Solid organ transplant recipients are at heightened risk of death from cancer. Indeed, the immunosuppressive treatment, which is paramount to avoid transplant rejection, heightens the risk of death from cancer among solid organ transplant recipients (1). Yet, evidence-based data regarding their cancer treatment are scarce because transplant recipients are usually excluded from clinical trials, and registries are limited (2, 3).
In recent years, the development of immune checkpoint inhibitors (ICIs), including cytotoxic T-lymphocyte-associated protein 4 (CTLA4) inhibitors and inhibitors of programmed cell death protein 1 or its ligand (PD1, PDL1), has considerably improved the survival of patients with certain advanced cancers (4). These monoclonal antibodies foster immune responses against malignancies, but sometimes lead to off-target immune adverse drug reactions (ADRs) (5). ICI unleash the breaks on the immune system and may trigger allograft rejection (AR) (6, 7).
Based on their similar mechanisms of action, different ICI classes are though to mediate the same effect on AR, however this has never been investigated (8). Besides, although AR associated with ipilimumab and pembrolizumab are mentioned in drug labels from both the Food and Drug Administration (FDA) (9, 10) and the European Medicines Agency (EMA) (11, 12), AR is only addressed in the FDA’s drug label for cemiplimab (13, 14). Similarly, regarding PDL1 inhibitors, AR risk is inconsistently mentioned in the FDA’s drug labels, and does not appear in the EMA’s drug labels. Therefore, we aimed to clarify the association of AR with the different ICI classes, through a disproportionality analysis of the pharmacovigilance database of the World Health Organization (WHO).
2 Materials and methods2.1 DatabaseThe Uppsala Monitoring Center (UMC) manages the WHO Safety Database, also known as VigiBase® (15). Since 1967, VigiBase® collects Individual Case Safety Reports from over 172 countries’ national pharmacovigilance networks. These spontaneous reports are submitted after drugs’ marketing, and may be issued by healthcare professionals, patients, and pharmaceutical companies. The anonymity of both patients and reporters is preserved. Each report includes demographic details such as the country of origin, qualifications of the reporter, patient characteristics, information about the drugs involved (for instance indication, start and stop dates, dosage, and any other medications taken simultaneously), and information about ADR (including the effects, seriousness, onset, and outcome of the reaction).
2.2 QueryVigibase® was queried for all reports of the High Level Term (HLT) “Transplant Rejection” involving either the CTLA4 inhibitor (ipilimumab), PD1 inhibitors (cemiplimab, emiplimab, dostarlimab, nivolumab, pembrolizumab, retifanlimab, tislelizumab, toripalimab), or PDL1 inhibitors (atezolizumab, avelumab, durvalumab). In the Medical Dictionary for Regulatory Activities (MedDRA, version 26.1), a HLT is a grouping of related Preferred Terms (PTs) based upon anatomy, pathology, physiology, etiology or function (16). A PT is defined as the distinct descriptor for a single medical concept. For instance, the “Transplant Rejection” HLT includes PTs such as “Kidney transplant rejection”, “Liver transplant rejection” or “Heart transplant rejection” inter alia.
Quantitative variables were described in terms of medians with interquartile ranges (IQR) and/or minimum–maximum ranges (min–max). Qualitative variables were described with numbers and proportions.
2.3 Disproportionality analysisDisproportionality analysis is a statistical technique used in pharmacovigilance to identify potential signals regarding ADRs. It consists of comparing the proportion of reports with a specific ADR and a given drug (cases) to the proportion of reports with the same ADR and other drugs (non-cases). If the proportion of cases is higher than that of non-cases, it suggests a possible association between the drug and the ADR.
Disproportionality can be assessed through the Information Component (IC [95% confidence interval]). This measure derives from a Bayesian confidence propagation neural network, and is validated by the Uppsala Monitoring Centre (UMC). The IC compares the observed and expected number of reports for a specific ADR-drug combination. It offers a more accurate detection of potential pharmacovigilance signals, reducing the risk of false positive signals compared to other disproportionality measures. A positive lower end of the 95% confidence interval of the IC is commonly used as the threshold for identifying potential safety signals (see Supplementary Material) (17).
We calculated the IC to assess whether transplant rejections (HLT “Transplant Rejection”) are disproportionately reported in association with ICIs. Specifically, we also calculated the IC for the association between each class of ICIs (CTLA4, PD1, or PDL1 inhibitors) and the occurrence of AR. Then, we calculated the comparative reporting odds ratio (ROR [95% confidence interval]) for AR with PD1 inhibitors compared to PDL1 inhibitors. ROR approximates the odds ratio of case-control studies and is specific to case-non-case studies. A ROR > 1 suggests that the ADR is more frequently reported with the drug of interest than with the comparator. The higher the ROR, the more statistically relevant is the potential pharmacovigilance signal. The ROR was expressed as a point estimate with a 95% Confidence Interval, using Woolf’s method (see Supplementary Material) (18).
3 Results3.1 Characteristics of the reportsAs of 8 February 2024, 159 reports accounting for the HLT “Transplant Rejection” involving an ICI were gathered, accounting for 0.1% of the 171,230 cases reported with ICI in VigiBase®. The first case has been reported in 2015 (Figure 1). Most patients were male (n=105, 66.0%), with a median age of 63 years (IQR 52-70, min-max 14-85). Most cases were reported in the United States of America (89, 56.0%) and in France (21, 13.2%), mostly by healthcare professionals (152, 95.6%).
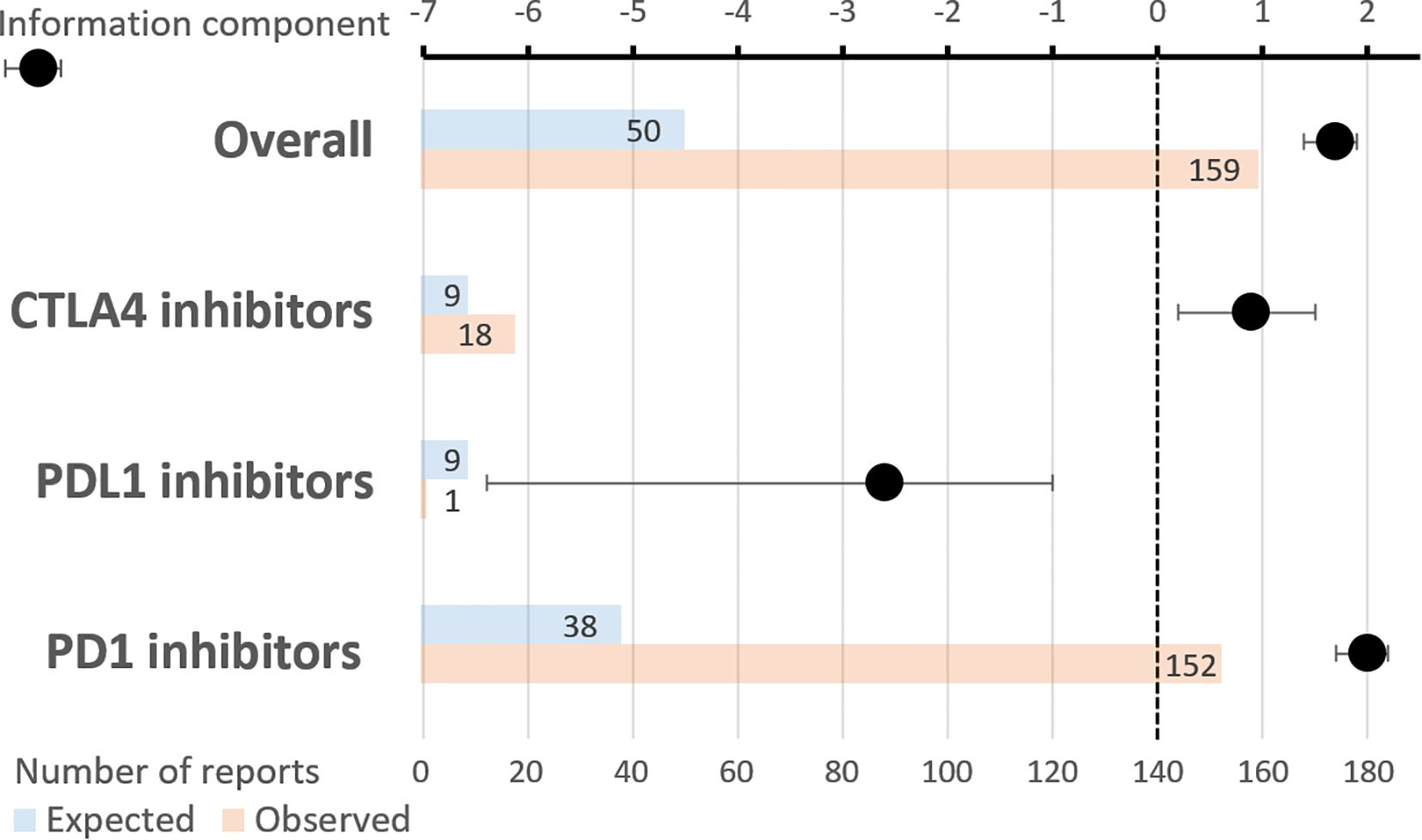
Figure 1. Temporal trends in transplant rejections involving immune checkpoint inhibitors reported to VigiBase. Vertical axis: percentage of the total number of reports. Horizontal axis: year of reporting.
The most frequently involved ICIs were PD1 inhibitors, especially nivolumab (n=73, 45.9%) and pembrolizumab (n=62, 39.0%). The CTLA4 inhibitor, ipilimumab, was involved in 18 reports (11.3%). One report (0.6%) involved a PDL1 inhibitor, avelumab. Combined PD1 and CTLA4 inhibitors were used in 12 reports (7.5%). The most co-reported active ingredients were mycophenolic acid (n=31, 10.7%), prednisone (n=28, 10.1%), tacrolimus (n=26, 7.5%), sirolimus (n=17, 5.7%), ciclosporin, and everolimus (n=10 each, 5.0%).
3.2 Characteristics of the adverse drug reactionsAR mostly concerned kidney transplants (n=87, 54.7%), then liver transplants (25, 15.7%). The three most frequently co-reported PTs were “malignant neoplasm progression”, “acute kidney injury”, and “intentional product use issue”, with 12 reports each (7.5%).
The median time to onset between ICI introduction and the occurrence of AR was 28 days (IQR 16-60, minimum 11 days, maximum 9 months), corresponding to a median of 1 dose of ICI. A fatal outcome was mentioned in 36 reports (22.6%), mostly in patients with liver transplant rejection (n=15, 41.7% of fatal outcomes). Patients recovered or were recovering from AR in 34 reports (21.4%), and recovered with sequelae in 4 reports (2.5%).
3.3 Disproportionality analysisAs a whole, ICIs were significantly associated with AR, with an IC of 1.7 [1.4;1.9], with 159 reports observed as compared to 50 expected (Figure 2). Specifically, PD1 inhibitors exhibited an IC of 2.0 [1.7;2.2], with 152 reports observed as compared to 38 expected. CTLA4 inhibitors showed an IC of 0.9 [0.2;1.5], with 18 reports observed as compared to 9 expected. By contrast, the IC of PDL1 inhibitors did not reach statistical significance: -2.6 [-6.4;-1.0], with 1 report observed as compared to 9 expected. The comparative ROR of PD1 inhibitors, as compared to PDL1 inhibitors, was 33.7 [4.7;240.9] (p=0.0005).

Figure 2. Reports of transplant rejection involving immune checkpoint inhibitors. Upper horizontal axis: information component with its 95% confidence interval. Lower horizontal axis: number of reports (expected and observed). Combined PD1 and CTLA4 inhibitors were used in 12 reports.
4 DiscussionThis analysis of real-world data, issued from the world pharmacovigilance database confirms that treatment of solid organ transplant recipients with ICIs is significantly associated with the risk of AR. This result seems plausible, due to the mechanisms of action of ICIs (19). It is also consistent with the results of previous studies (6, 7, 20). Our results suggest that AR occurs at an early stage of treatment and is associated with poor outcomes. Most reports or AR occurred in kidney transplant recipients, mostly because kidney transplantation is more prevalent than other solid organ transplantations. Moreover, clinicians might be less reluctant to use ICIs on kidney transplant recipients, because renal replacement therapy by dialysis is available. By contrast, liver (or heart) transplant rejection can be life-threatening on a short term.
In the literature, almost all reported cases occur in patients who have been transplanted for more than one year, and sometimes for more than 10 years, before the initiation of an ICI (20). This may be because clinicians are aware of the increased risk of rejection in the early post-transplantation period. Moreover, most patients probably undergo thorough screening for potential malignancies before being transplanted, so that it is rarely required to treat advanced cancer shortly after transplantation. According to the literature, when AR occurs, most patients receive high-dose corticosteroids, sometimes supplemented with intravenous immunoglobulins (in the case of antibody-mediated rejection) (21). For some patients, tacrolimus and/or mycophenolate are initiated or their dose are increased (20). Data from a systematic review report a mortality rate of 57.8% with a longer follow-up duration (median overall survival: 36 weeks) (7). Most deaths were due to cancer progression, although AR significantly impacted survival, particularly in liver recipients.
Interestingly, only one case of AR involving PDL1 inhibitors was reported in the WHO pharmacovigilance database, which is surprisingly low. Moreover, whereas CTLA4 and PD1 inhibitors were associated with statistically significant safety signals for AR in VigiBase®, no signal was found with PDL1 inhibitors. The IC for PDL1 inhibitors was even negative, as 9 reports of ARs were expected considering the total number of reports with this class and with this ADR. Our findings are supported by a multicenter retrospective study, which focused on the safety and efficacy of ICIs in kidney transplant recipients. Among the 6 patients treated with PDL1 inhibitors, none developed AR, while almost half of the patients treated with PD1 inhibitors did (21). This class seemed associated with a lower risk of rejection, though the number of patients in this non-randomized cohort was too limited to draw definite conclusions.
Debate is still ongoing regarding the different safety profiles of PD1 and PDL1 inhibitors. Both molecules are involved in immune tolerance in transplant recipients (22). Therefore, the potentially lowered risk of AR in patients treated with PDL1 inhibitors might be underpinned by the fact that few lymphocytes are trafficking in the transplanted organ at a baseline state. Hence, PD1 inhibitors could potentially activate a larger proportion of lymphocytes overall, while PDL1 inhibitors may primarily activate lymphocytes at the tumor site, where PDL1 expression is significantly higher than in the graft (23). Another hypothesis is that the intensity of checkpoint inhibition may vary depending on the chosen class. This suggests a potential interest of therapeutic drug monitoring for ICIs, to reduce the risk of overexposure in at-risk patients (24).
Besides, PDL1 inhibitors do not disrupt the interaction between PD1 and PDL2, unlike PD1 inhibitors. As a result, the PDL2 pathway remains efficient in patients receiving PDL1 inhibitors, as a potential protective mechanism for the transplant (25). In fact, PDL2 is expressed on the human kidney and helps to regulate CD8 proliferation, while promoting Treg functions (26–30). From a different perspective, belatacept, a costimulation blocker targeting the CTLA4 pathway, is increasingly used in transplant recipients. A deeper understanding of the physiology of immune checkpoint blockade could, therefore, clear the path for harnessing these pathways to enhance allograft tolerance.
However, it is necessary to guard against overly speculative assertions. In fact, this study cannot definitely conclude that PDL1 inhibitors decrease the risk of AR, as compared to other ICIs. Indeed, limitations inherent to spontaneous reporting systems and pharmacovigilance approaches are hindering factors. Lacking data are frequent, preventing thorough causality assessment for each reported case of AR. Furthermore, we do not have access to the transplant biopsies and the details on the management of AR. The WHO safety database does not provide data regarding the interval between transplantation and the initiation of an ICI. The lack of case validation can induce false positives (31). Several confounding factors could not be addressed either, such as the indications of the treatment with ICIs, or the overall immunological risk status of the patients. Then, the available data do not allow for a distinction between cancer-related mortality and the direct consequences of rejection. The number of reports is low for some classes, with, for instance, only one report of AR involving PDL1 inhibitors in the WHO safety database. This may be due to PD-L1 inhibitors being less frequently used than other classes of ICIs. However, the disproportionality approach aims at mitigating the possible impact of varying prescribing rates (32, 33). In fact, when a drug is prescribed in a larger number of patients, the occurrence of all ADRs is expected to increase. Thus, the disproportionate reporting of a given ADR does not directly depend on the number of patients exposed to the drug. Anyway, reporting rates cannot be interpreted as incidence rates, hence the need for further confirmatory studies.
All in all, our exploratory study paves the way to further prospective studies investigating whether PDL1 inhibitors decrease the risk of AR compared to other classes of ICIs. Besides, the underlying mechanisms involved in this discrepancy warrant further investigation. If these data are confirmed, PDL1 inhibitors could become the preferred class of immune checkpoint inhibitors in solid organ transplant recipients.
Data availability statementThe datasets generated during and/or analyzed during the current study are not publicly available because data are owned by UMC, who manages VigiBase®, but are available from the corresponding author on reasonable request. Requests to access these datasets should be directed tocGhhcm1hY292aWdpbGFuY2VAY2h1LW5pY2UuZnI=.
Ethics statementEthical approval was not required for the study involving humans in accordance with the local legislation and institutional requirements. Written informed consent to participate in this study was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and the institutional requirements.
Author contributionsAG: Writing – original draft, Writing – review & editing. DM: Writing – original draft, Writing – review & editing. JB: Writing – review & editing. AD: Writing – review & editing. DB: Writing – review & editing. CG: Writing – review & editing. FR: Writing – review & editing. MA: Writing – review & editing. C-HM: Writing – review & editing. HM: Writing – review & editing. M-DD: Writing – original draft, Writing – review & editing. AS: Writing – original draft, Writing – review & editing.
FundingThe author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
AcknowledgmentsThe authors acknowledge the UMC who manages and provided the data used in the present study. Access to VigiBase® is available without fees to Dr. FR and the pharmacovigilance center of Nice.
Conflict of interestThe authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statementThe author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s noteAll claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Author DisclaimerThe views expressed in this article are the authors’ personal views and may not be understood or quoted as being made on behalf of or reflect the position of the ANSM, the EMA, the WHO, or one of their committees or working parties.
Supplementary materialThe Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2025.1514033/full#supplementary-material
References1. Re Sartò GV, Alfieri C, Cosmai L, Brigati E, Campise M, Regalia A, et al. Post-kidney transplant cancer: A real-world retrospective analysis from a single italian center. Transpl Int. (2024) 37:13220. doi: 10.3389/ti.2024.13220
PubMed Abstract | Crossref Full Text | Google Scholar
2. Haanen J, Ernstoff MS, Wang Y, Menzies AM, Puzanov I, Grivas P, et al. Autoimmune diseases and immune-checkpoint inhibitors for cancer therapy: review of the literature and personalized risk-based prevention strategy. Ann Oncol. (2020) 31:724–44. doi: 10.1016/j.annonc.2020.03.285
PubMed Abstract | Crossref Full Text | Google Scholar
3. Ferrándiz-Pulido C, Leiter U, Harwood C, Proby CM, Guthoff M, Scheel CH, et al. Immune checkpoint inhibitors in solid organ transplant recipients with advanced skin cancers—Emerging strategies for clinical management. Transplantation. (2023) 107:1452. doi: 10.1097/TP.0000000000004459
PubMed Abstract | Crossref Full Text | Google Scholar
6. Abdel-Wahab N, Safa H, Abudayyeh A, Johnson DH, Trinh VA, Zobniw CM, et al. Checkpoint inhibitor therapy for cancer in solid organ transplantation recipients: an institutional experience and a systematic review of the literature. J Immunother Cancer. (2019) 7:106. doi: 10.1186/s40425-019-0585-1
PubMed Abstract | Crossref Full Text | Google Scholar
7. d’Izarny-Gargas T, Durrbach A, Zaidan M. Efficacy and tolerance of immune checkpoint inhibitors in transplant patients with cancer: A systematic review. Am J Transplant Off J Am Soc Transplant Am Soc Transpl Surg. (2020) 20:2457–65. doi: 10.1111/ajt.15811
PubMed Abstract | Crossref Full Text | Google Scholar
8. Abdelrahim M, Esmail A, Abudayyeh A, Saharia A, Ghobrial RM. 502 Transplant oncology: Utilization of immunotherapy in the peri-transplant setting. J Immunother Cancer. (2022) 10. doi: 10.1136/jitc-2022-SITC2022.0502
Crossref Full Text | Google Scholar
17. Bate A, Evans SJW. Quantitative signal detection using spontaneous ADR reporting: QUANTITATIVE SIGNAL DETECTION. Pharmacoepidemiol Drug Saf. (2009) 18:427–36. doi: 10.1002/pds.1742
PubMed Abstract | Crossref Full Text | Google Scholar
19. Kawashima S, Joachim K, Abdelrahim M, Abudayyeh A, Jhaveri KD, Murakami N. Immune checkpoint inhibitors for solid organ transplant recipients: clinical updates. Korean J Transplant. (2022) 36:82–98. doi: 10.4285/kjt.22.0013
PubMed Abstract | Crossref Full Text | Google Scholar
20. Nguyen LS, Ortuno S, Lebrun-Vignes B, Johnson DB, Moslehi JJ, Hertig A, et al. Transplant rejections associated with immune checkpoint inhibitors: A pharmacovigilance study and systematic literature review. Eur J Cancer. (2021) 148:36–47. doi: 10.1016/j.ejca.2021.01.038
PubMed Abstract | Crossref Full Text | Google Scholar
21. Murakami N, Mulvaney P, Danesh M, Abudayyeh A, Diab A, Abdel-Wahab N, et al. A multi-center study on safety and efficacy of immune checkpoint inhibitors in cancer patients with kidney transplant. Kidney Int. (2021) 100:196–205. doi: 10.1016/j.kint.2020.12.015
PubMed Abstract | Crossref Full Text | Google Scholar
22. Ito T, Ueno T, Clarkson MR, Yuan X, Jurewicz MM, Yagita H, et al. Analysis of the role of negative T cell costimulatory pathways in CD4 and CD8 T cell-mediated alloimmune responses. vivo J Immunol Baltim Md 1950. (2005) 174:6648–56. doi: 10.4049/jimmunol.174.11.6648
PubMed Abstract | Crossref Full Text | Google Scholar
23. Waeckerle-Men Y, Starke A, Wüthrich RP. PD-L1 partially protects renal tubular epithelial cells from the attack of CD8 + cytotoxic T cells. Nephrol Dial Transplant. (2007) 22:1527–36. doi: 10.1093/ndt/gfl818
PubMed Abstract | Crossref Full Text | Google Scholar
24. Liu D, Hu L, Shao H. Therapeutic drug monitoring of immune checkpoint inhibitors: based on their pharmacokinetic properties and biomarkers. Cancer Chemother Pharmacol. (2023) 92:165–79. doi: 10.1007/s00280-023-04541-8
PubMed Abstract | Crossref Full Text | Google Scholar
25. Latchman Y, Wood CR, Chernova T, Chaudhary D, Borde M, Chernova I, et al. PD-L2 is a second ligand for PD-1 and inhibits T cell activation. Nat Immunol. (2001) 2:261–8. doi: 10.1038/85330
PubMed Abstract | Crossref Full Text | Google Scholar
27. Habicht A, Kewalaramani R, Vu MD, Demirci G, Blazar BR, Sayegh MH, et al. Striking dichotomy of PD-L1 and PD-L2 pathways in regulating alloreactive CD4+ and CD8+ T cells. In Vivo Am J Transplant. (2007) 7:2683–92. doi: 10.1111/j.1600-6143.2007.01999.x
PubMed Abstract | Crossref Full Text | Google Scholar
28. Hurrell BP, Helou DG, Howard E, Painter JD, Shafiei-Jahani P, Sharpe AH, et al. PD-L2 controls peripherally induced regulatory T cells by maintaining metabolic activity and Foxp3 stability. Nat Commun. (2022) 13:5118. doi: 10.1038/s41467-022-32899-5
PubMed Abstract | Crossref Full Text | Google Scholar
29. Nie X, Chen W, Zhu Y, Huang B, Yu W, Wu Z, et al. B7-DC (PD-L2) costimulation of CD4+ T-helper 1 response via RGMb. Cell Mol Immunol. (2018) 15:888–97. doi: 10.1038/cmi.2017.17
PubMed Abstract | Crossref Full Text | Google Scholar
30. Xavier PDP, Oliveira JGG. Co-inhibitors of second signal of lymphocyte response in human renal transplants: PD-L2, GITR, and ILT-2/3/5 positive cells from aspiration biopsies associate with acute rejection-freedom. Open J Nephrol. (2021) 11:58–77. doi: 10.4236/ojneph.2021.111006
Crossref Full Text | Google Scholar
31. Montastruc J-L, Sommet A, Bagheri H, Lapeyre-Mestre M. Benefits and strengths of the disproportionality analysis for identification of adverse drug reactions in a pharmacovigilance database. Br J Clin Pharmacol. (2011) 72:905–8. doi: 10.1111/j.1365-2125.2011.04037.x
PubMed Abstract | Crossref Full Text | Google Scholar
32. Khouri C, Petit C, Tod M, Lepelley M, Revol B, Roustit M, et al. Adverse drug reaction risks obtained from meta-analyses and pharmacovigilance disproportionality analyses are correlated in most cases. J Clin Epidemiol. (2021) 134:14–21. doi: 10.1016/j.jclinepi.2021.01.015
PubMed Abstract | Crossref Full Text | Google Scholar
33. Merino D, Gérard AO, Destere A, Saidessalam H, Askenazy F, Montastruc F, et al. Cardiac and metabolic safety profile of antipsychotics in youths: A WHO safety database analysis. Psychiatry Res. (2024) 334:115786. doi: 10.1016/j.psychres.2024.115786
留言 (0)