Trifluridine/tipiracil (FTD/TPI) is an oral combination of trifluridine (FTD), a cytotoxic thymidine–based nucleoside analog, and tipiracil hydrochloride, a thymidine phosphorylase inhibitor that prevents degradation of and improves systemic exposure to FTD (1). FTD/TPI is approved for the third- or later-line treatment of metastatic colorectal cancer (mCRC), either as monotherapy or in combination with bevacizumab, based on results from the phase 3 RECOURSE and SUNLIGHT trials, respectively (2–4), with the combination recommended in the National Comprehensive Cancer Network (NCCN) guidelines and the mCRC Living Guidelines of the European Society for Medical Oncology for patients previously treated with fluoropyrimidines, oxaliplatin, irinotecan, and biologics (5, 6).
The rationale for combining FTD/TPI with bevacizumab is based on their independent mechanisms of action, along with preclinical evidence showing that bevacizumab increases FTD accumulation in tumor cell DNA (7, 8). Clinically, FTD/TPI has shown efficacy and acceptable tolerability in combination with bevacizumab in first-line (SOLSTICE) and later-line (SUNLIGHT) mCRC, as well as in several phase 2 trials (4, 9–12). In the phase 3 SOLSTICE trial, conducted in patients who were not candidates for intensive therapy, the primary endpoint was not met. First-line FTD/TPI plus bevacizumab showed similar progression-free survival to capecitabine plus bevacizumab [median, 9.4 vs. 9.3 months; hazard ratio (HR), 0.87; 95% confidence interval (CI), 0.75–1.02; p = 0.0464] (9); median overall survival was also similar between treatment arms (19.7 vs. 18.6 months; HR, 1.06; 95% CI, 0.90–1.25) (13). However, in SUNLIGHT, treatment with FTD/TPI plus bevacizumab resulted in significantly longer overall survival (median, 10.8 vs. 7.5 months; HR, 0.61; 95% CI, 0.49–0.77; p < 0.001) and progression-free survival (5.6 vs. 2.4 months; HR, 0.44; 95% CI, 0.36–0.54; p < 0.001) than FTD/TPI in patients who had received no more than two prior chemotherapy regimens (4). Safety findings from the SOLSTICE and SUNLIGHT trials showed that treatment-emergent adverse events (TEAEs) with FTD/TPI plus bevacizumab were consistent with the known safety profiles of FTD/TPI and bevacizumab individually, the most common being hematologic toxicities, gastrointestinal adverse events (AEs), fatigue, and hypertension (7, 10). In SOLSTICE, compared with capecitabine plus bevacizumab, first-line FTD/TPI plus bevacizumab was associated with a higher rate of neutropenia but a lower rate of hand-foot syndrome (9). Grade ≥3 neutropenia, but not febrile neutropenia, was more common with FTD/TPI plus bevacizumab than with FTD/TPI alone in SUNLIGHT (4).
The objectives of the current analysis were to further characterize the overall safety of FTD/TPI plus bevacizumab in previously untreated or refractory patients with mCRC and to compare the safety of the combination in first- and later-line patient populations.
2 Methods2.1 Patients and study designsThis analysis included data from patients with mCRC who received at least one dose of FTD/TPI plus bevacizumab in SOLSTICE (NCT03869892) or SUNLIGHT (NCT04737187). Full details of the study designs and eligibility criteria have been published previously (4, 9, 14, 15). Both studies were global, open-label, randomized, phase 3 trials that enrolled adult patients with histologically confirmed, unresectable adenocarcinoma of the colon or rectum, known RAS mutation status, adequate organ function, and an estimated life expectancy of ≥12 weeks. Eligibility for SOLSTICE required an Eastern Cooperative Oncology Group performance status (ECOG PS) of 0–2, whereas, in SUNLIGHT, an ECOG PS of 0 or 1 was allowed. In SOLSTICE, patients were previously untreated and were not candidates for intensive combination chemotherapy with irinotecan or oxaliplatin per investigator judgment, due to clinical (e.g., ECOG PS, comorbidities, and age >70 years) and/or nonclinical (e.g., low tumor burden and patient preference) conditions. In SUNLIGHT, patients must have received no more than two prior chemotherapy regimens containing fluoropyrimidines, irinotecan, oxaliplatin, a vascular endothelial growth factor inhibitor, and/or (in patients with RAS wild-type tumors) an epidermal growth factor receptor inhibitor and have had disease progression or intolerance to the last regimen.
Patients were randomized (1:1) to either FTD/TPI 35 mg/m2 orally twice daily on days 1 through 5 and days 8 through 12, plus bevacizumab 5 mg/kg intravenously on days 1 and 15 of each 28-day cycle (SOLSTICE and SUNLIGHT); or capecitabine 1,250 or 1,000 mg/m2 orally twice daily on days 1 through 14, plus bevacizumab 7.5 mg/kg intravenously on day 1 of each 21-day cycle (SOLSTICE); or FTD/TPI alone (SUNLIGHT). Treatment continued until disease progression, unacceptable toxicity, or withdrawal of consent.
Both trials were performed in accordance with the principles of the Declaration of Helsinki, good clinical practice, and applicable regulatory requirements. The study protocols were approved by the institutional review board(s) and/or independent ethics committee(s) at each participating center. All enrolled patients provided written informed consent.
2.2 Safety assessmentsThe pooled safety analysis included data collected up to the data cutoff dates of 9 June 2021 for SOLSTICE and 5 July 2022 for SUNLIGHT. AEs were coded using the Medical Dictionary for Regulatory Activities version 25.0 and graded per the National Cancer Institute Common Terminology Criteria for Adverse Events (NCI-CTCAE) version 4.03 (SOLSTICE) or 5.0 (SUNLIGHT). Assessment of hematologic AEs was conducted on the basis of NCI-CTCAE definitions and laboratory parameters. For SOLSTICE, NCI-CTCAE version 5.0 was used for laboratory parameters in the pooled analysis, based on numeric criteria alone, without additional clinical information. AEs were summarized as those occurring from the initiation of treatment administration to 30 days after the last dose. Details of the protocol-defined management of AEs, including dose modifications and supportive care interventions, are provided as Supplementary Material (Supplementary Table 1).
2.3 Post-hoc and statistical analysisAll safety data are presented descriptively. No formal hypothesis testing was performed. Post-hoc analyses included assessment of the timing of onset and resolution of grade ≥3 hematologic AEs, calculated using Kaplan–Meier methodology, with 95% CIs based on the Greenwood formula. Subgroup analyses of treatment-related AEs (TRAEs) by age and ECOG PS were also conducted.
3 Results3.1 Patients and treatmentThe pooled safety population comprised 669 patients who received FTD/TPI plus bevacizumab in SOLSTICE (n = 423) and SUNLIGHT (n = 246; Table 1). The median age was 69.0 years, 53.8% of patients were men, and 61.4% were enrolled from the European Union. A greater proportion of the SOLSTICE patient population was aged ≥75 years compared with that of the SUNLIGHT population.
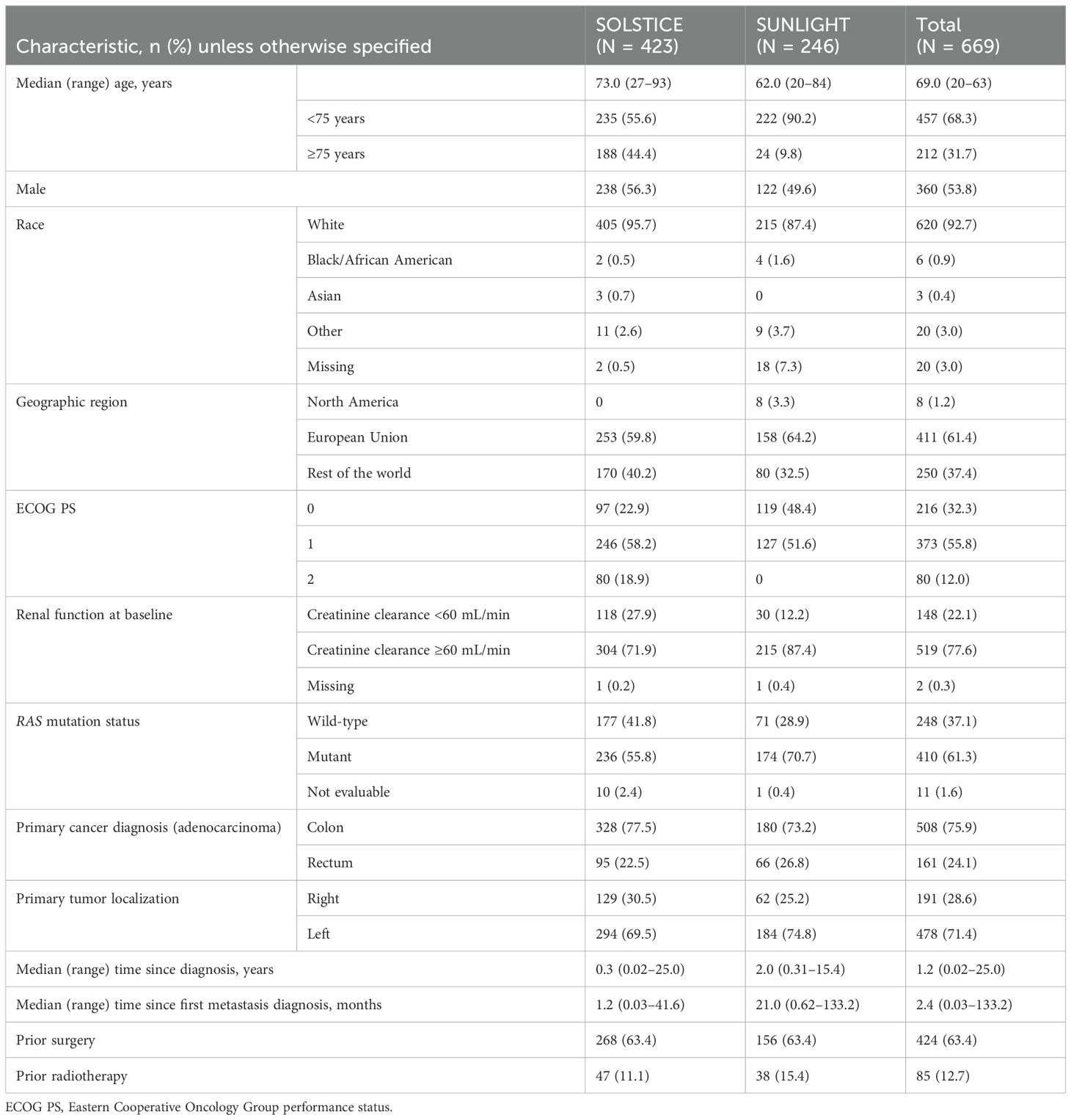
Table 1. Baseline characteristics of patients receiving FTD/TPI plus bevacizumab in the SOLSTICE, SUNLIGHT, and pooled safety populations.
3.2 Overall safetyThe median (range) duration of treatment was 8.2 (0.3–24.4) months in SOLSTICE and 5.0 (0.1–18.5) months in SUNLIGHT.
In the pooled safety population, TEAEs were reported by 98.5% of patients (Table 2). The most common, regardless of causality, were neutropenia, anemia, nausea, diarrhea, asthenia, fatigue, and decreased appetite. Grade ≥3 TEAEs were reported more frequently in SOLSTICE than in SUNLIGHT (86.8% and 72.4%, respectively). The most common grade ≥3 TEAEs in the pooled safety population were neutropenia (60.2%) and anemia (11.2%; Table 2).
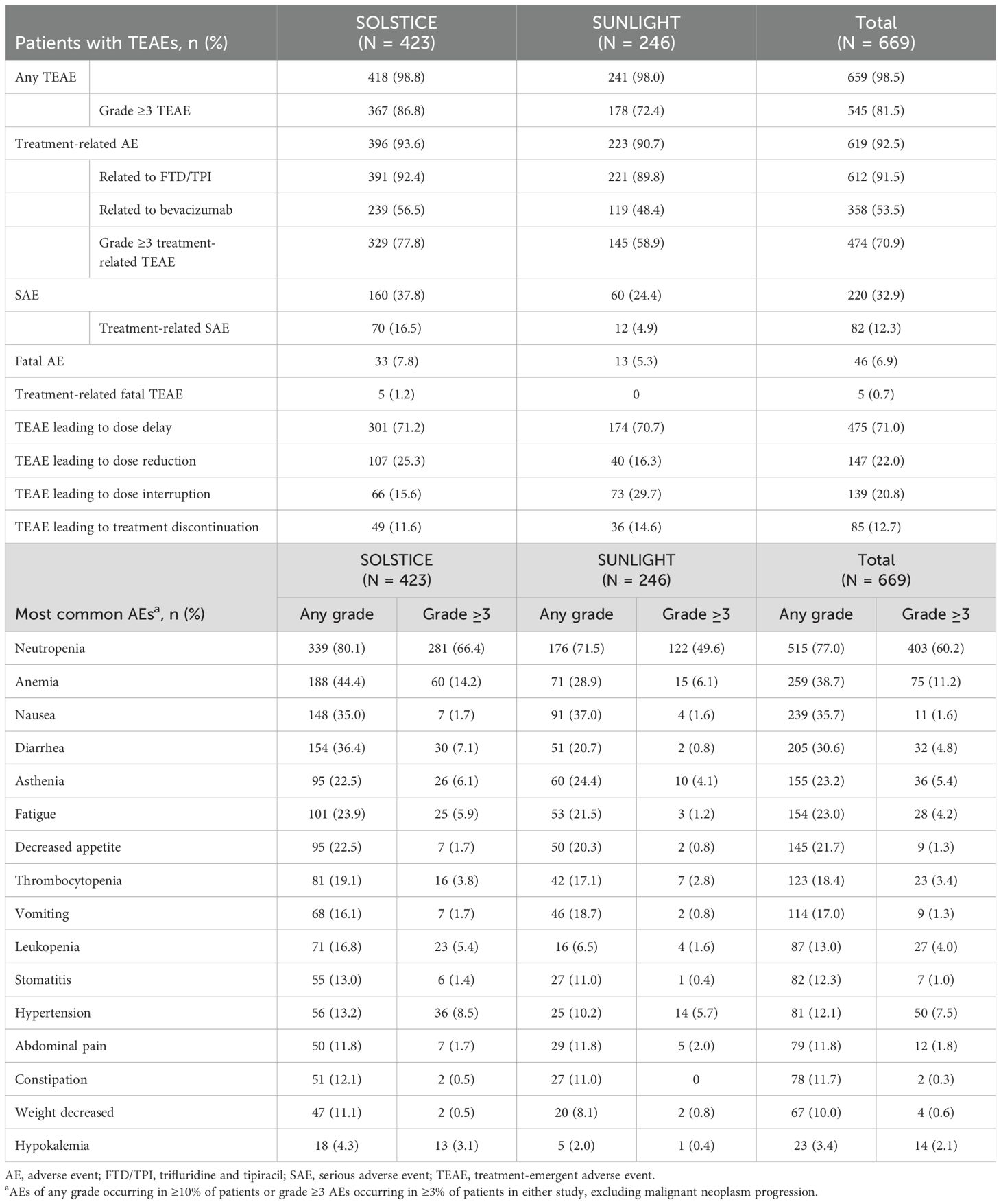
Table 2. Overall safety summary and most common any-grade/grade ≥3 TEAEs among patients receiving FTD/TPI plus bevacizumab in the SOLSTICE, SUNLIGHT, and pooled safety populations.
Most patients (92.5%) had TEAEs that were considered related to the combination of FTD/TPI plus bevacizumab, the most common of which were neutropenia (86.8%), anemia (35.0%), nausea (31.9%), and diarrhea (31.0%) in SOLSTICE and neutropenia (74.0%), nausea (33.3%), anemia (25.2%), and asthenia (19.1%) in SUNLIGHT. Bevacizumab-related hypertension was reported in 8.3% and 7.3% of patients in SOLSTICE and SUNLIGHT, respectively. Cardiac disorders related to bevacizumab were reported in nine (2.1%) patients in SOLSTICE, including cardiac failure in three patients and atrial fibrillation in two patients; no bevacizumab-related cardiac disorders were reported in SUNLIGHT.
Serious AEs (SAEs; 37.8% vs. 24.4%), treatment-related SAEs (16.5% vs. 4.9%), and AEs leading to dose reduction (25.3% vs. 16.3%) were more common in SOLSTICE; AEs leading to dose interruption (15.6% vs. 29.7%) or treatment discontinuation (11.6% vs. 14.6%) were more common in SUNLIGHT. Five (1.2%) patients in SOLSTICE died due to TRAEs [Dieulafoy’s vascular malformation and gastric hemorrhage; urosepsis; pulmonary embolism and pulmonary hemorrhage; chronic cardiac failure; and cardiorespiratory arrest (all n = 1)]; there were no treatment-related deaths in SUNLIGHT.
3.3 Analyses of hematologic AEs in the pooled safety populationIn total, 563 (84.2%) patients had at least one hematologic TEAE, including neutropenia (77.0%), anemia (38.7%), and thrombocytopenia (18.4%). Grade ≥3 hematologic TEAEs with onset in cycles 1 and 2 included neutropenia (36.8%), anemia (5.4%), and thrombocytopenia (0.7%). In SOLSTICE and SUNLIGHT, respectively, median time to onset (range) of grade ≥3 hematologic TEAEs was 88 days (3–710) and 90 days (13–309) and that of grade ≥3 neutropenia was 110 days (14–710) and 112 days (14–281) (Figure 1). The incidence of any-grade neutropenia was highest in the first four cycles (Supplementary Table 2). Grade ≥3 hematologic TEAEs and grade ≥3 neutropenia both resolved to grade ≤2 within a median of 8 days (Figure 1).
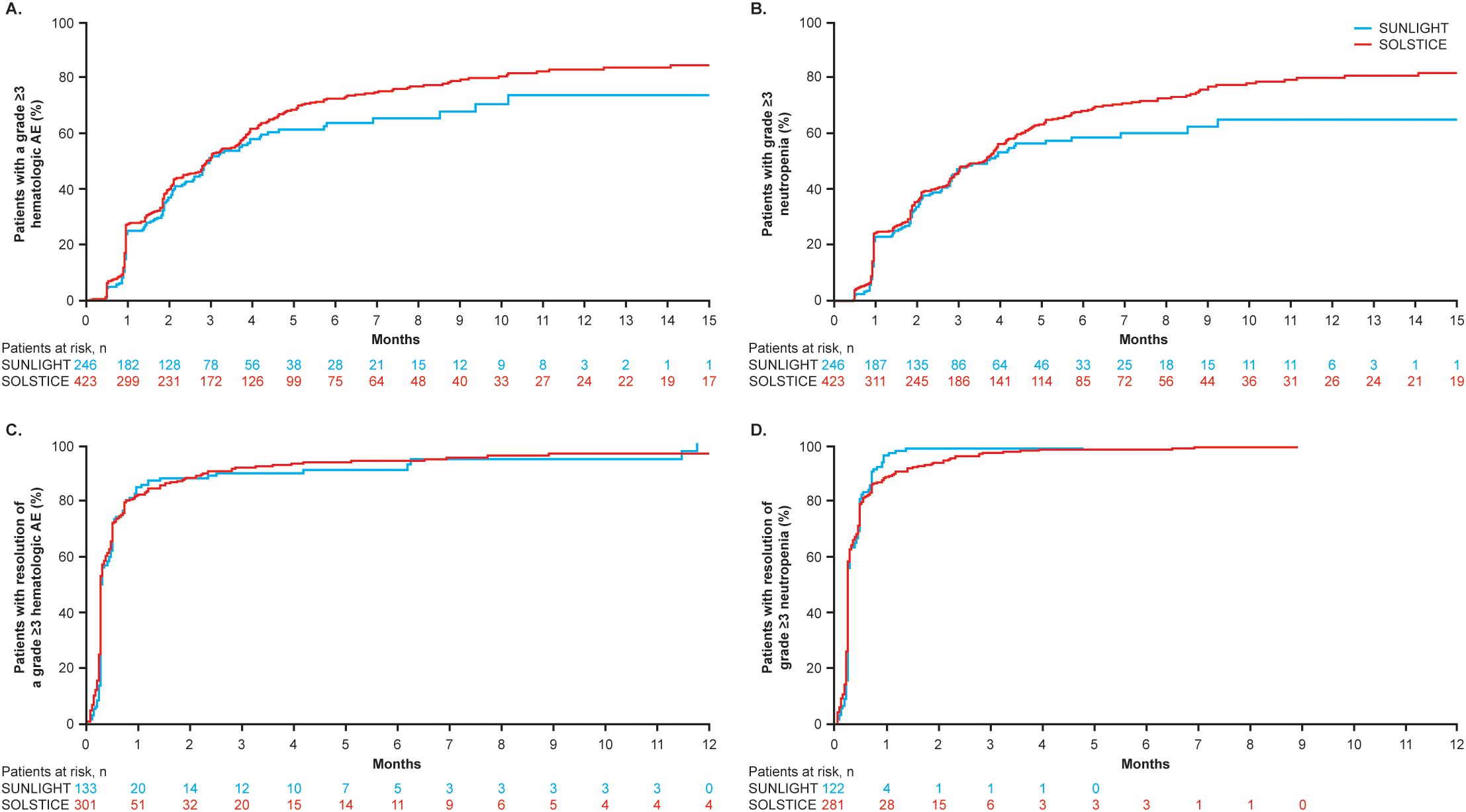
Figure 1. Time to (A) onset of grade ≥3 hematologic AEs and (B) grade ≥3 neutropenia and to (C) resolution of grade ≥3 hematologic AEs and (D) grade ≥3 neutropenia to grade ≤2 in the SOLSTICE and SUNLIGHT populations.
Overall, 66.5% of patients had a dose modification for neutropenia across the treatment period, most commonly (64.1%) dose delays (Supplementary Table 3). In the pooled safety population, 30.6% of patients received at least one concomitant granulocyte colony-stimulating factor (G-CSF) treatment, including 14.5% of patients who received G-CSF within the first two cycles. Median time to use of G-CSF was 34 days in SOLSTICE and 25.5 days in SUNLIGHT. Overall, G-CSF was mostly administered for secondary prophylaxis, and nonpegylated G-CSF was used more frequently than pegylated formulations (28.7% vs. 3.4%).
Hematologic TEAEs resulted in treatment discontinuation in 13 (1.9%) patients overall. There were no fatal hematologic AEs.
3.4 Subgroup analyses of grade ≥3 TRAEsGrade ≥3 TRAEs were more frequent in patients aged ≥75 years than in those aged <75 years, and the frequencies of the most common grade ≥3 TRAEs were generally higher in the older age group (Table 3). In the individual SOLSTICE and SUNLIGHT studies and the pooled population, the overall frequencies of grade ≥3 TRAEs tended to be numerically higher in patients with a baseline ECOG PS of 0 compared with those with an ECOG PS of either 1 or 2 individually. There were no clear trends in the frequencies of other common grade ≥3 TRAEs with respect to ECOG PS.
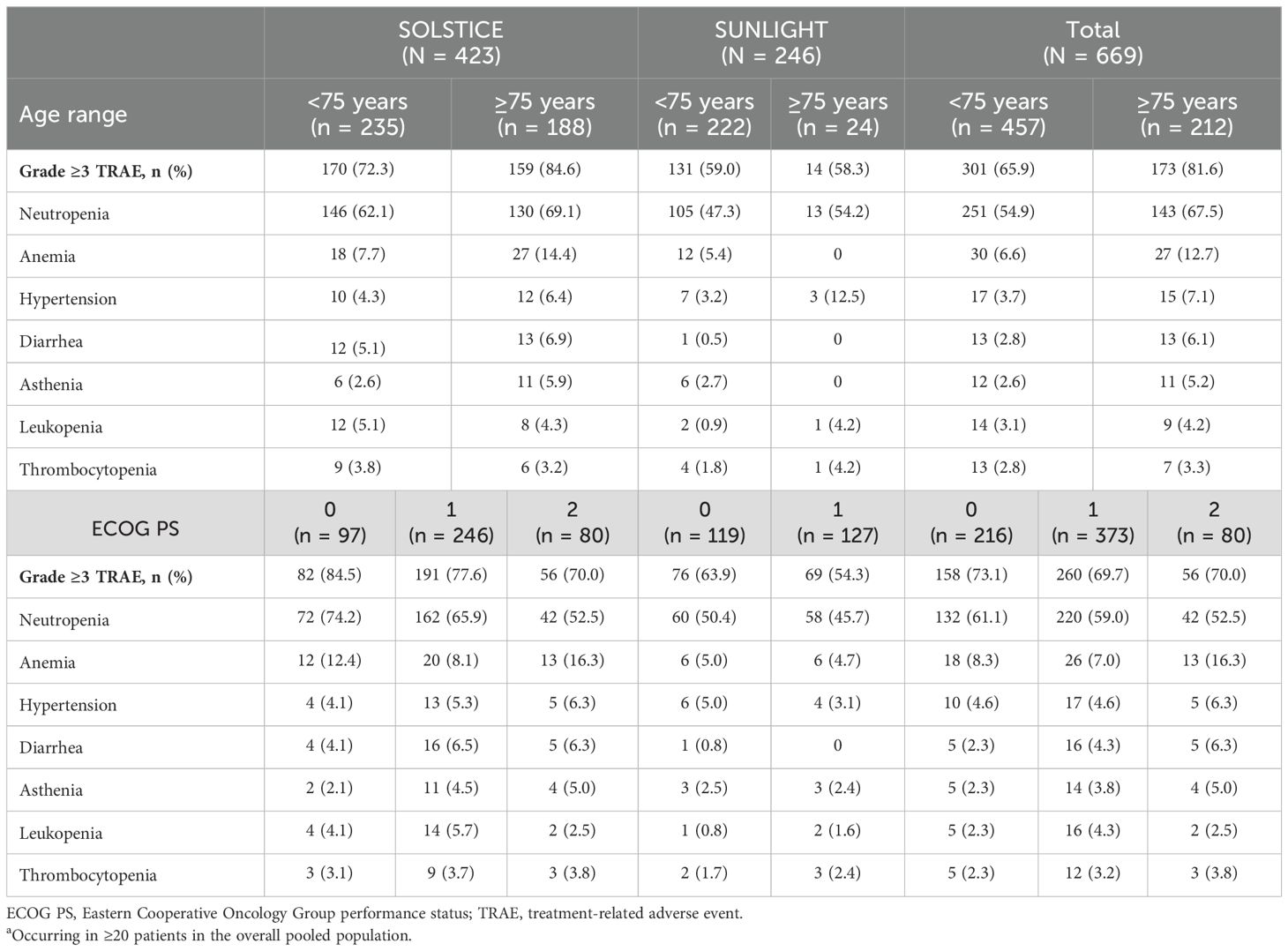
Table 3. Most commona grade ≥3 TRAEs by age and ECOG PS.
4 DiscussionIn this pooled safety analysis, FTD/TPI plus bevacizumab demonstrated a predictable and manageable safety profile across all lines of therapy in the continuum of care. Results were consistent across the two phase 3 trials and similar to those reported in phase 2 trials of FTD/TPI plus bevacizumab in first-, second-, and third-line mCRC and several real-world studies (10–12, 16–19). The most common TEAEs were hematologic AEs (e.g., neutropenia and anemia), gastrointestinal toxicities (e.g., nausea and diarrhea), and fatigue. Neutropenia was the most common any-grade/grade ≥3 TEAE in the individual and pooled SOLSTICE/SUNLIGHT populations. Grade ≥3 TEAEs, SAEs, and treatment-related SAEs occurred more frequently in SOLSTICE than in SUNLIGHT, likely reflecting the longer treatment duration in the SOLSTICE study, as well as the fact that, compared with SUNLIGHT, the study population in SOLSTICE tended to be older (median age of 73 vs. 62 years), more frail (19% with an ECOG PS of 2 vs. 0%), and/or had comorbidities that rendered them ineligible for intensive chemotherapy. Additionally, investigators may have been reluctant to enroll patients in SUNLIGHT who had experienced significant toxicity with a previous line of fluoropyrimidine-based therapy.
Any-grade neutropenia was most frequent in the first few treatment cycles and lasted a median of 8 days. Median time to onset of grade ≥3 hematologic AEs was similar in both SOLSTICE and SUNLIGHT, suggesting that prior treatment with chemotherapy and targeted therapy in SUNLIGHT did not predispose patients to earlier-onset neutropenia. Grade ≥3 hematologic AEs, including neutropenia, resolved to grade ≤2 in 8 days, indicating effective management was achieved with dose modifications and/or supportive care interventions, including G-CSF.
Nonpegylated G-CSF was used more commonly than pegylated formulations, and G-CSF was mainly used as secondary prophylaxis, which is consistent with consensus recommendations that primary prophylactic G-CSF use is reserved for chemotherapy regimens with a high (≥20%) risk of febrile neutropenia, and secondary prophylaxis is used in patients who experience febrile neutropenia or dose-limiting neutropenia in a previous treatment cycle (20, 21).
Toxicities considered to be related to bevacizumab occurred less frequently in the SUNLIGHT study than reported in the first-line SOLSTICE study (48.4% vs. 56.5% of patients), potentially reflecting the fact that approximately 72% of patients in the FTD/TPI plus bevacizumab arm had previously received a vascular endothelial growth factor inhibitor before enrollment in SUNLIGHT (4). Among the most frequently reported bevacizumab-related TEAEs was hypertension, which, although usually asymptomatic, can result in cardiovascular complications if unmanaged (22–25). In this pooled analysis, bevacizumab-related hypertension was observed in 8.3% of patients overall, although cardiac events related to bevacizumab treatment were relatively infrequent in SOLSTICE (2.1% in the FTD/TPI plus bevacizumab arm and 1.9% in the capecitabine plus bevacizumab arm) and absent in SUNLIGHT. As the patient population was older in SOLSTICE than in SUNLIGHT, a higher frequency of bevacizumab-related hypertension might have been expected; however, this was found to be similar in both studies. A possible explanation for this could be the slightly higher use of antihypertensives among patients who received FTD/TPI plus bevacizumab in the SOLSTICE trial vs. the SUNLIGHT trial before and during the treatment period (37.3% and 48.1% vs. 30.9% and 37.8%, respectively). In general, the most common grade ≥3 TRAEs occurred more frequently in patients aged ≥75 years than in younger patients, possibly because older patients tend to have more comorbidities, as well as age-associated immune dysfunction that renders them more susceptible to myelosuppression and infections (26). The finding that grade ≥3 TRAEs tended to be more frequent in patients with an ECOG PS of 0 compared with those with a higher score is possibly the result of patients with a lower ECOG PS remaining on treatment for longer, and/or receiving a higher dose intensity of FTD/TPI. A recent retrospective study of FTD/TPI plus bevacizumab as second- or later-line treatment in vulnerable patients with mCRC (median age 79 years)—among whom were several factors associated with intolerance to intensive therapy, including older age (65%), serious concomitant disease (26%), and poor ECOG PS (20%)—found the combination to have an acceptable safety profile (27). Together, the data suggest that that poor functional status may not be predictive of toxicity with FTD/TPI plus bevacizumab, and the combination has acceptable tolerability in vulnerable patients with mCRC.
A limitation of the pooled analysis was that it was not conducted on a matched patient population and there were differences in patient demographics and baseline characteristics between the SUNLIGHT and SOLSTICE trials, including patient age, prior treatment, time on treatment, and ECOG PS. Furthermore, enrolled patients were predominantly White and other ethnicities were underrepresented in both trial populations. In addition, the post-hoc nature of the subgroup analyses limits the conclusions that can be drawn from these analyses.
5 ConclusionsOverall, the results described herein provide further evidence that FTD/TPI, with or without bevacizumab, is well tolerated. Grade ≥3 hematologic TEAEs were mostly reversible with appropriate management, including dose modifications and prophylactic G-CSF use. The safety profile of the combination was consistent and manageable across first and later lines of treatment, with no new safety concerns.
Data availability statementStudy-level clinical data from this study will be made available upon reasonable request from a qualified medical or scientific professional for the specific purpose laid out in that request and may include deidentified individual participant data. The data for this request will be available after a data access agreement has been signed.
Ethics statementThe studies involving humans were approved by the institutional review boards/independent ethics committees at participating centers. All studies were designed and conducted in compliance with the ethical principles of Good Clinical Practice and in accordance with the Declaration of Helsinki. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributionsJuT: Conceptualization, Funding acquisition, Investigation, Methodology, Validation, Visualization, Writing – original draft, Writing – review & editing. MF: Conceptualization, Funding acquisition, Investigation, Methodology, Validation, Visualization, Writing – original draft, Writing – review & editing. GL: Conceptualization, Funding acquisition, Investigation, Methodology, Validation, Visualization, Writing – original draft, Writing – review & editing. GP: Conceptualization, Funding acquisition, Investigation, Methodology, Validation, Visualization, Writing – original draft, Writing – review & editing. EV: Conceptualization, Funding acquisition, Investigation, Methodology, Validation, Visualization, Writing – original draft, Writing – review & editing. FC: Conceptualization, Funding acquisition, Investigation, Methodology, Validation, Visualization, Writing – original draft, Writing – review & editing. NA: Conceptualization, Formal analysis, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. EC: Conceptualization, Data curation, Formal analysis, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. ML: Conceptualization, Data curation, Formal analysis, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. LR: Conceptualization, Data curation, Formal analysis, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. JoT: Conceptualization, Funding acquisition, Investigation, Methodology, Validation, Visualization, Writing – original draft, Writing – review & editing. TA: Conceptualization, Methodology, Validation, Visualization, Writing – original draft, Writing – review & editing.
FundingThe author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by Servier International Research Institute and Taiho Oncology, Inc. The sponsors were involved in the design and conduct of the study; collection, analysis, and interpretation of the data; writing of the manuscript; and the decision to submit the manuscript for publication.
AcknowledgmentsWe thank and acknowledge all the participants, their families, and study personnel for participating in the studies. Medical writing assistance was provided by Envision Pharma Group and funded by Taiho Oncology, Inc.
Conflict of interestJuT has received fees for advisory/consultancy roles from Amgen, HalioDX SAS, Lilly, Merck Serono, MSD, Novartis, Pierre Fabre, Roche, Sanofi, and Servier. MF has received fees for advisory/consultancy roles from AbbVie, AstraZeneca, Bayer, Bristol Myers Squibb, Eisai, Entos Inc., Janssen, Merck, Mirati, Nouscom, Pfizer/Genentech, and Taiho Oncology, and his institution has received grants from Agenusbio, Bristol Myers Squibb, Genentech/imCore, and Verastem. GL has received honoraria for educational activities from Nutricia AS and Servier. GP has received advisory/consultancy fees from Arcus, Amgen, AstraZeneca, Bayer, Beigene, Bristol Myers Squibb, CECOG, Incyte, Lilly, Merck, MSD, Novartis, Pierre Fabre, Roche, Sanofi, Servier, Taiho Oncology, and Takeda. EVC has received advisory/consultancy fees from AbbVie, ALX, Amgen, Array, Astellas, AstraZeneca, Bayer, BeiGene, Boehringer Ingelheim, Bristol Myers Squibb, Daiichi Sankyo, GSK, Incyte, Ipsen, Lilly, Merck Sharp & Dohme, Merck KGaA, Mirati, Novartis, Nordic, Pierre Fabre, Pfizer, Roche, Seattle Genetics, Servier, Takeda, Terumo, Taiho, and Zymeworks, and his institution has received research grants from Amgen, Bayer, Boehringer Ingelheim, Bristol Myers Squibb, Ipsen, Lilly, Merck Sharp & Dohme, Merck KGaA, Novartis, Roche, and Servier. FC has received grants from Pfizer, Pierre Fabre, and Roche, and payment or honoraria from Bayer, Merck KGaA, MSD, Pierre Fabre, Roche, and Servier. NA and LR are employees of Servier. EC and ML are employees of Taiho Oncology. JoT has received fees for advisory/consultancy roles from Alentis Therapeutics, AstraZeneca, Boehringer Ingelheim, Cardiff Oncology, CARSgen Therapeutics, Chugai, Daiichi Sankyo, F. Hoffmann-La Roche Ltd, Genentech, Inc., hC Bioscience, Ikena Oncology, Immodulon Therapeutics, Inspirna, Inc., Lilly, Menarini, Merck Serono, Merus, MSD, Mirati, Neophore, Novartis, Ona Therapeutics, Orion Biotechnology, Peptomyc, Pfizer, Pierre Fabre, Sanofi, Scandion Oncology, Scorpion Therapeutics, Seattle Genetics, Servier, Sotio Biotech, Taiho Oncology, Takeda Oncology, and Tolremo Therapeutics; has stocks in 1TRIALSP, Alentis Therapeutics, Oniria Therapeutics, and Pangaea Oncology; and has participated in educational collaboration with Medscape Education and the PeerView Institute for Medical Education and Physicians Education Resource. TA reports attending advisory board meetings for and receiving consulting fees from AbbVie, Astellas, Bristol Myers Squibb, Gritstone Oncology, GamaMabs Pharma SA, GlaxoSmithKline, Merck & Co, Seagen, Servier, and Takeda; receiving honoraria from AstraZeneca, Bristol Myers Squibb, GlaxoSmithKline, Merck & Co, Pierre Fabre, Roche, Ventana Medical Systems, Sanofi, and Servier; and receiving support for meetings from Bristol Myers Squibb, Merck & Co, and Servier.
Generative AI statementThe author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s noteAll claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary materialThe Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2024.1506075/full#supplementary-material
AbbreviationsAE, adverse event; ECOG PS, Eastern Cooperative Oncology Group performance status; FTD/TPI, trifluridine/tipiracil; G-CSF, granulocyte colony-stimulating factor; mCRC, metastatic colorectal cancer; NCI-CTCAE, National Cancer Institute Common Terminology Criteria for Adverse Events; SAE, serious adverse event; TEAE, treatment-emergent adverse event; TRAE, treatment-related adverse event.
References1. Lenz HJ, Stintzing S, Loupakis F. TAS-102, a novel antitumor agent: a review of the mechanism of action. Cancer Treat Rev. (2015) 41:777–83. doi: 10.1016/j.ctrv.2015.06.001
Crossref Full Text | Google Scholar
2. LONSURF® (trifluridine and tipiracil) tablets, for oral use [Prescribing information]. Princeton, NJ: Taiho Oncology, Inc (2023).
3. Mayer RJ, Van Cutsem E, Falcone A, Yoshino T, Garcia-Carbonero R, Mizunuma N, et al. Randomized trial of TAS-102 for refractory metastatic colorectal cancer. N Engl J Med. (2015) 372:1909–19. doi: 10.1056/NEJMoa1414325
Crossref Full Text | Google Scholar
4. Prager GW, Taieb J, Fakih M, Ciardiello F, Van Cutsem E, Elez E, et al. Trifluridine-tipiracil and bevacizumab in refractory metastatic colorectal cancer. N Engl J Med. (2023) 388:1657–67. doi: 10.1056/NEJMoa2214963
Crossref Full Text | Google Scholar
5. Cervantes A, Martinelli E, ESMO Guidelines Committee. Updated treatment recommendation for third-line treatment in advanced colorectal cancer from the ESMO Metastatic Colorectal Cancer Living Guideline. Ann Oncol. (2024) 35:241–3. doi: 10.1016/j.annonc.2023.10.129
Crossref Full Text | Google Scholar
6. Benson AB, Venook AP, Al-Hawary MM, Arain MA, Chen YJ, Ciombor KK, et al. Colon cancer, version 2.2021, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. (2021) 19:329–59. doi: 10.6004/jnccn.2021.0012
Crossref Full Text | Google Scholar
7. Yeku OO, Longo DL. Combination therapy, including bevacizumab, for advanced colorectal cancer. N Engl J Med. (2023) 388:1711–4. doi: 10.1056/NEJMe2300385
Crossref Full Text | Google Scholar
8. Tsukihara H, Nakagawa F, Sakamoto K, Ishida K, Tanaka N, Okabe H, et al. Efficacy of combination chemotherapy using a novel oral chemotherapeutic agent, TAS-102, together with bevacizumab, cetuximab, or panitumumab on human colorectal cancer xenografts. Oncol Rep. (2015) 33:2135–42. doi: 10.3892/or.2015.3876
Crossref Full Text | Google Scholar
9. André T, Falcone A, Shparyk Y, Moiseenko F, Polo-Marques E, Csöszi T, et al. Trifluridine-tipiracil plus bevacizumab versus capecitabine plus bevacizumab as first-line treatment for patients with metastatic colorectal cancer ineligible for intensive therapy (SOLSTICE): a randomised, open-label phase 3 study. Lancet Gastroenterol Hepatol. (2023) 8:133–44. doi: 10.1016/S2468-1253(22)00334-X
Crossref Full Text | Google Scholar
10. Van Cutsem E, Danielewicz I, Saunders MP, Pfeiffer P, Argilés G, Borg C, et al. Trifluridine/tipiracil plus bevacizumab in patients with untreated metastatic colorectal cancer ineligible for intensive therapy: the randomized TASCO1 study. Ann Oncol. (2020) 31:1160–8. doi: 10.1016/j.annonc.2020.05.024
Crossref Full Text | Google Scholar
11. Kuboki Y, Nishina T, Shinozaki E, Yamazaki K, Shitara K, Okamoto W, et al. TAS-102 plus bevacizumab for patients with metastatic colorectal cancer refractory to standard therapies (C-TASK FORCE): an investigator-initiated, open-label, single-arm, multicentre, phase 1/2 study. Lancet Oncol. (2017) 18:1172–81. doi: 10.1016/S1470-2045(17)30425-4
Crossref Full Text | Google Scholar
12. Pfeiffer P, Yilmaz M, Möller S, Zitnjak D, Krogh M, Petersen LN, et al. TAS-102 with or without bevacizumab in patients with chemorefractory metastatic colorectal cancer: an investigator-initiated, open-label, randomised, phase 2 trial. Lancet Oncol. (2020) 21:412–20. doi: 10.1016/S1470-2045(19)30827-7
Crossref Full Text | Google Scholar
13. Andre T, Falcone A, Shparyk YV, Moiseenko FV, Polo E, Csoszi T, et al. Overall survival results for trifluridine/tipiracil plus bevacizumab vs capecitabine plus bevacizumab: results from the phase 3 SOLSTICE study. J Clin Oncol. (2023) 41:3512. doi: 10.1200/JCO.2023.41.16_suppl.3512
Crossref Full Text | Google Scholar
14. André T, Saunders M, Kanehisa A, Gandossi E, Fougeray R, Amellal NC, et al. First-line trifluridine/tipiracil plus bevacizumab for unresectable metastatic colorectal cancer: SOLSTICE study design. Future Oncol. (2020) 16:21–9. doi: 10.2217/fon-2019-0786
Crossref Full Text | Google Scholar
15. Tabernero J, Taieb J, Prager GW, Ciardiello F, Fakih M, Leger C, et al. Trifluridine/tipiracil plus bevacizumab for third-line management of metastatic colorectal cancer: SUNLIGHT study design. Future Oncol. (2021) 17:1977–85. doi: 10.2217/fon-2020-1238
Crossref Full Text | Google Scholar
16. Kuboki Y, Terazawa T, Masuishi T, Nakamura M, Watanabe J, Ojima H, et al. The TRUSTY study: a randomized phase 2/3 study of trifluridine/tipiracil plus bevacizumab versus irinotecan and fluoropyrimidine plus bevacizumab as second-line treatment in patients with metastatic colorectal cancer. J Clin Oncol. (2021) 39:3507. doi: 10.1200/JCO.2021.39.15_suppl.3507
Crossref Full Text | Google Scholar
17. Kuboki Y, Terazawa T, Masuishi T, Nakamura M, Watanabe J, Ojima H, et al. Trifluridine/tipiracil+bevacizumab (BEV) vs. fluoropyrimidine-irinotecan+BEV as second-line therapy for metastatic colorectal cancer: a randomised noninferiority trial. Br J Cancer. (2023) 128:1897–905. doi: 10.1038/s41416-023-02212-2
Crossref Full Text | Google Scholar
18. Yoshida Y, Yamada T, Kamiyama H, Kosugi C, Ishibashi K, Yoshida H, et al. Combination of TAS-102 and bevacizumab as third-line treatment for metastatic colorectal cancer: TAS-CC3 study. Int J Clin Oncol. (2021) 26:111–7. doi: 10.1007/s10147-020-01794-8
Crossref Full Text | Google Scholar
19. Yoshino T, Taieb J, Kuboki Y, Pfeiffer P, Kumar A, Hochster HS. Trifluridine/tipiracil with or without bevacizumab in metastatic colorectal cancer: results of a systematic review and meta-analysis. Ther Adv Med Oncol. (2023) 15:17588359221146137. doi: 10.1177/17588359221146137
Crossref Full Text | Google Scholar
20. Klastersky J, de Naurois J, Rolston K, Rapoport B, Maschmeyer G, Aapro M, et al. Management of febrile neutropaenia: ESMO Clinical Practice Guidelines. Ann Oncol. (2016) 27:v111–v8. doi: 10.1093/annonc/mdw325
Crossref Full Text | Google Scholar
21. Griffiths EA, Roy V, Alwan L, Bachiashvili K, Baird J, Cool R, et al. NCCN guidelines® Insights: hematopoietic growth factors, version 1.2022. J Natl Compr Canc Netw. (2022) 20:436–42. doi: 10.6004/jnccn.2022.0026
Crossref Full Text | Google Scholar
22. Li M, Kroetz DL. Bevacizumab-induced hypertension: clinical presentation and molecular understanding. Pharmacol Ther. (2018) 182:152–60. doi: 10.1016/j.pharmthera.2017.08.012
Crossref Full Text | Google Scholar
23. Brandes AA, Bartolotti M, Tosoni A, Poggi R, Franceschi E. Practical management of bevacizumab-related toxicities in glioblastoma. Oncologist. (2015) 20:166–75. doi: 10.1634/theoncologist.2014-0330
Crossref Full Text | Google Scholar
24. AVASTIN® (bevacizumab) injection, for intravenous use [Prescribing information]. South San Francisco, CA: Genetech, Inc (2009).
25. Arora N, Gupta A, Singh PP. Biological agents in gastrointestinal cancers: adverse effects and their management. J Gastrointest Oncol. (2017) 8:485–98. doi: 10.21037/jgo.2017.01.07
Crossref Full Text | Google Scholar
26. Gilmore N, Loh KP, Liposits G, Arora SP, Vertino P, Janelsins M. pigenetic and inflammatory markers in older adults with cancer: a Young International Society of Geriatric Oncology narrative review. J Geriatr Oncol. (2024) 15:101655. doi: 10.1016/j.jgo.2023.101655
Crossref Full Text | Google Scholar
27. Kito Y, Kawakami H, Mitani S, Nishina S, Matsumoto T, Tsuzuki T, et al. Trifluridine/tipiracil plus bevacizumab for vulnerable patients with pretreated metastatic colorectal cancer: a retrospective study (WJOG14520G). Oncologist. (2024) 9:e330–e6. doi: 10.1093/oncolo/oyad296
留言 (0)