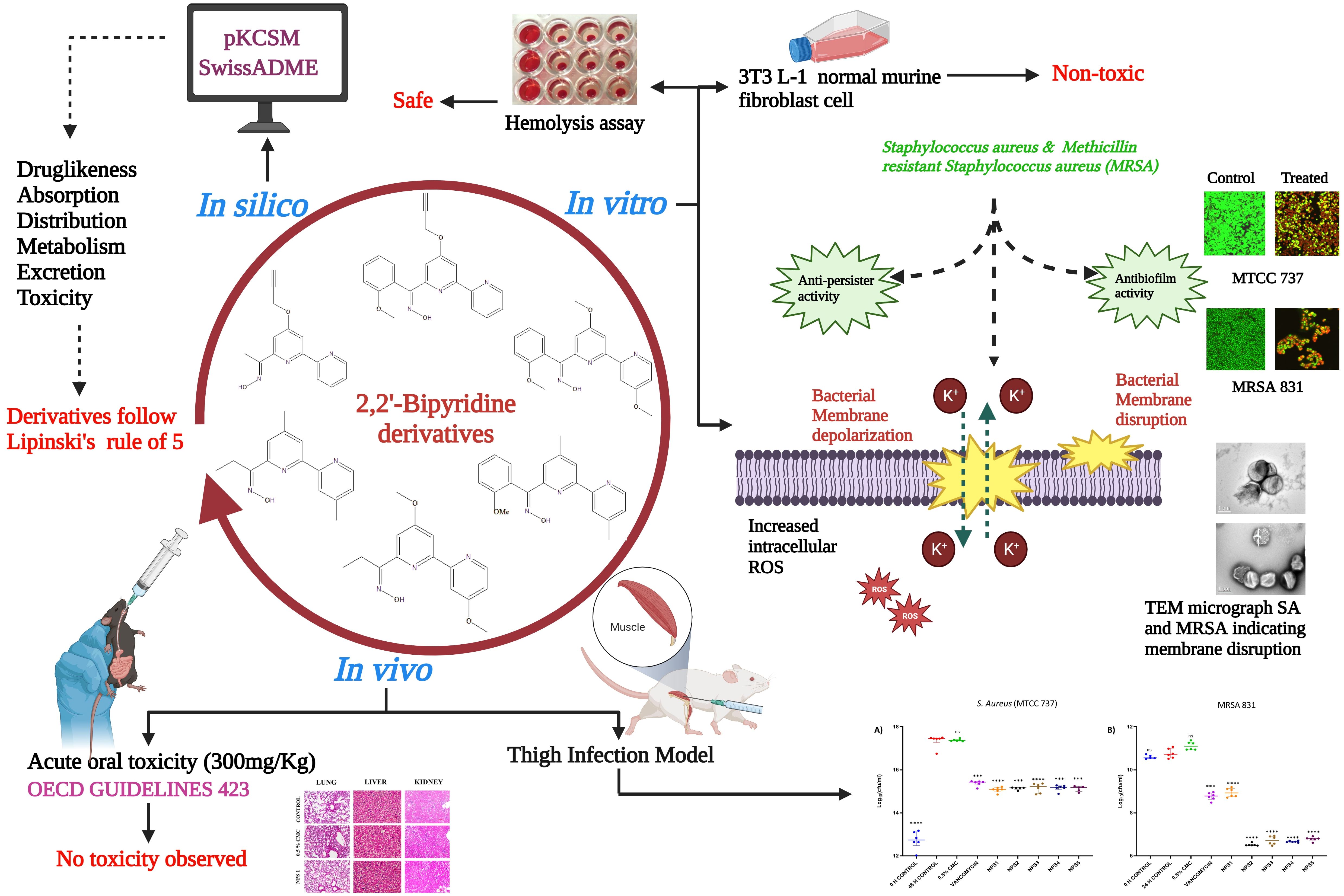
Graphical Abstract. Schematic illustration of the experimental protocol depicting different activities of 2,2’-Bipy derivatives.
1 IntroductionAccording to WHO, Antimicrobial resistance (AMR) is among the top 10 worldwide public health hazards to humanity and develops when microbes evolve and stop responding to medications, making infections more difficult to cure and raising the risk of disease spread, serious illness, and death. The WHO has declared Staphylococcus aureus (S. aureus) as a “priority pathogen” due to its threat to public health (WHO, 2017). S. aureus is associated with a range of human illnesses, from superficial skin infections to potentially fatal systemic infections such as sepsis, endocarditis, pleuropneumonia, and osteoarthritis (Tong et al., 2015; Turner et al., 2019). Methicillin-resistant S. aureus (MRSA) is a subtype that is resistant to beta-lactam antibiotics such as penicillin, oxacillin, and amoxicillin (Vestergaard et al., 2019). The emergence of vancomycin-resistant strains led to failure rates of vancomycin treatment for MRSA infections (Shariati et al., 2020b). In both community and hospital settings, S. aureus is a significant opportunistic human pathogen that frequently causes disease (van Dalen et al., 2020). In addition to its ability to acquire resistance, they are able to evade antibiotics by switching to a non-growing dormant bacterial state, known as persisters, and evidence suggests that persisters are present in significant numbers in biofilms and are responsible for the relapse of biofilm-associated infections (Grassi et al., 2017; Kim et al., 2018a; Niu et al., 2024). Antibiotics targeting bacterial membranes bypass the reliance on cell permeability, making them effective against persisters, and can act as synergistic agents to enhance the efficacy of other antibiotics (Mehta et al., 2021; Zhang and Ma, 2019). The growing prevalence of multidrug-resistant strains of methicillin-resistant S. aureus underscores an urgent and unmet clinical need for innovative approaches to treat these infections (Miller et al., 2020).
2,2’-Bipyridine (2,2’-Bipy) is among the most critical and favored bidentate ligands and consists of two linked pyridine units, which bind to metal ions at two different sites via neutral nitrogen atoms (Constable and Housecroft, 2019). The 2,2’-Bipy ligand is particularly suitable as a metal chelating ligand because of its high redox stability and ease of functionalization (Kaes et al., 2000). Additionally, 2,2’-Bipy forms the distinct molecular scaffold of bioactive natural products such as caerulomycins (CAEs) and collismycins (COLs) (Pang et al., 2021). In previous studies by our collaborators, Caerulomycin A (Cae A) was isolated, purified, and characterized from Actinoalloteichus spitiensis, a novel species of actinomycete. A patent was filed for using 2,2’-Bipy compounds, and studies by our co-authors have demonstrated the immunosuppressive and iron-chelating properties of these derivatives in autoimmune and iron overload diseases (Singla et al., 2007, 2014; Gurram et al., 2014; Kaur et al., 2015; Jolly et al., 2018). Different 2,2’-Bipy complexes exhibit antiviral, antifungal, antimicrobial, and potent antitumor activities (Lee et al., 2017; Lahoum et al., 2019; Burgart et al., 2022; Nasiri et al., 2023). The biological evaluation of the bipyridine complexes exhibited antibacterial activity against MRSA strains, though only at high concentrations. However, the studies were limited, with a minimum investigation into biofilm inhibition, persistence, and resistance development and no assessment of in vivo efficacy in murine models (Wang et al., 2021; Aragoni et al., 2023). Therefore, we developed novel 2,2’-Bipy derivatives with ketoxime substituents instead of aldoximes, a class of compounds with >C-N=O-H moiety. Oxime moieties have been reported in the literature to have excellent antimicrobial properties with less toxicity (Yong Hong et al., 1997; Syed, 2014; Hughes, 2017; Kojo et al., 1988; Souza et al., 2016; Chauhan, 2013; Liu et al., 2014). The synthesis and characterization of these novel derivatives are comprehensively detailed in the PhD thesis of Dr. Bhavna Vaid, who is also a co-author of this manuscript (Vaid Bhavna, 2018). The key objective of this study is to evaluate the biological activity of these novel 2,2’-Bipy derivatives, hypothesizing that they are potential candidates for new drug therapies against various types of microbial infections, including drug-resistant strains.
2 Materials and methods2.1 ChemicalsPotato Dextrose Agar (PDA), Nutrient Agar (NA), Mueller Hinton Broth (MHB), and Mueller Hinton Agar (MHA) were sourced from HiMedia. Dimethyl sulphoxide (DMSO), MTT [3-(4,5-dimethyl-thiazol-2-yl)-2,5-diphenyltetrazolium bromide], Polymyxin B, Valinomycin, DCFDHA (2′,7′-dichlorofluorescein diacetate, Sigma), Propidium iodide (PI), N-phenyl naphthylamine (NPN), Triton X-100 and Carboxy-methylcellulose (CMC) were procured from Sigma. Cell culture reagents 1X DMEM, 1X DPBS, Penicillin-Streptomycin (100 units), 0.25% Trypsin EDTA, and Foetal Bovine Serum (FBS) were acquired from GIBCO.
2.2 Cell cultureThe murine fibroblast 3T3 L-1 cell line was procured from the National Centre for Cell Science (NCCS), Pune, India. The 3T3 L-1 cells were incubated at 37°C in an incubator maintained at 95% humidity and 5% CO2 in DMEM medium complemented with 10% fetal bovine serum (FBS) and 1% penicillin-streptomycin.
2.3 Bacterial strains and growth conditionsStaphylococcus aureus (MTCC 737), Escherichia coli (MTCC 739), Pseudomonas aeruginosa (MTCC 1934), Micrococcus luteus (MTCC 106), Vibrio cholerae (MTCC 3906) and Klebsiella pneumoniae (MTCC 618) were attained from Microbial Type Culture Collection (MTCC) of CSIR-IMTECH, Chandigarh, India. The MRSA 831, a clinical strain, was a kind gift from Dr. Hemraj Nandanwar, CSIR-IMTECH, Chandigarh. All bacterial strains were cultured overnight at 37°C on nutrient agar and experimental procedures were performed using MHB and MHA.
2.4 ADMET analysisADMET profiling (absorption, distribution, metabolism, excretion, and toxicity) of newly synthesized compounds and their derivatives must be determined before their use in preclinical and clinical trials (Agrwal et al., 2022; Alesawy et al., 2022). Numerous in silico tools and servers can predict the ADMET profiles of these chemical entities. Therefore, 2,2’-Bipy derivatives (NPS1-6) were screened based on crucial features, including the rules of Lipinski, Ghose, Veber, and Egan and their pharmacokinetic and toxic profiles using the web servers pkCSM (Pires et al., 2015) and SwissADME (Daina et al., 2017; Kharb et al., 2023; Kumar et al., 2023; Knoll et al., 2022).
2.5 Hemolytic activityBlood from New Zealand White rabbits was collected in EDTA-coated vials. The blood was centrifuged at 1000 rpm and washed thrice with ice-cooled phosphate buffer saline (1X PBS, pH 7.4), and then resuspended at a 2% (v/v) concentration in PBS. The hemolytic assay was done using varying concentrations (0.156-400 µg/ml in two-fold dilutions) of NPS (1-6) bipyridine derivatives as described previously (Dubey et al., 2021). A known hemolytic agent, Triton-X 100, which lyses the RBCs, was taken as standard control, while 0.1% DMSO and PBS were used as vehicle and negative controls, respectively. 1X PBS without RBCs was used as the blank for this experiment, and absorbance was recorded at 570 nm. Percent hemolytic activity was calculated using a formula:
Hemolysis (%)=Absorbance (Test-Bank)Absorbance (Positive control-Bank)×1002.6 Cytotoxicity (MTT assay)Synthesized derivatives were evaluated for cytotoxicity in a 3T3-L1 cell line using the MTT assay (Ernawati et al., 2022). 8000 cells/well were added in 96-well tissue culture flat-bottom microtiter plates for 24 h and then incubated with synthesized molecules (0-400 µg/ml) for 48 h at 37°C in a CO2 incubator. DMSO was used as solvent at a concentration of 0.1% to ensure that any observed effects were not attributable to the solvent itself, whereas 1 µg/ml doxorubicin was used in toxicity tests as a positive control. Subsequently, the media was replaced with MTT solution (100 µl) diluted in fresh media (0.5 mg/ml), and incubated for 4 h. After incubation, 100 µl of stop solution (50% DMF with 20% SDS) was added to dissolve the formazan crystals, and the plates were further incubated for 1 h. Absorbance was measured at 570 nm using BioTEK Synergy multi-plate reader (Mosmann, 1983; Pratelli et al., 2023). Percent cell viability was calculated using the following formula:
Cell viability (%)=Absorbance (Untreated - blank) - Absorbance (Test sample- blank)Absorbance (Untreated - blank)×1002.7 Antibacterial activityThe antimicrobial activity of 2,2’-bipyridine derivatives was evaluated against a range of bacterial strains, including Gram-positive bacteria, S. aureus (MTCC 737), M. luteus (MTCC 106), and methicillin-resistant S. aureus (MRSA 831), as well as Gram-negative bacteria, E. coli (MTCC 739), P. aeruginosa (MTCC 1934), V. cholerae (MTCC 3906), and K. pneumoniae (MTCC 618). The assessments were conducted using the agar well diffusion method at a concentration of 50 µg/ml (Hassan et al., 2018). Clinical & Laboratory Standards Institute (CLSI) procedures were followed to determine minimum inhibitory concentrations (MICs) of the compounds in 96-well microplates against S. aureus (MTCC 737) and MRSA 831 using the broth-micro dilution assay in MHB (Ding et al., 2022; Sari et al., 2020). Briefly, 10 mg/ml master stocks and drugs were 2-fold diluted to final concentrations ranging from 0-200 µg/ml in the 96-well microtiter plate. Each well was then inoculated with an equal volume of bacterial suspension, having 1 × 105 CFU/ml (0.01 OD600), and incubated for 18 h. Controls included media control, DMSO (0.5%), positive (vancomycin and ampicillin), and culture controls. The MICs of the derivatives were determined as the lowest concentrations at which no visual turbidity was observed.
2.8 Killing kinetics assayMTCC 737 and MRSA 831 were grown in MHB medium overnight at 37°C with shaking at 200 rpm. In 96-well plates, cultures with a final concentration of 1 × 105 to 1 × 106 CFU/ml were added and incubated with 0.5 to 4 × MIC concentrations of 2,2’-Bipy derivatives. Untreated cells served as the negative control, while 0.5% DMSO and vancomycin were taken as vehicle and positive controls, respectively. The bacterial densities were recorded at OD600 at intervals of 2 h, starting from 0 h up to 18 h (Thakare et al., 2017; Thulshan Jayathilaka et al., 2021), and plotted as Log CFU/ml.
2.9 Biofilm inhibition and eradication assay2.9.1 Biofilm inhibition assayMTCC 737 and MRSA 831 grown overnight and diluted to 0.01 OD with fresh MHB medium, with or without 2,2’-Bipy derivatives and vancomycin, to evaluate biofilm inhibition. In 96-well plates, 180 µl of culture (1 × 105 to 1 × 106 CFU/ml) was incubated at 37°C for 24 h with 0.5 - 4 × MIC of the derivatives. After incubation, cells were washed with 1X PBS, and stained with 100 µl of Crystal Violet dye for 15 min, and rinsed again with 1X PBS. The retained dye was dissolved in 100 µl of 95% ethanol, and absorbance was recorded at 595 nm (She et al., 2019).
2.9.2 Biofilm inhibition imaging using confocal scanning laser microscopyThe bacterial cultures were grown using the procedure mentioned above (She et al., 2019). Biofilm adhesion was ensured by use of petri-dishes coated with poly-L-lysine. One milliliter of the bacterial suspension was dispensed into each polystyrene tissue culture plate (Himedia) on the coated side. The cultures were treated with 2,2’-Bipy derivatives (100 µg/ml) and vancomycin and incubated in a static environment for 24 h. Untreated wells were used as a control. After treatment, the media was disposed of, and the wells were washed three times with 0.85% saline. The wells were then stained for 35 to 40 min. Using SYTO 9 and PI dyes. Excess stain was removed by rinsing the wells three times, and the plates were dried in laminar airflow for a short while. Inverse confocal laser scanning microscopy (CLSM) [Nikon AI(R)] was used to examine the slides at 488 nm and 561 nm excitation and capture the representative images. NIS-Elements Viewer 5.21 was used to capture and process the images. Two independent experiments were done in triplicate.
2.9.3 Biofilm eradication assayFor biofilm-eradication, 180 µl cultures were incubated in the 96-well plates at 37°C for 24 h to follow biofilm formation, and then 0.5 - 4 × MIC of the 2,2’-Bipy derivatives were added biofilm and incubated for another 24 h. Biofilm was washed with 1X PBS and analyzed using Crystal Violet staining following previously established protocol (She et al., 2019; Song et al., 2020).
2.10 Anti-persister activity2.10.1 Persister killing assayTo evaluate the persister-killing activity of 2,2’-Bipy derivatives, MTCC 737 and MRSA 831 cells were grown overnight to the stationary phase at 37°C (Kim et al., 2018b). The cultures were washed twice with 1X PBS, added to the 96-well plate with OD600 = 0.2, and incubated with 0.5 - 4 × MIC of 2,2’-Bipy derivatives with vancomycin used as positive control. The bacterial densities of persisters were recorded at OD600 at hourly intervals from 0 to 6 h.
2.10.2 Biofilm persisters killing assayTo evaluate the effect of these derivatives on persister cells associated with the biofilm, a biofilm persister killing assay was executed, as reported by De Breij et al., 2018 with slight changes. MTCC 737 and MRSA 831 cells were grown to an OD600 of 0.5 at 37°C, then centrifuged at 2000 rpm, and diluted in fresh MHB medium containing 1 × 108 CFU/ml. The same general procedure was followed as in the killing kinetics assay, and absorbance was recorded at 600 nm at hourly intervals up to 18 h. The number of live bacteria was determined depending on the absorbance readings.
2.11 Drug resistance screeningDrug resistance in MTCC 737 and MRSA 831 to 2,2’-Bipy derivatives was evaluated using sequential passage method. Cultures were grown overnight, adjusted to OD600 of 0.3 with MHB medium, and treated with MIC and 0.5 × MIC of all 2,2’-Bipy derivatives. After 24 h, absorbance was recorded. A 50 µl bacterial suspension was transferred to new 96-well plates with fresh medium and the same concentrations of derivatives. After 24 h again, absorbance was recorded, and the process was repeated every 24 h for 19 days (Sarkar et al., 2023). MICs were compared to the initial MIC, and a graph was plotted to illustrate the results.
2.12 Antibacterial mechanismIn our experiments, we found that NPS (1-5) derivatives exhibited excellent antibacterial activity. To further elucidate the mechanism behind their antibacterial potential, we conducted additional experiments.
2.12.1 Transmission electron microscopyMTCC 737 and MRSA 831 were cultured to an OD600 of 0.5 at 37°C, with shaking at 200 rpm. The cultures were then treated with 100 µg/ml 2,2’-Bipy derivatives and vancomycin (as the positive control) for 12 h. Untreated bacteria served as the negative control, while 0.5% of the DMSO was taken as vehicle control. After treatment, the bacterial cultures were centrifuged, dissolved in fresh 1X PBS (pH 7.4), loaded onto a carbon grid, and further processed for transmission electron microscopy (She et al., 2020).
2.12.2 PI uptake assayMTCC 737 and MRSA 831 were grown, washed, and resuspended in 5 mM HEPES (pH 7.2), with the OD600 normalized to 0.5. The cultures were then treated with 100 µg/ml of 2,2’-Bipy derivatives for 1 h. Polymyxin B (10 μM), a membrane destabilizer, was used as a reference control. Drug-free and vehicle controls (cells treated with 0.5% DMSO) were included to analyze changes in fluorescence. After 1 h of treatment, cultures were centrifuged and stained with 5 µM PI dye for 15 min. Following staining, the cultures were washed twice to remove the extra dye, and fluorescence was recorded at 485 nm excitation and 525 nm emission wavelengths (Zhou et al., 2020).
2.12.3 N-phenyl naphthylamine (NPN) uptake assayAn N-phenyl naphthylamine (NPN) absorption assay was performed to assess the permeability of the inner membrane. MTCC 737 and MRSA 831 cultures were grown and diluted to an OD600 of 0.05. The NPN dye (8 µM) was added to the cultures and incubated with 100 µg/ml of 2,2’-Bipy derivatives for 15 min. Controls included Triton X- 100 (0.1%) as positive control and DMSO (0.1%) as a negative control. Fluorescence data were recorded with an excitation of 340 nm and an emission wavelength of 405 nm (Elliott et al., 2020).
2.12.4 Membrane depolarization assayThe membrane depolarization assay was conducted as described by (Zhou et al., 2020) with some modifications. MTCC 737 and MRSA 831 cells in the mid-log growth phase were washed and resuspended in 5 mM HEPES buffer, with the absorbance adjusted to 0.05. The cells were then incubated with 0.1 M KCl and 0.4 µM Disc3(5) (Invitrogen) at room temperature in the dark for 1 h. The plates were then incubated with 100 μg/ml 2,2’-Bipy derivatives for 30 min. Fluorescence intensity was measured with excitation and emission wavelengths of 622 nm and 670 nm, respectively. Valinomycin (16 µg/ml) and 0.1% DMSO (She et al., 2020) were used as positive and negative controls, respectively.
2.12.5 Reactive oxygen species measurementThe experiment was conducted as reported previously (Song et al., 2020) with slight modifications. The level of reactive oxygen species (ROS) in MTCC 737 and MRSA 831, incubated with 2,2’-Bipy derivatives, was analyzed using DCFH-DA dye (2′,7′-dichlorofluorescein diacetate). MTCC 737 and MRSA 831 were grown overnight, washed, and resuspended in 1X PBS with OD600 of 0.05. The cultures were incubated with 0.4 μM DCFH-DA dye for 30 min. in the dark. After 30 min., 10 μl of 2,2’-Bipy derivatives (at a concentration of 100 µg/ml) were added to a black 96-well plate and incubated for an additional 30 min. Fluorescence intensity was measured at an excitation of 488 nm and an emission of 525 nm. Increased fluorescence corresponds to increased intracellular ROS levels compared to untreated cells and the vehicle control.
2.13 Animal studiesFemale BALB/c and C57BL/6 mice, aged 6 to 8 weeks (19-25 g), were acclimatized for one week prior to experiments. Animals were housed in individually ventilated cages with controlled humidity (30-70%), a photoperiod of 12:12, and temperature was maintained at 24-25°C. Food pellets and sterile water were available at all times. All the procedures on animals were done following the guidelines of the Committee for the Control and Supervision of Experiments on Animals (CCSEA), India. The use of mice for acute oral toxicity studies and the murine thigh infection model, as well as rabbit blood for hemolysis assays, was approved by the Institutional Animal Ethics Committee (IAEC) of the CSIR-Institute of Microbial Technology, Chandigarh, under protocol numbers IAEC/19/19 and IAEC/22/07.
2.13.1 Acute oral toxicity in miceAcute oral toxicity was performed in mice to evaluate the in vivo toxicity of 2,2’-Bipy derivatives according to OECD guideline 423 (OECD, 2001). In this study, a starting dose of 300 mg/kg body weight was used, as the derivatives were insoluble at 2000 mg/kg. Mice (n=3) were divided into eight groups - Group 1 (control), Group 2 (vehicle), and Groups 3-8 (2,2’-Bipy derivatives NPS1, NPS2, NPS3, NPS4, NPS5, and NPS6, respectively). Prior to dosing, animals were fasted for 3-4 h. A single dose of 2,2’-Bipy derivatives (dissolved in 0.5% CMC) was administered orally by gavage and post-dosing, the mice were kept fasted for an additional 1- 2 h. According to OECD guideline 423, symptoms and behavior such as alterations in the mucous membranes, eyes, skin and fur, respiration, salivation, diarrhea, tremors, convulsions, sleep, coma, lethargy, and death were observed. After treatment, the toxic symptoms, time of onset, duration of recovery, and death of the animals were observed initially for 4 h and then daily for up to 14 days. Body weight was recorded weekly. On day 15, blood was collected by retroorbital method for hematological as well as biochemical analysis. and mice of different groups were euthanized by cervical dislocation after deep isoflurane anesthesia, and a necropsy was performed. Vital organs, including the brain, lungs, heart, liver, spleen, and kidneys, were collected for histological analysis. These organs were fixed in 10% neutral buffered formalin, sectioned, and further embedded in paraffin wax before the tissues were cut into 5 micron sections and stained with hematoxylin and eosin (H&E). Histopathological examination was performed under a light microscope.
2.13.2 Thigh infection model in miceThe efficacy of 2,2’-Bipy derivatives against MTCC 737 and MRSA 831, a clinical isolate, was investigated using a thigh infection model in mice. Female BALB/c mice (n=6) were administered with two doses of cyclophosphamide, 150 mg/kg and 100 mg/kg, administered 4 days and 1 day prior to infection to induce neutropenia. The mice were then infected intramuscularly in the right thigh muscle with 1 × 106 CFU/ml MTCC 737 and 1 × 108 CFU/ml MRSA 831. In MTCC 737 infected group, all mice received an oral dose of 2,2’-Bipy derivatives (30 mg/kg) and vancomycin (30 mg/kg) 4 h after infection. Vehicle and control groups were treated with 0.5% CMC and saline, respectively. After 4 h of infection, one of the control group of mice was sacrificed, and thigh muscle was collected. The muscle tissue was homogenized, and the CFU in the muscle was determined by spreading the homogenate on MHA plates using serial dilution in PBS. After 48 h, the remaining mice from all groups were sacrificed, and CFU per thigh was calculated (Ling et al., 2015). A similar experiment was performed with the MRSA 831 group, where treatment was given 2 h after infection, and the mice were sacrificed after 24 h of treatment (Choi et al., 2020).
2.14 Statistical analysisData analysis was done using GraphPad Prism software (version 9.2), p-values were analyzed using Student’s-tests for comparisons between two-groups and one-way or two-way ANOVA tests for comparisons among multiple groups. Each experiment included three biological replicates, with three technical replicates. Data are presented as Mean ± SD. Statistical significance was defined as *p<0.05, and **p<0.01, ***p<0.001 and ****p<0.0001.
3 Results3.1 Chemical structures2,2’-Bipy derivatives were synthesized using commercially available 2,2’-bipyridine (Sigma). Oxime moieties enhancing the functionality of antibiotics exhibit cytotoxic properties, making them effective against bacterial, fungal, and viral infections. One such example is Ceftobiprole medocaril, where oximes can address resistance issues and enhance efficacy. This underscores the importance of oxime modifications in advancing antibiotic therapies and overcoming resistance challenges (Hughes, 2017) and, alkyl or aryl groups were added to the oxime moiety further to modulate hydrophobicity of the molecule. Allyloxy and propargyloxy groups enhance antibacterial activity by improving compounds lipophilicity and targeting bacterial respiration, demonstrating effectiveness against resistant strains like MRSA and VRE, which exhibit low cytotoxicity, making them promising candidates for next-generation antimicrobials (Li et al., 2016; Kisiel-Nawrot et al., 2022). The molecules used in this study are NPS1 (N-[1-(4-allyloxy[2,2’-bipyridin]-6-yl)ethyldiene]hydroxylamine), NPS2 (N-[(4-propargyloxy-[2,2’-bipyridin]-6-yl)(2-methoxyphenyl) methylidene] hydroxylamine), NPS3 (N-[(4,4’-dimethoxyl[2,2’-bipyridin]-6-yl)(2-methoxyphenyl) methylidene] hydroxylamine), NPS4 (N-[(4,4’-dimethyl[2,2’-bipyridin]-6-yl)(2-methoxyphenyl) methylidene] hydroxylamine), NPS5 (N-[1-(4,4’-dimethoxy[2,2’-bipyridin]-6-yl)propyldiene]hydroxylamine), and NPS6 (N-[1-(4,4’-dimethoxy[2,2’-bipyridin]-6-yl)propyldiene]hydroxylamine) respectively with the structures shown in Supplementary Figure S1.
3.2 In silico studiesIn silico analysis was performed to corroborate the physiochemical and ADMET (absorption, distribution, metabolism, excretion, and toxicity) properties of 2,2’-Bipy derivatives.
3.2.1 Drug likenessDrug likeness was assessed based on Lipinski’s Rule of 5, which considers molecular weight, hydrogen bond acceptors, hydrogen bond donors, and log P (octanol/water partition coefficient). According to this rule, compounds should have a molecular weight less than 500 Da, hydrogen bond acceptors ≤10, hydrogen bond donors ≤5, and log P ≤5. All 2,2’-Bipy derivatives complied with Lipinski’s rules, as well as those of Ghose, Egan, and Veber, except vancomycin, which exceeds the molecular weight threshold and has higher hydrogen bond donors and acceptors. Ampicillin did not meet Egan’s rule. A summary of these findings is presented in Table 1. Although predictions are not always experimentally validated, the calculated masses of these derivatives closely matched the experimental values obtained via mass spectrometry.
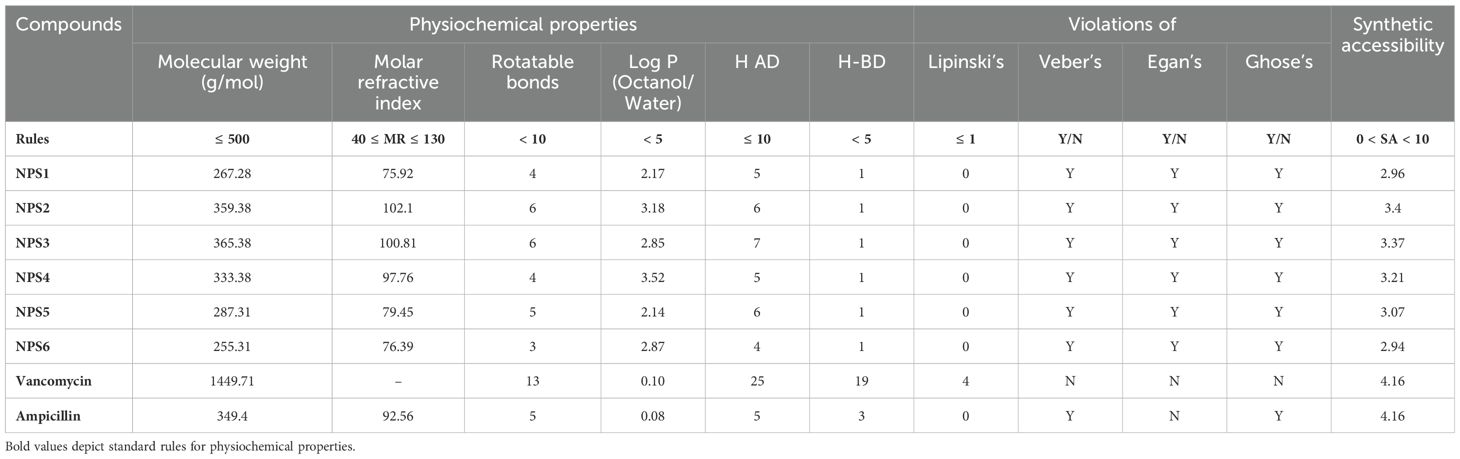
Table 1. Physicochemical and drug-likeness properties of 2,2’- Bipy derivatives based on SwissADME and pKCSM.
3.2.2 ADMET predictionADMET profiles for the 2,2’-Bipy derivatives were analyzed using the pkCSM tool (Table 2). The derivatives showed over 90% intestinal absorption, suggesting effective oral bioavailability. They also met the threshold for effective transdermal delivery with log Kp values <-2.5. Water solubility ranged from 0 to -4.203, which is favorable for good absorption and distribution.
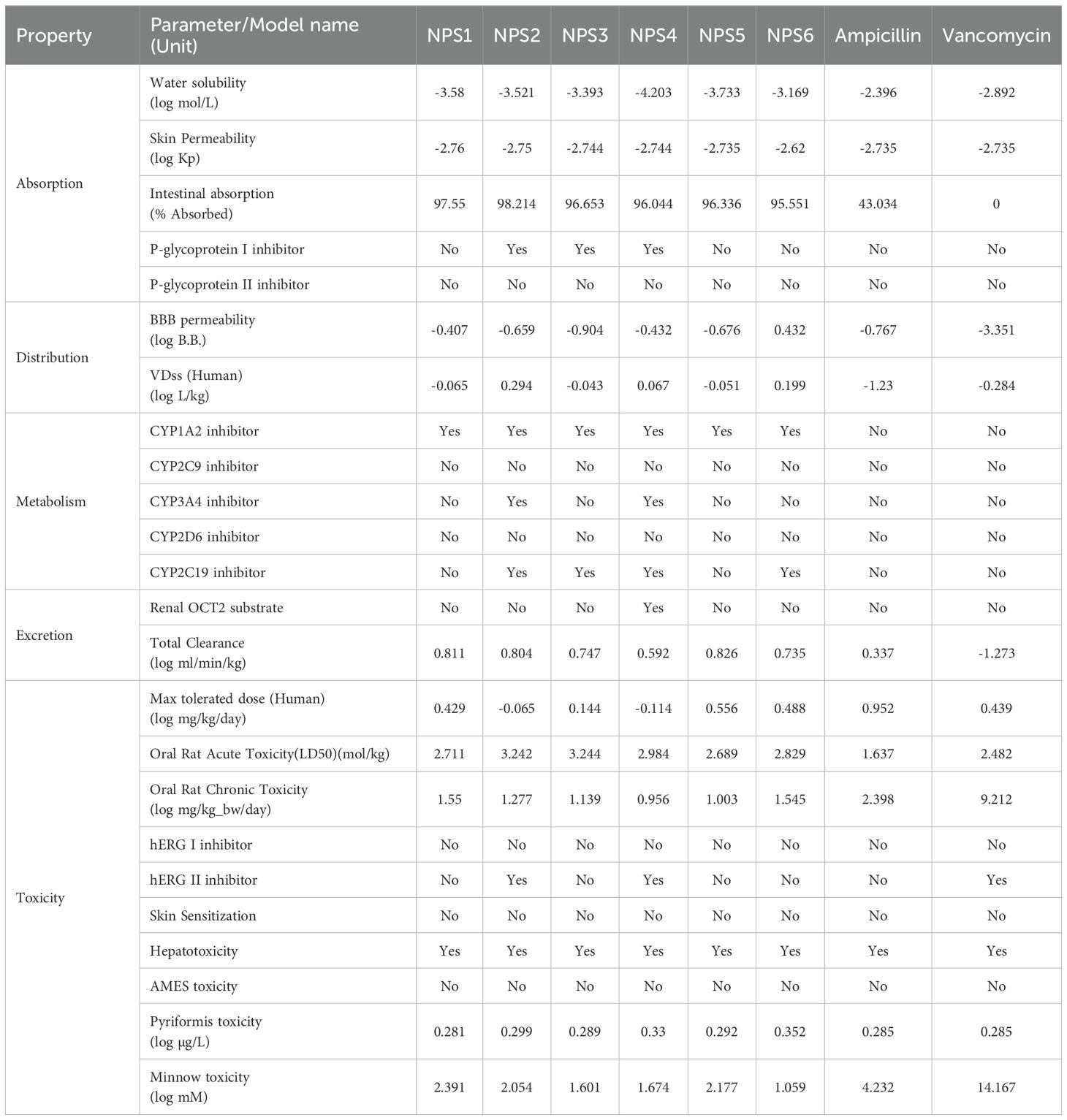
Table 2. ADMET properties of 2,2’-Bipy derivatives.
P-glycoprotein interaction varied among the derivatives: NPS1, NPS5, and NPS6 had no effect, while NPS2, NPS3, and NPS4 inhibited P-glycoprotein I but not P-glycoprotein II. This selective inhibition influenced the overall effect on P-glycoprotein, as determined by the in silico absorption analysis. The volume of distribution (VDss) for all derivatives, except vancomycin and ampicillin, fell between -0.15 and 0.45 log L/kg, indicating balanced tissue distribution. All derivatives, including ampicillin, crossed the blood-brain barrier (log BB >-1.0), unlike vancomycin (log BB -3.351).
Regarding cytochrome P450 interactions, the ADMET analysis revealed that all 2,2’-Bipy derivatives inhibit CYP 1A2, which plays a significant role in the metabolism of various drugs. This inhibition could potentially affect the pharmacokinetics of co-administered drugs that are substrates of CYP 1A2. Importantly, the derivatives were non-inhibitors of CYP 2D6 and CYP 2C9, which are involved in the metabolism of a wide range of drugs, suggesting that these compounds have a lower likelihood of causing drug-drug interactions mediated by these enzymes. Furthermore, NPS2 and NPS4 were found to inhibit both CYP 2C19 and CYP 3A4, two isoforms involved in metabolizing numerous therapeutic agents. While this interaction could suggest potential metabolic interference with drugs processed by these enzymes, the selective inhibition of these isoforms may be leveraged to enhance the efficacy of the derivatives in specific therapeutic contexts. The fact that these interactions are selective further supports the potential of the 2,2’-Bipy derivatives as candidates for drug development with tailored metabolic profiles.
The derivatives showed higher overall clearance rates than vancomycin and ampicillin and did not inhibit renal OCT2. AMES toxicity tests indicated no mutagenic potential, and none of the derivatives inhibited hERG channels, making them suitable for topical application.
3.3 In vitro studies3.3.1 Hemolysis assayThe ability of all six derivatives (NPS1-6) to lyse RBCs was assessed using hemolysis assay. In this assay, none of the derivatives exhibited hemolysis of rabbit red blood cells across a concentration range of up to 400 μg. In contrast, the positive control, Triton X-100, showed severe hemolysis at a concentration of 0.1% (Figure 1A). Therefore, findings of hemolytic assay revealed that all 2,2’-Bipy derivatives are non-hemolytic/non-toxic.
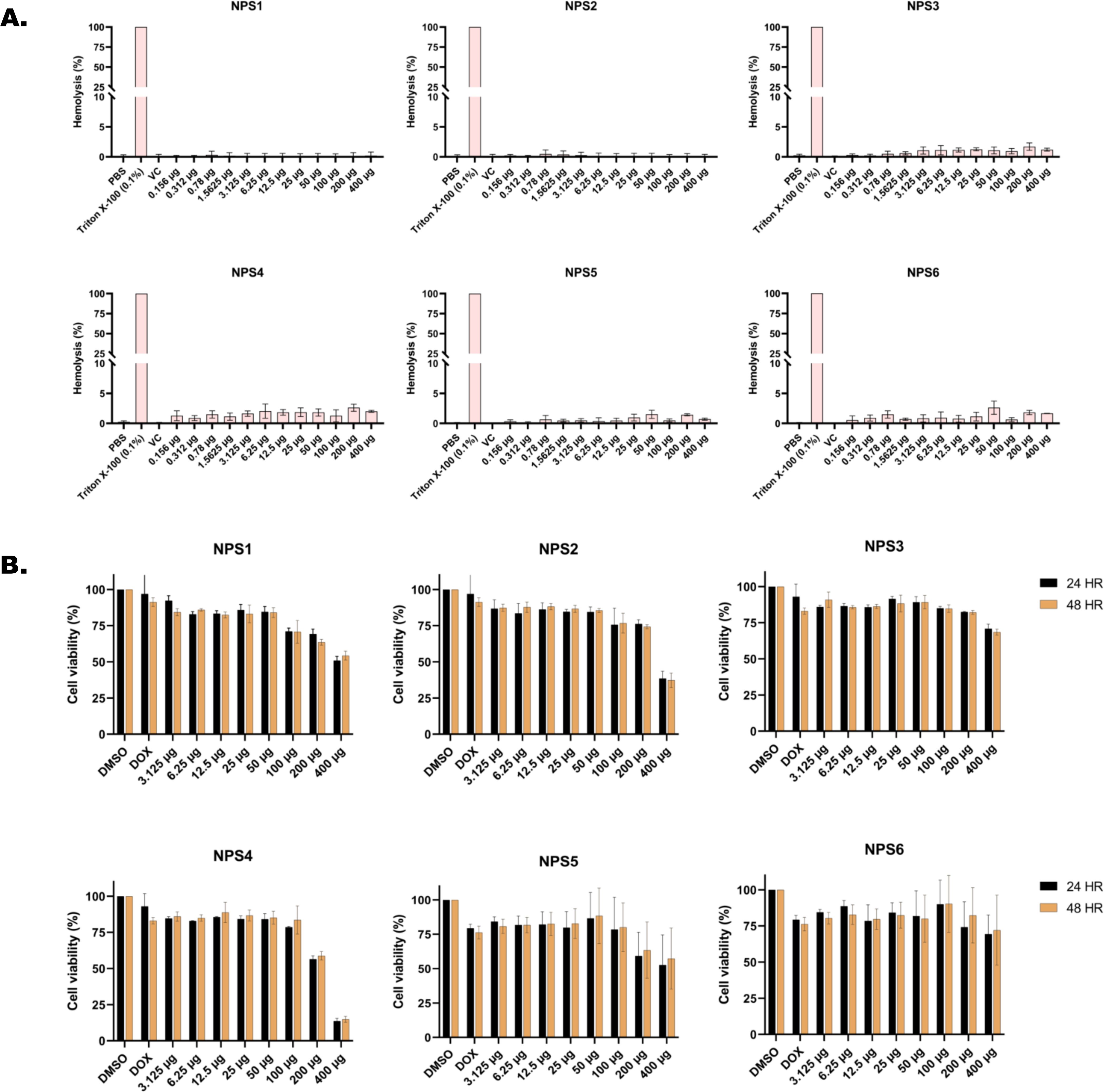
Figure 1. In vitro toxicity assessment of 2,2’-Bipy derivatives (A) Hemolytic assay against rabbit RBCs (Triton X-100 was used as positive control) (B) Cell viability assay on 3T3L-1 cell line (normal murine fibroblast cell line) at 24 h and 48 h using MTT assay (Doxorubicin and DMSO-treated cells were considered positive and negative controls). The cumulative data of three independent experiments (n=3) was plotted in the graph, and data is represented as Mean ± SD.
3.3.2 Cytotoxicity assay in normal murine fibroblast cell line (3T3L-1 cell line)Cytotoxicity of the 2,2’-Bipy derivatives was assessed using an MTT assay on 3T3L-1 cells. All derivatives exhibited more than 75% cell viability at 24 h and 48 h (Figure 1B). However, at a concentration of 400 μg/ml, the viability decreased to 50% or less. The calculated IC50 (µg/ml) values differed among derivatives and were 151.2, 1712, 253, 278.8, 151.5, and 168.1 for 24 h, and 137.6, 1341, 274.3, 239.6, 157.2, and 685.4 at 48 h for NPS1, NPS2, NPS3, NPS4, NPS5, and NPS6.
3.3.3 Antimicrobial activityThe antimicrobial activity of NPS1-NPS5 was evaluated against battery of strains including Gram-positive as well as Gram-negative strains as mentioned in section 2.7, using the agar well diffusion method (Figure 2A). The zone of inhibition for the inhibitory activity of NPS (1-5) at 50 µg/ml was measured in millimeters (mm) and is presented in Table 3. At this concentration, NPS6 exhibited poor diffusion due to its low solubility. NPS4 and NPS5 showed the highest zone of inhibition (14 mm) against MRSA 831. The minimum inhibitory concentrations (MICs) were determined using broth microdilution assay, and it was confirmed that all derivatives were effective against the tested bacteria (Table 4). NPS1 and NPS4 were found to be more effective than positive control against V. cholerae, and all of them showed activity against clinical MRSA 831. Therefore, we decided to proceed with S. aureus and MRSA 831 for further experiments. The therapeutic index (TI) calculated from 24-hour IC50 values was ≥3, indicating the safety of these derivatives (Figure 2B).
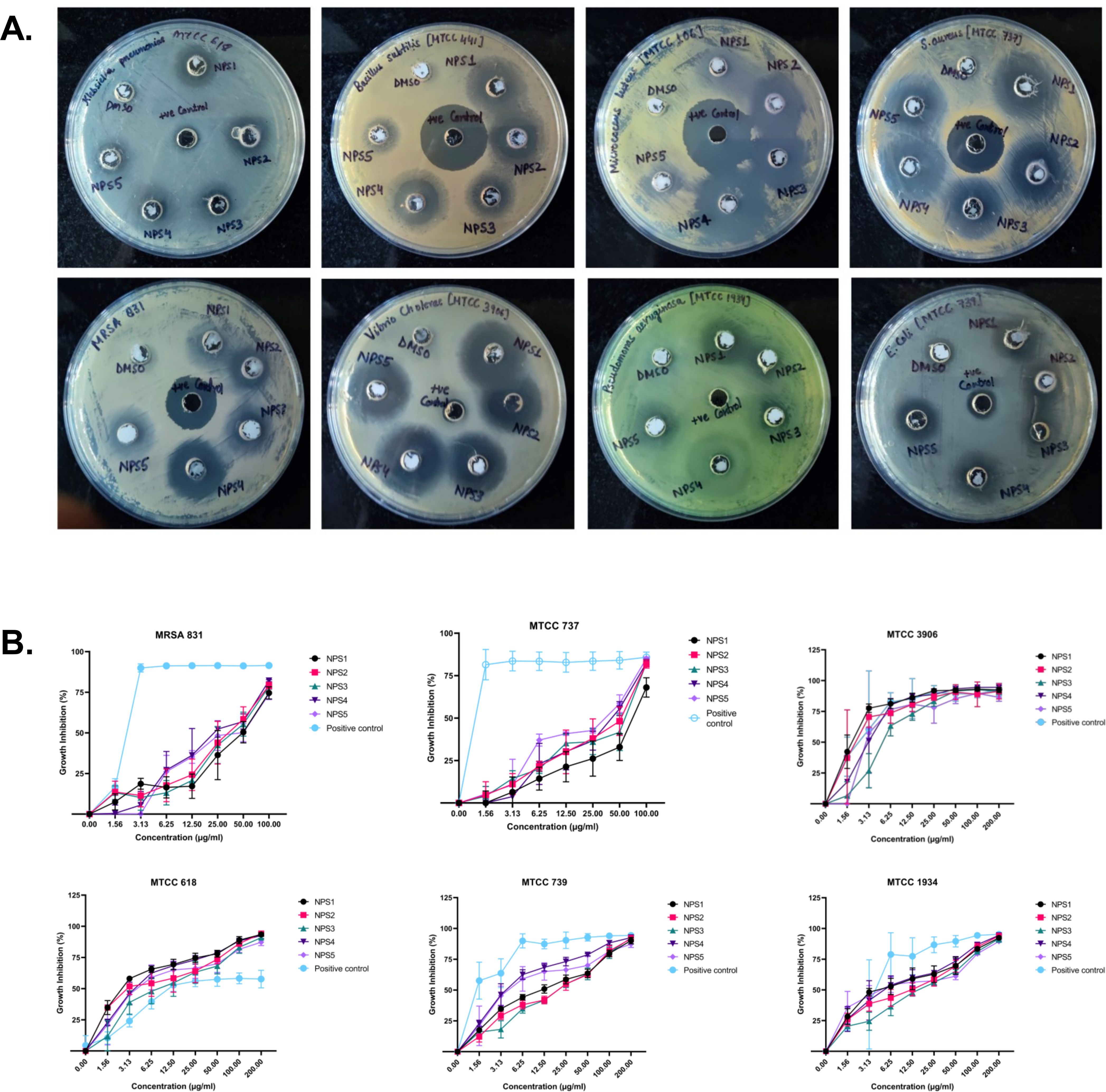
Figure 2. Antibacterial activity of 2,2’-Bipy derivatives (A) Representative images of Agar well diffusion assay against Gram-positive and Gram-negative bacteria, (B) Growth inhibition of bacteria. Vancomycin, Ampicillin, and PBS were included as the reference control. The cumulative data of three independent experiments (n=3) was plotted in the graph, and data is represented as Mean ± SD.
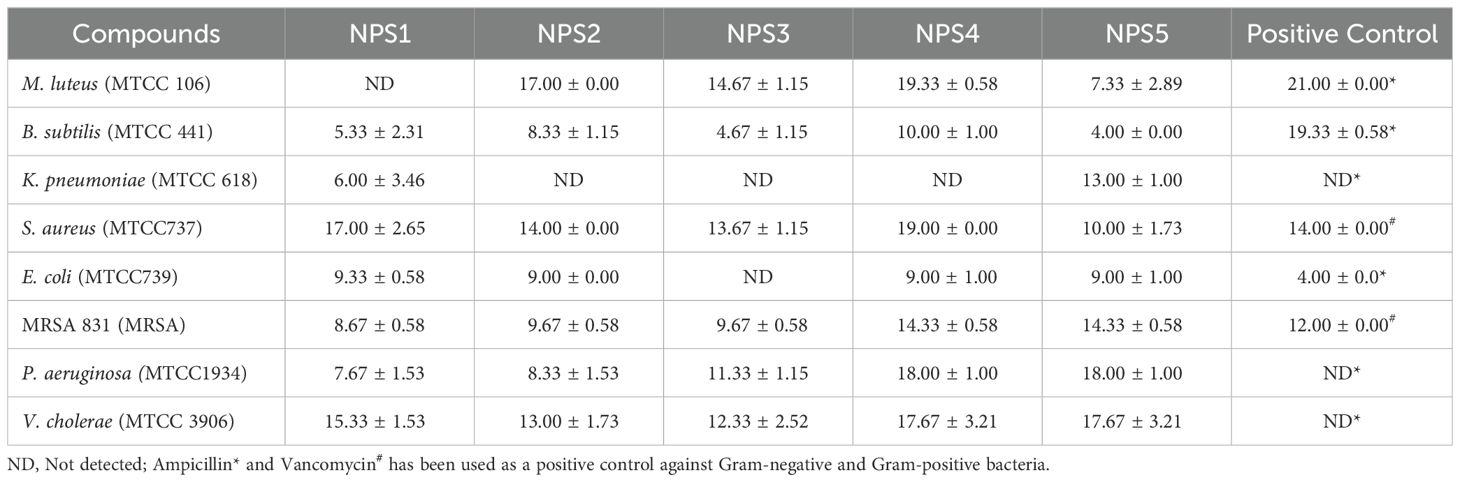
Table 3. Zone of inhibition (in mm) of 2,2’-bipyridine derivatives against various microbial strains.
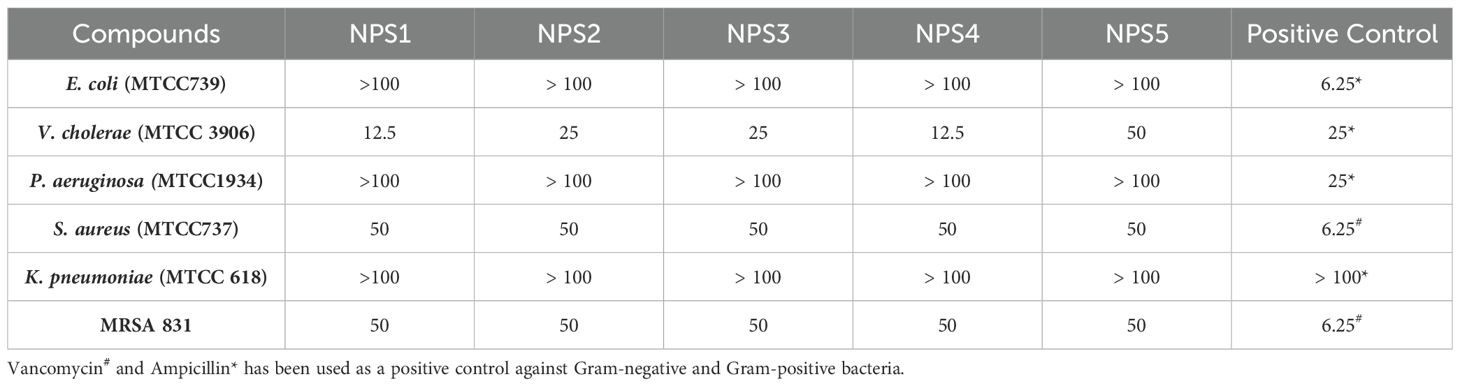
Table 4. Minimum inhibitory concentration values (µg/ml) of 2,2’-Bipy derivatives and standard controls against bacterial strains.
3.3.4 Time kill-kineticsThe derivatives exhibit antibacterial activity against S. aureus and MRSA 831. A time kill-kinetics assay was performed to evaluate their bactericidal effect. The assay demonstrated that derivatives NPS (1-5) were bactericidal against both S. aureus and MRSA 831, showing a dose- and time-dependent effect at concentrations of 0.5 to 4 × MIC. Significant reductions in bacterial counts, from 6 to 2.6-2 Log10 CFU/ml, were observed after 2 h of treatment (Figures 3A, B).
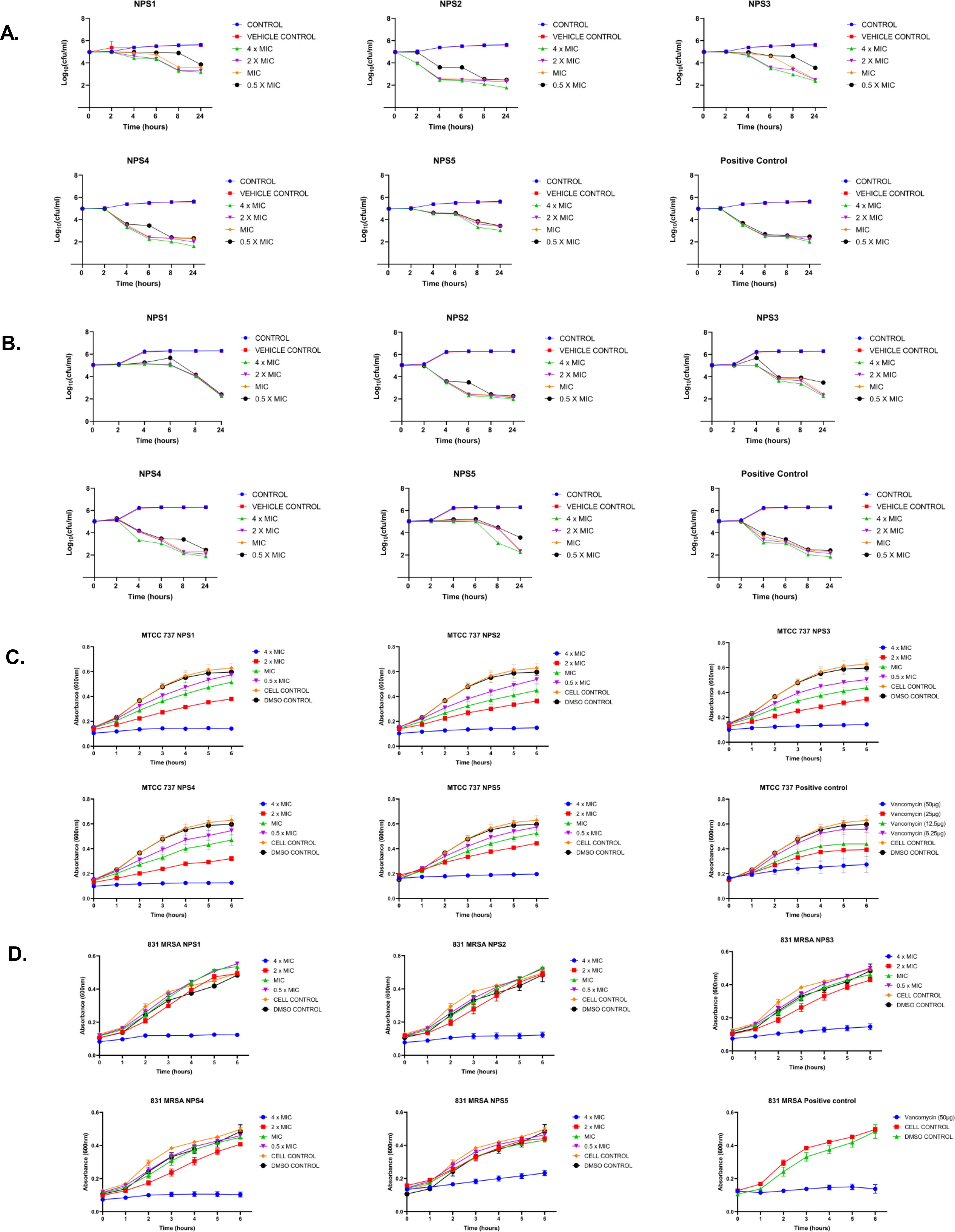
Figure 3. Time kill kinetics of (A) MTCC 737 and (B) MRSA 831 at four different concentrations 0.5 - 4 × MIC of 2,2’-Bipy derivatives and vancomycin (Positive control). Persister kill kinetics of (C) MTCC 737 and (D) MRSA 831 at four different concentrations 0.5- 4 X MIC of 2,2’-Bipy derivatives and vancomycin (Positive control). Control (untreated cells) and vehicle control were used to monitor normal growth. The cumulative data of three independent experiments (n=3) was plotted in the graph, and data is represented as Mean ± SD.
3.3.5 2,2’-Bipy derivatives show activity against persister cellsA time-kill kinetics assay was conducted on S. aureus and MRSA 831 persister cells, both of which have high biofilm-forming potential. At a 4-fold MIC, the derivatives effectively reduced persister cell counts compared to vancomycin at the same concentration. Treatment with NPS3 and NPS4 with a 4-fold MIC significantly reduced the number of persister cells in the MRSA strain. Although the same concentration of derivatives decreased persister cell counts in S. aureus, in contrast, vancomycin showed similar activity only at a concentration of 100 µg/ml (Figures 3C, D).
3.3.6 Biofilm inhibition and eradication2,2’- Bipy have been shown to inhibit the growth of S. aureus and MRSA 831. Additionally, we aimed to test the inhibitory potential of these derivatives against biofilm formation. Biofilms, which are collections of microorganisms adhering to a surface matrix pose significant health risks by increasing antibiotic resistance and causing severe infections in humans. We evaluated the anti-biofilm activity of 2,2’-Bipy derivatives against Gram-positive bacteria (MTCC 737, MRSA 831) and Gram-negative bacteria (MTCC 739, MTCC 1934, MTCC 3906, and MTCC 618) at concentrations ranging from 0.5 to 4 times the MIC. These derivatives inhibited biofilm formation in all strains in a dose-dependent manner, achieving up to 75% inhibition at 4 × MIC (Figures 4A, B; Supplementary Figures S2A–D), indicating effectiveness of these derivatives. NPS1 and NPS4 are highly effective against S. aureus when used at MIC or higher concentrations, while the remaining derivatives exhibited 50% inhibition at half their respective MICs. When tested against resistant strain MRSA 831, all the derivatives showed inhibition comparable to vancomycin, highlighting their potent anti-staphylococcal activity. Further, to check if these derivatives are effective in disrupting a pre-formed biofilm, a biofilm eradication assay was conducted using MTCC 737, MRSA 831, MTCC 1934, and MTCC 3906. The derivatives NPS (1-5) effectively eradicated the biofilms in a dose-dependent manner (Figures 4C, D; Supplementary Figures S3A, B). As depicted in the figures, all derivatives show up to 75% eradication of pre-formed biofilms of S. aureus and demonstrated increased efficacy against MRSA biofilms compared to the positive control, vancomycin. These results highlight the potential of these derivatives as alternatives to traditional antibiotics for treating resistant bacterial strains. Furthermore, the findings confirmed the efficacy of 2,2’-Bipy derivatives against Staphylococcus biofilms, as well as their significant anti-biofilm activity against both Gram-positive and Gram-negative bacteria.
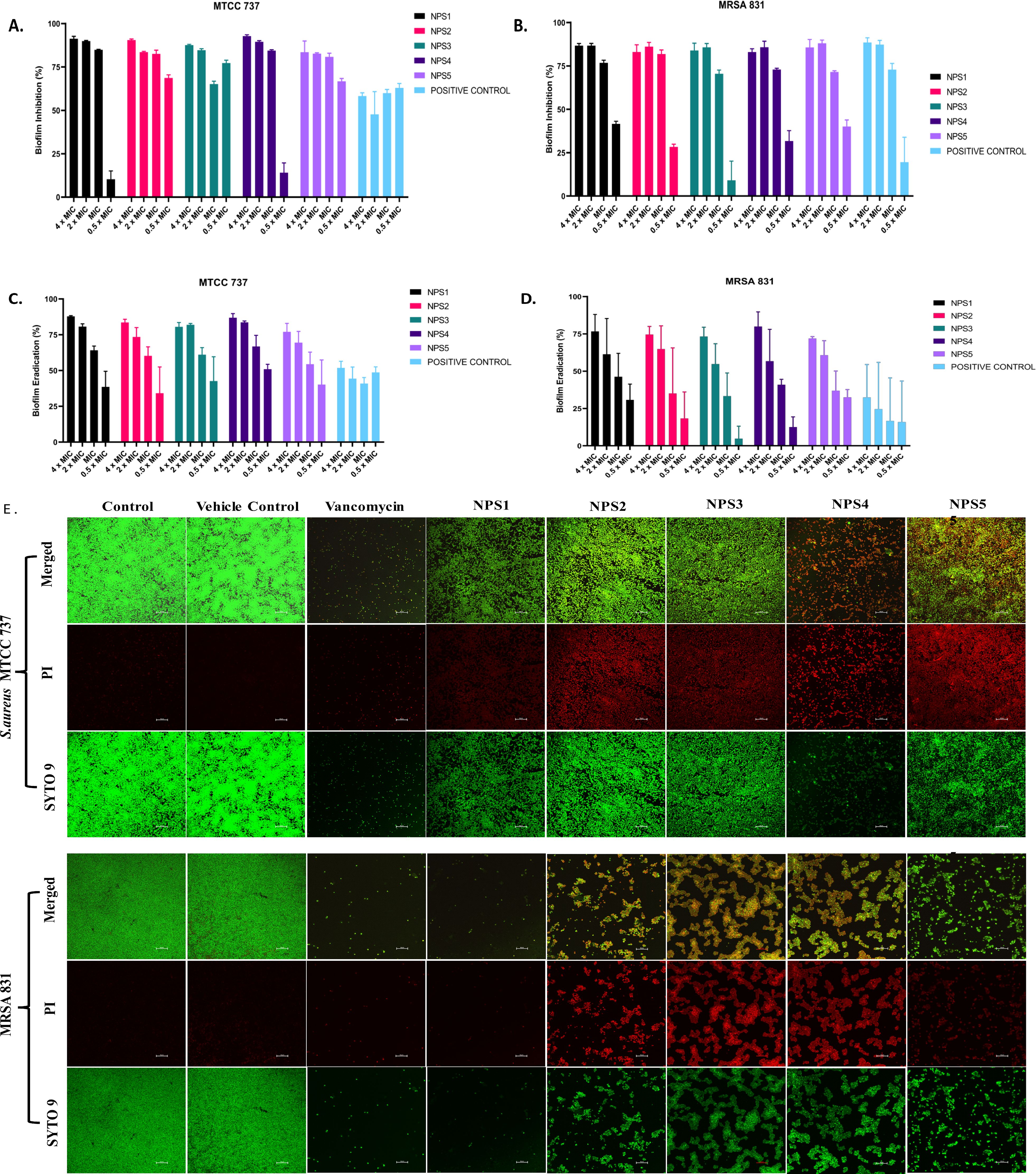
Figure 4. Effect of 2,2’-Bipy derivatives on biofilms. (A, B) Biofilm inhibition against MTCC 737 and MRSA 831; (C, D) Biofilm eradication against MTCC 737 and MRSA 831 at four different concentrations 0.5 - 4 × MIC of 2,2’-Bipy derivatives and vancomycin. The cumulative data of three independent experiments (n=3) was plotted in the graph, and data is represented as Mean ± SD. (E) Representative CLSM images of MTCC 737 and MRSA 831 after incubation with SYTO 9 (green fluorescence) and PI (red fluorescence) indicate the live and dead cells. Two independent experiments were performed in triplicate.
A CLSM assay was performed to visualize the effect of these derivatives on the biofilms of MTCC 737 and MRSA 831 using SYTO 9 dye and propidium iodide. SYTO 9 is a fluorescent dye that stains living and damaged cells, while propidium iodide penetrates the dead or membrane-compromised cells. In Figure 4E, the untreated and vehicle controls exhibited an intact biofilm lawn with high SYTO 9 and low PI fluorescence, indicating live cells and biomass of MTCC 737 and MRSA 831. Vancomycin, the positive control, showed a low number of cells, indicating effective biofilm disruption and severe cell damage. 2,2’-Bipy derivatives showed varying efficacy in inhibiting biofilms, of MTCC 737 and MRSA 831. In the biofilms of MTCC 737, NPS4 caused significant disruption and damage, while the other derivatives show only slight disruption. In MRSA 831, derivatives NPS (1-5) effectively disrupted the biofilms, with the remaining cells retaining both SYTO9 and PI dye, indicating membrane-damaged cells. The results demonstrated that NPS4 efficiently inhibits both biofilms, while the remaining derivatives were more effective against the biofilm of MRSA 831. The existence of persister cells, which are dormant and extremely resistant to antibiotics, intensifies the challenges posed by biofilm-associated infections. Since these derivatives suppressed the growth of the persister cells, the inhibition of the persister cells associated with the biofilms was also investigated. As shown in Figure 5, derivatives NPS (1–5) could inhibit 50% of persister cells attached to the biofilm in a dose and time-dependent manner. The results show that 2,2’-Bipy derivatives effectively inhibit persister cells, disrupt mature biofilms, and prevent biofilm development in Gram-positive and Gram-negative bacteria. These compounds are intriguing options for treating infections linked to biofilms because of their broad-spectrum activity and dose-dependent efficacy. Further investigation into their molecular mechanisms, including interactions with biofilm-specific targets, could enhance understanding and support their clinical translation.
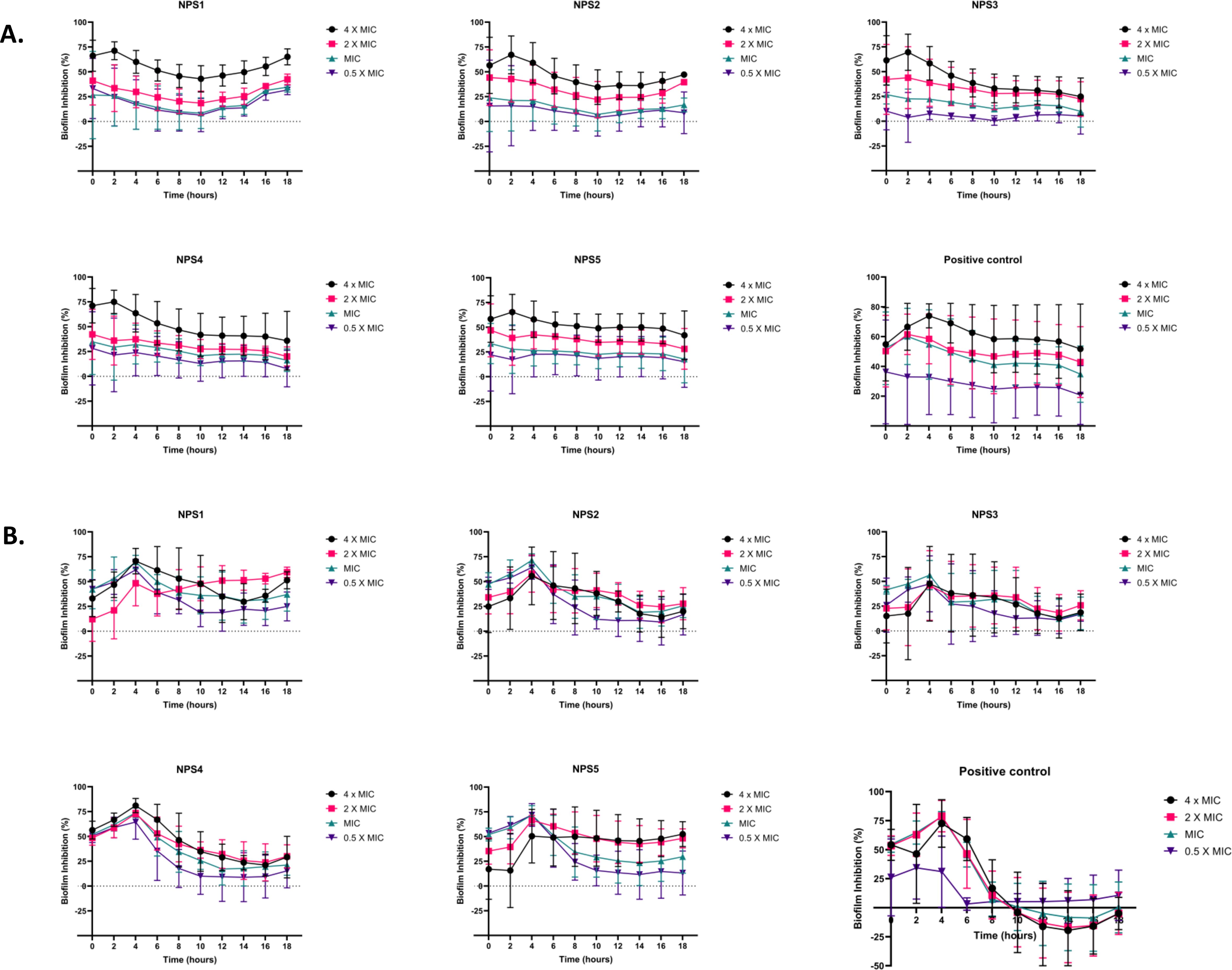
Figure 5. Effect of 2,2’-Bipy derivatives in inhibiting persister’s biofilm developed by (A) MTCC 737 and (B) MRSA 831, at four different concentrations 0.5 - 4 × MIC of 2,2’-Bipy derivatives and vancomycin. Data is represented as Mean ± SD and the cumulative data of three independent experiments (n=3) was plotted in the graph.
3.3.7 Antibacterial mechanismTransmission electron microscopy (TEM) was performed to visualize the changes in cell morphology. TEM micrographs of untreated and 0.5% DMSO (vehicle control)-treated bacterial cells of S. aureus and MRSA 831 demonstrated typical morphology. These cells were normally spherical, with intact and smooth cell surfaces, a homogeneous shape, an intact inner membrane with its waved pattern, a uniformly thin periplasmic space, and a complete outer surface. Cells treated with 100 µg/ml vancomycin exhibited distorted morphology, wrinkled surfaces with some appendages, blebbing, buds, and a slight increase in size and irregular shape. Cells treated with derivatives at 2-fold MIC (100 µg/ml) showed symptoms of cell damage, such as missing or cracked cell walls leading to a distorted shape. Cell death commenced as the cell membrane gradually broke down, and the cytoplasm began to leak out. Certain cells appeared to be destroyed, and the majority showed clear signs of damaged and distorted membranes, with a rougher surface than the control group. Significant morphological alterations were observed in the cells treated with NPS 4 (Figures 6A, B). Following the detection of changes in the cell membranes of MTCC 737 and MRSA 831, the dye propidium iodide (PI) dye was utilized to investigate the antibacterial mechanism of action in order to check the membrane integrity. Disruption of bacterial membranes is one of the key steps in inhibiting bacterial growth. PI is a cell impermeable dye that penetrates the cell when the membrane is damaged. Polymyxin B, a membrane destabilizer used as a positive control, showed higher fluorescence (Figures 6C, D) at an excitation of 490 nm and 635 nm emission than the 2,2’-Bipy derivatives, except for NPS1, which exhibited fluorescence indicating membrane instability of the MTCC 737 and MRSA 831 bacterial cell membrane. The membrane permeability of the 2,2’-Bipy derivatives to the inner membrane of MTCC 737 and MRSA 831 was examined using NPN, a hydrophobic dye. When the derivatives were administered to the cells, the fluorescence decreased in comparison to the positive control, Triton X-100 (Figures 6E, F), suggesting that the derivatives don’t permeabilize the cell membrane, possibly due to limited exposure to the derivatives. Bacteria are inherently negatively charged and allow an exchange of positive ions. The nature of the charge on the bacterial membrane changes due to depolarization. The cationic dye binds to the negatively charged membrane of the polarized cells, and depolarization prevents the binding and release of dye into the medium. As a result, the control cells and the vehicle control-treated cells (not treated with drugs) produce a weak signal. In contrast, depolarized cells produced high fluorescence (Figures 6G, H). When derivatives were added to the cells at a concentration of 100 µg/ml (about twice the MIC), the cells exhibited intense fluorescence emission, indicating depolarization of the cell membrane. DCFH-DA is a cell-permeable dye that binds to reactive oxygen species (ROS) and produces fluorescence when read in a fluorescence reader. DCFH-DA fluorescence increased when the bacterial cells of MTCC 737 and MRSA 831 were treated with the 2,2’-Bipy derivatives compared to the vehicle control and untreated cells, indicating increased intracellular ROS (Figures 6I, J). Consequently, it turns out that an increase in intracellular ROS causes a shift in membrane polarity, which triggers membrane disruption by the NPS derivatives (1-5).
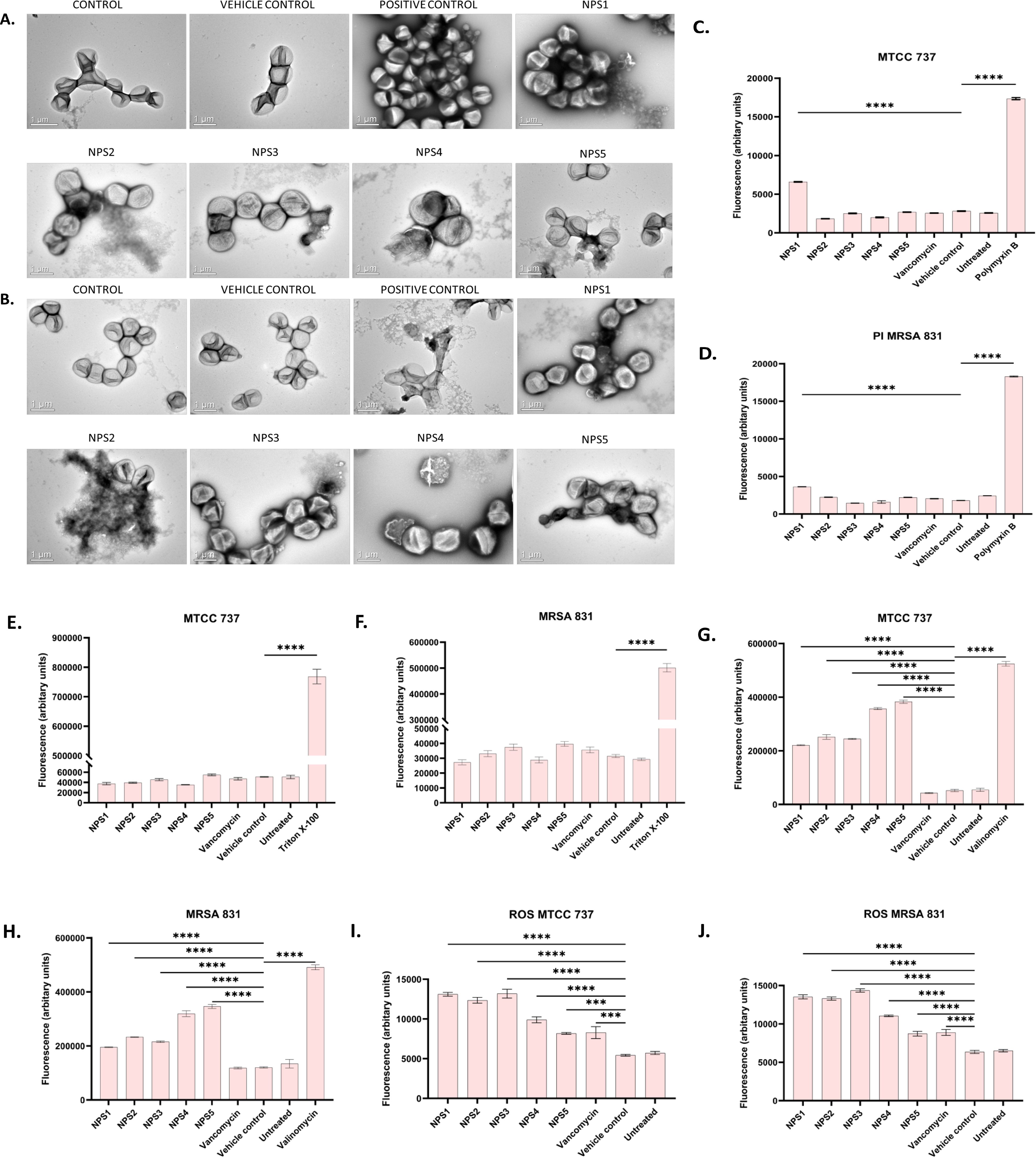
Figure 6. (A, B) Representative TEM micrographs of MTCC 737 and MRSA 831; (C, D) Bacterial membrane permeability of MTCC 737 and MRSA 831 using PI dye; (E, F) Assessment of membrane permeability with NPN dye; (G, H) 2,2’-Bipy derivatives depolarize the bacterial membrane of MTCC 737 and MRSA 831; (I, J) Total ROS accumulation in MTCC 737 and MRSA 831. Results are the cumulative data of three independent experiments (n=3) that are plotted in the graph, expressed as Mean ± SD. The p-values of 0.001 (***) and 0.0001 (****) considered highly significant.
3.3.8 Resistance development in strains MTCC 737 and MRSA 831The results of resistance development using the sequential passage technique are shown in Figure 7. MTCC 737 and MRSA 831 were cultured for 18-19 days in the presence of 2,2’-Bipy derivatives and vancomycin. Fold change in MIC was calculated to assess resistance development. For 2,2’-Bipy derivatives, the fold change in MIC remained nearly 1 after 19 days, indicating no significant resistance development. In contrast, vancomycin treatment did not show any resistance until the 9th day in both strains; after that, the fold change became neither linear nor consistent. Vancomycin resistance in various strains is well-documented in the literature. Our findings also indicate that the cells did not acquire resistance to the compounds NPS (1-5) for up to 19 days, highlighting the potential of 2,2’-Bipy derivatives in combating bacterial infections without promoting resistance. The bacteria showed no evidence of resistance, highlighting the potential of 2,2’-Bipy derivatives for managing bacterial infections, particularly in an era where antimicrobial resistance is a growing global threat.
留言 (0)