Periodontitis is a bacterially induced oral inflammatory disorder, which eventually causes the degradation of periodontal tissues and tooth loss (Pihlstrom et al., 2005). It is a major oral health issue in public health, with high costs to society, ranking as the sixth most common infectious disease globally, and in the United States, 42% of adults over 30 have a form of periodontitis (Eke et al., 2018). The collective research to date supports that in scenarios where the overall balance among the resident periodontal polymicrobial bacterial communities is disturbed, a dysbiotic environment will result, and some of the species might exhibit potential pathogenic characteristics, leading to an inflammatory response in the periodontal tissues, which together with factors related to the host susceptibility promotes the degradation (Darveau, 2010; Hajishengallis and Lamont, 2021; Lamont et al., 2023). Typically, periodontitis affecting adolescents and young individuals has been referred to as aggressive periodontitis and is at present defined as stage IV and grade C, indicating rapid progress (Tonetti et al., 2018). There is a wide geographical spread with an enhanced incidence in North and West Africa (Bouziane et al., 2020; Yoshida et al., 2021; Kissa et al., 2022). Leukotoxin (LtxA) belongs to the repeats-in-toxin (RTX) family of toxins and is a major virulence factor of the Gram-negative species Aggregatibacter actinomycetemcomitans. As far as known, LtxA is produced by all hitherto recognized wild-type strains (Fine et al., 2020). A highly leukotoxin-producing genotype (JP2) is associated with the periodontal disease progress in these geographical regions in Africa (Haubek et al., 2008; Höglund Åberg et al., 2014). The JP2 genotype strains of this organism carry a deletion of 530 base pairs (bp) in the ltxCABD promoter (Brogan et al., 1994), which could be responsible for the enhanced production of leukotoxin in these strains by containing a potential transcriptional terminator sequence (Sampathkumar et al., 2017). To the best of our knowledge, few studies executed in the northern and western geographical regions of Africa have been reported regarding the relation between periodontitis and the progression of this disease in young individuals, and concomitantly focusing on bacterial species other than A. actinomycetemcomitans (Dahlén et al., 2014; Yoshida et al., 2021), thus including the Gram-positive, anaerobic organism Filifactor alocis.
F. alocis has recently been identified in the oral microbiome via metagenome DNA sequencing. It is culturable in the laboratory and is considered an emerging oral pathogen implicated in the etiology of periodontal (Aruni et al., 2014; Greenwood et al., 2020), peri-implantitis (Sanz-Martin et al., 2017), and endodontic (Zehnder et al., 2017) infections. It is a potential biomarker for active disease in young children (Aruni et al., 2014). F. alocis seems to have a synergistic relationship with A. actinomycetemcomitans in periodontitis (Fine et al., 2013; Wang et al., 2013). However, A. actinomycetemcomitans appears thereafter to be outcompeted by F. alocis in the deeper pockets (Dahlén et al., 2014). This suggests that F. alocis is able to adapt and drive the disease process forward in the absence of A. actinomycetemcomitans via as yet unknown mechanisms.
Approximately 50% of isolated strains of F. alocis encode a recently identified RTX protein, FtxA (Oscarsson et al., 2020; Bao et al., 2022; Ozuna et al., 2022). We recently assessed 2-year longitudinal data from a study population of adolescents in Ghana (Höglund Åberg et al., 2012; Höglund Åberg et al., 2014). This first revealed a possible correlation between deep periodontal pockets measured at the 2-year follow-up, the presence of the ftxA gene, and a high quantity of F. alocis (Razooqi et al., 2022). To further understand the contribution of F. alocis and FtxA in periodontal disease, we then quantified the carriage loads of F. alocis and the prevalence of its ftxA gene in subgingival plaque specimens, sampled at baseline from this Ghanaian cohort (Razooqi et al., 2024). Comparing these results with the recorded clinical attachment loss (CAL) longitudinal progression data from the 2-year follow up, we concluded that carriers of ftxA-positive F. alocis typically exhibited higher loads of the bacterium. Moreover, high carriage loads of F. alocis and the concomitant presence of the ftxA gene were two factors that were associated with an enhanced prevalence of CAL progression. Interestingly, CAL progression appeared to be further promoted upon the simultaneous presence of F. alocis and the non-JP2 genotype of A. actinomycetemcomitans (Razooqi et al., 2024). These results hence were consistent with the notion that F. alocis and its ftxA gene promotes CAL during periodontal disease.
Our previous work focused on adolescents while here we wanted to confirm the possible significance of F. alocis and its FtxA in association with periodontal disease in a wider age range, in a different study population, and in different disease severities. Hence, we have in the present work assessed saliva samples from a collection of patients all diagnosed with periodontitis (n=156; ages 26-86), collected at two private periodontal specialist practices in Perth, Western Australia. Here, we report on correlations between the presence and loads of F. alocis and its ftxA with clinical and demographic parameters, and with the presence of A. actinomycetemcomitans.
2 Materials and methods2.1 Study populationAll the clinical specimens used in the present work are salivary samples from a population of periodontally diseased patients (n=156; ages 26-86; 73 men, and 83 women) in Perth, Western Australia (Khzam et al., 2024). Extensive clinical parameters and demographic data on this population have been described (Khzam et al., 2024). The graphical representation of the means of periodontal pocket depth (PPD) across tooth groups in this population demonstrates a clear trend where molars exhibit the highest PPD (Figure 1).
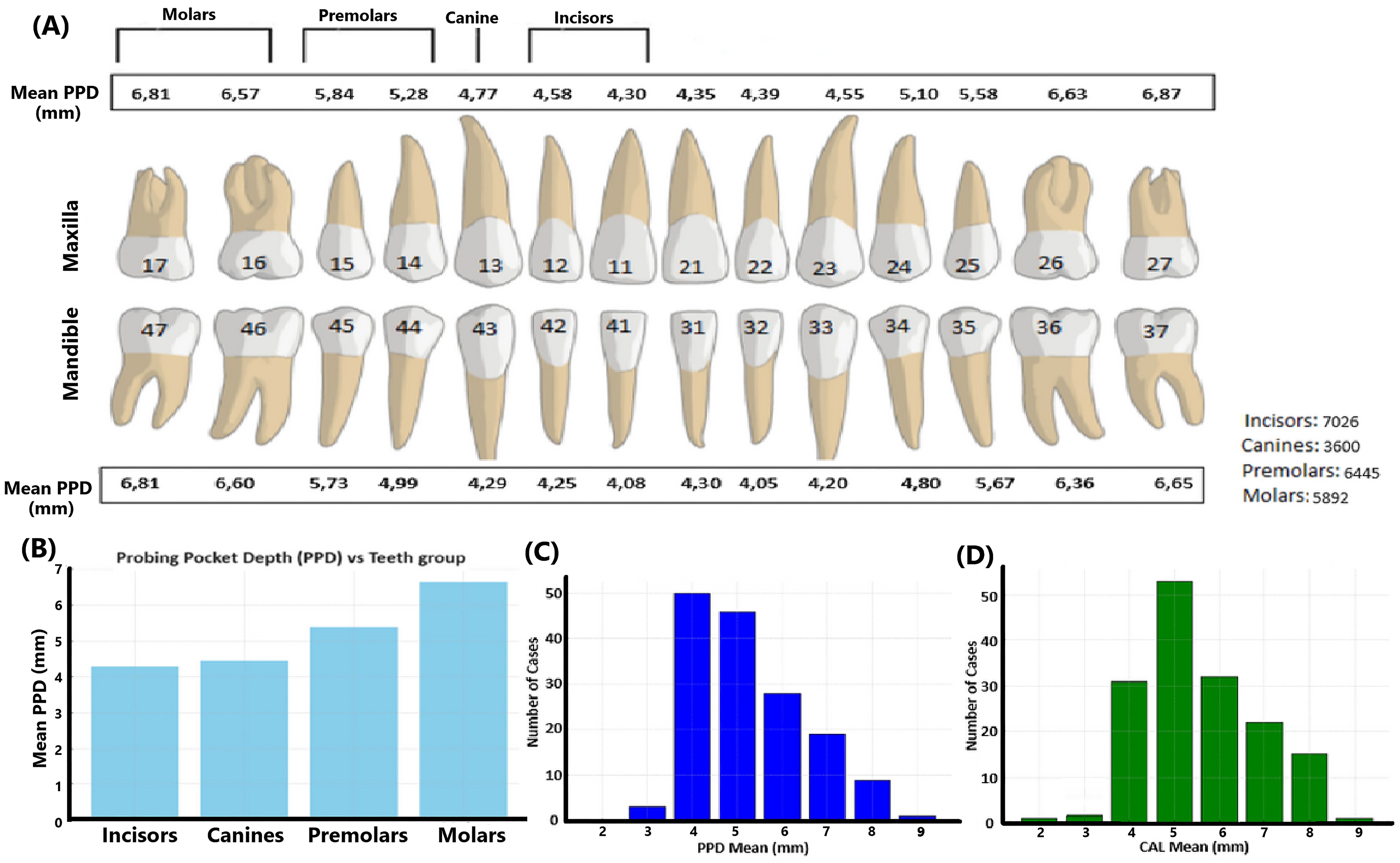
Figure 1. Mean periodontal pocket depth data of the present study population. (A) Illustration of the PPD measurements across the dentition in millimeters. The maxillary and mandibular teeth are represented with corresponding mean PPD values for each tooth type, including incisors, canines, premolars, and molars. The total number of teeth in each category is also provided, indicating the distribution of the cases studied. (B) Mean PPD values across the four groups of teeth: incisors, canines, premolars, and molars. (C) Distribution of cases based on the PPD mean values based on all dental sites assessed in each patient. (D) Distribution of cases based on the CAL mean values based on all dental sites assessed in each patient. Numbers on teeth are according to the documentation used in dentistry in Sweden.
2.2 DNA extractionThe saliva samples were subject to genomic DNA isolation using a GXT NA Extraction Kit® (Hain Lifesience, GmBH, Nehren, Germany), and an Arrow extraction instrument (Liaison IXT, DiaSorin Ltd., Fort Henry, Ireland) using procedures described previously (Razooqi et al., 2022). In brief, for pre-treatment, 400 μl of each saliva sample was mixed with 400 μl lysis buffer. To the mixture 20 μl proteinase K (20 mg/ml) was added, and then the samples were incubated at 55°C for 30 min. The DNA was extracted from 550 μl of the mix, eluted in a volume of 100 μl, and stored at 4°C prior to use.
2.3 Quantitative PCR analysisFor this, DNA samples from saliva were used as templates in qPCR for each of the individuals (n=156). The KAPA SYBR® FAST qPCR Kit (Kapa Biosystems, Wilmington, MA, USA) was used, with cycling conditions as described previously (Razooqi et al., 2022), and with a forward (5’-CAGGTGGTTTAACAAGTTAGTGG-3’), and a reverse (5’-CTAAGTTGTCCTTAGCTGTCTCG-3’) oligonucleotide primer, respectively, targeting the F. alocis 16s rRNA gene (Siqueira and Rocas, 2003). These primers have been found to be very efficient for the determination of F. alocis loads by qPCR in our previous work, and assays were standardized based on serially diluted samples of F. alocis cells (Razooqi et al., 2022, Razooqi et al., 2024). Loads of F. alocis were determined as numbers of cells/ml of the sample as described previously (Razooqi et al., 2022). Cut-off values of 10,000 and 100 F. alocis cells/ml of the sample were then used to divide the individuals as carriers of “high” (>10,000), and “low” (100<10,000) loads of F. alocis per sample, respectively. Loads <100 cells/sample were determined as negative for this bacterium. The presence of A. actinomycetemcomitans in the saliva samples was determined by qPCR, using a forward primer (5’-CTAGGTATTGCGAAACAATTTG-3’), and a reverse primer (5’-CCTGAAATTAAGCTGGTAATC-3’), and the cycling conditions were as described previously (Kirakodu et al., 2008). A load of 100 A. actinomyctemcomitans cells per ml of the sample was set as a positive result, in line with our recent work (Khzam et al., 2024).
2.4 PCR determination of presence of the ftxA gene of F. alocisA forward (5′-GGCTCAGATACCTACTTCTTC-3′) and a reverse (5′-GAAGGCTATGATTTGATTGTTTCC-3′) oligonucleotide primer were used to amplify a 798-base pair (bp) internal fragment of the ftxA gene, as described previously (Oscarsson et al., 2020; Razooqi et al., 2022). Results were scored as positive or negative for ftxA when this amplicon was obtained or not, as visualized on agarose gels.
2.5 Statistical analysisData analyses were performed using the Chi-square and Fisher’s exact tests, using SPSS 22.0 (SPSS Inc., Chicago, IL, USA) or Microsoft Excel (version 16.80). Input microbial data were (i) presence, and levels of F. alocis (i.e., present/absent, and high, low, or negative, respectively), (ii) presence of ftxA (yes or no), and (iii) presence of A. actinomycetemcomitans (yes or no), determined as described above. Microbial data were initially assessed for associations between F. alocis and/or ftxA, and A. actinomycetemcomitans, and then with the respective clinical parameters. These are summarized in Table 1. Significant differences between sample groups were examined using the Mann–Whitney U test or t-test. Results were estimated by an odds ratio (OR) with a 95% confidence interval (CI).
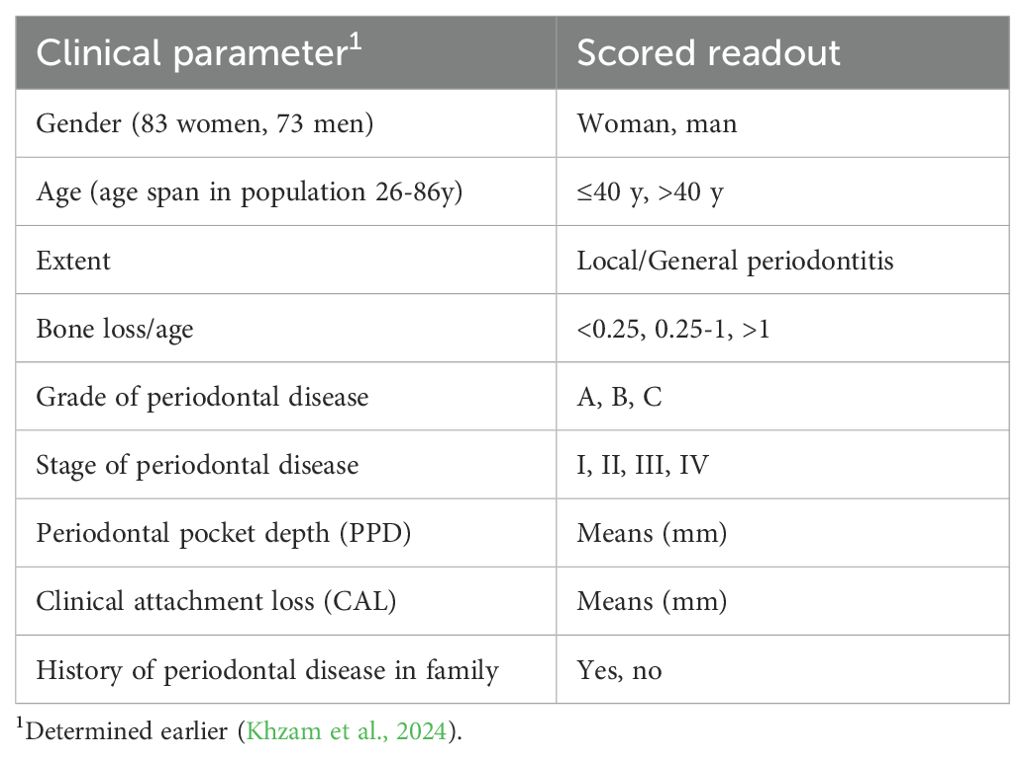
Table 1. Microbial data and clinical parameters of the study population (n=156) assessed in the present work.
2.6 Ethics statementThe studies in the present work were approved by the ethical committee of the University of Western Australia (Ref no. 2022/ET000252; June 13, 2022; and updated April 5, 2023), and by the Swedish Ethical Review Authority (dnr 2024-01704-01).
3 Results3.1 Carriers of ftxA+F. alocis exhibit higher loads of this bacteriumA flow chart summarizing the outline of the present study is shown in Figure 2. The qPCR analysis revealed that 137 of 154 (89.0%) assessed patients carried F. alocis (i.e., exhibited a load of >100 F. alocis per sample). Among these F. alocis-positive patients, 113 (82.5%) exhibited high levels of this bacterium (>10,000 per sample). Moreover, of the 153 assessed patients, 66 (43.1%) were found to be ftxA-positive using PCR. Interestingly, a majority of these 66 ftxA-positive individuals, 55 (83.3%), had high loads (>10,000 cells of F. alocis per sample; Figure 3). This supports the idea that ftxA is associated with high loads of F. alocis among carriers, and that high levels of F. alocis is a property of a majority of the carriers of an ftxA-positive F. alocis.
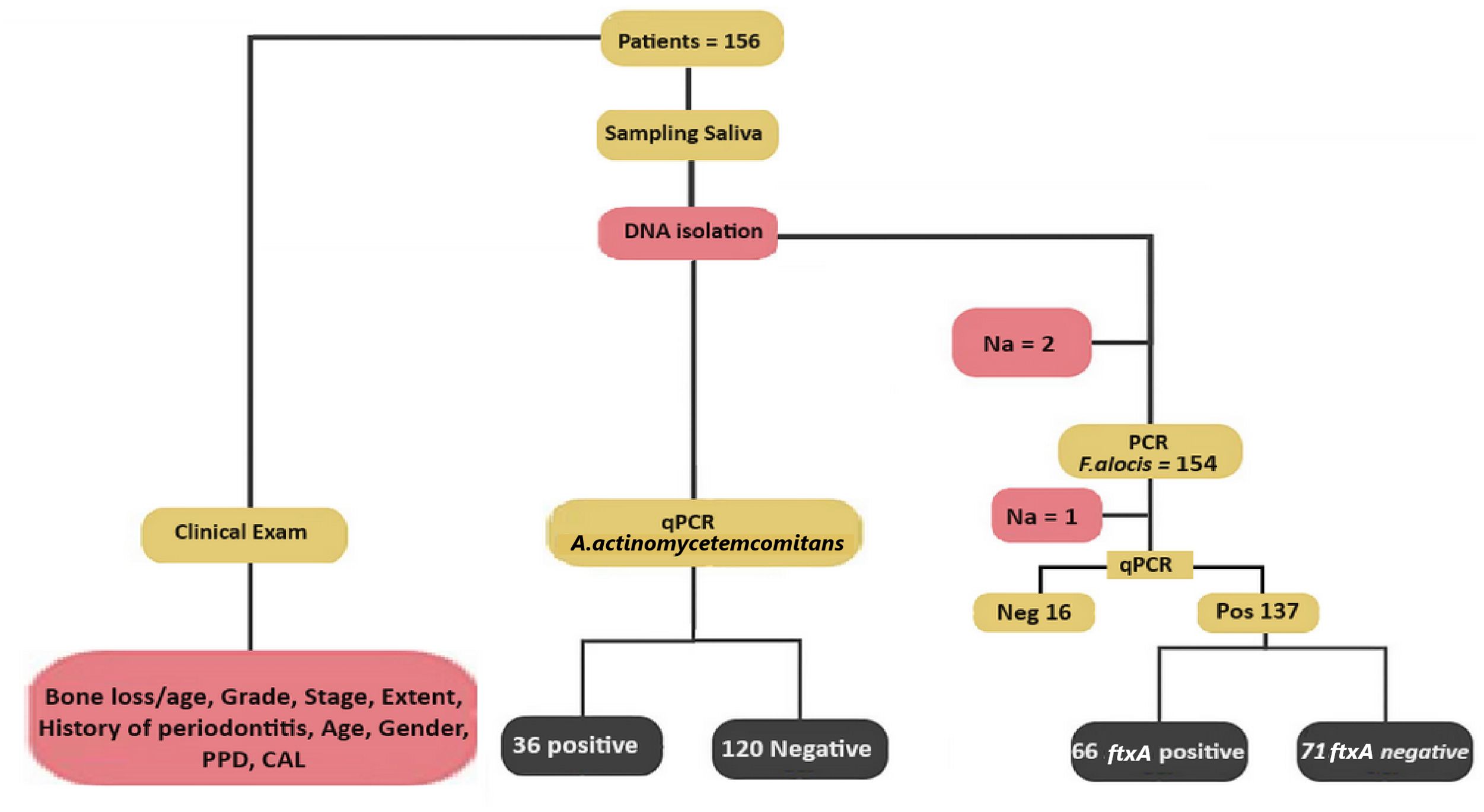
Figure 2. Scheme illustrating the design of the present study. Multiple clinical parameters from each of the 156 patients were recorded previously (Khzam et al., 2024). In the saliva samples, F. alocis levels were determined by qPCR, and the samples considered positive for F. alocis were genotyped for the presence or absence of the ftxA gene using PCR. The presence of A. actinomyetemcomitans in the samples was also set as positive or negative, deduced via qPCR. Also indicated is the number of non-analyzed samples (Na), which represent samples that were not analyzed due to a lack of material.
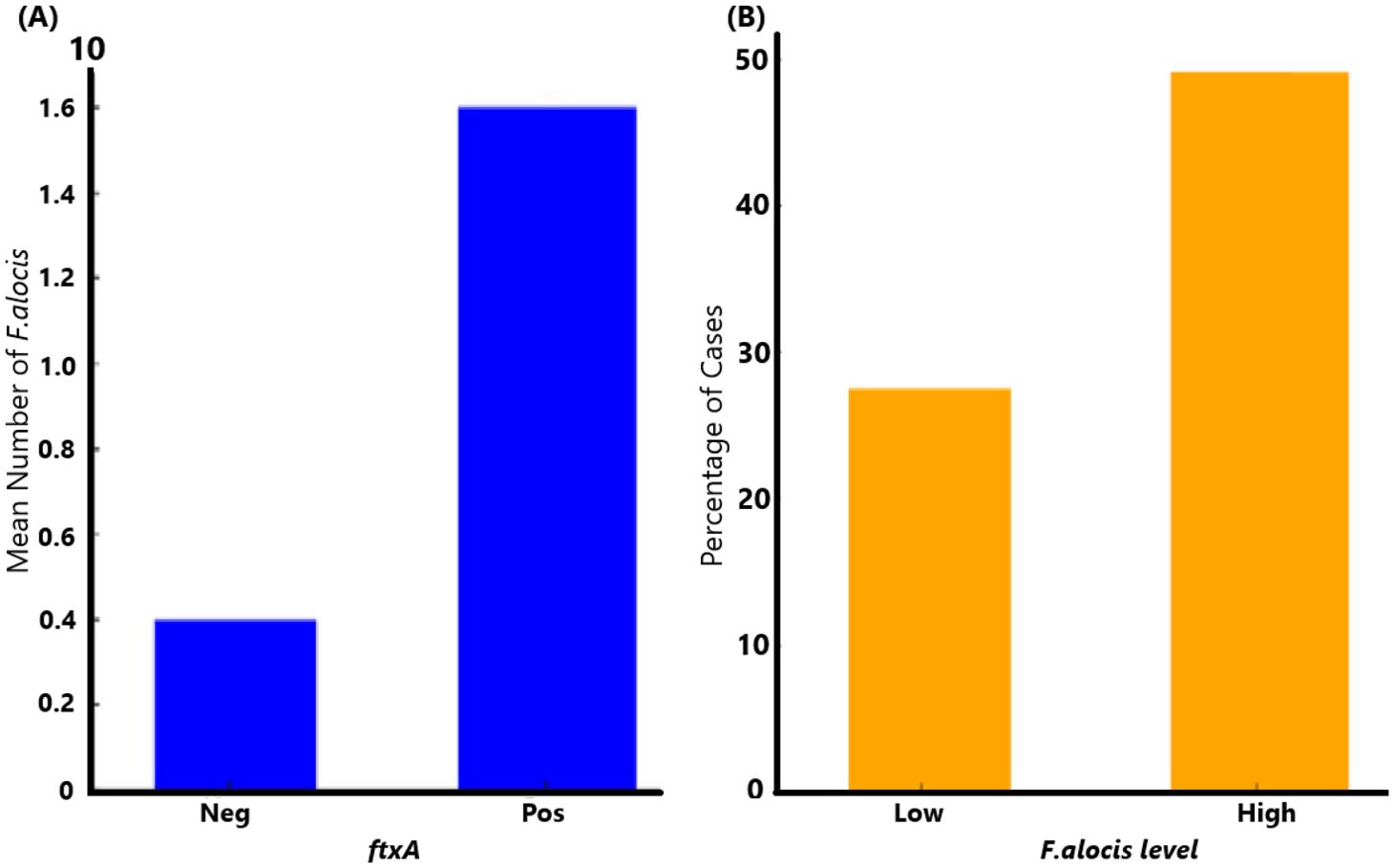
Figure 3. Association of ftxA with high loads of F. alocis among carriers. (A) Comparison of mean F. alocis (FA) counts (cells per sample) between ftxA-negative (Neg), and positive (Pos) cases (p=0.001). (B) High levels of F. alocis were a property of a majority of the carriers of an ftxA-positive F. alocis (p=0.018).
3.2 Co-existence of A. actinomycetemcomitans with F. alocis and ftxA in the study populationAccording to qPCR, A. actinomycetemcomitans was present in 36 (23.1%) of 156 analyzed samples (Figure 2). Of these 36 samples, 34 (94.4%) carried F. alocis, 28 (77.8%) had high loads of the bacterium (>10,000 cells/sample), and 20 (55.6%) were ftxA-positive (Figure 4).
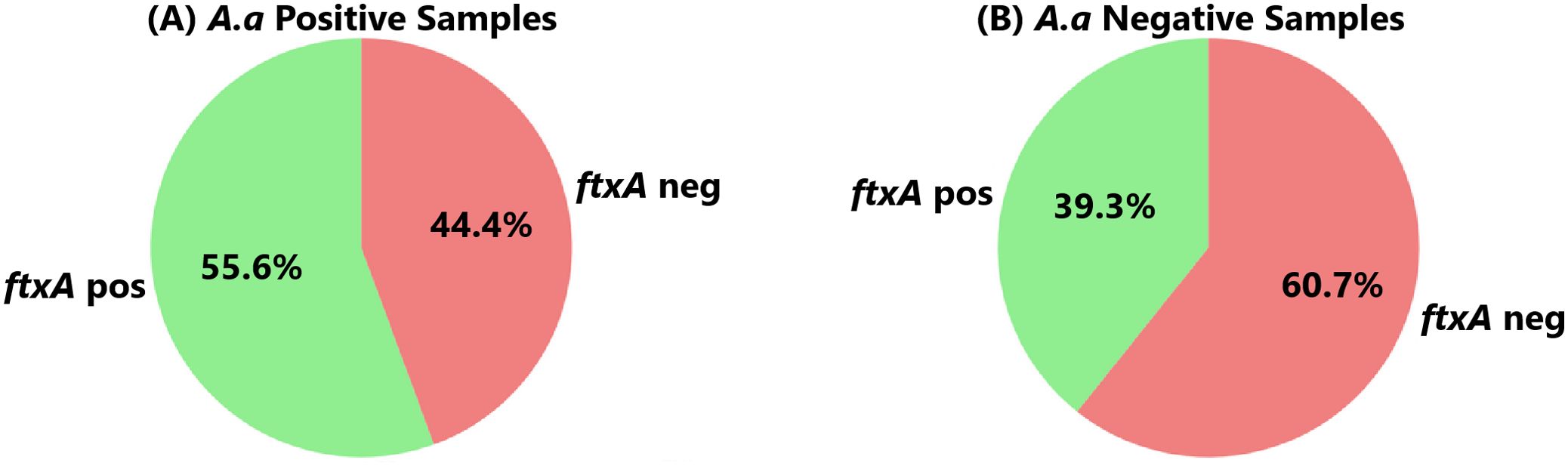
Figure 4. Co-carriage of an ftxA-positive F. alocis in (A) the 36 individuals who carried A. actinomycetemcomitans (A.a. Positive), and in (B) the 136 who lacked this bacterium (A.a. Negative), respectively. (p=0.085).
A. actinomycetemcomitans-negative samples (n=120), however, exhibited a similar prevalence to F. alocis (104 were positive; 86.7%). Of these 104, 85 (70.8%) exhibited high loads of F. alocis, and 47 (39.3%) were ftxA-positive (Figure 4B). Thus, these results revealed no apparent trend regarding the presence of F. alocis in the samples regardless of whether A. actinomycetyemcomitans was present or not, albeit with a potential association with co-presence of A. actinomycetemcomitans with ftxA, in this periodontally diseased population.
3.3 F. alocis loads and ftxA-presence in relation to clinical parametersThese bacterial parameters were assessed in relation to the clinical parameters listed in Table 1.
3.3.1 Bone loss/ageIt was observed that the proportion of F. alocis-positive cases was consistently high across both levels of bone loss (Figure 5A). Where bone loss was in the range of 0.25-1 or greater than 1, respectively, >80% of the cases were positive for F. alocis, suggesting a strong and persistent association between F. alocis presence and bone loss. Comparing the same bone loss parameters with the presence of ftxA revealed that 40% of the cases tested positive for ftxA when bone loss was in the range of 0.25-1 (Figure 5B). This percentage increased slightly when bone loss exceeded 1, with nearly 45% of cases being ftxA positive, suggesting a possible correlation between increased bone loss and ftxA positivity. Finally, these bone loss parameters were compared with simultaneous carriage of high levels of F. alocis and ftxA (Figure 5C). Approximately 30% of the cases with lower bone loss (0.25-1) were found to be positive for both high F. alocis loads and ftxA. The number of cases increased to approximately 40% when bone loss was greater than 1. This trend highlights a potential cumulative effect of high F. alocis levels and ftxA positivity in cases with severe bone loss, albeit with no significant positive association.
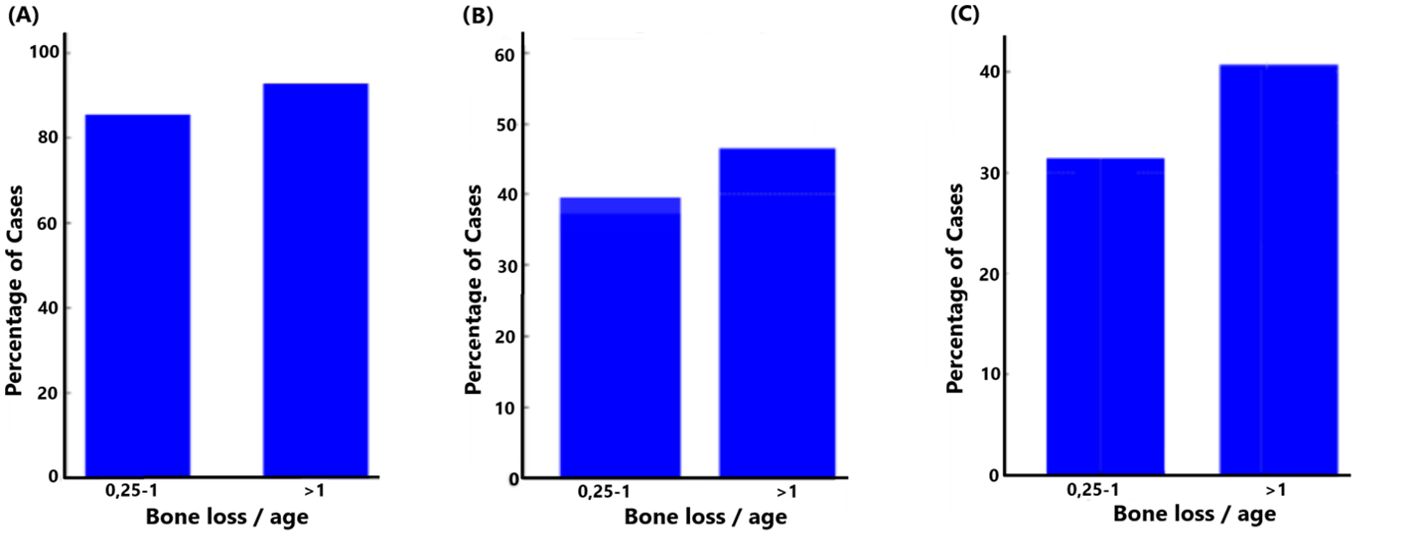
Figure 5. Relationship between bone loss/age and the presence of F. alocis (A); p=0.177), ftxA (B; p=0.309), and combined high F. alocis loads with ftxA positivity (C; p=0.191).
3.3.2 Age groupThe prevalence of F. alocis was assessed among two age groups, i.e., individuals aged 40 years or younger and those older than 40 years, respectively. In both age groups, a majority of individuals tested positive for F. alocis, but a slightly higher percentage of the ≤40 years group tested positive for F. alocis (Figure 6A). This suggests a potential trend that age may not be a significant factor governing the prevalence of F. alocis within the population studied. However, assessing the distribution of ftxA across these two age groups (Figure 6B) revealed that, among individuals aged ≤40 years, approximately 25% tested ftxA-positive. A similar distribution was observed in the >40 years age group, with a clear increase in the proportion of ftxA-positive cases to approximately 50%. These results suggest a potential age-related trend in ftxA-positivity, with older individuals showing a higher frequency of carriage of ftxA-positive F. alocis. Assessing the distribution of individuals with high loads of F. alocis and simultaneous presence of ftxA across the two age groups (Figure 6C) revealed that among the ≤40 years, approximately 19% were identified as carriers of high F. alocis loads and ftxA-positivity. In contrast, the proportion of high F. alocis levels and co-presence of ftxA increased markedly in the group >40 years, with approximately 40%. This supports a clear age-related trend, where older individuals exhibit a notably higher percentage of high loads of F. alocis and a high simultaneous presence of ftxA, suggesting a potential increased susceptibility or exposure in this demographic. Finally, we addressed the distribution of A. actinomycetemcomitans between the two age groups (Figure 6D). For the ≤40 years, 43% of cases were positive for A. actinomycetemcomitans. In contrast, the percentage of carriers of this bacterium decreased significantly in the >40 years age group (approximately 19%). This suggests the possibility of a higher prevalence of carriage of A. actinomycetemcomitans among the younger individuals, and a potential age-related decline in the detection of this bacterium over time.
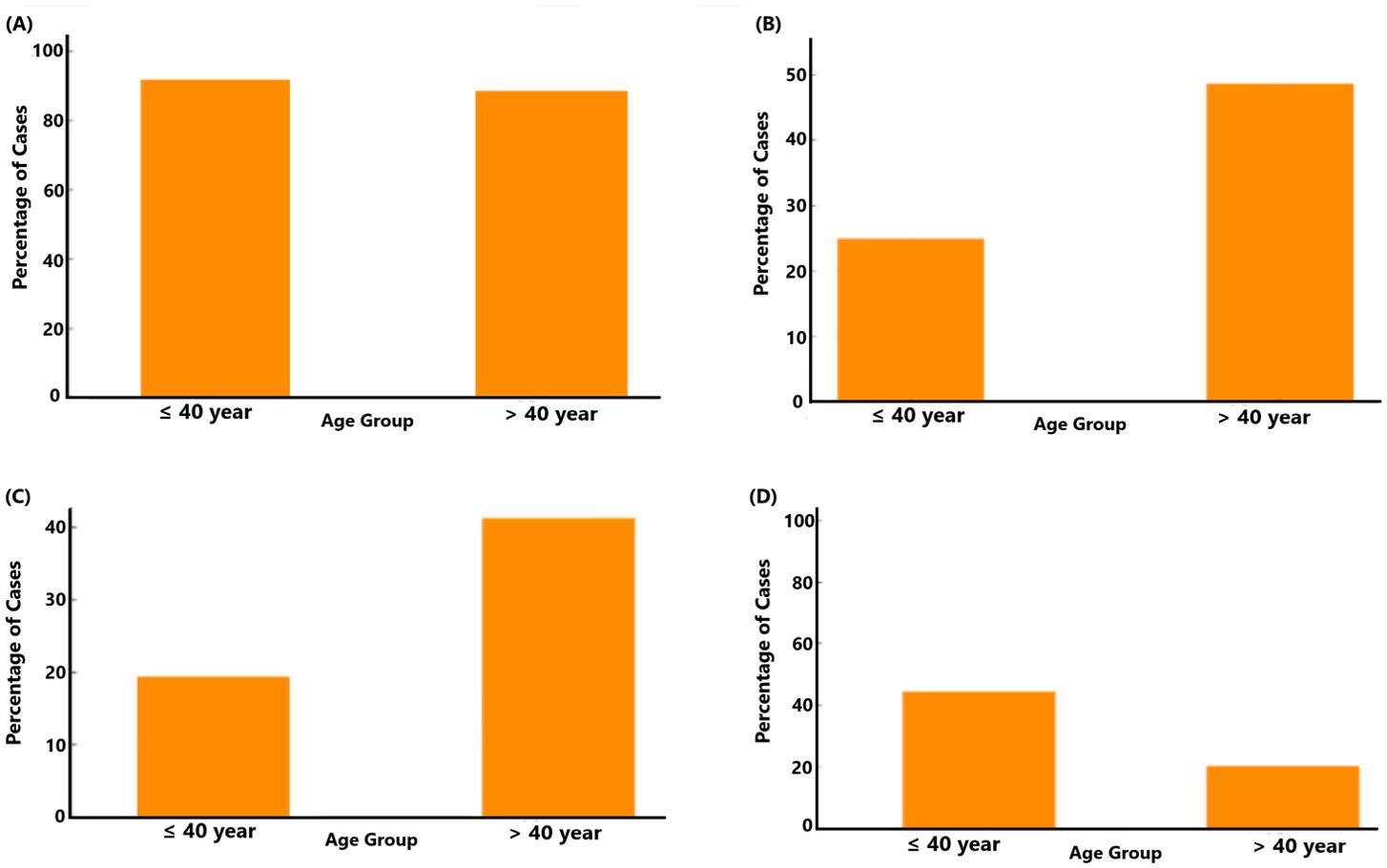
Figure 6. Relationship between age group (>40 years; n=36, and ≤40 years; n=117) and the presence of F. alocis (A; p=0.645), ftxA (B; p=0.012), combined high F. alocis loads and ftxA positivity (C; p=0.017), and A. actinomycetemcomitans (D; p=0.22).
3.3.3 Presence of A. actinomycetemcomitans by CALIn the present study population (n=156; 23.1% testing positive for A. actinomycetemcomitans), all subjects were assessed across different levels of CAL measured in millimeters (Figure 7). The results indicate that upon increasing CAL, the presence of A. actinomycetemcomitans decreased. Approximately 30% of the individuals with a CAL of 2-4 mm tested positive for A. actinomycetemcomitans. This frequency was lower, i.e., ≈22% for the individuals with a CAL of 5-7. This trend continued with 18% of A. actinomycetemcomitans-positive individuals exhibiting a CAL of 8-10 mm. These data together suggest that A. actinomycetemcomitans is more common in individuals with less attachment loss, reflecting a potential trend that this organism may be less present in deeper periodontal pockets.
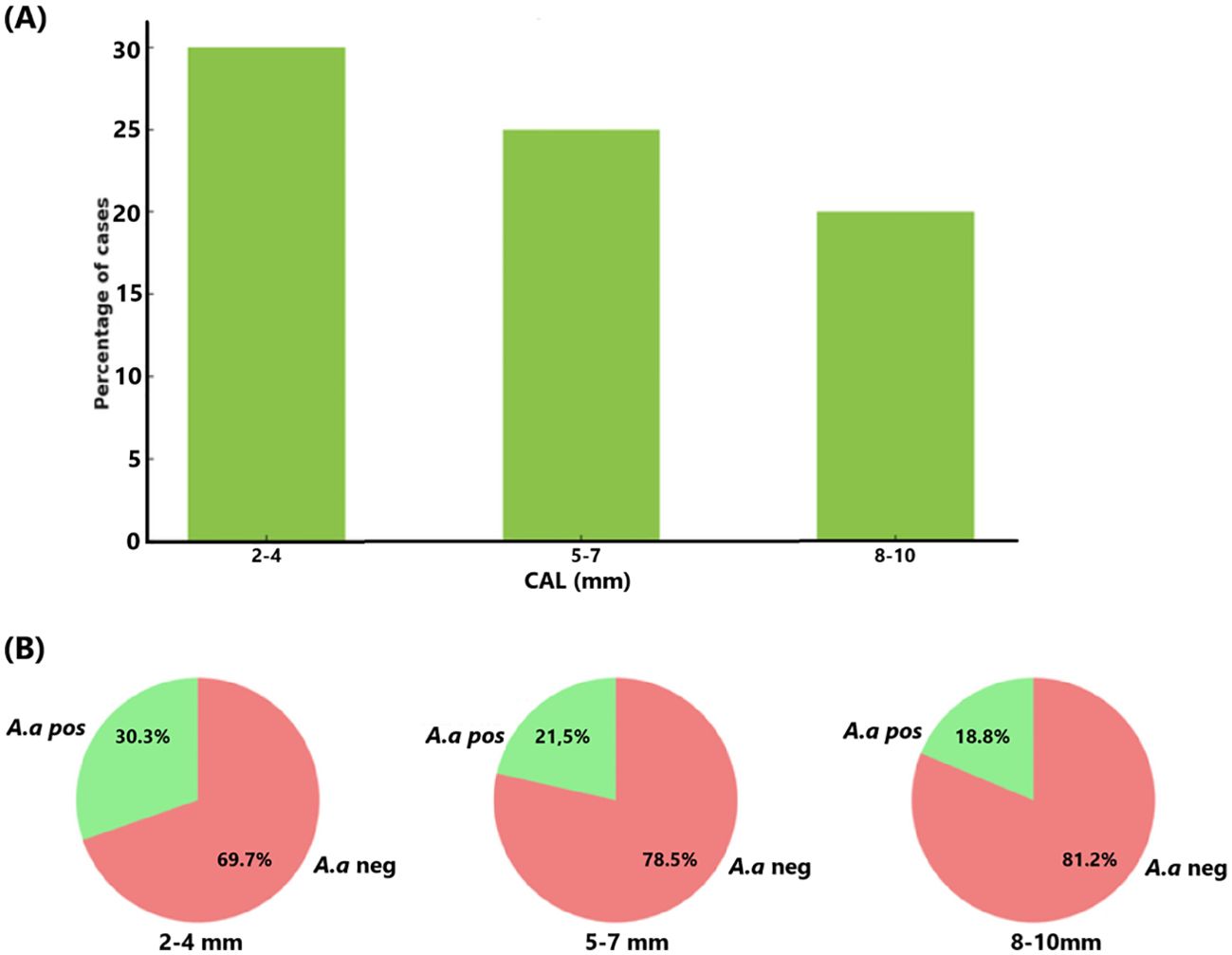
Figure 7. Relationship between CAL (mm) and the presence of A. actinomycetemcomitans (A.a) in the studied population (n=156) (A). The proportion (%) of A. actinomycetemcomitans-positive cases reduces as CAL increases (B).
3.3.4 Grade and stage of periodontitisGrade and stage were classified according to the established classification criteria (Kornman and Papapanou, 2020). Assessing ftxA-positivity among the 156 samples in relation to disease grade (Figure 8A) revealed that the percentage of ftxA-positive cases increased from approximately 39% in Grade B (representing 43 cases of 111) to ≈53% (23 cases of 42) in Grade C. This indicates an increase in ftxA-positivity for Grade C, suggesting a potential correlation between higher grades and ftxA presence. A similar potential trend was observed when monitoring carriage of ftxA across different stages of the disease, with stages II and III combined, and compared against stage IV (Figure 8B). The frequency of ftxA-positive carriers was approximately 40% (48 cases) in the combined stages II and III, and this percentage rose to nearly 51% (18 of 35 cases) in stage IV. This indicates an increase in ftxA-positivity in more advanced stages of the disease, indicating that the presence of this RTX protein may be more prevalent as the disease progresses to later stages.
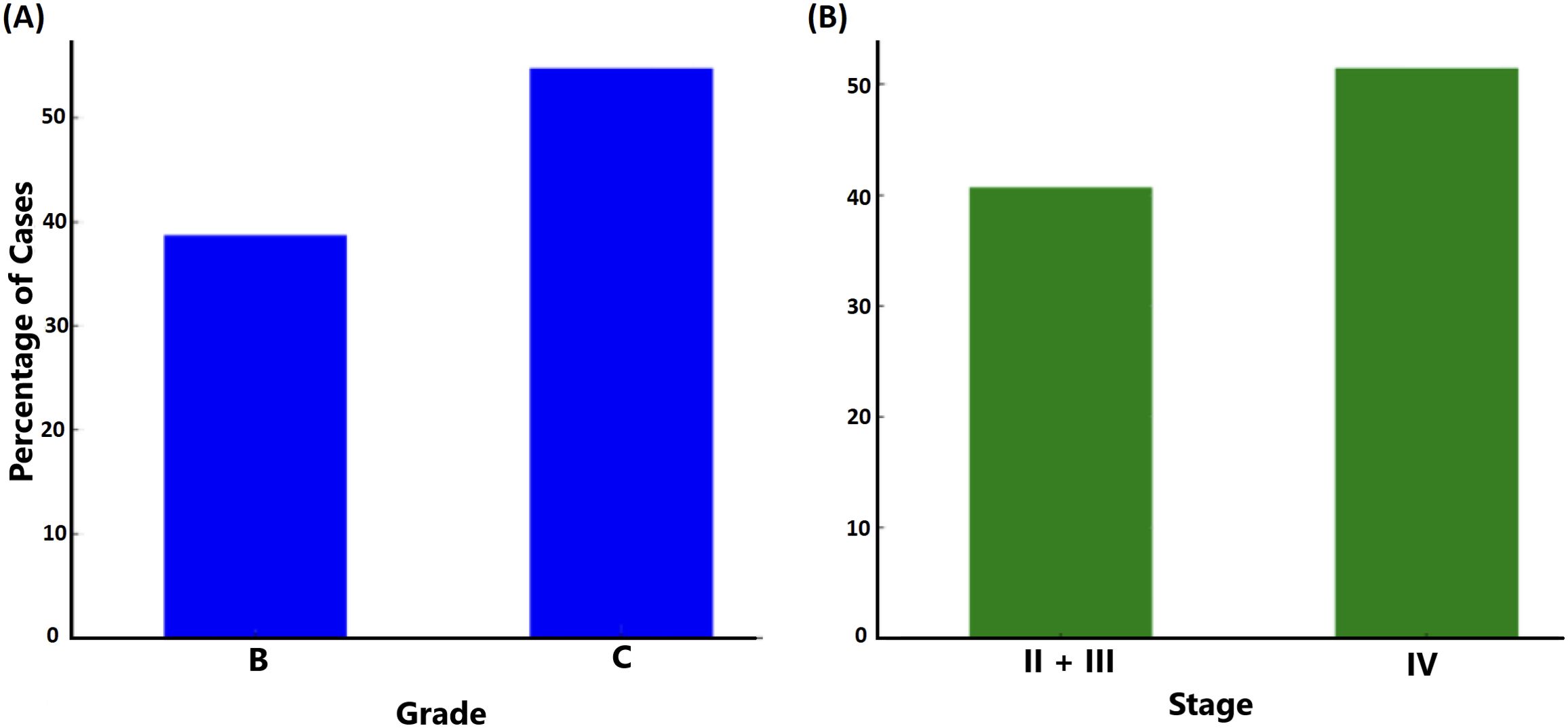
Figure 8. Relationship between grade (A; p=0.075) and stage (B; p=0.259) of periodontal disease and carriage of ftxA (% of carriers).
3.3.5 Extent of periodontitis (local and generalized)The extent of periodontitis was assessed regarding possible associations with the presence of A. actinomycetemcomitans, ftxA, and with high loads of F. alocis in combination with the presence of ftxA, respectively. As shown in Figure 9A, the distribution of A. actinomycetemcomitans-positive cases showed no difference between localized and generalized forms of periodontitis. This indicates no association between the presence of A. actinomycetemcomitans and the form of periodontal disease. Moreover, ftxA positivity was more frequently observed in localized forms of the disease. Assessment of the extent of periodontitis and presence of ftxA (Figure 9B) revealed a higher percentage of ftxA-positive cases with localized periodontitis (≈64%; 16 cases), compared to those with generalized periodontitis (39%; 50 ftxA-positive cases). Finally, cases with both high loads of F. alocis and ftxA were concluded to be more prevalent when diagnosed with localized periodontitis (56%, 14 cases) compared to generalized periodontitis (32%, 41 cases; Figure 9C). Thus, taken together, these results indicate that F. alocis and its ftxA can play a role in the extent of periodontitis.
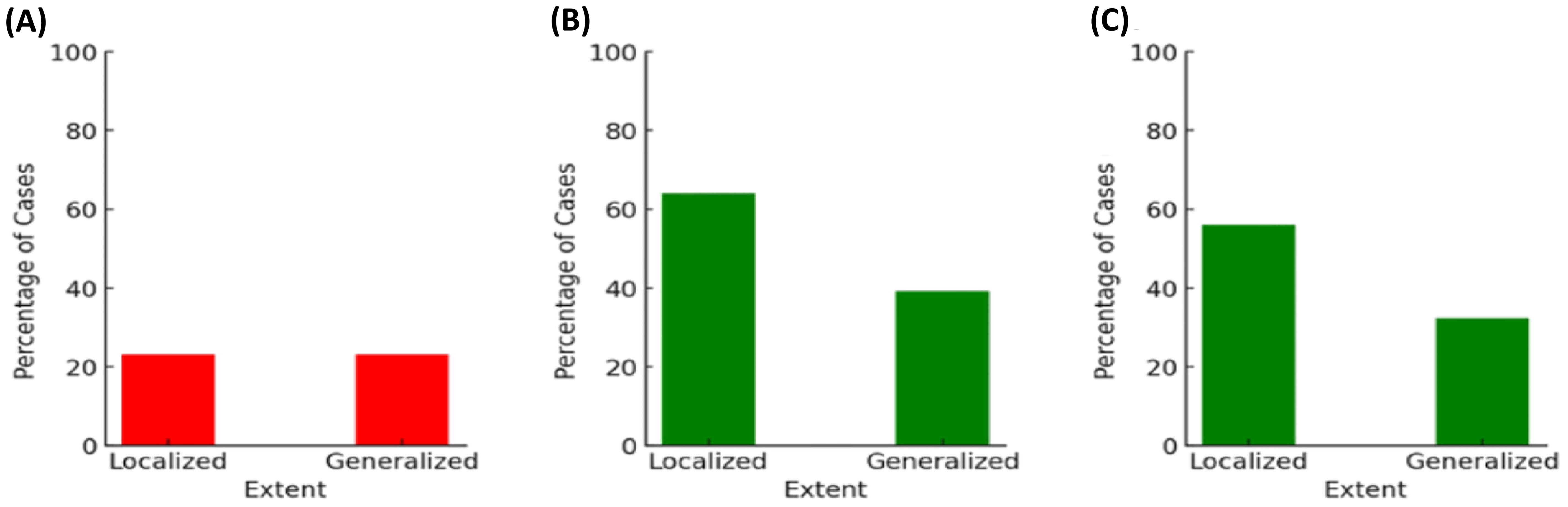
Figure 9. The extent of periodontitis analyzed regarding possible associations with the presence of A. actinomycetemcomitans (A; p=1.00), ftxA (B; p=0.027), and high loads of F. alocis in combination with the presence of ftxA (C; p=0.025), respectively. The percentages of cases (%) are on the basis of the 156 assessed individuals for A. actinomycetemcomitans, and 153 for F. alocis loads and ftxA, respectively.
3.3.6 History of periodontitisOur present data indicate that individuals with a history of periodontal disease in the family exhibited a higher prevalence of ftxA-positivity, representing nearly 50% (n=42) of the cases (Figure 10). In contrast, only ≈35% (20 cases) of individuals without a history of periodontitis were found to be ftxA-positive. This suggests a strong link between a history of periodontitis in the family and carriage of an ftxA-positive F. alocis. Moreover, ≈45% (37 cases) of the individuals with a family history of periodontitis appeared to exhibit both high levels of F. alocis and the presence of ftxA, compared to those without a history of periodontitis (25%, 14 cases). This suggests a strong link between a family history of periodontitis and the presence of ftxA, and/or simultaneous carriage of high loads of F. alocis and the presence of ftxA.
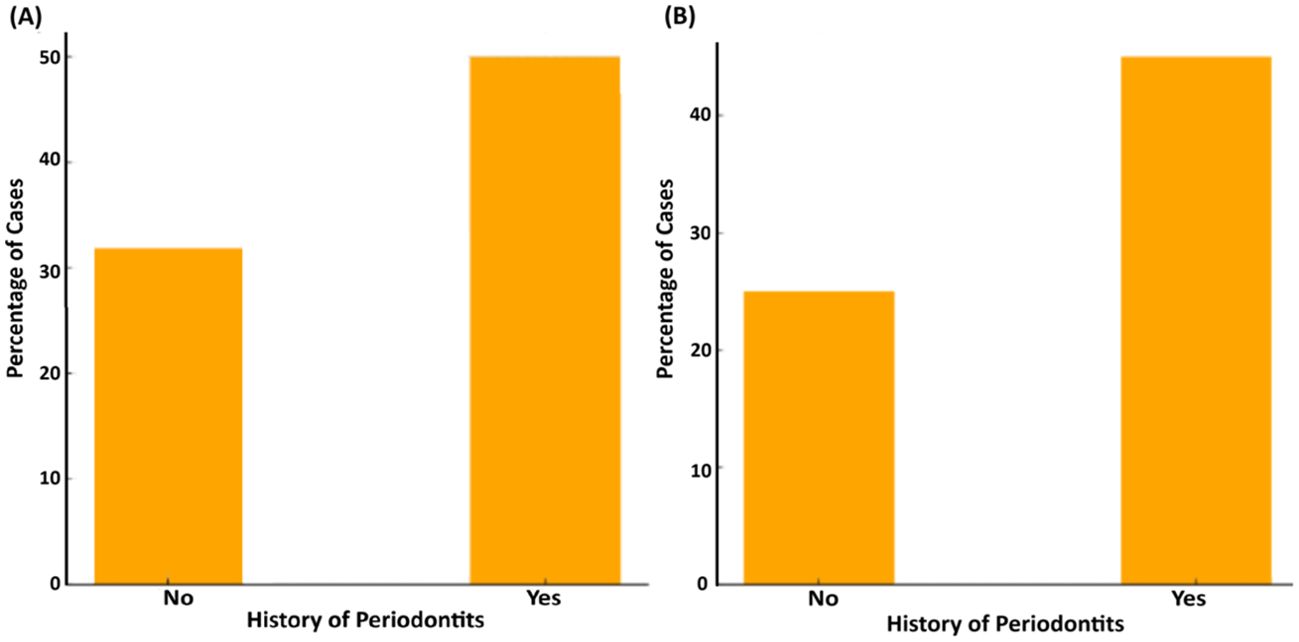
Figure 10. Association between history of periodontitis in the family and the presence of ftxA (A; p=0.061), and the combined presence of high F. alocis loads and ftxA (B; p=0.016). Frequency of cases is displayed as a percentage (%) of the total number of assessed cases (n=153).
3.3.7 GenderFinally, we addressed the loads of F. alocis (high versus low) related to gender in the sampled population (n=153; Figure 11). This revealed that for the carriers of low F. alocis levels, men represented a higher proportion of cases (26; 65%), compared to women (14 cases; 35%). Among the carriers of high F. alocis loads, women represented a higher proportion with 69 cases (61%) compared to men (44; 39%). These results highlight potential gender-related differences related to the levels of F. alocis within the studied population.
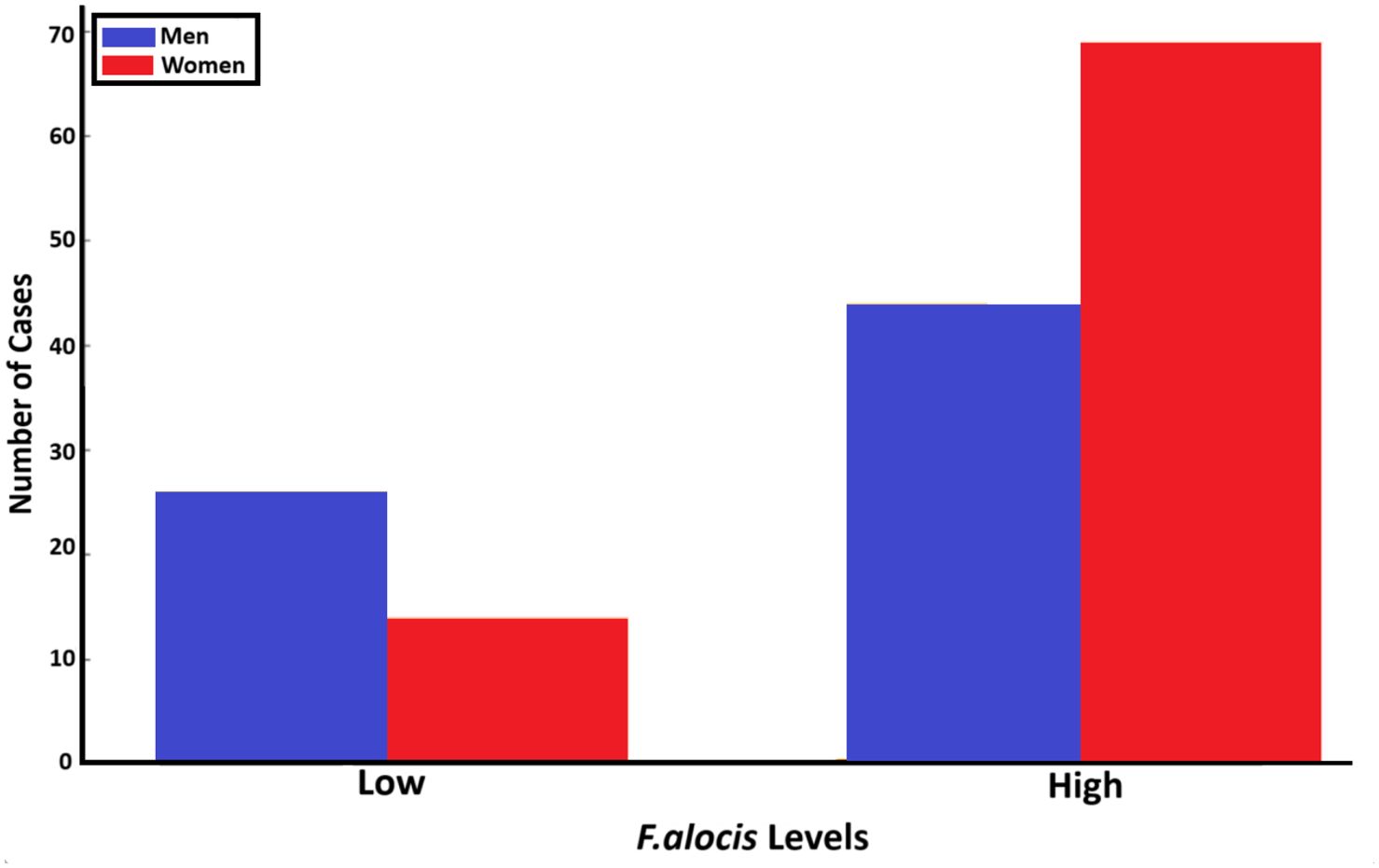
Figure 11. Relationship between the gender of individuals in the studied population (n=153) and levels of F. alocis carried (low and high, respectively) (p=0.011).
4 DiscussionIn the present work, we used qPCR and PCR to characterize the prevalence and carriage loads of the emerging oral pathogen F. alocis, and its RTX-toxin-encoding gene ftxA, respectively, in salivary specimens previously sampled from 156 patients (aged 26-86 years), all diagnosed with periodontitis (Khzam et al., 2024). The obtained results were thereafter compared with our qPCR data on the presence or absence of A. actinomycetemcomitans, and earlier recorded clinical and demographic data for eight parameters (including age, bone loss/age, extent, grade and stage of periodontitis, PPD, and CAL) from these individuals (Khzam et al., 2024).
Despite periodontitis being a complex multifactorial disease, involving multiple host factors and complex microbiota, our focus was here, in the present approach, essentially on a single bacterial organism, F. alocis, to expand our knowledge of the virulence characteristics of individual bacteria linked to clinical parameters. F. alocis has attracted further interest recently after the discovery of genotypes that express FtxA, via its gene ftxA, which is the hitherto second identified RTX toxin in the oral cavity after the well-studied leukotoxin of the periodontopathogen A. actinomycetemcomitans (Johansson, 2011; Oscarsson et al., 2020; Bao et al., 2022; Ozuna et al., 2022). The functional mechanism(s) of FtxA are not yet known. However, interestingly, a potential role of FtxA in the virulence of F. alocis in periodontal disease was recently demonstrated upon assessment of the longitudinally followed Ghana adolescent cohort, revealing that carriers of ftxA-positive F. alocis typically exhibited higher loads of the bacterium and also had an enhanced prevalence of CAL progression (Razooqi et al., 2022; Razooqi et al., 2024). The rationale for selecting a population with all individuals (n=156) diagnosed with periodontitis in the present work was its suitability in allowing further assessment of the role of F. alocis and its expressed FtxA in periodontal disease.
The presence of F. alocis perse in the samples appeared not to be clearly associated with any clinical or demographic parameter, which most likely was due to the high prevalence of this bacterium in the present study population (89%), which could mask any potential differences. However, consistent with our studies on the Ghanaian adolescent cohort (Razooqi et al., 2022; Razooqi et al., 2024), there was also a strong association in a recently tested West Australian population between ftxA positivity and elevated F. alocis levels. This suggests a potential role of FtxA in enhancing the pathogenicity of F. alocis. Whether this may be via enhancing the proliferation of F. alocis in the periodontal pocket and/or by augmenting its virulence via host cell modulation is not known. The correlation between ftxA positivity and enhanced bone loss, and moreover with the diagnosed extent of periodontitis (local and generalized), support the notion that presence of this RTX toxin gene increases the risk for more severe disease among carriers. As might be expected, the simultaneous presence of F. alocis at high levels and ftxA in samples revealed a cumulative effect as evidenced by a significantly greater disease severity, again as measured by bone loss and extent of periodontitis. These correlations suggest that ftxA could serve as a biomarker for identifying individuals at higher risk for rapid progression of periodontitis, highlighting its potential value in clinical diagnostics.
A potentially synergistic behavior between F. alocis and A. actinocytetemcomitans was initially implicated based on observations using the human oral microbe infection microarray (HOMIM), concluding that A. actinomycetemcomitans, Streptococcus parasanguinis, and F. alocis together may represent a consortium in the causation of localized aggressive periodontitis, by indicating sites of future bone loss (Fine et al., 2013). This notion has since then been further supported by us and others by analyzing the Ghanaian longitudinal adolescent cohort, reflecting that in a potential scenario in which these species are co-present in the periodontal pocket that A. actinomycetemcomitans may be successively out-competed by F. alocis (Dahlén et al., 2014; Razooqi et al., 2022; Razooqi et al., 2024). This was supported by our present observations of clinical readouts reflecting CAL. Moreover, in the present work, it was revealed that among the individuals carrying A. actinomycetemcomitans, a majority also exhibited an ftxA-positive F. alocis. Regarding the age groups, it became apparent that ftxA-positivity and combined high loads of F. alocis were both clearly associated with the older group (>40 years) in the present study population. This suggests that FtxA might contribute to enhancing the survival chances of this bacterium within the periodontal pocket during progression of the disease.
This was also evident when assessing the association of these parameters with the presence of A. actinomycetemcomitans. These observations further support the potential use of implementing diagnostic procedures based on the detection of the simultaneous presence of ftxA-positive F. alocis and A. actinomycetemcomitans. Finally, the observation that women in the assessed population exhibited a higher prevalence and higher loads of F. alocis compared to men may indicate that hormonal regulation may affect the behavior of this species in the periodontal or oral environment in general (Sharma et al., 2018; Gil-Montoya et al., 2021; Park et al., 2023).
5 ConclusionsWe conclude that high loads of F. alocis and the presence of its ftxA gene together are associated with the occurrence and severity of periodontitis. This emphasizes that F. alocis and the expression of its ftxA gene are associated with the progression of the disease. A further observation of ecological significance is that ftxA-positive F. alocis strains more frequently co-existed with A. actinomycetemcomitans, and an observation of diagnostic potential is that these ecological changes were associated with periodontal tissue destruction (i.e., increased CAL). These findings pave the way for more studies on the pathogenic potential, risk assessment value, and diagnostic utility of F. alocis and its FtxA, potentially also based on the simultaneous detection of ftxA and A. actinomycetemcomitans.
Data availability statementThe datasets presented in this article are available. Requests to access the datasets should be directed to amFuLm9zY2Fyc3NvbkB1bXUuc2U=.
Ethics statementThe studies in the present work were approved by the ethical committee of University of Western Australia (Ref no. 2022/ET000252; June 13, 2022; and updated April 5, 2023), and by Swedish Ethical Review Authority (dnr 2024-01704-01). The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributionsZR: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. NK: Conceptualization, Data curation, Investigation, Resources, Visualization, Writing – original draft, Writing – review & editing. ML: Formal analysis, Investigation, Visualization, Writing – original draft, Writing – review & editing. GB: Funding acquisition, Writing – original draft, Writing – review & editing. AJ: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Supervision, Visualization, Writing – original draft, Writing – review & editing. JO: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing.
FundingThe author(s) declare financial support was received for the research, authorship, and/or publication of this article. This research was funded by TUA grants from the County Council of Region Västerbotten, Sweden (to JO; grant number 7003766, and to AJ; 7003193), and by grants from the Swedish Research Council (to GB; grant number 2022-01014), and the Medical Faculty of Umeå University (Insamlingsstiftelsen; to JO and AJ, respectively). This work was in addition supported by funds from Anna Cederbergs stiftelse för medicinsk forskning (to ZR), and from The Kempe Foundation (to ZR).
AcknowledgmentsWe are grateful to Björn Tavelin, a statistician at the Department of Radiation Sciences, Umeå University, for help with the statistical calculations. We also wish to thank Carina Öhman, and Ewa Strömqvist Engbo for important technical assistance, and Rolf Claesson for valuable scientific discussions.
Conflict of interestThe authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s noteAll claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
ReferencesAruni, W., Chioma, O., Fletcher, H. M. (2014). Filifactor alocis: the newly discovered kid on the block with special talents. J. Dent. Res. 93, 725–732. doi: 10.1177/0022034514538283
PubMed Abstract | Crossref Full Text | Google Scholar
Bao, K., Claesson, R., Gehrig, P., Grossmann, J., Oscarsson, J., Belibasakis, G. N. (2022). Proteomic characterization of the oral pathogen Filifactor alocis reveals key inter-protein interactions of its RTX toxin: FtxA. Pathog. 11, 590. doi: 10.3390/pathogens11050590
PubMed Abstract | Crossref Full Text | Google Scholar
Bouziane, A., Hamdoun, R., Abouqal, R., Ennibi, O. (2020). Global prevalence of aggressive periodontitis: A systematic review and meta-analysis. J. Clin. Periodontol 47, 406–428. doi: 10.1111/jcpe.13266
PubMed Abstract | Crossref Full Text | Google Scholar
Brogan, J. M., Lally, E. T., Poulsen, K., Kilian, M., Demuth, D. R. (1994). Regulation of Actinobacillus actinomycetemcomitans leukotoxin expression: analysis of the promoter regions of leukotoxic and minimally leukotoxic strains. Infect. Immun. 62, 501–508. doi: 10.1128/iai.62.2.501-508.1994
PubMed Abstract | Crossref Full Text | Google Scholar
Dahlén, G., Claesson, R., Åberg, C. H., Haubek, D., Johansson, A., Kwamin, F. (2014). Subgingival bacteria in Ghanaian adolescents with or without progression of attachment loss. J. Oral. Microbiol. 6, 23977. doi: 10.3402/jom.v6.23977
PubMed Abstract | Crossref Full Text | Google Scholar
Eke, P. I., Thornton-Evans, G. O., Wei, L., Borgnakke, W. S., Dye, B. A., Genco, R. J. (2018). Periodontitis in US adults: national health and nutrition examination survey 2009-2014. J. Am. Dent. Assoc. 149, 576–588 e6. doi: 10.1016/j.adaj.2018.04.023
PubMed Abstract | Crossref Full Text | Google Scholar
Fine, D. H., Markowitz, K., Fairlie, K., Tischio-Bereski, D., Ferrendiz, J., Furgang, D., et al. (2013). A consortium of Aggregatibacter actinomycetemcomitans, Streptococcus parasanguinis, and Filifactor alocis is present in sites prior to bone loss in a longitudinal study of localized aggressive periodontitis. J. Clin. Microbiol. 51, 2850–2861. doi: 10.1128/JCM.00729-13
PubMed Abstract | Crossref Full Text | Google Scholar
Fine, D. H., Schreiner, H., Velusamy, S. K., Aggregatibacter, A. (2020). Low abundance pathobiont that influences biogeography, microbial dysbiosis, and host defense capabilities in periodontitis: the history of A bug, and localization of disease. Pathog. 9, 179. doi: 10.3390/pathogens9030179
PubMed Abstract | Crossref Full Text | Google Scholar
Gil-Montoya, J. A., Garrido-Martinez, M., Barrios-Rodriguez, R., Ramos-Garcia, P., Lenouvel, D., Montes-Castillo, C., et al. (2021). Association between low bone mineral density and periodontitis in generally healthy perimenopausal women. J. Periodontol 92, 95–103. doi: 10.1002/JPER.20-0029
留言 (0)