Dupuytren's contracture is a common localized fibrotic condition that often affects the fourth digit and fifth digit. It is more prevalent in Northern Europe, typically starting between the ages of 50 and 70, with men being three times more susceptible than women (1). Initially, firm nodules develop on the palms of the hand, which later evolve into fibrous collagen cords extending toward the fingers (2, 3). As the condition progresses, these cords thicken, mature, and contract, leading to permanent finger flexion deformity. The patient's table top test is positive. Surgical intervention is often necessary for individuals who sustain severe Dupuytren's contracture. The severity of Dupuytren's contracture is graded based on the Tubiana staging system, with severe cases falling under Tubiana stages Ⅲ and IV (4). In cases of severe contracture, local soft tissue defects with exposed tendons, nerves, and vessels may occur after removing the diseased palmar fascial tissue. These defects should be promptly repaired with a suitable flap (5).
The perforators of the ulnar palmar artery in the fifth digit are situated approximately 1.3 cm above the head of the fifth metacarpal bone. They travel between the superficial hypothenar tendon and the fifth metacarpal bone subcutaneously, forming connections directly or indirectly with the descending dorsal branch of the ulnar artery. Along its path, small blood vessels branch out to supply nearby soft tissues (5). A perforator flap from the ulnar palmar digital artery of the fifth digit is a suitable option for repairing local soft tissue defects on the palm and the fifth digit (6).
This study aims to investigate the design of a perforator flap based on the ulnar palmar artery perforator of the fifth digit for repairing soft tissue defects in the palm and fifth digit.
2 Materials and methodsIn this study, patients who met the following criteria were included: (1) patients suffering from Dupuytren’s contracture in Tubiana stage III or IV; (2) patients with a soft tissue defect of the palm after surgery and repaired using a perforator flap from the ulnar palmar artery. (3) Age and gender were not limited. The exclusion criteria were as follows: (1) clinical data were incomplete; (2) patients with severe scar constitution and diabetes; (3) patients with Raynaud syndrome.
All the procedures in this study were approved by the Ethics Committee of the Second Hospital of Tangshan and in accordance with the ethical standards of the Institutional Research Committee and with the Declaration of Helsinki. All patients signed a written informed consent form for the operation.
2.1 Surgical techniqueThe ulnar palmar artery was preoperatively identified by high-frequency Doppler ultrasound and marked on the surface of the ulnar palm. The patient was placed in the supine position and underwent surgery under a brachial plexus nerve block with tourniquet control. Loupe magnification and an operating microscope were also needed. Following hand disinfection, the contracture site was palpated and the incision location was labeled. Subsequently, the skin, subcutaneous tissue, and fascia were incised along the scar marks on the palm. Care was taken not to injure the deep nerves and arteries when cutting the contracture band. Even after the complete removal of the contracture tissue, flexion of the affected finger may still be observed. To address this, an incision near the proximal interphalangeal (PIP) joints was designed to release the joints. The resected contracture tissue was pathologically examined. Skin and soft tissue defects, with exposed nerves, tendons, and vessels, were visible after the dissection. These defects, located either on the ulnar side of the palm or the palmar side of the fifth digit, were repaired using a perforator flap of the ulnar palm. The perforator flap of the ulnar palmar digital artery was designed according to the size, shape, and position of the wound. The rotation point of the flap is usually located around the metacarpophalangeal (MP) joint. The axis of the flap generally aligns with the line between the pea bone and the midpoint of the hypothenar striae. The flap size should be greater than 10%–20% of the wound dimensions. The ulnar margin of the flap was first incised, identifying the perforator entering the flap from the ulnar palmar digital artery of the fifth digit (Figure 1A). During the cutting of the flap pedicle, 0.5 cm of fascial tissue around the perforator was preserved on both sides of the axis. The flap was dissected from the superficial layer of the hypothenar muscle from the proximal to the distal up to the rotation point. The tourniquet was then released, ensuring normal blood supply to the flap. The flap was transferred to cover the wound through the open tunnel which was formed between the donor and recipient sites. The flap donor site was directly sutured (Figure 1B).
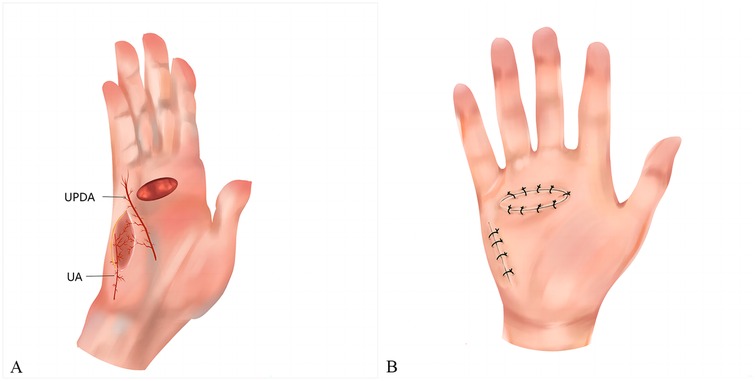
Figure 1. (A) The perforator flap was harvested at the ulnar palm and pedicled through the perforator of the ulnar palmar digital artery of the little finger. (B) The wound was repaired with the perforator flap and the donor site was closed directly. UPDA, ulnar palmar digital artery; UA, ulnar artery.
Postoperative management included drainage strip placement, sterile dressing application, and elevation of the affected limb to prevent venous congestion. Skin flap color, temperature, and capillary refill were monitored closely for 7 days. Stitches were removed after 2 weeks, followed by the rehabilitation exercises with the aid of a physical therapist.
2.2 Evaluation of outcomesData acquisition and assessment were performed by a single senior hand surgeon (HuW). All patients completed the visual analog scale (VAS) and disability of the arm, shoulder, and hand (DASH) questionnaires preoperatively, postoperatively, and at the final follow-up (7). Concurrently, the MP and PIP joint contracture levels and the two-point discrimination of the flap were assessed by the same physician. Furthermore, the appearance of the flap and donor site was evaluated based on the Michigan Hand Outcomes Questionnaire (MHQ) (very satisfied = 5 points, satisfied = 4 points, average = 3 points, dissatisfied = 2 points, very dissatisfied = 1 point) (8). At the final follow-up, the total joint motion of the patient post-treatment was assessed using the total active motion (TAM) scale. The TAM scores of the affected hands are classified as excellent for more than 220°, as good for 180°–220°, and as poor for less than 180°.
Data were analyzed with the SPSS 25.0 statistical software (IBM, Armonk, NY, USA). A paired T-test was conducted to compare the recovery of the affected fingers at the final follow-up with the degree of contracture before surgery. It was also conducted to compare the contracture degree of the affected fingers at the final follow-up with the healthy contralateral hand. The Kruskal–Wallis (K–W) test was used to compare the VAS and DASH scores at the final follow-up, after surgery, and before surgery. P < 0.05 was considered statistically significant.
3 Typical caseA 73-year-old female patient presented to our hospital suffering from severe Dupuytren's contracture in her left palm (Figure 2A). Upon examination, hardened skin was observed on the ulnar side of the palm and the volar side of the proximal section of the fifth digit, along with localized skin depressions. A firm cord-like structure was palpable from the palm to the proximal section of the fifth digit (Figure 2B). The MP joint of the fifth digit had a flexion angle of 90°, the table top test yielded positive results (Figure 2C), the Tubiana stage was classified as III, and the patient opted for surgical intervention.
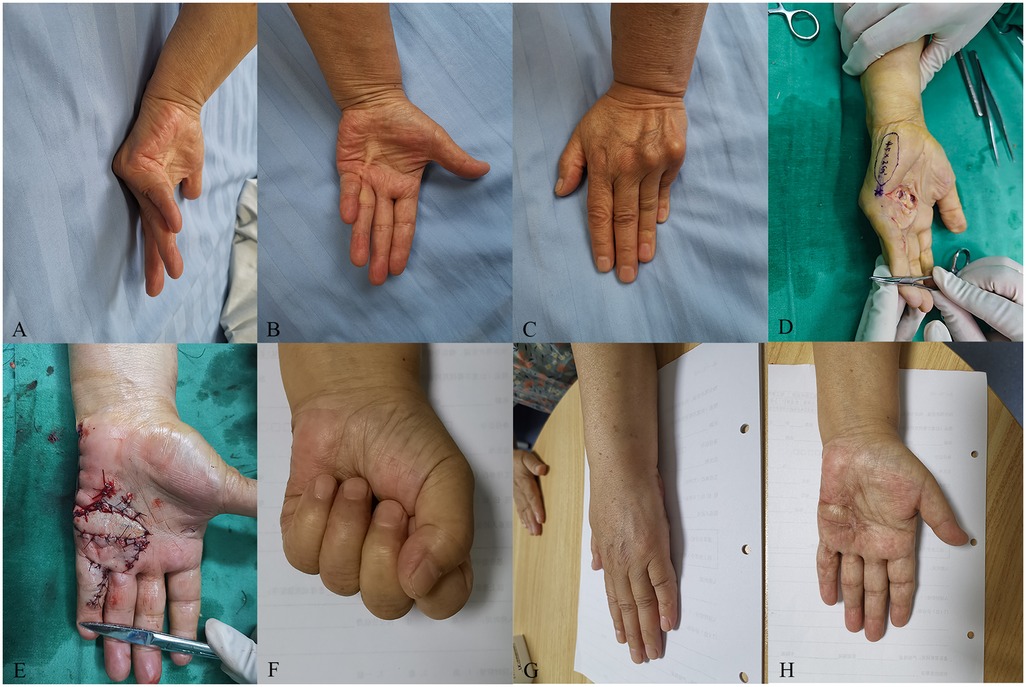
Figure 2. A 73-year-old female patient who suffered from severe Dupuytren’s contracture in her left hand was treated with the perforator flap of the ulnar palm. (A) The preoperative MP joint of the ring and little fingers had a flexion angle of 70° and 90°, respectively. (B) A cord-like object can be seen on the ulnar palm. (C) The patient’s table top test was positive. (D) Design of the flap during the operation. (E) The wound was covered by the flap and the donor site was closed directly. (F) The flap appearance was satisfactory and the active extension of the affected ring and little fingers were normal at the 22-month follow-up. (G) The table top test was negative at the 22-month follow-up. (H) The active flexion of the affected ring and little fingers was normal at the 22-month follow-up.
Prior to surgery, high-frequency Doppler ultrasound was used to locate and mark the perforators of the ulnar palmar digital artery in the fifth digit. The surgery was performed with a tourniquet applied to the brachium (250 mmHg) under a brachial plexus block. Following palmar aponeurotomy and soft tissue release, a palmar wound was created, with the size of 30 mm × 15 mm, exposing the nerves, tendons, and blood vessels. A perforator flap 45 mm in length and 20 mm in width was designed to cover the wound (Figure 2D). The donor site of the flap was directly sutured (Figure 2E). Postoperatively, extension at the MP joint was 0°. The flap survived and the wound healed primarily. At the final follow-up, MP joint extension was maintained at 0°, flexion activity was normal, and the table top test was negative (Figures 2F,G). The patient satisfaction score regarding the flap appearance was 5. The skin color and texture of the flap were similar to the nearby tissue, and there was only a linear scar in the donor area (Figure 2H).
4 ResultsOf the 25 patients, 20 (80%) were men and 5 (20%) were women with an average age of 60.64 (48–81) years. There were 25 hands involved that were treated with a flap. The size of the soft tissue defects ranged from 1.6 to 3.0 cm (mean, 2.1 cm) in length and 1.5 to 4.4 cm (mean, 3.2 cm) in width. The flaps ranged in dimension from 1.8 cm × 3.0 cm to 2.6 cm × 4.7 cm (mean, 2.4 cm × 3.5 cm). The details of the patients are displayed in Table 1.
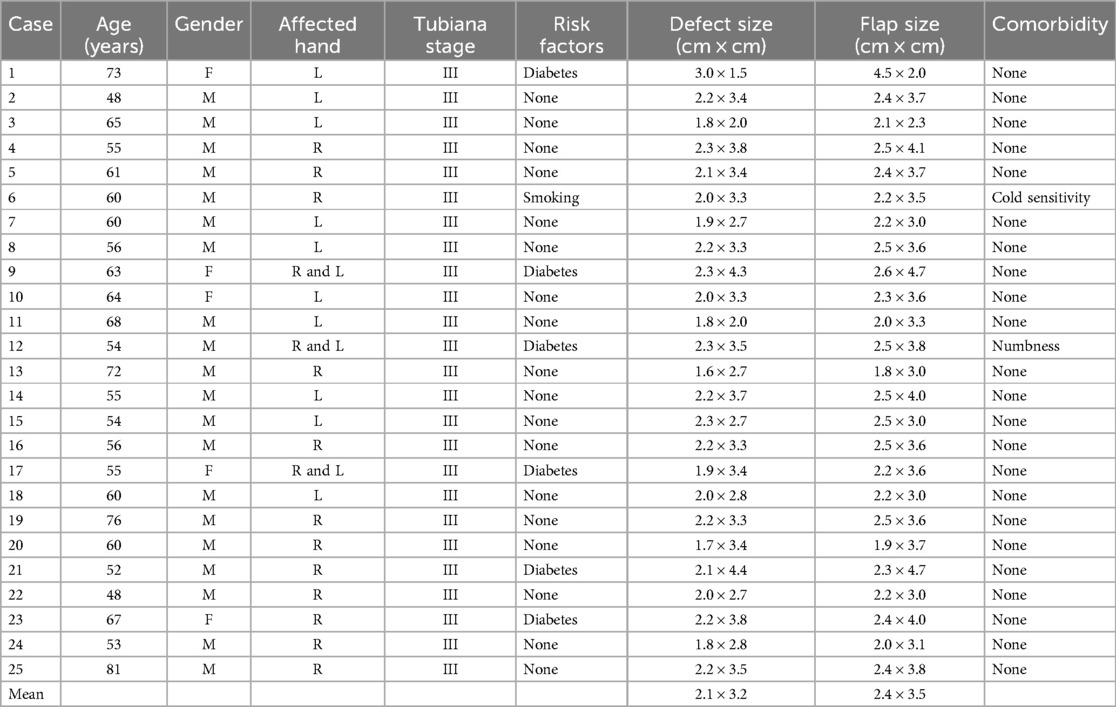
Table 1. Demographic details of the 25 patients.
4.1 Preoperative dataPreoperative observation showed unilateral and bilateral contractures in 21 (84%) and 4 cases (16%), respectively. Furthermore, 13 patients (52%) presented with contractures in a single digit, while 12 patients (48%) had contractures in multiple digits, most commonly involving the fourth and fifth digits. All patients presented with MP joint flexion contracture, with average values of contracture in the third, fourth, and fifth digits of 86.67°, 87.00°, and 86.32°, respectively.
In addition, 33 digits exhibited PIP joint flexion contracture, with average contracture degrees in the third, fourth, and fifth digits measuring 70.00°, 81.88°, and 74.64°, respectively.
4.2 Outcomes of postoperative and final follow-upThe mean follow-up time was 20.48 months (ranging from 15 to 26 months). Immediately postoperatively, the average MP joint flexion contracture degrees in the third, fourth, and fifth digits were 3.33°, 2.50°, and 4.21°, respectively, with complete extension observed at the final follow-up. The average PIP joint flexion contracture degrees in the third, fourth, and fifth digits post-surgery were 1.67°, 7.19°, and 3.93°, respectively. In the final follow-up period, the average PIP joint flexion contracture degrees in the third, fourth, and fifth digits were 1.33°, 3.13°, and 3.21°, respectively. Compared to flexion contracture at the MP joint, flexion contracture at the PIP joint showed less improvement post-surgery. The average values of the MP and PIP flexion contractures preoperatively, immediately postoperatively, and at the final follow-up are shown in Table 2.
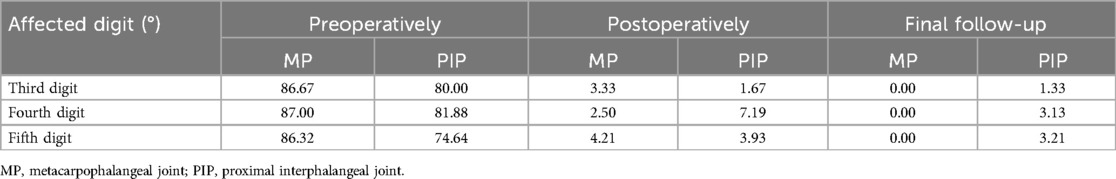
Table 2. The average flexion contracture of the MP and PIP joints.
At the last follow-up, the average MP joint contracture degree in all patients was 0°, matching that of the healthy contralateral hand. Comparison of the PIP joint contracture degrees in the third, fourth, and fifth digits with the healthy hand showed no statistically significant differences (P > 0.05) (Table 3).
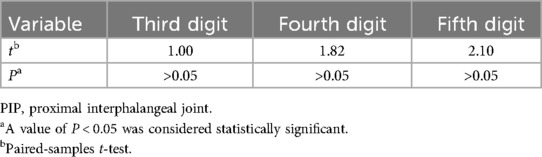
Table 3. The comparison results about the PIP joints of the affected digits and the contralateral digits at final follow-up.
A paired T-test was also conducted to compare the recovery of the affected digits at the final follow-up with the degree of contracture before surgery (Table 4). The contracture of the MP joint in the third, fourth, and fifth digits showed a significant difference at the final follow-up compared to the preoperative values (P < 0.05). Similarly, the contracture of the PIP joint in the third, fourth, and fifth digits at the final follow-up had a statistically significant difference compared to the preoperative values (P < 0.05).
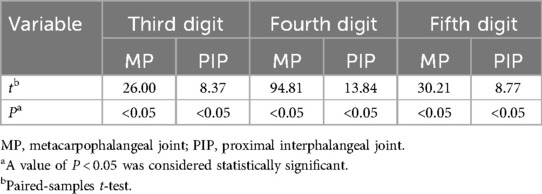
Table 4. The comparison results of the affected digits before and after the operation.
Based on the results (Table 5), the trend of the patient’s pain severity demonstrated a significant decrease in the final follow-up compared to the preoperative levels (P < 0.05). This result suggests the effectiveness of the procedure in treating Dupuytren's contracture. Moreover, the functional status of the affected hands improved following the surgical intervention (P < 0.05), which also confirmed the above conclusion.
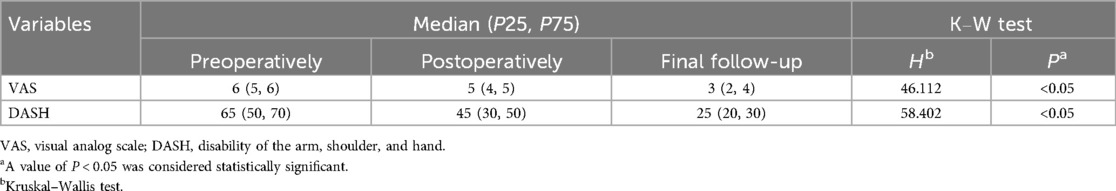
Table 5. Visual analog scale, disabilities of the arm shoulder and hand information.
Upon final assessment, the hands exhibited an esthetically pleasing appearance with only linear scars visible in the donor flap and flap areas, matching the color and texture of the surrounding skin. The table top tests of the patients were negative. The average two-point discrimination value of the flap was 5.52 ± 0.97 mm. The results of the TAM assessment were classified as excellent in 19 cases and good in 6 cases, yielding an average TAM of 229° ± 16.75°. The outcomes of the MHQ indicated that 19 cases reported being very satisfied with the appearance, 5 cases were satisfied, and 1 case was deemed normal. The average satisfaction score for the skin flap appearance was 4.72.
5 DiscussionThere is currently no cure for Dupuytren's contracture, and the treatment focuses on improving hand function by reducing joint contracture. In the early stages of the disease, various non-surgical treatments can be employed, such as drug therapy with vitamin E or steroids, and non-invasive options such as physical therapy and radiation (9, 10). For late-stage disease, a broader array of treatment options is available, including percutaneous needle fasciotomy (PNF), collagenase Clostridium histolyticum (CCH) injection, or surgical excision to remove the diseased tissue through limited fasciectomy (LF) or cutaneous fasciectomy.
Research indicates that non-invasive treatments for Dupuytren's contracture have limited effectiveness and are often associated with a high recurrence rate (11). In contrast, invasive procedures are linked to a lower risk of recurrence, although they necessitate a longer postoperative recovery period.
Multiple studies have demonstrated that PNF improves flexion deformities and allows for quicker recovery post-surgery compared to fasciectomy; however, it has a higher 5-year recurrence rate of approximately 30%, compared to 6% for limited fasciectomy (12). The PNF technique is considered safe, although potential complications include flexor tendon rupture and nerve damage. As a minimally invasive therapy, CCH injection is easy to perform and facilitates faster postoperative recovery with minimal disruption to the patient’s daily activities. Nonetheless, complications such as hematoma, nerve injury, and impaired wound healing have been observed, with an incidence rate of 23% (13). A randomized controlled trial comparing PNF and CCH injections found that PNF is comparable to CCH in correcting flexion and contraction deformities, although with a potentially higher risk of recurrence (14).
The current standard treatment is LF (15). It is associated with a high complication rate (approximately 20.9%) over the subsequent 5 years due to increased wound skin tension and larger scars (16, 17). To address this issue, flap coverage of soft tissue defects due to LF is being considered as it may reduce surgical risks and recurrence rates while maintaining the benefits of the resection. Commonly utilized skin extension techniques for the surgical treatment of Dupuytren's contracture include YV-plasty and Z-plasty. However, these methods may prove challenging for achieving adequate skin extension in cases where the preoperative contracture is severe, the skin defect is extensive, the defect is difficult to cover, or when the PIP contracture exceeds 30° (18). Following YV- or Z-plasty, the tension in the skin after the release of the contracted joint may be excessively high. Patients with severe Dupuytren's contracture often present challenges in achieving complete extension through conventional surgical techniques, with insufficient skin extension being a significant contributing factor (19). This lack of skin extension not only impedes early active recovery in the direction of extension but also diminishes the overall effectiveness of postoperative recovery. In light of this issue, surgeons frequently opt to utilize flaps to cover the soft tissue defect following a fasciectomy, thereby addressing the problem of inadequate skin extension that arises from direct suturing. The use of the first dorsal metacarpal artery island flap for repairing soft tissue defects from Dupuytren's contracture may not be ideal for men with hairy skin on the back of their hands (20), as the skin on the back of the hand differs esthetically and sensuously from the palmar skin, with variations in touch and tactile corpuscle density (21, 22). When utilizing the first dorsal metacarpal artery island flap to address soft tissue defects caused by LF, the resulting appearance and texture of the treated area may differ from that of unaffected sites. The free forearm flap is effective for repairing hand defects; however, it imposes high technical demands on the operator and may result in significant defects at the donor site (23). In addition, the excess tissue of the excised skin flap may necessitate a secondary procedure for reduction. In some instances, the thenar flap has been utilized to transpose and suture palm defects caused by the operation (24). It is important to recognize that Dupuytren's contracture predominantly affects the ulnar side, with higher contracture rates observed in the ring finger and little finger compared to the thumb and index finger (25). Following the principle of proximity in skin flap repair, a perforator flap from the ulnar palm presents a viable option for addressing soft tissue defects on the distal palm and the palmar aspect of the little finger (6).
The design of the flap is simple, the flap donor area is easy to close, the flap blood supply is good, and the flap is rich in adipose tissue and does not easily atrophy after the operation (26). Researchers such as Bakhach et al. have leveraged the dorsal blood supply of the ulnar artery to opt for an ulnar parametacarpal flap (27, 28). Other researchers such as Hirase et al. have successfully employed the ulnar palmar digital artery as a perforator (29), yielding satisfactory outcomes in treating soft defects on the palmar side of the little finger. Notably, this flap can also serve as a rotational flap to cover the palmar ulnar MP joints (29). In addition, the position of the efferent deep fascia of the perforator branch emanating from the ulnar palmar artery is relatively constant. It penetrates between the superficial hypothenar muscles and the fifth metacarpal bone to the subcutaneous tissue and anastomoses with the small blood vessels emanating from the descending dorsal branch of the ulnar artery to form the vascular chain nutrient flap, which increases the possibility of the survival of the flap and enlarges the size of the flap. In cases where the longitudinal scar contracture in Dupuytren's contracture involves the skin of the fingers, flexion contracture is likely to occur. The flap is harvested from the skin on the lateral aspect of the ulnar palm, minimizing contact with the contracture tissue of the Dupuytren's contracture. This creates a transverse barrier across the palm, effectively reducing the recurrence rate (30). Given that the donor area of the flap was situated on the volar ulnar side, it is essential to pay careful attention to the palmar-dorsal junction, which is located away from the hypothenar region, during the flap removal process. This precaution is necessary to prevent complications such as grip discomfort or sensory loss following the operation (31). The flap used in this procedure provides skin that closely resembles the palm in texture, appearance, and feeling (32). It allows for a relatively long flap to be harvested compared to other flaps in the hand (33), but may not be suitable for large wounds (34). This flap is effective for repairing medium-sized defects, particularly when the flap width does not exceed 25 mm, allowing for direct closure of the donor site with minimal scarring (34). A previous study suggests that the choice of a non-fingertip defect reconstruction flap depends on the defect size, with small defects being less than 4 cm2, medium defects ranging between 4 and 8 cm2, and large defects usually exceeding 8 cm2 (35). The ulnar palmar flap can be used to cover the flexor tendons and neurovascular bundles, thus preventing flexor tendon adhesions and neurological impairment. However, caution must be taken to prevent venous congestion when using this flap (36). Post-surgery, flap monitoring, and adherence to rehabilitation nursing are recommended. Using a heat lamp may help to promote blood circulation in the flap and improve its survival rate. It is advised to engage in appropriate rehabilitation exercises with the aid of professional physical therapists.
The advantages of the flap include a reliable blood supply, matching skin texture, a thin flap, absence of swelling in the recipient area, esthetic appeal, and no adverse effects on hand grip function. In addition, the flap causes minimal collateral damage due to direct suturing in the donor area, resulting in only a slight linear scar on the wound surface. It is also easy to obtain and provides effective coverage for palmar defects without significant functional impairment or complications in the upper limb. However, it is not suitable for wounds on the middle finger or large-sized defects.
The limitations of this study include the following aspects. First, it employs a retrospective design with a relatively small sample size, which presents certain constraints. Second, the absence of a control group limits the ability to compare the advantages of this treatment with other methods through specific data analysis. Third, the follow-up period is short; further long-term follow-up is necessary to assess the effectiveness of this flap in treating skin defects caused by Dupuytren's contracture and to evaluate the incidence of long-term complications. Fourth, patient-reported evaluations may influence the actual outcomes. Finally, the patient population is limited in terms of ethnicity.
6 ConclusionThe perforator flap of the ulnar palm is particularly effective for skin and soft tissue defects on the ulnar side of the palm or the palmar side of the fifth digit, making it suitable for treating severe Dupuytren's contracture. Based on the discussion of the advantages of this flap and the limitations of this study, it is necessary to conduct a comparative study and long-term follow-up of this flap and other methods for the treatment of skin defects caused by surgical treatment of Dupuytren's contracture.
Data availability statementThe original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Ethics statementThe studies involving humans were approved by the Ethics Committee of the Second Hospital of Tangshan. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributionsJD: Writing – original draft. HuW: Writing – review & editing. HaW: Data curation, Writing – review & editing. XS: Methodology, Writing – review & editing. YZ: Supervision, Writing – review & editing.
FundingThe author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interestThe authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statementThe author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher's noteAll claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References2. Layton TB, Williams L, Nanchahal J. Dupuytren's disease: a localised and accessible human fibrotic disorder. Trends Mol Med. (2023) 29(3):218–27. doi: 10.1016/j.molmed.2022.12.001
PubMed Abstract | Crossref Full Text | Google Scholar
4. Murphy A, Lalonde DH, Eaton C, Denkler K, Hovius SER, Smith AA, et al. Minimally invasive options in Dupuytren’s contracture: aponeurotomy, enzymes, stretching, and fat grafting. Plast Reconstr Surg. (2014) 134(5):822e–9e. doi: 10.1097/PRS.0000000000000603
PubMed Abstract | Crossref Full Text | Google Scholar
5. Facchin F, Sonda R, Faccio D, Stecco C, Angelini A, Ruggieri P, et al. Multi-dorsal metacarpal artery perforator adipofascial turnover flap for index to little finger reconstruction: anatomical study and clinical application. Hand Surg Rehabil. (2021) 40(2):177–82. doi: 10.1016/j.hansur.2020.11.004
PubMed Abstract | Crossref Full Text | Google Scholar
6. Uchida R, Matsumura H, Imai R, Tanaka K, Watanabe K. Anatomical study of the perforators from the ulnar palmar digital artery of the little finger and clinical uses of digital artery perforator flaps. Scand J Plast Reconstr Surg Hand Surg. (2009) 43(2):90–3. doi: 10.1080/02844310802556976
PubMed Abstract | Crossref Full Text | Google Scholar
7. Yazdani A, Nasri P, Baradaran Mahdavi S. The effects of shock wave therapy on the symptoms and function of individuals with Dupuytren disease: a systematic review. Arch Phys Med Rehabil. (2024) 105(10):1985–92. doi: 10.1016/j.apmr.2024.05.030
PubMed Abstract | Crossref Full Text | Google Scholar
8. Chung KC, Pillsbury MS, Walters MR, Hayward RA. Reliability and validity testing of the Michigan hand outcomes questionnaire. J Hand Surg Am. (1998) 23(4):575–87. doi: 10.1016/S0363-5023(98)80042-7
PubMed Abstract | Crossref Full Text | Google Scholar
9. van Rijssen AL, Ter Linden H, Werker PMN. Five-year results of a randomized clinical trial on treatment in Dupuytren's disease: percutaneous needle fasciotomy versus limited fasciectomy. Plast Reconstr Surg. (2012) 129(2):469–77. doi: 10.1097/PRS.0b013e31823aea95
PubMed Abstract | Crossref Full Text | Google Scholar
10. Peimer CA, Blazar P, Coleman S, Kaplan FT, Smith T, Tursi JP, et al. Dupuytren contracture recurrence following treatment with collagenase clostridium histolyticum (CORDLESS study): 3-year data. J Hand Surg Am. (2013) 38(1):12–22. doi: 10.1016/j.jhsa.2012.09.028
PubMed Abstract | Crossref Full Text | Google Scholar
11. De Ketele A, Degreef I. Full-thickness skin grafting in preventing recurrence of Dupuytren’s disease: a systematic review. Hand Surg Rehabil. (2023) 42(4):273–83. doi: 10.1016/j.hansur.2023.06.002
PubMed Abstract | Crossref Full Text | Google Scholar
13. Goto A, Komura S, Kato K, Maki R, Hirakawa A, Tomita H, et al. C-X-C domain ligand 14-mediated stromal cell-macrophage interaction as a therapeutic target for hand dermal fibrosis. Commun Biol. (2023) 6(1):1173. doi: 10.1038/s42003-023-05558-8
PubMed Abstract | Crossref Full Text | Google Scholar
14. Strömberg J, Ibsen Sörensen A, Fridén J. Percutaneous needle fasciotomy versus collagenase treatment for Dupuytren contracture: a randomized controlled trial with a two-year follow-up. J Bone Joint Surg Am. (2018) 100(13):1079–86. doi: 10.2106/JBJS.17.01128
PubMed Abstract | Crossref Full Text | Google Scholar
15. Räisänen MP, Leppänen OV, Soikkeli J, Reito A, Malmivaara A, Buchbinder R, et al. Surgery, needle fasciotomy, or collagenase injection for Dupuytren contracture: a randomized controlled trial. Ann Intern Med. (2024) 177(3):280–90. doi: 10.7326/M23-1485
Crossref Full Text | Google Scholar
16. Kan HJ, Selles RW, van Nieuwenhoven CA, Zhou C, Khouri RK, Hovius SER. Reply: percutaneous aponeurotomy and lipofilling (PALF) versus limited fasciectomy in patients with primary Dupuytren's contracture: a prospective, randomized, controlled trial. Plast Reconstr Surg. (2017) 140(2):358e–9e. doi: 10.1097/PRS.0000000000003537
PubMed Abstract | Crossref Full Text | Google Scholar
17. Gardeil L, Matter Parrat V, Portenard AC, Coquerel D, Bonmarchand A, Auquit Auckbur I. Segmental aponeurectomy with Z-plasty as a treatment option in Dupuytren’s disease: a retrospective cohort study. Orthop Traumatol Surg Res. (2019) 105(8):1627–31. doi: 10.1016/j.otsr.2019.08.016
PubMed Abstract | Crossref Full Text | Google Scholar
18. Lukas B, Lukas M. Lappenplastiken bei fortgeschrittenem Morbus Dupuytren [flap plasty in advanced Dupuytren’s disease]. Oper Orthop Traumatol. (2016) 28(1):20–9. German. doi: 10.1007/s00064-015-0435-3
PubMed Abstract | Crossref Full Text | Google Scholar
19. Yamaguchi K, Kaji Y, Nakamura O, Tobiume S, Nomura Y, Oka K, et al. Skin extension with a digito-lateral flap and early active finger extension training for Dupuytren contracture: a retrospective study. Medicine (Baltimore). (2022) 101(33):e30107. doi: 10.1097/MD.0000000000030130
PubMed Abstract | Crossref Full Text | Google Scholar
20. Ozdemir O, Coskunol E, Ozalp T. An alternative approach in the treatment of Dupuytren’s contracture skin defects: first dorsal metacarpal artery island flap. Tech Hand Up Extrem Surg. (2004) 8(1):16–20. doi: 10.1097/00130911-200403000-00005
PubMed Abstract | Crossref Full Text | Google Scholar
21. Brammer AJ, Sutinen P, Das S, Pyykkö I, Toppila E, Starck J. Quantitative test for sensory hand symptoms based on mechanoreceptor-specific vibrotactile thresholds. J Acoust Soc Am. (2010) 127(2):1146–55. doi: 10.1121/1.3270395
PubMed Abstract | Crossref Full Text | Google Scholar
23. Hao PD, Zhuang YH, Zheng HP, Yang XD, Lin J, Zhang CL, et al. The ulnar palmar perforator flap: anatomical study and clinical application. J Plast Reconstr Aesthet Surg. (2014) 67(5):600–6. doi: 10.1016/j.bjps.2013.12.048
PubMed Abstract | Crossref Full Text | Google Scholar
27. Ali SN, McMurtrie A, Rayatt S, Roberts JO. Ulnar-based skin flap for Dupuytren’s fasciectomy. Scand J Plast Reconstr Surg Hand Surg. (2006) 40(5):307–10. doi: 10.1080/02844310600836794
PubMed Abstract | Crossref Full Text | Google Scholar
28. Bakhach J, Saint Cast Y, Gazarian A, Martin D, Comtet JJ, Baudet J. Le lambeau paramétacarpien ulnaire. Etude anatomique et application clinique [Ulnar parametacarpal flap. Anatomical study and clinical application]. Ann Chir Plast Esthet. (1995) 40(2):136–47.7574391
PubMed Abstract | Google Scholar
29. Sugiyama Y, Naito K, Igeta Y, Kaneko K, Obayashi O. Ulnar para-metacarpal flap for recurrence of Dupuytren’s disease with skin ulcer: a case report. J Orthop Case Rep. (2014) 4(4):60–3. doi: 10.13107/jocr.2250-0685.229
PubMed Abstract | Crossref Full Text | Google Scholar
30. Anwar MU, Al Ghazal SK, Boome RS. The lateral digital flap for Dupuytren’s fasciectomy at the proximal interphalangeal joint–a study of 84 consecutive patients. J Hand Surg Eur Vol. (2009) 34(1):90–3. doi: 10.1177/1753193408093560
PubMed Abstract | Crossref Full Text | Google Scholar
31. Usami S, Inami K, Hirase Y, Mori H. An ulnar parametacarpal perforator flap for volar digital soft tissue reconstruction. J Hand Surg Eur Vol. (2020) 45(8):842–8. doi: 10.1177/1753193420939379
PubMed Abstract | Crossref Full Text | Google Scholar
32. Han HH, Choi YS, Kim IB, Kim SH, Jun YJ. A perforator from the ulnar artery and cutaneous nerve of the hypothenar area: an anatomical study for clinical application. Microsurgery. (2017) 37(1):49–56. doi: 10.1002/micr.22463
PubMed Abstract | Crossref Full Text | Google Scholar
33. Tapan M, İğde M, Yıldırım AR, Balı YY, Yılancı S, Ünlü RE. Hypothenar island flap: a safe and excellent choice for little finger defects. Indian J Plast Surg. (2015) 48(3):288–92. doi: 10.4103/0970-0358.173127
PubMed Abstract | Crossref Full Text | Google Scholar
34. Seo BF, Kwon BY, Han HH. Sensate hypothenar perforator free flap for resurfacing large volar surface defect of the digit. Microsurgery. (2018) 38(4):419–22. doi: 10.1002/micr.30102
留言 (0)