Spinal cord injury without radiographic abnormalities (SCIWORA) was first proposed by Burke in 1974 (1) and subsequently defined by Pang and Wilberger in 1982 as an acute spinal cord injury (TSCI) (2) caused by trauma, with no evidence of vertebral fractures or dislocations on x-ray or CT scans. SCIWORA accounts for 13%–42% of all pediatric spinal cord injuries, with the majority of cases occurring in children aged 3–10 years (3). This study retrospectively analyzed the clinical data of four SCIWORA patients treated at our hospital from November 2022 to June 2024, exploring their injury mechanisms, clinical features, spinal cord imaging changes, treatment, and prognosis to provide a reference for the management of SCIWORA.
2 Subjects and methodsClinical data of children diagnosed with SCIWORA admitted to our hospital from November 2022 to June 2024 were collected, including age, gender, cause of injury, injury site, diagnostic imaging methods, treatment strategies, neurological function grading, and prognosis.
Upon emergency admission, all patients underwent neurological examinations to assess their motor and sensory functions. The neurological status was evaluated according to the American Spinal Injury Association (ASIA). classification. x-ray and CT were used to assess fractures, subluxations, dislocations, deformities, and soft tissue injuries. Routine magnetic resonance imaging (MRI) was further used to evaluate spinal cord and soft tissue injuries.
3 General informationAll four cases had a clear history of thoracolumbar trauma, with three females and one male; their ages were 3 years and 2 months, 5 years and 1 month, 5 years and 6 months, and 8 years (Table 1). The mechanisms of injury were: one case of lower back injury, one case of lumbar sprain, and two cases of low falls. Before the injuries, none of the patients exhibited any neurological dysfunction. Two patients experienced immediate symptoms of lower limb weakness, sensory loss, and bladder and bowel dysfunction, while the other two developed symptoms a few hours to a few days post-injury. All four patients underwent decompression surgery and termination of the filum terminale. During surgery, significant edema of the conus medullaris and thickening of the filum terminale were observed (Figures 1, 2, 3).
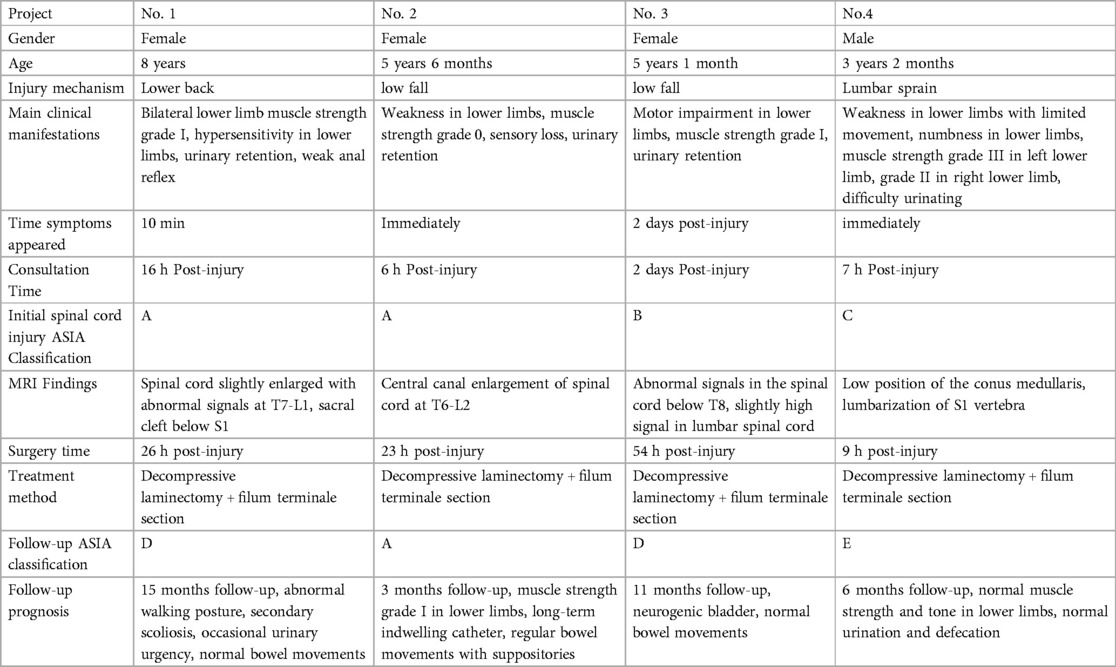
Table 1. General information.
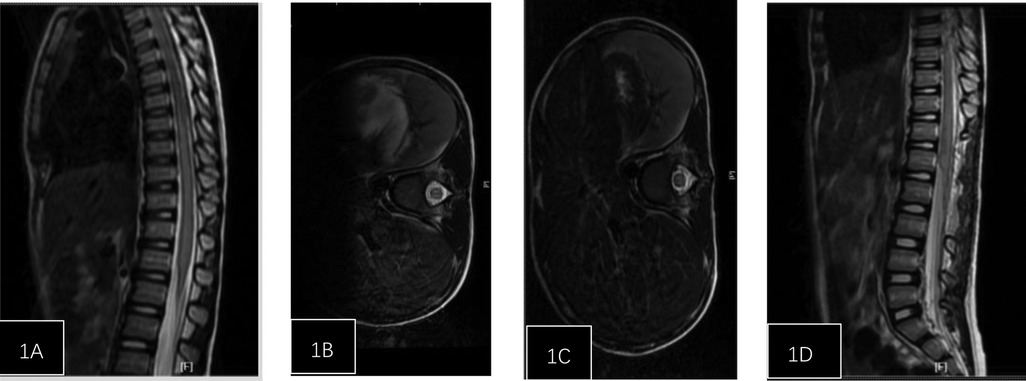
Figure 1. Patient, female, 5 years and 1 month, injured from a fall. (A) Post-injury spinal MRI shows edema of the spinal cord from T6 to L2 and dilatation of the central canal. (B) Axial T2-weighted imaging shows high signal intensity. (C) One week post-surgery follow-up spinal MRI shows reduced edema compared to pre-treatment, with no central canal dilation observed. (D) Axial T2-weighted imaging.
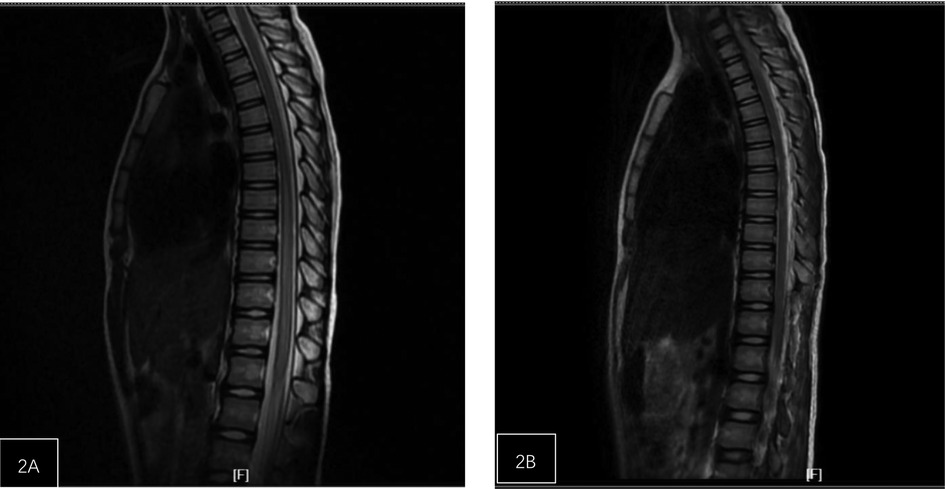
Figure 2. Patient, female, 8 years old, with lower back injury. (A) The spinal cord at the T7-L1 level is slightly enlarged with abnormal signal. (B) One-week post-operation, the range of abnormal signals in the spinal cord at the t7-L1 level has significantly decreased.
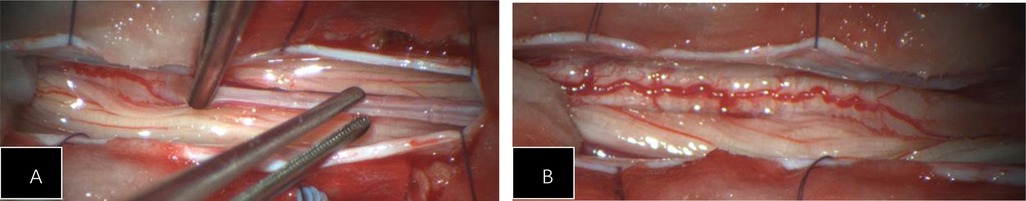
Figure 3. (A) During the surgery, significant edema of the conus medullaris was observed, with thickening and high tension of the filum terminale. (B) The spinal cord is congested and edematous, with poor pulsation.
4 Follow-up resultsOver a follow-up period of 3–24 months, three patients showed varying degrees of recovery in muscle strength and/or sensory function, as well as bladder and bowel function, while one showed no significant improvement. One patient developed scoliosis, and another developed neurogenic bladder.
5 DiscussionThe incidence of SCIWORA in children is between 8% and 32%, with rates of 9.4%in the 3–12 age group and 5% in the 13–20 age group (4). In a meta-analysis by Jazayeri et al., traffic accidents were the most common cause of TSCI injuries in children from developed countries, primarily involving cervical spinal cord injuries, while in developing countries, sports-related injuries were most common, primarily affecting the thoracic spinal cord (5), In this group, all four cases involved thoracolumbar injuries, with ages ranging from 3 to 8 years, and the injuries were caused by accidental trauma to the lumbar region. Intraoperative findings in all four pediatric cases revealed spinal cord tethering, with evidence of fatty or fibrous degeneration involving the filum terminale.
SCIWORA typically occurs following excessive extension of the spine. Early symptoms may include pain, numbness, or weakness in the lower back or legs. Although most children can stand and walk immediately after the injury, symptoms may rapidly worsen within hours, presenting with typical spinal cord injury symptoms below the level of injury, such as loss of sensory and motor function, as well as urinary and bowel dysfunction. The level of neurological damage is often between T4 and T12, with the most common lesions located above and below T10 (6). In this group, symptoms resulted in paralysis below the T10 level, accompanied by incontinence.
The mechanisms of SCIWORA injuries include longitudinal traction, excessive flexion, excessive extension, direct compression injuries, or a combination of these, as well as ischemic spinal cord injuries (7).
Spinal Cord Tethering Syndrome (TCS) encompasses a spectrum of skeletal, neurological, and urogenital symptoms due to the fixation of inelastic tissue on the conus medullaris, resulting in cord stretching and restricted mobility within the spinal canal. Characteristic findings on lumbar magnetic resonance imaging (MRI) may include a low-lying conus medullaris or a pathologic filum terminale that is thickened and/or fatty. MRI often reveals additional pathologies, such as congenital or acquired lumbosacral anomalies. The emergence of TCS symptoms typically coincides with pediatric growth spurts, attributed to the disparity in growth rates between the musculoskeletal components of the spinal column and the neuroaxis, leading to excessive strain on the tethered spinal cord (8).
The elasticity of the pediatric spine is greater than that of the spinal cord; under the same external force, the spine may not show obvious damage, while the spinal cord could suffer severe injury (9). Excessive spinal extension, such as during backward bending, can result in vascular damage, bleeding, and edema in the spinal cord, potentially causing obstruction of venous return. This may lead to increased venous pressure in the spinal cord and a decrease in the arterial-venous pressure gradient (10). This mechanism could be due to compression of the inferior vena cava by abdominal organs, coupled with increased pressure in the epidural venous plexus caused by the Valsalva maneuver, collectively exacerbating the obstruction to venous outflow from the spinal cord. Such venous return obstruction may further trigger insufficient spinal cord blood perfusion, venous thrombosis, and increased vascular permeability, damaging the blood-spinal cord barrier, thereby exacerbating secondary spinal cord injury and, in severe cases, leading to spinal cord infarction and atrophy (11,12). Furthermore, spinal cord injury (SCI) can interfere with the normal cerebrospinal fluid (CSF) circulation or impact the microcirculation within the spinal cord parenchyma, leading to ischemia and localized hypoxia. Chen et al. reported that among 74 pediatric SCI cases, 20 had occult spinal dysraphism, with 4 exhibiting fatty degeneration of the filum terminale (13). Liang et al. (7) reported three pediatric cases of SCIWORA complicated by fibrofatty filum terminale, suggesting that tight filum terminale (TFT) may be a predisposing factor for SCIWORA. They emphasized that chronic traction on the spinal cord could play a significant role in the pathogenesis of thoracolumbar SCIWORA in children after minor trauma. Patients who have not received treatment for TFT may be at higher risk for developing SCIWORA following minor trauma. Yuan et al. (14) also reported on eight cases of SCIWORA, suggesting that the presence of filum terminale syndrome, a form of tethered cord, may be associated with SCIWORA, and that early sectioning of the filum terminale might potentially reduce the risk of further spinal cord injury.
Additionally, even with the conus medullaris in a normal position, tethered cord syndrome cannot be ruled out, as tension in the filum terminale may lead to longitudinal traction on the spinal cord. This traction could exert pressure and cause injury to the spinal cord during body movements, especially during spinal flexion (15). Literature indicates that in asymptomatic SCI patients, over 50% of radiological findings may show suspected tethered cord syndrome and/or early signs of syringomyelia (16). Abnormalities in the filum terminale, such as increased width, lipomas, or fibrosis, may lead to a loss of elasticity, resulting in anchoring of the conus medullaris and pulling on the caudal portion of the spinal cord, worsening spinal cord injury (17). In this group, all four pediatric cases had fatty degeneration of the filum terminale confirmed during surgery. Preoperative MRIs did not show thickening of the filum terminale, but the conus medullaris displayed significant edema and morphological abnormalities, indicating some traction affecting the spinal cord. Therefore, we believe that even when the conus medullaris appears in a normal plane on imaging, it is essential to carefully evaluate the morphology of the conus, the filum terminale, and other spinal structures to determine if any biomechanical issues may lead to spinal cord injury, warranting timely surgical intervention.
The treatment of SCI in children and adolescents remains controversial. Current standard treatments include high-dose steroid pulse therapy and surgery. There is ongoing controversy regarding the safety of steroid treatment, and some literature indicates that high-dose methylprednisolone is not associated with improved outcomes (18). The biological principle behind early surgical decompression after acute spinal cord injury is to mitigate secondary damage. Data suggest that surgical decompression post-injury may alleviate secondary damage and improve neurological outcomes, with effects inversely related to the duration of compression (19, 20). Some scholars advocate for decompression surgery only in cases of spinal cord compression with neurological deficits, but a meta-analysis by Batchelor showed that early decompression improved functional outcomes by 35.1% (21). In a rat model of SCIWORA by Okimatsu et al., both acute and subacute decompression surgeries were effective, with earlier surgeries leading to faster recovery (22).
It has been reported that patients receiving early decompression within 24 h of SCI have 2.8 times greater likelihood of achieving at least a 2-level improvement on the ASIA impairment scale (AIS) at six months compared to those undergoing late decompression (≥24 h post-SCI). Patients with early decompression classified as AIS B, C, and D showed an additional 6.3 points increase in motor recovery compared to late decompression patients (23). Badhiwala et al. conducted a pooled analysis of individual patient data from four multicenter studies on spinal cord injury conducted between 1991 and 2017 (n = 1,548). They found that patients undergoing early decompression (within 24 h of injury) showed significantly faster recovery of motor and sensory functions compared to those receiving late surgery (≥24 h post-injury) and noted that surgery within 36 h post-SCI may still be beneficial (24).
In this case series, four pediatric patients received proactive surgical intervention within 48 h of symptom onset, and all cases confirmed the presence of spinal cord tethering intraoperatively. Postoperative MRI at one week showed a notable reduction in the extent of abnormal spinal cord signals. We believe that timely surgery, complete decompression, and resolution of tethering, followed by duroplasty to expand the spinal canal, can significantly alleviate intraspinal pressure and enhance spinal cord perfusion. Duroplasty improves radiological and physiological parameters by expanding the space around the injured spinal cord, reducing intra-spinal pressure (ISP), and increasing spinal cord perfusion pressure (SCPP), thus mitigating or alleviating secondary spinal cord injuries. This strategy can reduce the incidence of neurologic deficits, improve outcomes, and enhance prognosis. Except for one child with no significant improvement in this series, the remaining patients had favorable outcomes. We consider the lack of improvement in this case may be attributed to a short follow-up period and preoperative MRI indicating syringomyelia, suggesting more severe spinal cord injury and prolonged traction ischemia.
Spinal deformities are common complications, especially in children with skeletal dysplasia. Prior to peak growth, 97% of children with SCI develop scoliosis, and being younger than 14.6 years is a predictive factor for scoliosis (25). Urinary dysfunction is a major cause of disability and mortality in young patients, who may also experience hip abnormalities, osteoporosis, and hypercalcemia. In our group, complications included scoliosis and neurogenic bladder, necessitating further rehabilitation (26).
This study acknowledges several limitations. Firstly, due to the rarity of SCIWORA, the sample size evaluated in this study was relatively small and the follow-up period was short-term. Secondly, while the study presents outcomes of surgical decompression, it lacks a comparative analysis with conservative treatment approaches. Therefore, the findings presented here will necessitate validation through a comparative, prospective study.
In summary, tethered cord syndrome may be a potential underlying cause of SCIWORA. For children with SCIWORA accompanied by tethered cord syndrome, we recommend early surgical intervention for decompression and simultaneous release of the tether, which can aid in the recovery of neurological function.
Data availability statementThe original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Ethics statementWritten informed consent was obtained from the individual(s), and minor(s)' legal guardian/next of kin, for the publication of any potentially identifiable images or data included in this article.
Author contributionsYY: Data curation, Methodology, Writing – original draft. YS: Writing – review & editing. ZA: Methodology, Writing – review & editing. FG: Methodology, Writing – original draft. ZL: Formal analysis, Writing – original draft. WX: Conceptualization, Writing – original draft.
FundingThe author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interestThe authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statementThe author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher's noteAll claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References3. Liu R, Fan Q, He J, Wu X, Tan W, Yan Z, et al. Clinical characteristics analysis of pediatric spinal cord injury without radiological abnormality in China: a retrospective study. BMC Pediatr. (2024) 24(1):236. doi: 10.1186/s12887-024-04716-z
PubMed Abstract | Crossref Full Text | Google Scholar
4. Meira Goncalves J, Carvalho S, Silva AI, Pereira J, Polónia P. Real spinal cord injury without radiographic abnormality (SCIWORA) in pediatrics: a clinical case report and literature review. Cureus. (2023) 15(12):e50491. doi: 10.7759/cureus.50491
PubMed Abstract | Crossref Full Text | Google Scholar
5. Jazayeri SB, Kankam SB, Golestani A, Shobeiri P, Gholami M, Dabbagh Ohadi MA, et al. A systematic review and meta-analysis of the global epidemiology of pediatric traumatic spinal cord injuries. Eur J Pediatr. (2023) 182(12):5245–57. doi: 10.1007/s00431-023-05185-9
PubMed Abstract | Crossref Full Text | Google Scholar
6. Zeng L, Wang YL, Shen XT, Zhang ZC, Huang GX, Alshorman J, et al. Guidelines for management of pediatric acute hyperextension spinal cord injury. Chin J Traumatol. (2023) 26(1):2–7. doi: 10.1016/j.cjtee.2022.07.005
PubMed Abstract | Crossref Full Text | Google Scholar
7. Liang QC, Yang B, Song YH, Gao PP, Xia ZY, Bao N. Real spinal cord injury without radiologic abnormality in pediatric patient with tight filum terminale following minor trauma: a case report. BMC Pediatr. (2019) 19(1):513. doi: 10.1186/s12887-019-1894-8
PubMed Abstract | Crossref Full Text | Google Scholar
8. Tsiptsios D, Sysoev K, Ouranidis A, Rizos E, Tsamakis K. Occult tethered cord syndrome: a reversible cause of paraparesis not to be missed. Childs Nerv Syst. (2020) 36(9):1833–4. doi: 10.1007/s00381-020-04768-3
PubMed Abstract | Crossref Full Text | Google Scholar
9. Ren J, Zeng G, Ma YJ, Chen N, Chen Z, Ling F, et al. Pediatric thoracic SCIWORA after back bend during dance practice: a retrospective case series and analysis of trauma mechanisms. Childs Nerv Syst. (2017) 33(7):1191–8. doi: 10.1007/s00381-017-3407-0
PubMed Abstract | Crossref Full Text | Google Scholar
10. Qi C, Xia H, Miao D, Wang X, Li Z. The influence of timing of surgery in the outcome of spinal cord injury without radiographic abnormality (SCIWORA). J Orthop Surg Res. (2020) 15(1):223. doi: 10.1186/s13018-020-01743-1
PubMed Abstract | Crossref Full Text | Google Scholar
12. Gandhi J, Lee MY, Joshi G, Khan SA. Surfer’s myelopathy: a review of etiology, pathogenesis, evaluation, and management. J Spinal Cord Med. (2021) 44(1):2–7. doi: 10.1080/10790268.2019.1577057
PubMed Abstract | Crossref Full Text | Google Scholar
13. Liu G, Jiang W, Tang X, Tan S, Zhang M, Tao L, et al. Spina bifida occulta is a risk factor for spinal cord injury without fracture or dislocation for children performing a backbend during dance. Front Pediatr. (2022) 10:903507. doi: 10.3389/fped.2022.903507
PubMed Abstract | Crossref Full Text | Google Scholar
14. Yuan B, Wang HZ, Wang YQ, Li ZW, Wang GY, Zhu BX, et al. Clinical analysis of low-energy fracture-free dislocation spinal cord injury complex with tethered spinal cord in children. Chin J Neurosurg. (2021) 37(10):1019–21. doi: 10.3760/cma.j.cn112050-20210621-00298
Crossref Full Text | Google Scholar
16. Bratelj D, Stalder S, Capone C, Jaszczuk P, Dragalina C, Pötzel T, et al. Spinal cord tethering and syringomyelia after trauma: impact of age and surgical outcome. Sci Rep. (2023) 13(1):11442. doi: 10.1038/s41598-023-38565-0
PubMed Abstract | Crossref Full Text | Google Scholar
17. Michael MM, Garton ALA, Kuzan-Fischer CM, Uribe-Cardenas R, Greenfield JP. A critical analysis of surgery for occult tethered cord syndrome. Childs Nerv Syst. (2021) 37(10):3003–11. doi: 10.1007/s00381-021-05287-5
PubMed Abstract | Crossref Full Text | Google Scholar
18. Thomas AX, Riviello JJ Jr, Davila-Williams D, Thomas SP, Erklauer JC, Bauer DF, et al. Pharmacologic and acute management of spinal cord injury in adults and children. Curr Treat Options Neurol. (2022) 24(7):285–304. doi: 10.1007/s11940-022-00720-9
PubMed Abstract | Crossref Full Text | Google Scholar
19. Badhiwala JH, Wilson JR, Harrop JS, Vaccaro AR, Aarabi B, Geisler FH, et al. Early vs late surgical decompression for central cord syndrome. JAMA Surg. (2022) 157(11):1024–32. doi: 10.1001/jamasurg.2022.4454
PubMed Abstract | Crossref Full Text | Google Scholar
20. Liu JM, Long XH, Zhou Y, Peng HW, Liu ZL, Huang SH. Is urgent decompression superior to delayed surgery for traumatic spinal cord injury? A meta-analysis. World Neurosurg. (2016) 87:124–31. doi: 10.1016/j.wneu.2015.11.098
PubMed Abstract | Crossref Full Text | Google Scholar
21. Batchelor PE, Wills TE, Skeers P, Battistuzzo CR, Macleod MR, Howells DW, et al. Meta-analysis of pre-clinical studies of early decompression in acute spinal cord injury: a battle of time and pressure. PLoS One. (2013) 8(8):e72659. doi: 10.1371/journal.pone.0072659
PubMed Abstract | Crossref Full Text | Google Scholar
22. Okimatsu S, Furuya T, Miura M, Shiratani Y, Yunde A, Inoue T, et al. Early decompression promotes motor recovery after cervical spinal cord injury in rats with chronic cervical spinal cord compression. Sci Rep. (2022) 12(1):14400. doi: 10.1038/s41598-022-14723-8
PubMed Abstract | Crossref Full Text | Google Scholar
25. Wang JZ, Yang M, Meng M, Li ZH. Clinical characteristics and treatment of spinal cord injury in children and adolescents. Chin J Traumatol. (2023) 26(1):8–13. doi: 10.1016/j.cjtee.2022.04.007
PubMed Abstract | Crossref Full Text | Google Scholar
26. Eswara JR, Castellan M, González R, Mendieta N, Cendron M. The urological management of children with spinal cord injury. World J Urol. (2018) 36(10):1593–601. doi: 10.1007/s00345-018-2433-1
留言 (0)