Renal cell carcinoma (RCC) is a common cancer worldwide, with 81,610 new cases and 14,390 deaths expected in the United States in the year 2024 (Siegel et al., 2024). As for metastatic kidney cancer, the median overall survival of metastatic renal cell carcinoma (mRCC) patients currently treated with immune checkpoint inhibitors (ICIs) is estimated to be approximately 50 months (Park et al., 2024). To date, mRCC remains one of the most challenging aspects of renal cancer treatment. Targeted therapy for metastatic tumors, as an emerging approach, holds broader application prospects and is crucial for improving patient outcomes. The foundation of targeted therapy lies in identifying new therapeutic targets. The mechanisms driving RCC metastasis are intricate and involve multiple pathways and molecules, as reported in the literature (Ganesh and Massagué, 2021). At the molecular level, related studies have shown that tumor metastasis depends on clonal selection, the potential of metastatic cells to dynamically transition to different states, and the ability to exploit the immune environment (Gerstberger et al., 2023). Up to now, a few reviews have focused on the molecular mechanisms of RCC progression, but no reviews have focused on the summary of mechanisms of RCC metastasis and linked them to targeted therapy particularly. In this review, we summarized the definition and common molecular mechanisms of renal cell carcinoma metastasis and proposed new insights into its potential connection with targeted therapies, which may enlighten future targeted therapy drug development for improving the outcome of mRCC patients.
2 Clinical background of renal cell carcinomaClear cell renal carcinoma (ccRCC) accounts for 70%–80% of all pathologic types of RCC, with other types of RCC including papillary, chromophobe, medullary, collecting duct, microphthalmia (MiT) family translocation, succinate dehydrogenase–deficient, hereditary leiomyomatosis and syndrome-associated, and unclassified RCC (Barata et al., 2024; Wang et al., 2024). The most common symptoms of kidney cancer are hematuria or microscopic hematuria, lateral dorsal or flank pain, and palpable abdominal mass. However, due to its retroperitoneal location, RCC can grow significantly without causing symptoms. In some cases, metastatic lesions are detected before the primary kidney tumor is identified through systemic examination and pathology (Petejova and Martinek, 2016; Singla et al., 2022). The initial diagnosis of RCC relies on imaging modalities, and enhanced CT and MRI provide strong evidence for the detection of malignant mass (Bahadoram et al., 2022). Liquid biopsy has made significant progress in RCC through the use of circulating tumor cells (CTCs), ctDNA/cfDNA, cfRNAs, exosomes, and tumor-derived metabolites or proteins, with advancements in next-generation sequencing (NGS), droplet digital PCR (ddPCR), and methylation analysis enhancing the accuracy and applicability of ctDNA/cfDNA. However, challenges remain, including low CTC levels, difficulty distinguishing ctRNAs from cfRNAs, complex miRNA regulatory networks, and insufficient standardization of biomarker analysis (Li M. et al., 2023). Imaging diagnostics for RCC have seen advancements with agents like 68Ga-DPI-4452 and 68Ga-FAPI-PET/CT showing high sensitivity and specificity for detecting ccRCC, alongside CD8 PET imaging for predicting responses to immune checkpoint inhibitors (ICIs). Nonetheless, the long clearance time of high molecular weight tracers increases patient radiation burden, and small molecule tracers with rapid clearance are in need (Ali et al., 2024). While kidney biopsy is essential for histopathologic confirmation in certain cases, its use is controversial due to concerns about influencing treatment decisions, potential complications like bleeding and infection, and questions about accuracy and safety. Standard treatments like nephrectomy often provide the necessary histopathologic evidence (Bahadoram et al., 2022). Of all patients diagnosed with RCC, 20%–30% have metastasis at initial presentation, yet 20%–40% of patients with RCC still develop recurrence and metastasis after radical nephrectomy (Teishima et al., 2023; Vig et al., 2020).
For non-metastatic renal mass, the preferred treatment is surgical resection. For specific patients, a kidney-preserving partial nephrectomy prioritizing the achievement of negative surgical margins is recommended. Based on clinical indications, radical nephrectomy is indicated for patients with an increased oncologic risk as well as for patients scheduled for targeted therapy (Gray and Harris, 2019). In recent years, several agents have been developed to improve the prognosis of metastatic and recurrent RCC patients. These agents target the vascular endothelial growth factor (VEGF) such as bevacizumab and its receptor (VEGFR) such as sunitinib, mammalian target of rapamycin (mTOR) such as temsirolimus, immunocheckpoints programmed death-1 (PD-1) such as nivolumab and its ligand (PD-L1) such as atezolizumab, and cytotoxic T lymphocyte antigen 4 (CTLA-4) such as ipilimumab (Aurilio et al., 2019). Although advances have been made in some aspects of the diagnosis, screening, and treatment of RCC, the survival of patients with metastatic RCC remains unsatisfactory compared to other cancers (Ryalen et al., 2023; Wu et al., 2023a). In addition, drug resistance and side effects of drug therapy are shortcomings that cannot be ignored (Li X. et al., 2024). This means that more researches are still in need to discover new targets, optimize existing therapies, and ultimately improve the outcome and quality of life of mRCC patients.
3 Pathology of metastatic renal cell carcinomaHematogenous metastasis is one of the most common modes of metastasis for kidney cancer, and other common modes of RCC metastasis include lymphatic metastasis and direct extension (Huang et al., 2017). The most common sites of metastasis for renal cell carcinoma are the renal vein and its branches, the inferior vena cava, lung, bone, liver, lymph nodes, adrenal gland, and brain (Liu et al., 2021; Matuszczak et al., 2023). In recent years, metastasis of renal cell carcinoma to the larynx, mandible, eyelids, small intestine, appendix, and penis has also been reported in the literature (Ahmed et al., 2023; Bellouki et al., 2023; Cho et al., 2024; Elmusa et al., 2022; Huang and Yu, 2023; Ushakova and Ravakhah, 2023). The TNM staging system of renal cell carcinoma is considered to be the criterion for evaluating the metastasis of RCC, which contains tumor (T), node (N), and metastasis (M) classification. Localized RCC (LRCC) stands for RCC staging T1-2N0M0, advanced RCC consists of the remaining stages, while metastatic renal cell carcinoma is defined as tumor invasion through the perirenal fascia and/or presence of distant metastasis (Chen et al., 2021; Zhang et al., 2022). An interesting study showed that histological subtype, nuclear grade, and sarcomatoid differentiation are important predictors of metastasis in patients with RCC (Park, 2023). Besides, another retrospective cohort study showed that RCC WHO/ISUP grades are associated with metastasis, with the high-grade group (Grade 3–4) being more likely to metastasize than the low-grade group (Grade 1–2) (Fujita et al., 2022).
4 Molecular mechanisms of renal cell carcinoma metastasis4.1 Signaling pathway activation and inhibition4.1.1 Wnt/β-catenin signaling pathwayThe overexpression of centromere protein A (CENPA) would regulate the cell cycle and activate the Wnt/β-catenin signaling pathway, resulting in ccRCC proliferation and metastasis (Wang et al., 2021). Moreover, Sun et al. found the Follistatin-like 3 (FSTL3) regulates the GSK-3β/β-catenin and BMP1/SMAD pathways, enabling RCC proliferation and metastasis (Sun et al., 2021). In addition, a lncRNA named SLERCC inhibits RCC progression and metastasis by inhibiting the Wnt/β-catenin signaling pathway and directly binding to UPF1. However, high DNMT3A expression recruits DNMT3A to the SLERCC promoter region, inducing aberrant hypermethylation and ultimately inhibiting SLERCC expression (Mao et al., 2022).
4.1.2 PI3K/AKT signaling pathwayMao and colleagues reported that ciRS-7 can act as a sponge for miR-139-3p, a microRNA that inhibits RCC cell proliferation, migration, and invasion. However, based on targeting TAGLN, ciRS-7 can activate the PI3K/AKT signaling pathway by regulating the miR-139-3p/TAGLN axis, thus assisting the proliferation and metastasis of RCC cells (Mao et al., 2021). Additionally, DEP domain containing 1 (DEPDC1) promotes glycolysis in RCC via the AKT/mTOR/HIF1α pathway, which in turn affects tumor metastasis and TKI resistance (Di et al., 2024). Another study found that tescalcin promotes cell proliferation, migration, and invasion via the NHE1/pHi axis and AKT/NF-κB signaling pathway (Luo et al., 2019). Besides, AGK also promotes RCC metastasis through the PI3K/AKT pathway as mentioned above (Zhu et al., 2020). Moreover, centrosomal protein 55 (CEP55) could promote the upregulation of E-cadherin and downregulation of N-cadherin and ZEB1 via PI3K/AKT/mTOR pathway, resulting in RCC epithelial-mesenchymal transition (EMT), proliferation, and metastasis (Chen et al., 2019).
4.1.3 NF-κB signaling pathwayResearchers found that tumor necrosis factor α (TNFα) activates the NF-κB pathway in RCC cells, leading to p65 binding at the Rictor promoter and ultimately promoting cancer metastasis (Sun et al., 2016). Further, metastasis-associated gene 1 (MTA1) is overexpressed in RCC and regulates the expression of MMP2/MMP9 as well as E-calmodulin through the NF-κB signaling pathway, leading to RCC migration and invasion (Lv et al., 2020). Moreover, sphingosine 1-phosphate (S1P) promotes the proliferation, migration, and EMT of RCC cells through activation of its receptor S1PR3, thereby accelerating RCC carcinogenesis and metastasis. This process involves the S1PR3/Gi/p38/Akt/p65/cyclin D1-CDK4 signaling pathway to regulate cell proliferation, and the S1PR3/Gi/q/ERK/p38/p65 signaling pathway to regulate cell migration (Yan et al., 2022). As mentioned, Tescalcin is also associated with NF-κB signaling pathway (Luo et al., 2019).
4.1.4 Other pathwaysMetadherin (MTDH) activates SND1 to mediate ERK signaling and EMT, thereby promoting migration and metastasis (He et al., 2020). Additionally, MRCCAT1, a key lncRNA, inhibits NPR3 transcription by recruiting PRC2 to the NPR3 promoter region, therefore activating the p38-MAPK signaling pathway and promoting ccRCC metastasis (Li et al., 2017). Furthermore, ApoC1 could promote the activation of STAT3 and enhance the metastasis of ccRCC. Meanwhile, exosomes could transfer ApoC1 from the ccRCC cells to the vascular endothelial cells to promote tumor angiogenesis and metastasis via STAT3 pathway (Li et al., 2020).
4.2 Gene expression regulation4.2.1 Non-coding RNA regulationScientists have found that miR-148a-3p targets circUBAP2 in ccRCC, with its expression level negatively correlating with that of circUBAP2. The miR-148a-3p could reverse the inhibitory effect of circUBAP2 on ccRCC cell proliferation, migration, and invasion, and it could also target FOXK2 to affect ccRCC proliferation and metastasis (Sun et al., 2020). Dong’s team has found that lncRNA ZFAS1 could promote ccRCC growth and metastasis through the miR-10a/SKA1 pathway (Dong et al., 2019). Similarly, miR-100 promotes autophagy and inhibits migration and invasion of RCC cells by targeting NOX4 and inhibiting the mTOR signaling pathway (Liu et al., 2022). Besides, miR-139-3p would be inhibited by ciRS-7 to promote RCC metastasis (Mao et al., 2021).
Regarding lncRNAs, lncHILAR upregulates Jagged-1 and CXCR4 expression by acting as a ceRNA for miR-613/206/1-1-3p, thus activating the Jagged-1/Notch/CXCR4 signaling pathway and promoting RCC cell invasion and metastasis (Hu et al., 2021). Additionally, via binding to the promoter area of ERβ, lncRNA-SERB could regulate ERβ functions through transcriptional regulation of zinc finger E-box binding homeobox 1 (ZEB1), thus promoting vasculogenic mimicry (VM) formation (Tang et al., 2024).
In addition, circSDHC could protect CDKN3 from miR-127-3p inhibition by competitively binding to miR-127-3p, which in turn activated the E2F1 pathway and promoted RCC proliferation and invasion (Cen et al., 2021).
4.2.2 Transcription factor regulationLu et al. reported that KLF2 deficiency impairs the transcriptional repression of GPX4, inhibiting ferroptosis and thereby promoting ccRCC cell migration and invasion (Lu et al., 2021). Also, c-Myb could transcriptionally activate miR-520h, which would target MAGI1. Then, MAGI1 could stabilize the PTEN/MAGI1/β-catenin complex to modulate β-catenin signaling pathway, mediating RCC metastasis (Wang W. et al., 2019). Besides, HIF-2 transcriptionally targeted the hypoxia response element on the Polo-like kinase 1 (Plk1) promoter, which promoted Plk1 expression in ccRCC, leading to ccRCC growth, metastasis, and drug resistance (Dufies et al., 2021). Moreover, YBX1 could promote SPP1 expression by interacting with G3BP1, which in turn activates the NF-κB signaling pathway, ultimately leading to increased invasion and metastasis of RCC (Wang Y. et al., 2019). Interestingly, N-acetyltransferase 10 (NAT10) promotes ankyrin repeat and zinc finger peptidyl tRNA hydrolase 1 (ANKZF1) expression through N4-acetylcytidine (ac4C) modification, which in turn regulates YAP1 activity and activates the expression of pro-lymphangiogenic factors to promote lymphangiogenesis and tumor progression in ccRCC (Miao et al., 2024).
4.3 Protein modificationXu et al. found that circPOLR2A forms a UBE3C/circPOLR2A/PEBP1 protein-RNA ternary complex with UBE3C and PEBP1 proteins, which enhances UBE3C-mediated ubiquitination and degradation of PEBP1 proteins, which activates the ERK via ERK1/2 phosphorylation signaling pathway, thereby promoting angiogenesis (Xu et al., 2022). Acylglycerol kinase (AGK) activates the GSK3β S9 phosphorylation site via the PI3K/AKT pathway, leading to GSK3β inactivation, β-catenin stabilization, and subsequent promotion of RCC growth and metastasis (Zhu et al., 2020).
5 Mechanisms inhibiting renal cell carcinoma metastasis5.1 Signaling pathway regulationEF-hand domain family member D1 (EFHD1) binds to MCU through its N-terminal domain, suppressing mitochondrial Ca2+ uptake and thereby inactivating the Hippo/YAP signaling pathway (Meng et al., 2023). Similarly, leukemia inhibitory factor receptor (LIFR) attenuates ccRCC metastasis by upregulating Hippo signaling pathway kinase activity, which inhibits YAP expression (Lei et al., 2018). Yin’s team found that HOOK1 could inhibit RCC proliferation, metastasis, angiogenesis, and sunitinib resistance via TNFSF13B/VEGF-A signaling. Moreover, meletin, an agonist of HOOK1, demonstrates greater antitumor efficacy when combined with sunitinib or nivolumab compared to its use as a monotherapy (Yin et al., 2023). Additionally, MUC15 inhibits RCC cell invasion and metastasis through PI3K/AKT signaling (Yue et al., 2020). Nuclear receptor coactivator 7 (NCOA7) inhibits the MAPK/ERK pathway, regulating EMT and apoptosis and thereby inhibiting ccRCC progression and metastasis (Guo et al., 2023). Similarly, SH3BGRL2 inhibits ccRCC proliferation and metastasis by activating the LATS1/2-YAP-TEAD1 signaling pathway, and TEAD1 promotes EMT through TWIST1 upregulation (Yin et al., 2020). Zhang et al. found that thymoquinone induces autophagy in RCC cells by activating the AMPK/mTOR signaling pathway, which inhibits the EMT and metastasis of RCC cells (Zhang et al., 2018). Likewise, FOXC1 activates the AMPK signaling pathway and inhibits the mTOR signaling pathway by upregulating the expression of ABHD5 to inhibit the growth and metastasis of RCC cells (Li J. et al., 2024).
5.2 Transcription factor regulationThe cRAPGEF5 acts as a sponge of oncogenic miR-27a-3p, which targets the suppressor gene TXNIP, thus inhibiting RCC progression and metastasis (Chen et al., 2020). Additionally, melatonin reduces the DNA-binding activity of p65 and p52, thereby inhibiting MMP-9 transcriptionally and affecting its transcriptional activation and cell migration via Akt-mediated JNK1/2 and ERK1/2 signaling pathways. Besides, high MMP-9 expression correlates with a poorer RCC prognosis (Lin et al., 2016).
5.3 Protein modificationUbiquitin-specific peptidase 53 (USP53) prevents the inactivation of the NF-κB pathway by reducing ubiquitination of IκBα, thereby further inhibiting ccRCC proliferation and metastasis (Gui et al., 2021). Likewise, ubiquitin-specific peptidase 2 (USP2) downregulates the NF-κB pathway, inhibiting EMT in clear cell renal cell carcinoma metastasis (Duan et al., 2022). Luo and colleagues discovered that the outer mitochondrial membrane (OMM) protein MFN2 inhibits ccRCC tumor growth and metastasis by binding to the small GTPase Rab21, facilitating interaction with endocytosed EGFR in ccRCC. This process promotes docking of endocytosed EGFR to mitochondria, where it is subsequently dephosphorylated by OMM-resident tyrosine-protein phosphatase receptor type J (PTPRJ), leading to inactivation of the EGFR signaling pathway and attenuation of EGFR oncogenic signaling (Luo et al., 2023). Besides, EFHD1 would also upregulate STARD13 to enhance YAP protein phosphorylation at Ser-127 to suppress cell migration and metastasis (Meng et al., 2023). Moreover, IL6 mediates crosstalk between normal fibroblasts and RCC cells, promoting cell migration via the STAT3 pathway. Conversely, GATA3 reduces STAT3 phosphorylation, inhibiting RCC cell migration (Shi et al., 2020) (Table 1).
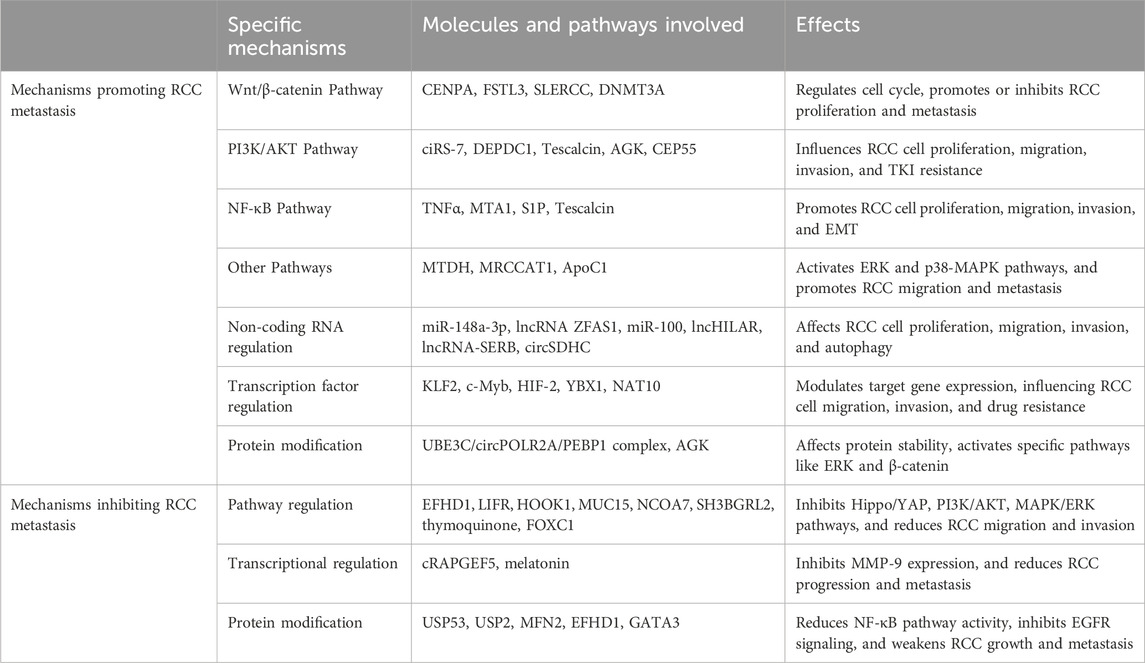
Table 1. Summary of the key mechanisms in promoting and inhibiting RCC metastasis.
6 Therapeutic approaches and current gapsCurrent major targeted therapies and potential drugs under investigation primarily focus on specific signaling pathways, including tyrosine kinase inhibitors (TKIs) and mTOR inhibitors. Pazopanib, a tyrosine kinase inhibitor, enhances the function of dendritic cells (DCs) by inhibiting the Erk/β-catenin pathway. This inhibition leads to the upregulation of maturation markers such as HLA-DR, CD40, and CCR7, reduces IL-10 production and endocytosis, and increases T-cell proliferation. Additionally, pazopanib downregulates PD-L1 expression. These effects collectively improve the antigen-presenting capability and immune-stimulating capacity of DCs, potentially augmenting the immune response in mRCC patients. Furthermore, pazopanib increases the population of circulating CD4+ T cells expressing CD137 (4-1BB), suggesting a potentially exploitable immunomodulatory effect that could be leveraged to improve responses when combined with immune checkpoint inhibitors (ICIs) in tailored treatment protocols (Zizzari et al., 2018). Besides, mTOR inhibitors have been the first-line treatment for mRCC. However, since the mTOR signaling axis is activated in only a subset of RCC patients, clinical trials involving mTOR inhibitors are ongoing (Zheng et al., 2021). A recent clinical trial revealed that the on-treatment tumor growth rate was 1.7 times higher for apitolisib (a dual PI3K/mTORC1/2 inhibitor) compared to everolimus (an mTORC1 inhibitor). The estimated half-life for the loss of treatment effect was 16.1 weeks for everolimus and 7.72 weeks for apitolisib, indicating a faster tumor regrowth rate for patients treated with apitolisib, possibly due to rapid resistance development (Moein et al., 2024). Another study found that MC-4, a novel Akt/PKM2 inhibitor, can potentially overcome the limitations of existing mTOR inhibitors. Therefore, combining MC-4 with current treatments may represent a promising new strategy for treating patients with rapidly progressing advanced RCC (Son et al., 2018). An intriguing study found that PI3K-mutant patient-derived xenograft (PDX) models exhibit significant resistance to TKI treatment. However, these PDX models seem to be sensitive to mTOR inhibitor treatment. Thus, combination therapies that incorporate drugs with different mechanisms of action, such as pairing mTOR inhibitors with TKI, could offer greater benefits to patients. Combination therapy strategies simultaneously target multiple signaling pathways and address potential resistance issues arising from monotherapy, thereby enhancing overall treatment effectiveness (Wu et al., 2023b). Currently, no therapeutic approaches specifically target NF-κB signaling in mRCC. However, inhibitors and monoclonal antibodies that target this pathway have been studied in other diseases and may represent a promising therapeutic approach for mRCC (Guo et al., 2024).
However, drug insensitivity is also tricky from molecular apects. A study found that the O-GlcNAcylation of RIPK1 at Ser331, Ser440, and Ser669 regulates its ubiquitination, thereby reducing the formation of the RIPK1/FADD/Caspase-8 complex. This alteration promotes NF-κB activation, ultimately inhibiting sunitinib-induced RIPK-dependent apoptosis in RCC (Zeng et al., 2024). Conversely, another intriguing study found that SEC14L3 not only emerges as a potential therapeutic target but also uncovers an SEC14L3/RPS3/NF-κB positive feedback loop that can inhibit ccRCC progression and sunitinib resistance. Modulating SEC14L3 expression to activate this feedback loop could offer new therapeutic strategies for ccRCC treatment (Jiang et al., 2024). Besides, ZZDHHC2 mediates AGK’s S-palmitoylation, promoting its translocation to the plasma membrane and activation of the PI3K-AKT-mTOR pathway in ccRCC, thereby modulating sunitinib sensitivity. This indicates that targeting ZDHHC2 could enhance the antitumor efficacy of sunitinib in ccRCC (Sun et al., 2023). Moreover, circPTEN suppresses ccRCC progression and resistance to mTOR inhibitors by enhancing PTEN expression through reduced methylation of the PTEN promoter and decreasing GLUT1 expression by lowering its m6A methylation (Zhan et al., 2023). Overall, these molecular mechanisms provide promising therapeutic avenues for enhancing sunitinib sensitivity and overcoming resistance in RCC, although they also carry the risk of drug insensitivity. Rational utilization of these mechanisms and molecules will contribute to the clinical translation of these fundamental discoveries.
7 Discussion and future perspectivesWhile the overall 5-year survival rate for renal cell carcinoma is approximately 76%, this rate plummets to 14% for patients with mRCC (Aldin et al., 2023). To date, over 800 clinical trials on advanced RCC have been registered on https://clinicaltrials.gov/, with the majority focusing on single-agent or multi-agent targeted therapies and immunotherapies. Nevertheless, while drug treatments—primarily targeted therapies and immunotherapies, administered as single agents or in combination—can extend patient overall survival (OS) in advanced kidney cancer, significant improvements in treatment efficacy remain necessary (Semenescu et al., 2023).
The molecular mechanisms underlying renal cell carcinoma metastasis are complex, diverse, and interconnected, with various pathways intersecting. Mutations in upstream genes associated with metastasis are relatively rare and exhibit high variability. In addition, the regulation of non-coding RNAs, the regulation of transcription factors, and protein modifications can be drivers of metastasis as well. However, certain downstream pathways, including the Wnt/β-catenin, PI3K/AKT, and NF-κB signaling pathways, share common features. These pathways regulate the cell cycle, apoptosis, autophagy, angiogenesis, and EMT, contributing to rapid tumor proliferation and metastasis (Ma et al., 2023; Mabeta and Steenkamp, 2022; Mirzaei et al., 2022; Wang, 2021) (Figure 1).
However, current research into the metastatic mechanisms of renal cancer predominantly focuses on RCC cells, with limited studies addressing the tumor microenvironment (TME) where these cells reside. Non-immune cells in the extracellular matrix are inextricably linked to the invasion and migration of cancer cells (Oxburgh, 2022). Immune cells within the TME differentiate and undergo functional changes as tumors progress, suppressing or depleting local tumor immunity and thereby fostering an environment conducive to renal cancer cell metastasis (Mei et al., 2024). A notable study reported that tumor-associated macrophages (TAMs) from VHL-deficient tumors displayed increased in vivo glucose uptake, enhanced phagocytic activity, and heightened inflammatory gene expression. Conversely, lymphocytes from Vhl-KO tumors exhibited decreased activation and reduced responsiveness to anti-programmed cell death 1 (anti-PD-1) therapy in vivo (Wolf et al., 2024).
Newly identified therapeutic targets offer hope for treating mRCC. Single-nucleus RNA sequencing revealed ceruloplasmin (CP) and proprotein convertase subtilisin/kexin type 6 (PCSK6) as promising ccRCC markers with potential diagnostic and prognostic significance (Wu et al., 2023c). Another study utilized a large sample size and multi-omics techniques to identify UCHL1 expression as one of the potential biomarkers for high-grade tumors with BAP1 mutations, genomic instability, or increased tumor hypermethylation, potentially influencing clinical and therapeutic management (Li Y. et al., 2023).
Future treatment modalities for metastatic renal cell carcinoma hold significant promise. Multi-drug combination therapies have already demonstrated improved patient outcomes compared to monotherapy, suggesting that integrating multiple targeted drugs or combining them with immunotherapy could be a promising direction for future development (Semenescu et al., 2023). Furthermore, genomics and proteomics analyses can predict patient sensitivity or resistance to specific drugs, while the presence or absence of certain biomarkers can guide personalized treatment choices, enabling more precise and individualized care plans (Elias et al., 2021). Moreover, continued in-depth investigation into the shared downstream pathways among different signaling pathways, regulatory mechanisms of gene expression, and cellular interactions within the TME of metastatic renal cell carcinoma could uncover additional therapeutic targets. Additionally, factors within the TME—such as inflammatory responses, hypoxic conditions, metabolic reprogramming, and mechanical stresses—further promote tumor metastasis. These interactions and environmental pressures contribute to genetic and epigenetic heterogeneity, highlighting the need for therapeutic targets that address these components. Importantly, given the relatively poor prognosis of metastatic renal cell carcinoma, developing reliable biomarkers to predict therapeutic response and monitor disease progression is crucial for guiding clinical decision-making. For example, co-deletions involving VHL alongside one or more of the three genes—PBRM1, BAP1, and SETD2—encoding proteins involved in chromatin modification and remodeling, are common and serve as significant co-drivers of tumorigenesis (Walton et al., 2023).
8 ConclusionIn conclusion, although significant progress has been made in targeted therapies for metastatic renal cell carcinoma, many challenges still exist. Future studies will require a deeper understanding of the biological process of this disease, which will contribute to the development of more effective treatments to improve patient survival and quality of life.
Author contributionsXL: Writing–original draft. WX: Writing–review and editing. ZX: Conceptualization, Funding acquisition, Supervision, Writing–review and editing. XZ: Conceptualization, Funding acquisition, Supervision, Writing–review and editing.
FundingThe author(s) declare that financial support was received for the research, authorship, and/or publication of this article. Our study was supported by the Key Research and Development Plan in China (Grant No. 2017YFB1303100), the National Natural Science Foundation of China (Grant No. 82202911, 82300786), Shenzhen Medical Research Fund (Grant No. B2302054), and Hubei Provincial Natural Science Foundation Projects (Grant No. 2023AFB210 and 2024AFB640) and Wuhan Talent Plan Funds (Grant No. 02.05.22030029).
Conflict of interestThe authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statementThe author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s noteAll claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
ReferencesAhmed, S. S., Barik, S. K., Adhya, A. K., Das, D. K., Parida, A. V., Mukherjee, P., et al. (2023). Metastatic renal cell carcinoma masquerading as a laryngeal tumor: a case report. Cureus 15 (5), e39229. doi:10.7759/cureus.39229
PubMed Abstract | CrossRef Full Text | Google Scholar
Aldin, A., Besiroglu, B., Adams, A., Monsef, I., Piechotta, V., Tomlinson, E., et al. (2023). First-line therapy for adults with advanced renal cell carcinoma: a systematic review and network meta-analysis. Cochrane Database Syst. Rev. 5 (5), Cd013798. doi:10.1002/14651858.CD013798.pub2
PubMed Abstract | CrossRef Full Text | Google Scholar
Ali, M., Eid, M., Saliby, R. M., Choi, S., McKay, R. R., Siva, S., et al. (2024). Emerging novel functional imaging and immunotherapy in renal cell carcinoma and current treatment sequencing strategies after immunotherapy. Am. Soc. Clin. Oncol. Educ. Book 44 (3), e438658. doi:10.1200/EDBK_438658
PubMed Abstract | CrossRef Full Text | Google Scholar
Aurilio, G., Piva, F., Santoni, M., Cimadamore, A., Sorgentoni, G., Lopez-Beltran, A., et al. (2019). The role of obesity in renal cell carcinoma patients: clinical-pathological implications. Int. J. Mol. Sci. 20 (22), 5683. doi:10.3390/ijms20225683
PubMed Abstract | CrossRef Full Text | Google Scholar
Bahadoram, S., Davoodi, M., Hassanzadeh, S., Bahadoram, M., Barahman, M., and Mafakher, L. (2022). Renal cell carcinoma: an overview of the epidemiology, diagnosis, and treatment. G. Ital. Nefrol. 39 (3).
PubMed Abstract | Google Scholar
Barata, P., Gulati, S., Elliott, A., Hammers, H. J., Burgess, E., Gartrell, B. A., et al. (2024). Renal cell carcinoma histologic subtypes exhibit distinct transcriptional profiles. J. Clin. Invest 134 (11), e178915. doi:10.1172/JCI178915
PubMed Abstract | CrossRef Full Text | Google Scholar
Bellouki, O., Ibrahimi, A., Soufiani, I., Boualaoui, I., El Sayegh, H., and Nouini, Y. (2023). Blepharoptosis revealing a metastatic renal cell carcinoma: a rare case report. Int. J. Surg. Case Rep. 112, 108910. doi:10.1016/j.ijscr.2023.108910
PubMed Abstract | CrossRef Full Text | Google Scholar
Cen, J., Liang, Y., Huang, Y., Pan, Y., Shu, G., Zheng, Z., et al. (2021). Circular RNA circSDHC serves as a sponge for miR-127-3p to promote the proliferation and metastasis of renal cell carcinoma via the CDKN3/E2F1 axis. Mol. Cancer 20 (1), 19. doi:10.1186/s12943-021-01314-w
PubMed Abstract | CrossRef Full Text | Google Scholar
Chen, H., Zhu, D., Zheng, Z., Cai, Y., Chen, Z., and Xie, W. (2019). CEP55 promotes epithelial-mesenchymal transition in renal cell carcinoma through PI3K/AKT/mTOR pathway. Clin. Transl. Oncol. 21 (7), 939–949. doi:10.1007/s12094-018-02012-8
PubMed Abstract | CrossRef Full Text | Google Scholar
Chen, J., Cao, N., Li, S., and Wang, Y. (2021). Identification of a risk stratification model to predict overall survival and surgical benefit in clear cell renal cell carcinoma with distant metastasis. Front. Oncol. 11, 630842. doi:10.3389/fonc.2021.630842
PubMed Abstract | CrossRef Full Text | Google Scholar
Chen, Q., Liu, T., Bao, Y., Zhao, T., Wang, J., Wang, H., et al. (2020). CircRNA cRAPGEF5 inhibits the growth and metastasis of renal cell carcinoma via the miR-27a-3p/TXNIP pathway. Cancer Lett. 469, 68–77. doi:10.1016/j.canlet.2019.10.017
PubMed Abstract | CrossRef Full Text | Google Scholar
Cho, D. Y., Kim, H. J., and Kim, J. Y. (2024). Renal cell carcinoma metastasis to the penis: a case report and literature review. Med. Kaunas. 60 (4), 554. doi:10.3390/medicina60040554
PubMed Abstract | CrossRef Full Text | Google Scholar
Choueiri, T. K., Powles, T., Albiges, L., Burotto, M., Szczylik, C., Zurawski, B., et al. (2023). Cabozantinib plus nivolumab and ipilimumab in renal-cell carcinoma. N. Engl. J. Med. 388 (19), 1767–1778. doi:10.1056/NEJMoa2212851
PubMed Abstract | CrossRef Full Text | Google Scholar
Di, S. C., Chen, W. J., Yang, W., Zhang, X. M., Dong, K. Q., Tian, Y. J., et al. (2024). DEPDC1 as a metabolic target regulates glycolysis in renal cell carcinoma through AKT/mTOR/HIF1α pathway. Cell Death Dis. 15 (7), 533. doi:10.1038/s41419-024-06913-1
PubMed Abstract | CrossRef Full Text | Google Scholar
Dong, D., Mu, Z., Wei, N., Sun, M., Wang, W., Xin, N., et al. (2019). Long non-coding RNA ZFAS1 promotes proliferation and metastasis of clear cell renal cell carcinoma via targeting miR-10a/SKA1 pathway. Biomed. Pharmacother. 111, 917–925. doi:10.1016/j.biopha.2018.12.143
PubMed Abstract | CrossRef Full Text | Google Scholar
Duan, J., Jin, M., Yang, D., Shi, J., Gao, J., Guo, D., et al. (2022). Ubiquitin-specific peptidase 2 inhibits epithelial-mesenchymal transition in clear cell renal cell carcinoma metastasis by downregulating the NF-κB pathway. Bioengineered 13 (2), 4455–4467. doi:10.1080/21655979.2022.2033403
PubMed Abstract | CrossRef Full Text | Google Scholar
Dufies, M., Verbiest, A., Cooley, L. S., Ndiaye, P. D., He, X., Nottet, N., et al. (2021). Plk1, upregulated by HIF-2, mediates metastasis and drug resistance of clear cell renal cell carcinoma. Commun. Biol. 4 (1), 166. doi:10.1038/s42003-021-01653-w
PubMed Abstract | CrossRef Full Text | Google Scholar
Elias, R., Tcheuyap, V. T., Kaushik, A. K., Singla, N., Gao, M., Reig Torras, O., et al. (2021). A renal cell carcinoma tumorgraft platform to advance precision medicine. Cell Rep. 37 (8), 110055. doi:10.1016/j.celrep.2021.110055
PubMed Abstract | CrossRef Full Text | Google Scholar
Elmusa, E., Raza, M. W., Hamza, A., Khokhar, H. T., and Butt, M. (2022). Metastatic jejunal renal cell carcinoma intussusception presenting as melena. Cureus 14 (12), e32554. doi:10.7759/cureus.32554
PubMed Abstract | CrossRef Full Text | Google Scholar
Fujita, K., Kimura, G., Tsuzuki, T., Kato, T., Banno, E., Kazama, A., et al. (2022). The association of tumor immune microenvironment of the primary lesion with time to metastasis in patients with renal cell carcinoma: a retrospective analysis. Cancers (Basel) 14 (21), 5258. doi:10.3390/cancers14215258
PubMed Abstract | CrossRef Full Text | Google Scholar
Gray, R. E., and Harris, G. T. (2019). Renal cell carcinoma: diagnosis and management. Am. Fam. Physician 99 (3), 179–184.
PubMed Abstract | Google Scholar
Grimm, M. O., Oya, M., Choueiri, T. K., Motzer, R. J., Schmidinger, M., Quinn, D. I., et al. (2024). Impact of prior cytoreductive nephrectomy on efficacy in patients with synchronous metastatic renal cell carcinoma treated with avelumab plus axitinib or sunitinib: post hoc analysis from the JAVELIN renal 101 phase 3 trial. Eur. Urol. 85 (1), 8–12. doi:10.1016/j.eururo.2023.09.016
PubMed Abstract | CrossRef Full Text | Google Scholar
Gui, D., Dong, Z., Peng, W., Jiang, W., Huang, G., Liu, G., et al. (2021). Ubiquitin-specific peptidase 53 inhibits the occurrence and development of clear cell renal cell carcinoma through NF-κB pathway inactivation. Cancer Med. 10 (11), 3674–3688. doi:10.1002/cam4.3911
PubMed Abstract | CrossRef Full Text | Google Scholar
Guo, J., Ke, S., Chen, Q., Zhou, J., Guo, J., and Qiu, T. (2023). NCOA7 regulates growth and metastasis of clear cell renal cell carcinoma via MAPK/ERK signaling pathway. Int. J. Mol. Sci. 24 (14), 11584. doi:10.3390/ijms241411584
PubMed Abstract | CrossRef Full Text | Google Scholar
Guo, Q., Jin, Y., Chen, X., Ye, X., Shen, X., Lin, M., et al. (2024). NF-κB in biology and targeted therapy: new insights and translational implications. Signal Transduct. Target Ther. 9 (1), 53. doi:10.1038/s41392-024-01757-9
PubMed Abstract | CrossRef Full Text | Google Scholar
He, A., He, S., Huang, C., Chen, Z., Wu, Y., Gong, Y., et al. (2020). MTDH promotes metastasis of clear cell renal cell carcinoma by activating SND1-mediated ERK signaling and epithelial-mesenchymal transition. Aging (Albany NY) 12 (2), 1465–1487. doi:10.18632/aging.102694
PubMed Abstract | CrossRef Full Text | Google Scholar
Hu, G., Ma, J., Zhang, J., Chen, Y., Liu, H., Huang, Y., et al. (2021). Hypoxia-induced lncHILAR promotes renal cancer metastasis via ceRNA for the miR-613/206/1-1-3p/Jagged-1/Notch/CXCR4 signaling pathway. Mol. Ther. 29 (10), 2979–2994. doi:10.1016/j.ymthe.2021.05.020
PubMed Abstract | CrossRef Full Text | Google Scholar
Huang, Q., Sun, Y., Ma, X., Gao, Y., Li, X., Niu, Y., et al. (2017). Androgen receptor increases hematogenous metastasis yet decreases lymphatic metastasis of renal cell carcinoma. Nat. Commun. 8 (1), 918. doi:10.1038/s41467-017-00701-6
PubMed Abstract | CrossRef Full Text | Google Scholar
Huang, X. F., and Yu, Z. L. (2023). Clear cell renal cell carcinoma metastatic to the mandible: a unique case report and literature review. Chin. J. Dent. Res. 26 (4), 265–270. doi:10.3290/j.cjdr.b4784061
PubMed Abstract | CrossRef Full Text | Google Scholar
Jiang, Z., Yang, G., Wang, G., Wan, J., Zhang, Y., Song, W., et al. (2024). SEC14L3 knockdown inhibited clear cell renal cell carcinoma proliferation, metastasis and sunitinib resistance through an SEC14L3/RPS3/NFκB positive feedback loop. J. Exp. Clin. Cancer Res. 43 (1), 288. doi:10.1186/s13046-024-03206-5
PubMed Abstract | CrossRef Full Text | Google Scholar
Lei, C., Lv, S., Wang, H., Liu, C., Zhai, Q., Wang, S., et al. (2018). Leukemia inhibitory factor receptor suppresses the metastasis of clear cell renal cell carcinoma through negative regulation of the yes-associated protein. DNA Cell Biol. 37 (8), 659–669. doi:10.1089/dna.2017.4102
PubMed Abstract | CrossRef Full Text | Google Scholar
Li, J., Chen, S., Xiao, J., Ji, J., Huang, C., and Shu, G. (2024b). FOXC1 transcriptionally suppresses ABHD5 to inhibit the progression of renal cell carcinoma through AMPK/mTOR pathway. Cell Biol. Toxicol. 40 (1), 62. doi:10.1007/s10565-024-09899-w
PubMed Abstract | CrossRef Full Text | Google Scholar
Li, J. K., Chen, C., Liu, J. Y., Shi, J. Z., Liu, S. P., Liu, B., et al. (2017). Long noncoding RNA MRCCAT1 promotes metastasis of clear cell renal cell carcinoma via inhibiting NPR3 and activating p38-MAPK signaling. Mol. Cancer 16 (1), 111. doi:10.1186/s12943-017-0681-0
PubMed Abstract | CrossRef Full Text | Google Scholar
Li, M., Li, L., Zheng, J., Li, Z., Li, S., Wang, K., et al. (2023a). Liquid biopsy at the frontier in renal cell carcinoma: recent analysis of techniques and clinical application. Mol. Cancer 22 (1), 37. doi:10.1186/s12943-023-01745-7
PubMed Abstract | CrossRef Full Text | Google Scholar
Li, X., Xiao, W., Yang, H., and Zhang, X. (2024a). Exosome in renal cell carcinoma progression and implications for targeted therapy. Front. Oncol. 14, 1458616. doi:10.3389/fonc.2024.1458616
PubMed Abstract | CrossRef Full Text | Google Scholar
Li, Y., Lih, T. M., Dhanasekaran, S. M., Mannan, R., Chen, L., Cieslik, M., et al. (2023b). Histopathologic and proteogenomic heterogeneity reveals features of clear cell renal cell carcinoma aggressiveness. Cancer Cell 41 (1), 139–163.e17. doi:10.1016/j.ccell.2022.12.001
PubMed Abstract | CrossRef Full Text | Google Scholar
Li, Y. L., Wu, L. W., Zeng, L. H., Zhang, Z. Y., Wang, W., Zhang, C., et al. (2020). ApoC1 promotes the metastasis of clear cell renal cell carcinoma via activation of STAT3. Oncogene 39 (39), 6203–6217. doi:10.1038/s41388-020-01428-3
PubMed Abstract | CrossRef Full Text | Google Scholar
Lin, Y. W., Lee, L. M., Lee, W. J., Chu, C. Y., Tan, P., Yang, Y. C., et al. (2016). Melatonin inhibits MMP-9 transactivation and renal cell carcinoma metastasis by suppressing Akt-MAPKs pathway and NF-κB DNA-binding activity. J. Pineal Res. 60 (3), 277–290. doi:10.1111/jpi.12308
PubMed Abstract | CrossRef Full Text | Google Scholar
Liu, X., Zhong, L., Li, P., and Zhao, P. (2022). MicroRNA-100 enhances autophagy and suppresses migration and invasion of renal cell carcinoma cells via disruption of NOX4-dependent mTOR pathway. Clin. Transl. Sci. 15 (2), 567–575. doi:10.1111/cts.12798
PubMed Abstract | CrossRef Full Text | Google Scholar
Liu, Z., Zhang, Q., Zhao, X., Zhu, G., Tang, S., Hong, P., et al. (2021). Inferior vena cava interruption in renal cell carcinoma with tumor thrombus: surgical strategy and perioperative results. BMC Surg. 21 (1), 402. doi:10.1186/s12893-021-01400-2
PubMed Abstract | CrossRef Full Text | Google Scholar
Lu, Y., Qin, H., Jiang, B., Lu, W., Hao, J., Cao, W., et al. (2021). KLF2 inhibits cancer cell migration and invasion by regulating ferroptosis through GPX4 in clear cell renal cell carcinoma. Cancer Lett. 522, 1–13. doi:10.1016/j.canlet.2021.09.014
留言 (0)