (t = 12.136, p = 0.001), and between the right precentral gyrus and right dorsal prefrontal cortex (t = 7.768, p = 0.002).
In addition to the above five networks, there were no significant inter- and intra-network differences between VAN, SC, and cerebellum networks before and after the intervention.
3.4 CorrelationCorrelation analyses demonstrated a significant negative correlation between changes in SOT scores and inter-network FC between the right dorsolateral prefrontal cortex (FPN) and right precentral gyrus (SMN) (r = −0.784, p = 0.012), suggesting that greater reductions in FC between these networks were associated with better balance performance. In contrast, a significant positive correlation was found between changes in DGI scores and intra-network FC within the SMN, specifically between the right precentral and right parietal lobule (r = 0.728, p = 0.026), suggesting that increases in connectivity within the somatosensory network were linked to improvements in gait performance. Details are shown in Table 3.
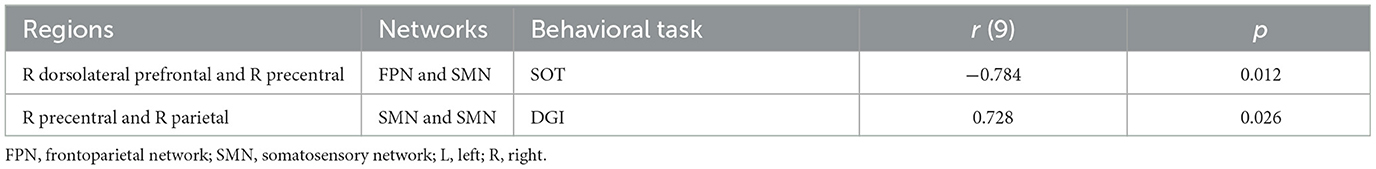
Table 3. The correlations between edge-based FC (post minus pre) and behavioral scores (post minus pre).
4 DiscussionAfter TLNS intervention on mmTBI patients, our study revealed significant increased intra-network FC at the VN, DMN, DAN, FPN, and SMN, and inter-network FC between the SMN and FPN. Additionally, mmTBI patients demonstrated significantly increased performance on the SOT and DGI tests after TLNS intervention compared to pre-TLNS intervention. There were significant positive correlations between the DGI and intra-network FC within the SMN, and significant negative correlations of the SOT and inter-network FC between the FPN and the SMN.
The SMN, which includes regions such as the precentral gyrus, dorsolateral and dorsomedial prefrontal cortex, temporoparietal junction (Kim et al., 2019; Kucyi et al., 2012), are responsible for functions such as motor, auditory, and somatosensory processing (Kim et al., 2019; Li et al., 2021). Post-TLNS intervention in our study showcased increased intra- and internetwork FC at the SMN, indicating that TLNS therapy may be effective in increasing motor perceptions, auditory functions, somatosensory processing, and vestibular-visual interactions that are crucial for regulating gait and balance. The VN encompasses the primary and second visual cortex that refers to visual attention (Macaluso et al., 2000), motor perception (Di Plinio and Ebisch, 2018; Li et al., 2021) and control monitoring (Brandi, 2014; Brandi et al., 2014). Post-TLNS intervention mmTBI exhibited significantly increased scores on SOT and DGI performances and increased VN FC, demonstrating improved abilities in sensory perception in motor and vision.
The post-TLNS intervention results also exhibited increased intra-network FC within the DAN, increased intra-network FC within the FPN, and increased inter-network FC between FPN and the SMN. The DAN is closely adjacent to the SMN and VN (Eklund et al., 2016; Li et al., 2021; Yan et al., 2019; Yeo et al., 2011) and is responsible for visuo-spatial processing (Li B. et al., 2013; Yan et al., 2019), containing neurons with spatially organized receptive fields (Posner et al., 2013; Wise et al., 2017) that are important to attentional shifting (Davey et al., 2012; Desseilles et al., 2009), goal-directed tasks (Fox et al., 2005) and visual-guided actions (Iwabuchi et al., 2015). Moreover, the DAN also plays a role in perceptual attention that is modulated by the FPN (Yan et al., 2019). The FPN includes regions such as dorsolateral prefrontal, parietal and occipital regions (Di Plinio and Ebisch, 2018) and it is important for executive control that refers to processes such as monitoring or flexible shifting in order to facilitate visual and movement interactions (Yan et al., 2019). Inter-network increased FC between FPN and SMN shown in our study after TLNS intervention may be especially effective in improving the cognitive functions that the FPN and SMN are responsible for.
The DMN has been widely studied with its alteration in TBI patients (D'Souza et al., 2020; Johnson et al., 2012). The DMN includes the regions such as the posterior cingulate, medial prefrontal and precuneus cortex (Washington and VanMeter, 2015), and it supports functions such as cognition (Cabeza et al., 2004; Hampson et al., 2006), attention regulation (Hampson et al., 2006; Leech et al., 2011), introspection, memory encoding, and error recognition (Mason et al., 2007; Zhou et al., 2012). The FC between dorsolateral prefrontal cortex and precuneus refers to the mental imagery, episodic memory and visuospatial imagery (Cavanna and Trimble, 2006; Margulies et al., 2009). The increased DMN FC after TLNS intervention in our study may reflect the improved ability of cognitive control or attentional regulation in mmTBI patients.
The intra-network FC changes (post- minus pre-intervention) in SMN was positively correlated to the changes of behavioral DGI testing (also the post- minus pre-intervention), which illustrates that more FC changes in SMN after intervention were associated with higher behavior improvements that are related to gait and stability. However, the inter-network FC changes between the FPN and SMN were negatively correlated to the changes of behavioral SOT testing, suggesting that fewer internetwork FC changes were associated with better performance. This possibly reflects the greater efficiency or automatization of functions such as somatosensory, vision, or vestibular balance involved after the TLNS intervention and less executive and attentional control by FPN. Moreover, the other potential network FCs or brain regions not included in Table 1 may have a compensatory role, or reflect the adaptive plasticity of functional connectivity as a result of TLNS intervention (Guvenc et al., 2018).
It is worth noting that in our previous study investigating the role of specific ROIs, no significant correlations were observed between the sensory/somatomotor, visual and cerebellar regions and the SOT and DGI (Hou et al., 2022). However, the current study showed significant correlations between SOT or DGI and network FC.
The limitations of the present study include a small sample size. To overcome this limitation, future studies should include larger sample sizes in order to improve generalizability and increase statistical power, as well as to discern gender differences between males and females. Additionally, participants in the main study received either a low- or high-frequency pulse (LFP or HFP) tongue stimulation, so a follow-up study of the specific effects of LFP or HFP on individuals will be beneficial. However, our study, while adding to our previous work (Hou et al., 2022) demonstrates that a network-based approach to study whole brain interactions yields promising results in terms of identifying significant relationships between functional connectivity changes and outcome measures.
In summary, the present study showed evidence that changes to brain network functional connectivity involved in gait, balance and motor control were produced by TLNS which improves functions of balance and gait in patients that have chronic symptoms from mild-moderate traumatic brain injury.
5 ConclusionThis study demonstrates that translingual neural stimulation (TLNS), in combination with physical therapy, leads to significant improvements in balance and gait in patients with mmTBI. These behavioral improvements, as measured by the SOT and DGI, are accompanied by increased intra- and inter-network RSFC in key brain networks, including the visual, default mode, dorsal attention, frontoparietal and somatosensory networks. The results suggest that TLNS may enhance brain network plasticity, offering a promising therapeutic approach for addressing mmTBI-related functional deficits. Future studies with larger sample sizes and longer follow-up periods are needed to validate these findings and further explore the mechanisms underlying TLNS-induced neuroplasticity.
Data availability statementThe raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statementThe studies involving humans were approved by Institutional Review Board at School of Medicine and Public Health, University of Wisconsin–Madison. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributionsDC: Writing – original draft, Writing – review & editing. JH: Formal analysis, Methodology, Software, Writing – original draft, Writing – review & editing. TH: Investigation, Writing – review & editing. VN: Conceptualization, Investigation, Project administration, Supervision, Validation, Writing – review & editing. NA: Investigation, Writing – review & editing. YD: Conceptualization, Data curation, Funding acquisition, Investigation, Project administration, Resources, Writing – review & editing. KK: Conceptualization, Data curation, Funding acquisition, Investigation, Project administration, Writing – review & editing. MM: Data curation, Investigation, Validation, Writing – review & editing. MT: Conceptualization, Data curation, Funding acquisition, Investigation, Project administration, Resources, Software, Supervision, Validation, Writing – review & editing. VP: Data curation, Funding acquisition, Investigation, Project administration, Resources, Validation, Writing – review & editing.
FundingThe author(s) declare financial support was received for the research, authorship, and/or publication of this article. Professional medical writing and editorial assistance were provided by Kelly M. Fahrbach, Ashfield Healthcare Communications, part of UDG Healthcare plc, funded by Helius Medical Technologies. This study is a part of the long-term clinical trial (NCT02158494), which was completed to investigate the efficacy of translingual neural stimulation (cranial nerve noninvasive neuromodulation). Drs. Tyler, Kaczmarek, Danilov, Hou, and Prabhakaran were awarded, Grant/Award Number: NHC-TBI-PoNS-RT001; Drs. Hou, Kulkarni, Nair, and Prabhakaran were awarded, Grant/Award Number: R01AI138647; Drs. Hou and Prabhakaran were awarded, Grant/Award Numbers: P01AI132132, R01NS105646; Mr. Chu was supported by MSTP, Grant/Award Number: T32 GM140935; National Center for Research Resources, Grant/Award Number: 1UL1RR025011; Drs. Meyerand, Prabhakaran, and Nair were awarded, Grant/Award Number: U01NS093650.
Conflict of interestMT, YD, and KK have a financial interest in Helius Medical Technologies and are also co-founders and co-owners of Advanced NeuroRehabilitation, LLC, which holds the intellectual property rights to the PoNS® technology. MT is a board member of Helius Medical Technologies.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's noteAll claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
ReferencesBassett, D. S., Nelson, B. G., Mueller, B. A., Camchong, J., and Lim, K. O. (2012). Altered resting state complexity in schizophrenia. Neuroimage 59, 2196–2207. doi: 10.1016/j.neuroimage.2011.10.002
PubMed Abstract | Crossref Full Text | Google Scholar
Bolognini, N., Pascual-Leone, A., and Fregni, F. (2009). Using non-invasive brain stimulation to augment motor training-induced plasticity. J. Neuroeng. Rehabil. 6:8. doi: 10.1186/1743-0003-6-8
PubMed Abstract | Crossref Full Text | Google Scholar
Brandi, M. L., Wohlschlager, A., Sorg, C., and Hermsdorfer, J. (2014). The neural correlates of planning and executing actual tool use. J. Neurosci. 34, 13183–13194. doi: 10.1523/JNEUROSCI.0597-14.2014
PubMed Abstract | Crossref Full Text | Google Scholar
Cabeza, R., Prince, S. E., Daselaar, S. M., Greenberg, D. L., Budde, M., Dolcos, F., et al. (2004). Brain activity during episodic retrieval of autobiographical and laboratory events: an fMRI study using a novel photo paradigm. J. Cogn. Neurosci. 16, 1583–1594. doi: 10.1162/0898929042568578
PubMed Abstract | Crossref Full Text | Google Scholar
Chao-Gan, Y., and Yu-Feng, Z. (2010). DPARSF: a MATLAB toolbox for “pipeline” data analysis of resting-state fMRI. Front. Syst. Neurosci. 4:13. doi: 10.3389/fnsys.2010.00013.eCollection.2010
Crossref Full Text | Google Scholar
Danilov, Y., Kaczmarek, K., Skinner, K., and Tyler, M. (2015). “Cranial nerve noninvasive neuromodulation: new approach to neurorehabilitation,” in: Brain Neurotrauma: Molecular, Neuropsychological, and Rehabilitation Aspects (ed) F. H. Kobeissy. Available at: https://www.ncbi.nlm.nih.gov/pubmed/26269928
Davey, C. G., Harrison, B. J., Yucel, M., and Allen, N. B. (2012). Regionally specific alterations in functional connectivity of the anterior cingulate cortex in major depressive disorder. Psychol. Med. 42, 2071–2081. doi: 10.1017/S0033291712000323
PubMed Abstract | Crossref Full Text | Google Scholar
Desseilles, M., Balteau, E., Sterpenich, V., Dang-Vu, T. T., Darsaud, A., Vandewalle, G., et al. (2009). Abnormal neural filtering of irrelevant visual information in depression. J. Neurosci. 29, 1395–1403. doi: 10.1523/JNEUROSCI.3341-08.2009
PubMed Abstract | Crossref Full Text | Google Scholar
Dosenbach, N. U., Nardos, B., Cohen, A. L., Fair, D. A., Power, J. D., Church, J. A., et al. (2010). Prediction of individual brain maturity using fMRI. Science 329, 1358–1361. doi: 10.1126/science.1194144
PubMed Abstract | Crossref Full Text | Google Scholar
D'Souza, M. M., Kumar, M., Choudhary, A., Kaur, P., Kumar, P., Rana, P., et al. (2020). Alterations of connectivity patterns in functional brain networks in patients with mild traumatic brain injury: a longitudinal resting-state functional magnetic resonance imaging study. Neuroradiol. J. 33, 186–197. doi: 10.1177/1971400920901706
PubMed Abstract | Crossref Full Text | Google Scholar
Eklund, A., Nichols, T. E., and Knutsson, H. (2016). Cluster failure: Why fMRI inferences for spatial extent have inflated false-positive rates. Proc. Natl. Acad. Sci. U.S.A. 113, 7900–7905. doi: 10.1073/pnas.1602413113
PubMed Abstract | Crossref Full Text | Google Scholar
Falcon, M. I., Riley, J. D., Jirsa, V., McIntosh, A. R., Chen, E. E., and Solodkin, A. (2016). Functional mechanisms of recovery after chronic stroke: modeling with the virtual brain. eNeuro 3:2. doi: 10.1523/ENEURO.0158-15.2016
PubMed Abstract | Crossref Full Text | Google Scholar
Fox, M. D., Snyder, A. Z., Vincent, J. L., Corbetta, M., Van Essen, D. C., and Raichle, M. E. (2005). The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc. Natl. Acad. Sci. U.S.A. 102, 9673–9678. doi: 10.1073/pnas.0504136102
PubMed Abstract | Crossref Full Text | Google Scholar
Guan, X. Y., Zheng, W. J., Fan, K. Y., Han, X., Li, X., Yan, Z. H., et al. (2022). Changes in a sensorimotor network, occipital network, and psychomotor speed within three months after focal surgical injury in pediatric patients with intracranial space-occupying lesions. BMC Pediatr. 22:321. doi: 10.1186/s12887-022-03348-5
PubMed Abstract | Crossref Full Text | Google Scholar
Guvenc, C., Dupont, P., Van den Stock, J., Seynaeve, L., Porke, K., Dries, E., et al. (2018). Correlation of neuropsychological and metabolic changes after epilepsy surgery in patients with left mesial temporal lobe epilepsy with hippocampal sclerosis. EJNMMI Res. 8:31. doi: 10.1186/s13550-018-0385-5
PubMed Abstract | Crossref Full Text | Google Scholar
Hampson, M., Driesen, N. R., Skudlarski, P., Gore, J. C., and Constable, R. T. (2006). Brain connectivity related to working memory performance. J. Neurosci. 26, 13338–13343. doi: 10.1523/JNEUROSCI.3408-06.2006
PubMed Abstract | Crossref Full Text | Google Scholar
Han, B. I., Song, H. S., and Kim, J. S. (2011). Vestibular rehabilitation therapy: review of indications, mechanisms, and key exercises. J. Clin. Neurol. 7, 184–196. doi: 10.3988/jcn.2011.7.4.184
PubMed Abstract | Crossref Full Text | Google Scholar
Hou, J., Mohanty, R., Chu, D., Nair, V. A., Danilov, Y., Kaczmarek, K. A., et al. (2022). Translingual neural stimulation affects resting-state functional connectivity in mild-moderate traumatic brain injury. J. Neuroimaging 32, 1193–1200. doi: 10.1111/jon.13029
PubMed Abstract | Crossref Full Text | Google Scholar
Iwabuchi, S. J., Krishnadas, R., Li, C., Auer, D. P., Radua, J., and Palaniyappan, L. (2015). Localized connectivity in depression: a meta-analysis of resting state functional imaging studies. Neurosci. Biobehav. Rev. 51, 77–86. doi: 10.1016/j.neubiorev.2015.01.006
PubMed Abstract | Crossref Full Text | Google Scholar
Johnson, B., Zhang, K., Gay, M., Horovitz, S., Hallett, M., Sebastianelli, W., et al. (2012). Alteration of brain default network in subacute phase of injury in concussed individuals: resting-state fMRI study. Neuroimage 59, 511–518. doi: 10.1016/j.neuroimage.2011.07.081
PubMed Abstract | Crossref Full Text | Google Scholar
Kim, J., Mawla, I., Kong, J., Lee, J., Gerber, J., Ortiz, A., et al. (2019). Somatotopically specific primary somatosensory connectivity to salience and default mode networks encodes clinical pain. Pain 160, 1594–1605. doi: 10.1097/j.pain.0000000000001541
PubMed Abstract | Crossref Full Text | Google Scholar
Kucyi, A., Hodaie, M., and Davis, K. D. (2012). Lateralization in intrinsic functional connectivity of the temporoparietal junction with salience- and attention-related brain networks. J. Neurophysiol. 108, 3382–3392. doi: 10.1152/jn.00674.2012
PubMed Abstract | Crossref Full Text | Google Scholar
Kuhn, S., Vanderhasselt, M. A., De Raedt, R., and Gallinat, J. (2012). Why ruminators won't stop: the structural and resting state correlates of rumination and its relation to depression. J. Affect. Disord. 141, 352–360. doi: 10.1016/j.jad.2012.03.024
PubMed Abstract | Crossref Full Text | Google Scholar
Leech, R., Kamourieh, S., Beckmann, C. F., and Sharp, D. J. (2011). Fractionating the default mode network: distinct contributions of the ventral and dorsal posterior cingulate cortex to cognitive control. J. Neurosci. 31, 3217–3224. doi: 10.1523/JNEUROSCI.5626-10.2011
PubMed Abstract | Crossref Full Text | Google Scholar
Li, B., Liu, L., Friston, K. J., Shen, H., Wang, L., Zeng, L. L., et al. (2013). A treatment-resistant default mode subnetwork in major depression. Biol. Psychiatry 74, 48–54. doi: 10.1016/j.biopsych.2012.11.007
PubMed Abstract | Crossref Full Text | Google Scholar
Li, L., Su, Y. A., Wu, Y. K., Castellanos, F. X., Li, K., Li, J. T., et al. (2021). Eight-week antidepressant treatment reduces functional connectivity in first-episode drug-naive patients with major depressive disorder. Hum. Brain Mapp. 42, 2593–2605. doi: 10.1002/hbm.25391
PubMed Abstract | Crossref Full Text | Google Scholar
Li, Q., Wang, X., Wang, S., Xie, Y., Li, X., Xie, Y., et al. (2018). Musical training induces functional and structural auditory-motor network plasticity in young adults. Hum. Brain Mapp. 39, 2098–2110. doi: 10.1002/hbm.23989
PubMed Abstract | Crossref Full Text | Google Scholar
Li, S., Zaninotto, A. L., Neville, I. S., Paiva, W. S., Nunn, D., and Fregni, F. (2015). Clinical utility of brain stimulati
留言 (0)