Peripheral Artery Disease (PAD) typically refers to a group of conditions characterized by arterial narrowing or occlusion in the lower extremities, excluding the coronary arteries, aorta, and intracranial segments (Bevan and White Solaru, 2020; Mehta et al., 2021; Tran, 2021). The diagnosis of PAD can be made by measuring the blood pressure in the posterior tibial artery and the brachial artery, which involves calculating the Ankle-Brachial Index (ABI), defined as the systolic pressure in the posterior tibial artery divided by the systolic pressure in the brachial artery (Makowsky et al., 2008; Conen et al., 2009; Barnes et al., 2020). PAD is diagnosed when the ABI is less than 0.9 (Hicks et al., 2023; Mandaglio-Collados et al., 2023). It is reported that over 200 million people worldwide suffer from PAD, with the prevalence increasing with age and affecting a significant proportion of the elderly population (Shu and Santulli, 2018; Mastracci et al., 2022). Individuals with PAD mainly exhibit symptoms such as intermittent claudication, rest pain, abnormal skin temperature, discoloration, and sensory abnormalities, with severe cases potentially leading to non-traumatic amputations and even death (Geiss et al., 2019; Mahe et al., 2022). This not only impacts the health of the elderly but also increases the disability rate in the population, imposing a substantial economic burden on families and society (Matsushita et al., 2022). Therefore, PAD is not just a health issue; it also involves public health challenges related to family happiness, economic prosperity, and social harmony. The etiology of PAD is diverse, including hypertension (Agnelli et al., 2020), diabetes (Krzyzanowska et al., 2006), hyperlipidemia (Selvaggio et al., 2020), obesity, inflammation (Chan et al., 2022), and lifestyle factors (Criqui et al., 2021) Among these, inflammation is a particularly intriguing area of research with implications for targeted treatment (Brevetti et al., 2010; Alnima et al., 2023).
HRR is recognized as an inflammatory marker associated with the incidence and adverse events of various diseases (Qu et al., 2021), such as diabetes (Wang et al., 2020; Xiu et al., 2022), non-small cell lung cancer (Chen et al., 2020), and atrial fibrillation (Wang et al., 2022). HRR is primarily composed of two hematological indicators: hemoglobin (HB) and red blood cell distribution width (RDW) (Zhao et al., 2022; Song et al., 2023). HB mainly reflects the degree of anemia (Gell, 2018), while RDW indicates the heterogeneity in red blood cell volume and is indirectly used for diagnosing anemia (Fava et al., 2019; Yang et al., 2023). Previous studies (Li et al., 2021; Guaní-Guerra et al., 2022) have shown that HRR undergoes dynamic changes in response to disease stimuli, and its level variations can serve as a sensitive measure of inflammation (Nan et al., 2024). Although HRR, as an inflammatory marker, is related to numerous diseases, the clinical relationship between HRR and PAD remains unclear. PAD is a disease associated with inflammation and immunity, while HRR is an indicator that reflects the levels of inflammation and immune metabolism in the body. Therefore, exploring the role of HRR in PAD is of great importance.
Given that PAD is a condition that may be associated with inflammation, there might be a unique link between HRR and PAD. However, the relationship between HRR and PAD is not clear in current research. Therefore, in this study, we extracted data on HRR and PAD from the National Health and Nutrition Examination Survey (NHANES) between 1999 and 2004, attempting to explore whether there is a specific correlation between HRR and the prevalence of PAD.
Materials and methodsStudy population and study designThe NHANES is a cross-sectional, nationally representative survey of the non-institutionalized civilian population in the United States (US). It includes interviews and standardized physical examinations, with a focus on collecting data through anthropometric measurements, blood samples, and self-report questionnaires. Additionally, at the outset of the NHANES, informed consent was obtained from each participant. This obviates the need for further provision of ethical clearance for this study. Figure 1 provides a detailed depiction of the participant selection process for this study (Figure 1). Initially, we extracted demographic, laboratory, physical examination, and questionnaire data from a total of 31,126 participants in the NHANES spanning from 1999 to 2004. Subsequently, since NHANES only included PAD-related examinations for individuals aged 40 and above, the first step was to exclude those lacking PAD-related information, resulting in the exclusion of 21,156 participants. Next, we removed 2,032 participants due to missing data required for the calculation of the HRR. To further enhance the credibility of the study, we proceeded to screen for potential confounding factors, leading to the exclusion of an additional 2,742 participants who had missing information regarding these factors. Specifically, 736 individuals were missing income level Poverty Impact Ratio (PIR) data, 301 lacked hypertension-related data, 85 had unknown education levels, 221 were without low-density lipoprotein (LDL) data, 384 did not provide marital status information, 186 had not had their systolic blood pressure measured, 238 lacked glycated hemoglobin data, 417 were missing fasting glucose data, and another 174 had no diagnosed diabetes-related information. After these exclusions, a final total of 5,196 participants were included in the study.

Figure 1. Research flowchart.
Measurement of ABI and PADLeveraging the examination data component of NHANES, one of its four main components, we assessed the ankle brachial blood pressure index (ABI) as a measure for evaluating lower extremity disease. Specifically, we measured the systolic blood pressure of the right brachial artery and the posterior tibial artery bilaterally at the ankle joints of the participants. In cases where participants’ right arms were not accessible for measurement, the left brachial artery systolic blood pressure was used as a substitute for the right brachial artery systolic blood pressure. Given that NHANES collected this data with varying measurement frequencies for different age groups, the calculation of ABI was stratified by age. 1) For individuals between the ages of 40–59, the ABI was calculated by dividing the average of two measurements of the posterior tibial artery systolic blood pressure bilaterally by the average of two brachial artery systolic blood pressure measurements. 2) For those aged 60 and above, who had only one measurement of the brachial artery systolic blood pressure, the ABI was determined by dividing the average of two measurements of the posterior tibial artery systolic blood pressure bilaterally by a single brachial artery systolic blood pressure measurement. According to previous studies, participants with an ABI less than 0.9 were defined as having PAD.
Definition of the HRRThe hemoglobin-to-red blood cell distribution width ratio (HRR) is calculated by dividing hemoglobin (HB) levels by the red blood cell distribution width (RDW), utilizing laboratory data from the NHANES. For subsequent analysis, HRR is stratified into quartiles.
CovariatesBuilding on previous related research, this study included waist circumference (WC), high-density lipoprotein (HDL), triglycerides (TG), low-density lipoprotein (LDL), total cholesterol (TC), age, poverty-income ratio (PIR), body mass index (BMI), systolic blood pressure (SBP), glycated hemoglobin (HbA1C), fasting plasma glucose (FPG), gender, marital status, race, education level, hypertension, and diabetes as covariates.
BMI is calculated from anthropometric data, specifically height (HT in meters, m) and weight (WT in kilograms, kg), using the formula BMI = WT/(HT) ^ 2.
Marital status is categorized into three main groups: 1) Married/cohabiting, 2) Widow/widower/separated, and 3) Unmarried.
Race is classified into five categories based on NHANES survey results: Mexican, Hispanic, White, Black, and Other Race.
Education level is primarily divided into three categories: Under high school, High school, and Over high school.
Hypertension is defined by three main criteria: 1) Systolic blood pressure of 140 mmHg or higher in anthropometric data, 2) Diastolic blood pressure of 90 mmHg or higher in anthropometric data, and 3) Participants who have been told by a doctor that they have hypertension, as reported in the blood pressure questionnaire. Meeting any one of these criteria is sufficient to be defined as having hypertension.
Diabetes is defined by four criteria: 1) FPG of 7 mmol/L or higher, 2) HbA1C of 6.5% or higher, 3) Participants who have been told by a doctor that they have diabetes, as reported in the questionnaire, and 4) Participants who have taken hypoglycemic medications, as reported in the questionnaire. Meeting any one of these criteria is sufficient to be defined as having diabetes.
Statistical analysisIn this study, we conducted a thorough processing and quality control of the data. Given the non-normal distribution of the data, continuous variables are expressed as medians with interquartile ranges (Q1, Q3), while categorical variables are presented as counts and percentages. Comparisons between the PAD and non-PAD groups were made using the Kruskal-Wallis test for continuous variables and the chi-squared test for categorical variables. A p-value less than 0.05 is considered to indicate a statistically significant difference.
Utilizing logistic regression analysis appropriate for the survey design, we explored the relationship between HRR and PAD. The robustness of the model was incrementally enhanced by adjusting for different covariates. Specifically, Model 1 is the most basic model, with no adjustments for any variables. Model 2 is adjusted for race, age, and gender. Finally, Model 3 includes adjustments for potential confounding factors such as gender, age, PIR, education level, race, BMI, HDL, TG, LDL, TC, SBP, HbA1C, FPG, WC, hypertension, and diabetes to further strengthen the robustness of the model.
ResultsDemographic and initial characteristics of participantsThe study selected a cohort of 5,196 adult Americans from the NHANES database spanning from 1999 to 2004, with a higher number of males, totaling 2,684 individuals, accounting for approximately 51.66% of the study population. Within this cohort, 316 individuals were diagnosed with PAD, representing 6.08% of the sample size. The majority of participants were identified as non-Hispanic whites, with 2,890 individuals making up 55.62% of the total study cohort. Table 1 summarizes the demographic and baseline characteristics of the study subjects, stratified by HRR quartiles (Table 1). Individuals with higher HRR tended to be younger (P < 0.001), have a higher proportion of males (P < 0.001), and were free from hypertension (P < 0.001). Additionally, this group was likely to exhibit lower HDL levels (P < 0.001) and higher TG levels in their blood biochemistry (P < 0.001). However, there were no significant differences in fasting plasma glucose (FPG) (P = 0.223) and diabetes (P = 0.163) across HRR quartiles.
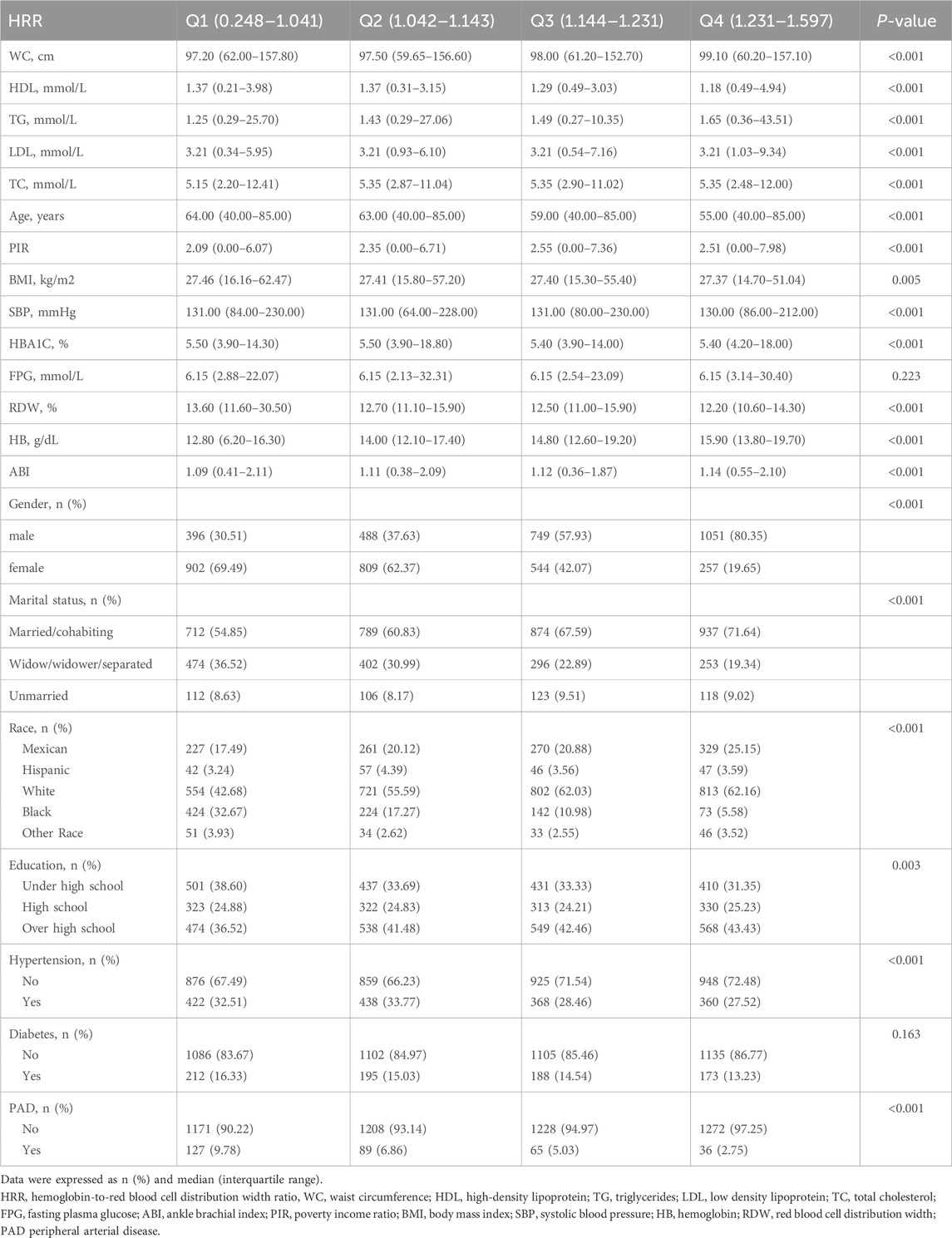
Table 1. Characteristics of the participants categorized by HRR.
Univariate logistic analyses revealed the associations between multiple variables with PADAs shown in Table 2, in the univariate analysis, HB (OR = 0.84, 95%CI: 0.78–0.90), and HRR (OR = 0.08, 95%CI: 0.04–0.15) were negatively associated with PAD (Table 2).
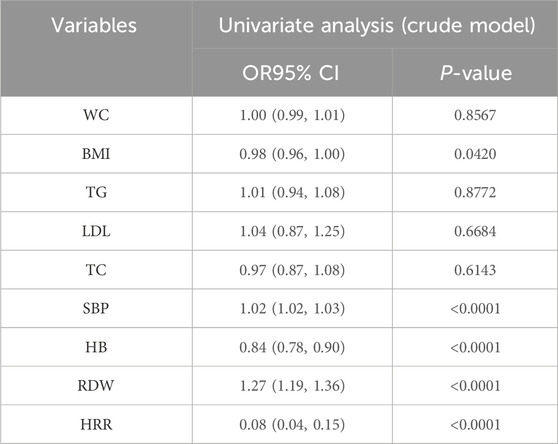
Table 2. Weighted univariate logistic analyses between Variables and PAD (odds ratios, 95% confidence intervals).
Receiver operating characteristic (ROC) analysis revealed HRR shows relatively better predictive performanceTo further elucidate the impact of the aforementioned factors on PAD, we conducted a ROC curve analysis. The ROC curves indicate that compared to HB (AUC = 0.574), BMI (AUC = 0.528), and TC (AUC = 0.511), HRR shows relatively better predictive performance. (AUC = 0.633) (Figure 2).
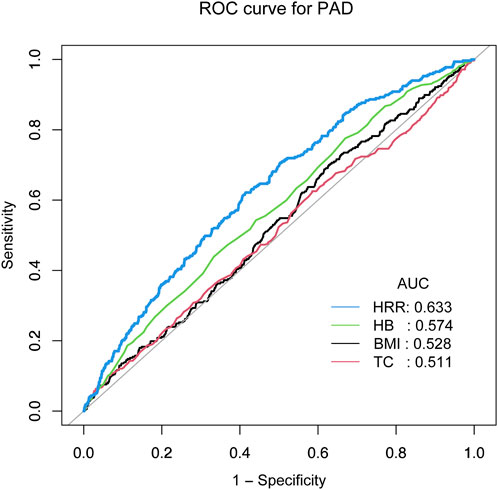
Figure 2. ROC analysis revealed HRR shows relatively better predictive performance.
The relationship between PAD and HRR quartilesReferencing Table 3, elevated HRR quartiles are linked to a decreased risk of PAD (Table 3). The unadjusted model reveals an inverse relationship between HRR quartiles and PAD prevalence (Model 1). This correlation retains its significance following partial adjustments in Model 2. With a comprehensive consideration of all potential confounding factors, the OR for PAD in relation to the second (Q2), third (Q3), and fourth (Q4) quartiles compared to the reference quartile (Q1) are approximately 0.71 (95% CI: 0.59–0.86), 0.62 (95% CI: 0.53–0.73), and 0.44 (95% CI: 0.29–0.68), respectively, for Model 3 (P < 0.001). Additionally, After adjusting for factors such as gender, age, PIR, education level, race, BMI, HDL, TG, LDL, TC, SBP, HbA1C, FPG, WC, hypertension, and diabetes, an increase of one unit in HRR corresponds to a 56% reduction in the probability of PAD, as demonstrated by the OR.

Table 3. Multivariate Cox regression analysis of HRR with PAD.
Utilizing smooth curve fitting and threshold effect analysis, we assessed the potential linear correlation between HRR and the risk of PAD. As shown in Figure 3, the findings indicated a substantial linear association between these variables (Figure 3). Table 4 details the outcomes from a standard linear regression model, revealing an 80% reduction in PAD risk for each one-unit increment in HRR (P < 0.0001). Two-piecewise linear regression models identified a critical point at an HRR value of 1.12. For HRR values at or below 1.12, an increment of one unit in HRR is associated with a 75% decrease in PAD odds (OR = 0.25, 95%CI = 0.10–0.64; P < 0.0036). Beyond an HRR of 1.12, an additional unit increase in HRR is linked to a 96% decrease in PAD odds (OR = 0.04, 95%CI = 0.00–1.54; P = 0.845), and the log-likelihood ratio test yields a P value of 0.364. Consequently, when synthesizing these findings, a definite linear relationship is observed between HRR and PAD risk.
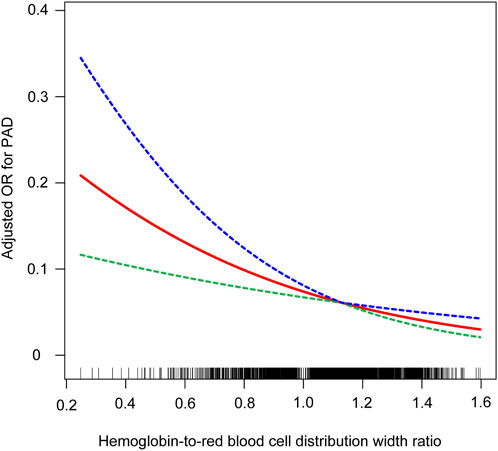
Figure 3. Relationship between HRR and PAD. the blue line represents the upper limit of the 95% confidence interval for the adjusted OR values, the green line represents the lower limit of the 95% confidence interval for the adjusted OR values, and the red line represents the adjusted OR values themselves.
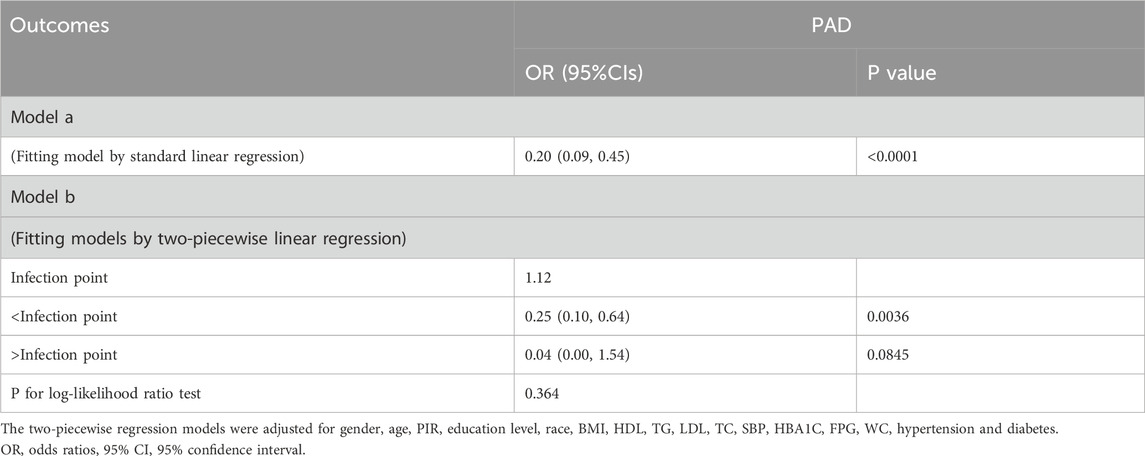
Table 4. Threshold and saturation effect analysis of HRR on PAD.
Stratified analysisAs indicated in Table 5, we conducted a series of stratified analyses to assess the robustness of the association between HRR and PAD and to uncover any potential differences among various demographic subgroups (Table 5). In the majority of these stratified subgroups, HRR emerged as a significant protective factor for PAD. Notably, as mentioned in Table 1, age has a substantial impact on PAD, and younger individuals tend to have higher HDL levels, which becomes evident in the stratified analysis. That is, the younger the population, the stronger the influence of HRR on PAD (P = 0.0002). Furthermore, regarding BMI, lower BMI values correspond to a reduced risk of PAD (P < 0.0001). These findings suggest that individuals who are obese, older, and have lower HRR values may be more susceptible to PAD.
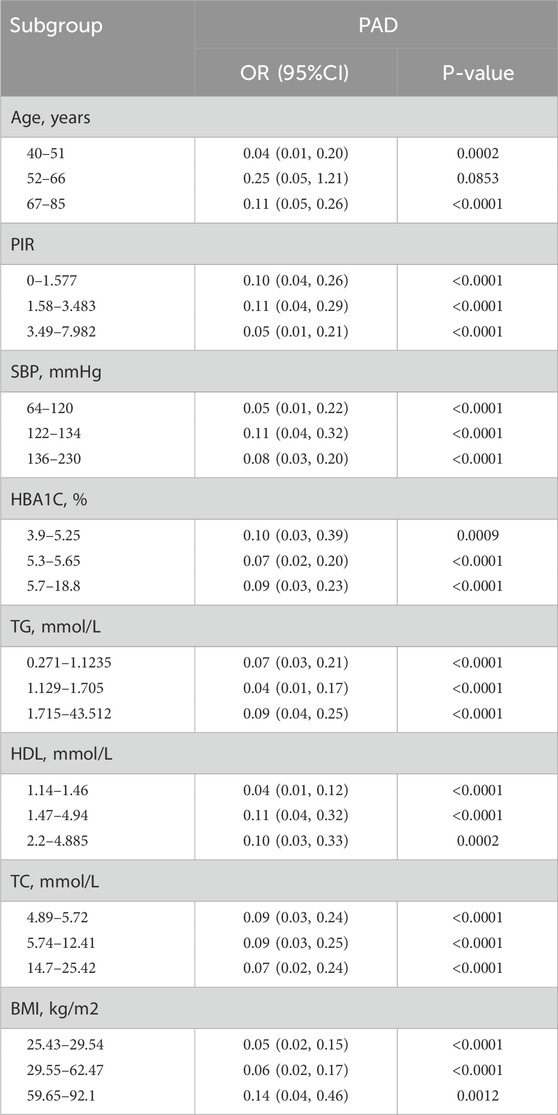
Table 5. Stratified analysis of the correlation between HRR and PAD in adults in the NHANES 1999–2004.
DiscussionPAD is a syndrome of peripheral circulatory dysfunction, with the broad sense of peripheral arterial disease referring to atherosclerotic changes caused by large arteries other than the coronary arteries and aorta, while the narrow sense refers specifically to lower extremity arterial diseases. Among the many etiologies of PAD, inflammation plays an extremely important role. By exploring data from the NHANES between 1999 and 2004, our study reveals an approximate linear association between HRR and PAD. This indicates a significant negative correlation between HRR and the likelihood of PAD.
Inflammation is a key element in the pathophysiology of PAD (Fort-Gallifa et al., 2017), a condition associated with inflammation (Tantry et al., 2020). RDW (Hu et al., 2020) and HRR (Liu and Wang, 2023), both implicated in inflammation, are considered novel inflammatory biomarkers (Xi et al., 2024). Previous studies have shown RDW to be useful in predicting mortality rates among PAD patients. In a study examining 1,039 consecutive outpatients from January 1997 to December 2007, with follow-up through September 2009, the cohort had an average age of 69.5 ± 12.0 years, with approximately 60.9% males and 97.6% whites. After adjusting for age, gender, cardiovascular risk factors, and comorbidities, patients in the highest RDW quartile (>14.5%) had a 66% higher risk of death compared to those in the lowest quartile (<12.8%, p < 0.0001); a 1% increase in RDW was associated with a 10% increase in the risk of all-cause mortality (hazard ratio 1.10, 95% confidence interval 1.08 to 1.12, p < 0.0001). RDW was ultimately found to be an independently available prognostic indicator for PAD patients (Ye et al., 2011). Zalawadiya et al., studying 6,950 participants in the NHANES database from 1999 to 2004, found that higher levels of RDW were independently associated with a higher risk of PAD and significantly improved risk prediction beyond the estimates of the American College of Cardiology’s PAD screening criteria (Zalawadiya et al., 2012). Studies on HRR have yielded different conclusions. A study including 3,745 adult COPD patients from the NHANES database between 1999 and 2018 explored the association between RDW and HRR levels and mortality. After adjusting for various potential confounders, higher RDW levels were positively associated with all-cause mortality risk (HR = 1.16, 95% CI = 1.11–1.21, P < 0.001) and cardiovascular disease (CVD) mortality risk (HR = 1.13, 95% CI = 1.06–1.21, P < 0.001). HRR was negatively associated with all-cause mortality risk (HR = 0.14, 95% CI = 0.08–0.25, P < 0.001) and CVD mortality risk (HR = 0.12, 95% CI = 0.05–0.31, P < 0.001), concluding that higher RDW levels in COPD patients were positively correlated with increased mortality risk, while HRR levels were negatively correlated with all-cause and CVD mortality risks (Liu et al., 2024). Bozkaya et al., in a retrospective screening of nasopharyngeal carcinoma (LANC) patients treated with chemotherapy and radiotherapy between October 2010 and June 2020, found that in the low HRR group, overall survival (OS) and disease-free survival (DFS) were 44.4 months (95% CI: 4.9–83.8) and 15.7 months (95% CI: 0.1–36.2), respectively, but not attainable in the high HRR group (p < 0.001). They concluded that HRR is an independent prognostic marker for OS and DFS in LANC patients treated with chemotherapy and radiotherapy (Bozkaya et al., 2023). Studies have been conducted on the association between red blood cell distribution width (RDW) and the ratio of RDW to platelet count (RPR) with cardiovascular disease (CVD), and it has been found that the association between RDW and the prevalence of CVD is more pronounced in women and smokers (all interaction p-values <0.05). It was concluded that there is statistical heterogeneity in the association between RDW, RPR distribution, and the prevalence of CVD across gender, smoking status, and age groups (Veeranna et al., 2013; Shah et al., 2017; Ainiwaer et al., 2023). Therefore, HRR may play different roles in various diseases. This study addresses this controversial point by exploring the potential link between HRR and PAD using a large dataset from NHANES. Notably, this study found that high levels of HRR are a protective factor for PAD, with each unit increase in HRR reducing the probability of PAD by 56%. The results differ from those of Bozkaya et al., possibly because their study focused on oncology patients who underwent chemotherapy and radiotherapy, leading to varying degrees of change in hemoglobin levels. This is one possible explanation, and further research is needed to confirm it.
Under rigorous inclusion criteria, this study carefully selected study indicators and specific populations from the reputable NHANES database to ensure a certain level of persuasiveness in the results. We made every effort to adjust for a range of covariates to further ensure the reliability of the findings. However, it must be acknowledged that there are several limitations. The study is cross-sectional, meaning it can only explore potential associations between HRR and PAD, not causal relationships. Additionally, while this study controlled for multiple covariates, there may be other confounding factors that could affect the relationship between HRR and PAD, and it was not possible to include all potential confounders in this study. Despite the aforementioned limitations, this study is novel in being the first to explore the possible role of HRR in PAD and finds that as HRR levels increase, the risk of PAD decreases. This provides a foundation and new insights for future research.
ConclusionThis study reveals a robust inverse relationship between the HRR and PAD, highlighting HRR as a significant protective factor against PAD. The findings underscore the importance of HRR in PAD risk assessment, particularly among demographic subgroups, and contribute to a deeper understanding of the role of inflammation in PAD pathogenesis.
Data availability statementPublicly available datasets were analyzed in this study. This data can be found here: 1999–2000: https://wwwn.cdc.gov/nchs/nhanes/ContinuousNhanes/Default.aspx?BeginYear=1999 2001–2002: https://wwwn.cdc.gov/nchs/nhanes/ContinuousNhanes/Default.aspx?BeginYear=2001 2003–2004: https://wwwn.cdc.gov/nchs/nhanes/ContinuousNhanes/Default.aspx?BeginYear=2003.
Author contributionsZY: Data curation, Formal Analysis, Methodology, Resources, Writing–original draft, Writing–review and editing. BL: Conceptualization, Formal Analysis, Methodology, Validation, Writing–review and editing. RH: Formal Analysis, Investigation, Software, Writing–original draft. CT: Data curation, Investigation, Project administration, Resources, Visualization, Writing–original draft, Writing–review and editing.
FundingThe author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interestThe authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statementThe author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s noteAll claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
ReferencesAgnelli, G., Belch, J. J. F., Baumgartner, I., Giovas, P., and Hoffmann, U. (2020). Morbidity and mortality associated with atherosclerotic peripheral artery disease: a systematic review. Atherosclerosis 293, 94–100. doi:10.1016/j.atherosclerosis.2019.09.012
PubMed Abstract | CrossRef Full Text | Google Scholar
Ainiwaer, A., Kadier, K., Abulizi, A., Hou, W. Q., Rehemuding, R., Maimaiti, H., et al. (2023). Association of red cell distribution width (RDW) and the RDW to platelet count ratio with cardiovascular disease among US adults: a cross-sectional study based on the National Health and Nutrition Examination Survey 1999-2020. BMJ Open 13 (3), e068148. doi:10.1136/bmjopen-2022-068148
PubMed Abstract | CrossRef Full Text | Google Scholar
Alnima, T., Meijer, R. I., Spronk, H. M. H., Warlé, M., and Cate, H. T. (2023). Diabetes-versus smoking-related thrombo-inflammation in peripheral artery disease. Cardiovasc Diabetol. 22 (1), 257. doi:10.1186/s12933-023-01990-6
PubMed Abstract | CrossRef Full Text | Google Scholar
Barnes, J. A., Eid, M. A., Creager, M. A., and Goodney, P. P. (2020). Epidemiology and risk of amputation in patients with diabetes mellitus and peripheral artery disease. Arterioscler. Thromb. Vasc. Biol. 40 (8), 1808–1817. doi:10.1161/ATVBAHA.120.314595
PubMed Abstract | CrossRef Full Text | Google Scholar
Bevan, G. H., and White Solaru, K. T. (2020). Evidence-based medical management of peripheral artery disease. Arterioscler. Thromb. Vasc. Biol. 40 (3), 541–553. doi:10.1161/ATVBAHA.119.312142
PubMed Abstract | CrossRef Full Text | Google Scholar
Bozkaya, Y., Dilber, M., Bilgili, A. M., and Aktaş, C. (2023). A new prognostic parameter associated with recurrence in patients with nasopharyngeal cancer treated with chemoradiotherapy: the ratio of the hemoglobin-to-red cell distribution width. Cureus 15 (6), e39907. doi:10.7759/cureus.39907
PubMed Abstract | CrossRef Full Text | Google Scholar
Brevetti, G., Giugliano, G., Brevetti, L., and Hiatt, W. R. (2010). Inflammation in peripheral artery disease. Circulation 122 (18), 1862–1875. doi:10.1161/CIRCULATIONAHA.109.918417
PubMed Abstract | CrossRef Full Text | Google Scholar
Chan, N. C., Xu, K., de Vries, T. A. C., Eikelboom, J. W., and Hirsh, J. (2022). Inflammation as a mechanism and therapeutic target in peripheral artery disease. Can. J. Cardiol. 38 (5), 588–600. doi:10.1016/j.cjca.2022.01.026
PubMed Abstract | CrossRef Full Text | Google Scholar
Chen, J. L., Wu, J. N., Lv, X. D., Yang, Q. C., Chen, J. R., and Zhang, D. M. (2020). The value of red blood cell distribution width, neutrophil-to-lymphocyte ratio, and hemoglobin-to-red blood cell distribution width ratio in the progression of non-small cell lung cancer. PLoS One 15 (8), e0237947. doi:10.1371/journal.pone.0237947
PubMed Abstract | CrossRef Full Text | Google Scholar
Conen, D., Rexrode, K. M., Creager, M. A., Ridker, P. M., and Pradhan, A. D. (2009). Metabolic syndrome, inflammation, and risk of symptomatic peripheral artery disease in women: a prospective study. Circulation 120 (12), 1041–1047. doi:10.1161/CIRCULATIONAHA.109.863092
PubMed Abstract | CrossRef Full Text | Google Scholar
Criqui, M. H., Matsushita, K., Aboyans, V., Hess, C. N., Hicks, C. W., Kwan, T. W., et al. (2021). Lower extremity peripheral artery disease: contemporary epidemiology, management gaps, and future directions: a scientific statement from the American heart association. Circulation 144 (9), e171–e191. doi:10.1161/CIR.0000000000001005
PubMed Abstract | CrossRef Full Text | Google Scholar
Fava, C., Cattazzo, F., Hu, Z. D., Lippi, G., and Montagnana, M. (2019). The role of red blood cell distribution width (RDW) in cardiovascular risk assessment: useful or hype? Ann. Transl. Med. 7 (20), 581. doi:10.21037/atm.2019.09.58
PubMed Abstract | CrossRef Full Text | Google Scholar
Fort-Gallifa, I., Hernández-Aguilera, A., García-Heredia, A., Cabré, N., Luciano-Mateo, F., Simó, J. M., et al. (2017). Galectin-3 in peripheral artery disease. Relationships with markers of oxidative stress and inflammation. Int. J. Mol. Sci. 18 (5), 973. doi:10.3390/ijms18050973
PubMed Abstract | CrossRef Full Text | Google Scholar
Geiss, L. S., Li, Y., Hora, I., Albright, A., Rolka, D., and Gregg, E. W. (2019). Resurgence of diabetes-related nontraumatic lower-extremity amputation in the young and middle-aged adult U.S. Population. Diabetes Care 42 (1), 50–54. doi:10.2337/dc18-1380
PubMed Abstract | CrossRef Full Text | Google Scholar
Guaní-Guerra, E., Torres-Murillo, B., Muñoz-Corona, C., Rodríguez-Jiménez, J. C., Macías, A. E., Scavo-Montes, D. A., et al. (2022). Diagnostic accuracy of the RDW for predicting death in COVID-19. Med. Kaunas. 58 (5), 613. doi:10.3390/medicina58050613
PubMed Abstract | CrossRef Full Text | Google Scholar
Hicks, C. W., Wang, D., McDermott, K., Matsushita, K., Tang, O., Echouffo-Tcheugui, J. B., et al. (2023). Associations of cardiac biomarkers with peripheral artery disease and peripheral neuropathy in US adults without prevalent cardiovascular disease. Arterioscler. Thromb. Vasc. Biol. 43 (8), 1583–1591. doi:10.1161/ATVBAHA.122.318774
PubMed Abstract | CrossRef Full Text | Google Scholar
Hu, Z. D., Lippi, G., and Montagnana, M. (2020). Diagnostic and prognostic value of red blood cell distribution width in sepsis: a narrative review. Clin. Biochem. 77, 1–6. doi:10.1016/j.clinbiochem.2020.01.001
PubMed Abstract | CrossRef Full Text | Google Scholar
Krzyzanowska, K., Mittermayer, F., Krugluger, W., Schnack, C., Hofer, M., Wolzt, M., et al. (2006). Asymmetric dimethylarginine is associated with macrovascular disease and total homocysteine in patients with type 2 diabetes. Atherosclerosis 189 (1), 236–240. doi:10.1016/j.atherosclerosis.2005.12.007
PubMed Abstract | CrossRef Full Text | Google Scholar
Li, X., Chen, Q., Bi, X., Zhao, J., Li, Z., Zhou, J., et al. (2021). Preoperatively elevated RDW-SD and RDW-CV predict favorable survival in intrahepatic cholangiocarcinoma patients after curative resection. BMC Surg. 21 (1), 105. doi:10.1186/s12893-021-01094-6
PubMed Abstract | CrossRef Full Text | Google Scholar
Liu, J., and Wang, J. (2023). Association between hemoglobin-to-red blood cell distribution width ratio and hospital mortality in patients with non-traumatic subarachnoid hemorrhage. Front. Neurol. 14, 1180912. doi:10.3389/fneur.2023.1180912
PubMed Abstract | CrossRef Full Text | Google Scholar
Liu, S., Zhang, H., Zhu, P., Chen, S., and Lan, Z. (2024). Predictive role of red blood cell distribution width and hemoglobin-to-red blood cell distribution width ratio for mortality in patients with COPD: evidence from NHANES 1999-2018. BMC Pulm. Med. 24 (1), 413. doi:10.1186/s12890-024-03229-w
PubMed Abstract | CrossRef Full Text | Google Scholar
Mahe, G., Brodmann, M., Capodanno, D., Ceriello, A., Cuisset, T., Delgado, V., et al. (2022). Current management and screening of peripheral and coronary artery disease in people with diabetes mellitus in Europe. The PADDIA/CADDIA survey. Diabetes Res. Clin. Pract. 184, 109214. doi:10.1016/j.diabres.2022.109214
PubMed Abstract | CrossRef Full Text | Google Scholar
Makowsky, M. J., McAlister, F. A., Galbraith, P. D., Southern, D. A., Ghali, W. A., Knudtson, M. L., et al. (2008). Lower extremity peripheral arterial disease in individuals with coronary artery disease: prognostic importance, care gaps, and impact of therapy. Am. Heart J. 155 (2), 348–355. doi:10.1016/j.ahj.2007.09.005
PubMed Abstract | CrossRef Full Text | Google Scholar
Mandaglio-Collados, D., Marín, F., and Rivera-Caravaca, J. M. (2023). Peripheral artery disease: update on etiology, pathophysiology, diagnosis and treatment. Med. Clin. Barc. 161 (8), 344–350. doi:10.1016/j.medcli.2023.06.005
PubMed Abstract | CrossRef Full Text | Google Scholar
Mastracci, T. M., Anand, S. S., and Aday, A. W. (2022). Peripheral artery disease: a high-risk yet understudied, underdiagnosed, and undertreated condition-A call to action. Can. J. Cardiol. 38 (5), 553–554. doi:10.1016/j.cjca.2022.03.016
PubMed Abstract | CrossRef Full Text | Google Scholar
Matsushita, K., Gao, Y., Sang, Y., Ballew, S. H., Salameh, M., Allison, M., et al. (2022). Comparative mortality according to peripheral artery disease and coronary heart disease/stroke in the United States. Atherosclerosis 354, 57–62. doi:10.1016/j.atherosclerosis.2022.04.029
PubMed Abstract | CrossRef Full Text | Google Scholar
Mehta, A., Dhindsa, D. S., Hooda, A., Nayak, A., Massad, C. S., Rao, B., et al. (2021). Premature atherosclerotic peripheral artery disease: an underrecognized and undertreated disorder with a rising global prevalence. Trends Cardiovasc Med. 31 (6), 351–358. doi:10.1016/j.tcm.2020.06.005
PubMed Abstract | CrossRef Full Text | Google Scholar
Nan, W., Li, S., Wan, J., and Peng, Z. (2024). Association of mean RDW values and changes in RDW with in-hospital mortality in ventilator-associated pneumonia (VAP): evidence from MIMIC-IV database. Int. J. Lab. Hematol. 46 (1), 99–106. doi:10.1111/ijlh.14192
PubMed Abstract | CrossRef Full Text | Google Scholar
Qu, J., Zhou, T., Xue, M., Sun, H., Shen, Y., Chen, Y., et al. (2021). Correlation analysis of hemoglobin-to-red blood cell distribution width ratio and frailty in elderly patients with coronary heart disease. Front. Cardiovasc Med. 8, 728800. doi:10.3389/fcvm.2021.728800
PubMed Abstract | CrossRef Full Text | Google Scholar
Selvaggio, S., Abate, A., Brugaletta, G., Musso, C., Di Guardo, M., Di Guardo, C., et al. (2020). Platelet-to-lymphocyte ratio, neutrophil-to-lymphocyte ratio and monocyte-to-HDL cholesterol ratio as markers of peripheral artery disease in elderly patients. Int. J. Mol. Med. 46 (3), 1210–1216. doi:10.3892/ijmm.2020.4644
PubMed Abstract | CrossRef Full Text | Google Scholar
Shah, N., Pahuja, M., Pant, S., Handa, A., Agarwal, V., Patel, N., et al. (2017). Red cell distribution width and risk of cardiovascular mortality: insights from national health and nutrition examination survey (NHANES)-III. Int. J. Cardiol. 232, 105–110. doi:10.1016/j.ijcard.2017.01.045
PubMed Abstract | CrossRef Full Text | Google Scholar
Shu, J., and Santulli, G. (2018). Update on peripheral artery disease: epidemiology and evidence-based facts. Atherosclerosis 275, 379–381. doi:10.1016/j.atherosclerosis.2018.05.033
PubMed Abstract | CrossRef Full Text | Google Scholar
Song, J., Yu, T., Yan, Q., Zhang, Q., and Wang, L. (2023). Association of hemoglobin to red blood cell distribution width-standard deviation (RDW-SD) ratio and 3-month readmission in elderly Chinese patients with heart failure: a retrospective cohort study. Int. J. Gen. Med. 16, 303–315. doi:10.2147/IJGM.S396805
PubMed Abstract | CrossRef Full Text | Google Scholar
Tantry, U., Cummings, C., Mackrell, P., Gonze, M., Ulloa, K., Bafford, R., et al. (2020). Synergistic influence of rivaroxaban on inflammation and coagulation biomarkers in patients with coronary artery disease and peripheral artery disease on aspirin therapy. Future Cardiol. 16 (2), 69–75. doi:10.2217/fca-2019-0091
留言 (0)