Total Knee Arthroplasty (TKA) is widely regarded as the most effective surgical intervention for managing end-stage knee osteoarthritis (KOA), offering significant pain relief and functional restoration (1, 2). Despite advancements in surgical techniques, prosthetic knee designs (3), and postoperative rehabilitation protocols, approximately 10–40% of patients may still develop postoperative cognitive dysfunction (POCD) (4, 5). POCD is a common postoperative syndrome characterized by cognitive impairments, including deficits in memory, intellectual ability, and executive function (6). This complex condition often persists beyond the expected recovery period from anesthesia, contributing to prolonged hospital stays, increased healthcare costs, greater care requirements, as well as elevated morbidity and mortality (7–9).
The precise mechanisms underlying POCD remain poorly understood, emerging evidence points to a potential role of central sensitization (CS) in its pathogenesis (10). CS, a pathological condition frequently observed in chronic pain patients, is marked by a heightened excitability of the central nervous system (CNS) triggered by persistent peripheral nociceptive stimuli, leading to exaggerated pain responses even from subthreshold or normal sensory input (11). CS has been shown to a significant risk factor for persistent pain and patient dissatisfaction after TKA (12, 13). However, CS not only intensifies pain perception in patients undergoing TKA, but also potentially compromises cognitive function through a cascade of neuroinflammatory processes (14, 15). Studies suggest that patients with CS exhibit a significantly lowered pain threshold, a phenomenon closely linked to microglial activation and the release of pro-inflammatory cytokines such as IL-6 and TNF-α within the CNS (16–18). These inflammatory mediators are thought to be key drivers of cognitive decline (19, 20). This suggests that CS is not only a primary mechanism responsible for chronic postoperative pain but also a contributor to POCD through sustained neuroinflammation and altered neuroplasticity.
Notably, the presence of CS in TKA patients is often accompanied by preoperative chronic pain and psychosocial factors, such as anxiety, depression, and sleep disturbances, all of which are themselves closely linked to the occurrence of POCD (21, 22). As such, CS is considered to be a critical risk factor for POCD following TKA. Although previous studies have explored the impact of CS on postoperative pain and functional recovery, systematic research into its relationship with POCD remains very limited.
Given this, the study aims to conduct a retrospective analysis to investigate the impact of preoperative CS on POCD in TKA patients. Patients will be stratified based on their preoperative Central Sensitization Inventory (CSI) scores, and the correlation between CS and POCD will be assessed. The findings from this study are expected to offer new insights for preoperative screening, and postoperative management, ultimately reducing the incidence of POCD and improving patient outcomes.
2 Materials and methods 2.1 Study designThis study is a multi-center, retrospective cohort investigation aimed at evaluating the impact of CS on POCD in patients undergoing TKA. Clinical data were sourced from medical records at the First Affiliated Hospital of Bengbu Medical College, the First Affiliated Hospital of Anhui University of Traditional Chinese Medicine, and Hefei Southeast Orthopedics Hospital. The study adhered to the STROBE guidelines and received ethical approval from the Institutional Review Board (2023-zj-40). As all data were de-identified, informed consent from patients was waived.
2.2 ParticipantsThis study retrospectively analyzed patients who underwent TKA at the aforementioned three hospitals between January 1, 2020, and May 31, 2024. The inclusion criteria were as follows: (1) age ≥ 55 years; (2) diagnosed with end-stage KOA; (3) undergoing primary unilateral TKA; and (4) having complete CSI scores. Exclusion criteria included: (1) simultaneous bilateral TKA or revision TKA; (2) a history of central nervous system or psychiatric disorders; (3) severe sensory impairments affecting vision or hearing; (4) severe comorbidities involving the heart, brain, or lungs; (5) preoperative Mini-Mental State Examination (MMSE) score ≤ 23; (6) use of cognitive-impairing medications pre- or post-surgery (e.g., sedatives, anti-inflammatory drugs, psychotropics); and (7) incomplete clinical records.
2.3 Data collectionWe collected comprehensive demographic and clinical data from patients meeting the study criteria, including age, gender, BMI, educational level, American Society of Anesthesiologists (ASA) classification, smoking, drinking, duration of KOA, and comorbidities such as hypertension, hyperlipidemia, diabetes, coronary heart disease, cerebrovascular disease, and chronic obstructive pulmonary disease. Data were collected at the following time points: preoperatively (T0), intraoperatively (T1), on postoperative day 1 (T2), postoperative day 3 (T3), postoperative day 7 (T4), and postoperative day 30 (T5). At T0, CSI scores were recorded to assess the state of CS, and preoperative hemoglobin levels were documented to evaluate the presence of anemia. At T1, the anesthesia methods, duration of anesthesia, duration of surgery, and intraoperative blood loss were documented. At T2-T5, MMSE scores were recorded to assess cognitive function and determine the incidence of POCD, while the Knee Injury and Osteoarthritis Outcome Score (KOOS) was used to evaluate postoperative recovery. Although MMSE is not universally a routine clinical assessment item for TKA patients, its inclusion in this study reflects institutional efforts to systematically monitor and manage POCD. The MMSE data were collected as part of the standard cognitive assessments implemented at the participating institutions and were consistently recorded in the hospitals’ medical records by trained clinical staff.
2.4 Assessment tools 2.4.1 Central sensitization assessmentThe Central Sensitization Inventory (CSI) was used preoperatively to assess the CS status of patients. The CSI is a validated, patient-reported questionnaire designed to evaluate symptoms associated with CS. It has been demonstrated to be a reliable, consistent, and effective tool for quantifying the severity of CS-related symptoms (23). The inventory includes 25 items, each scored from 0 to 4, with a total score ranging from 0 to 100. Based on established criteria (24, 25), a score of 40 or higher indicates the presence of CS, while a score below 40 suggests the absence of CS. In this study, patients were categorized into a CS group (CSI ≥ 40) and a non-CS group (CSI < 40).
2.4.2 Cognitive function assessmentThe MMSE scores was used to evaluated cognitive function (26). It primarily evaluates five cognitive domains: orientation, registration, attention and calculation, recall, and language abilities. The MMSE consists of 24 items with a total score of 30, where higher scores indicate better cognitive function. In this study, POCD was defined based on the changes in MMSE scores observed on postoperative day 30 (T5). Specifically, POCD was defined as a decline of 1 or more standard deviations (SD) in the postoperative MMSE score compared to the preoperative score. A reduction of 1–2 SD indicates postoperative mild neurocognitive disorder (NCD), while a reduction greater than 2 SD indicates postoperative major neurocognitive disorder (27).
2.4.3 Knee function assessmentThe KOOS is a patient-reported outcome measurement system used to evaluate short-term and long-term symptoms and function in individuals after TKA. The score consisted of five separately scored subscales: pain, symptoms, function in daily living (ADL), function in sport and recreation (Sport/Rec), and knee-related quality of life (QoL) (28). The KOOS scale consists of 42 items with a total score ranging from 0 to 100, where higher scores indicate better functional recovery.
2.5 Statistical analysisAll statistical analyses were performed using SPSS version 26.0. Continuous variables are presented as mean ± standard deviation (SD), while categorical variables are expressed as frequencies and percentages. For continuous variables measured at multiple time points (e.g., MMSE and KOOS scores at preoperative and various postoperative intervals), repeated measures analysis of variance (ANOVA) was employed to assess both within-group and between-group trends. Independent samples t-tests were conducted to compare mean values between two groups, whereas paired t-tests were utilized to evaluate changes within the same group at two distinct time points. Categorical variables were analyzed using the chi-square test. To identify potential risk factors for POCD, both univariate and multivariate logistic regression analyses were performed, and odds ratios (ORs) with corresponding 95% confidence intervals (CIs) were calculated. All statistical tests were conducted with a significance level set at p < 0.05.
3 Results 3.1 Patient demographics and clinical characteristicsA total of 294 patients scheduled for TKA were initially recruited for the study. However, 152 patients were excluded based on the following criteria: age < 55 years, revision TKA, non-end-stage KOA, preoperative MMSE score < 24, history of psychiatric disorders, hearing impairment, preoperative sedative use, and incomplete clinical data (n = 54). Ultimately, 142 patients were included in the final analysis. Figure 1 illustrates the patient selection flowchart.
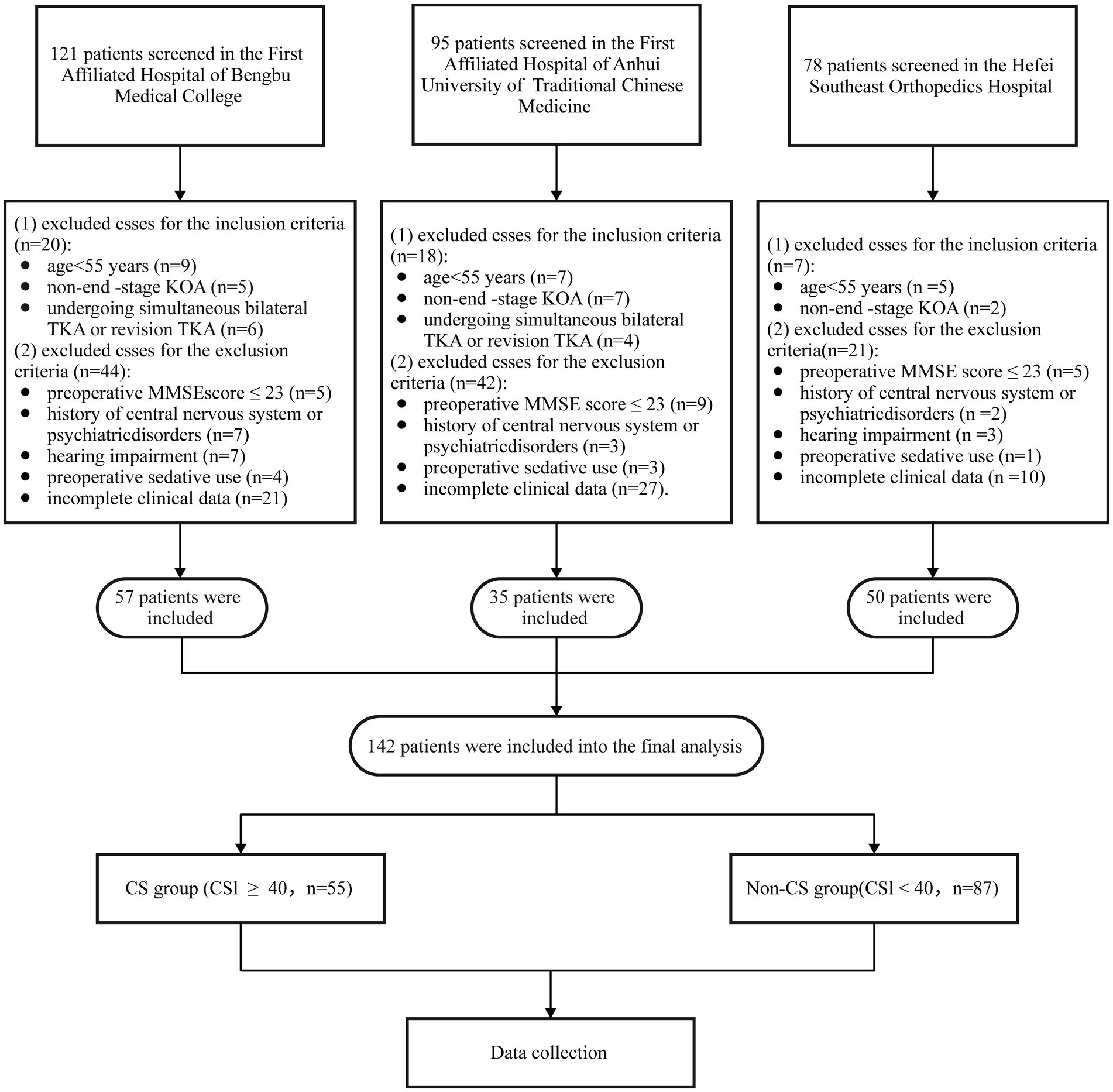
Figure 1. Flow chart of patient selection. TKA, total knee arthroplasty; KOA, Knee osteoarthritis; CS, central sensitization; MMSE, mini-mental state examination.
Among the 142 patients enrolled in the study, 55 were assigned to the CS group (CSI ≥ 40), and 87 to the non-CS group (CSI < 40). Analysis of baseline data revealed no significant differences between the groups in terms of age, gender, BMI, education level, smoking, drinking, ASA classification, duration of KOA, comorbidities, preoperative hemoglobin, anemia, preoperative MMSE, operation time, intraoperative hemorrhage, anesthesia type, and anesthesia time (all p > 0.05) (Table 1).
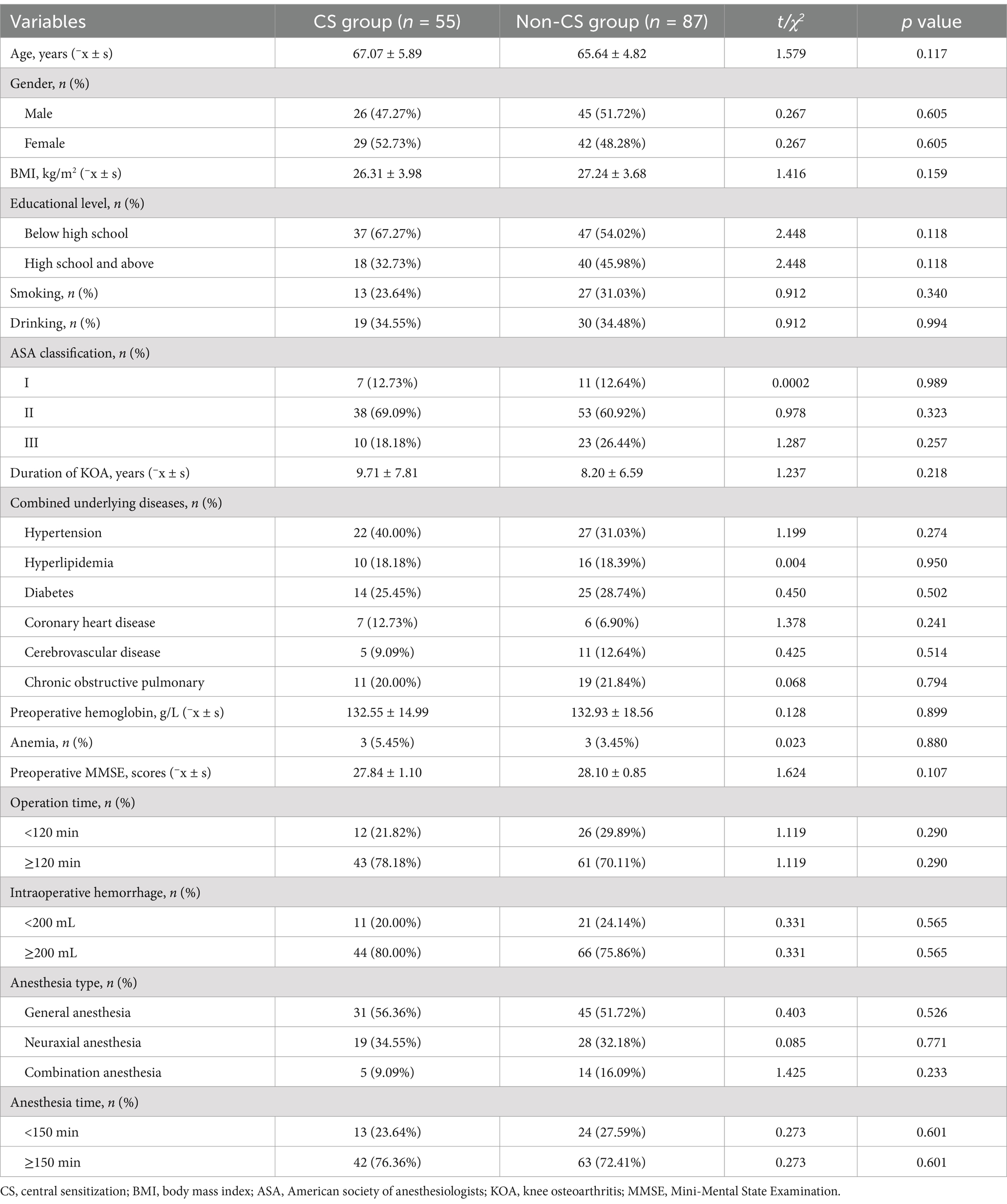
Table 1. Comparison of baseline demographic and clinical characteristics.
3.2 Comparison of the incidence of POCDThe overall incidence of POCD on 30 days postoperatively was 19.72% (28/142). The incidence in the CS group was 30.91% (17/55), significantly higher than 12.64% (11/87) in the non-CS group (p = 0.008), indicating a strong correlation between central sensitization and the overall occurrence of POCD. However, when examining the severity of POCD, no significant differences were observed between the two groups regarding the proportions of mild and major cases (p>0.05). In the CS group, 12 cases were classified as postoperative mild NCD and 5 as major, while in the non-CS group, 9 cases were mild and 2 were postoperative major NCD. These findings suggest that central sensitization primarily influences the overall incidence of POCD rather than its severity (Figure 2).
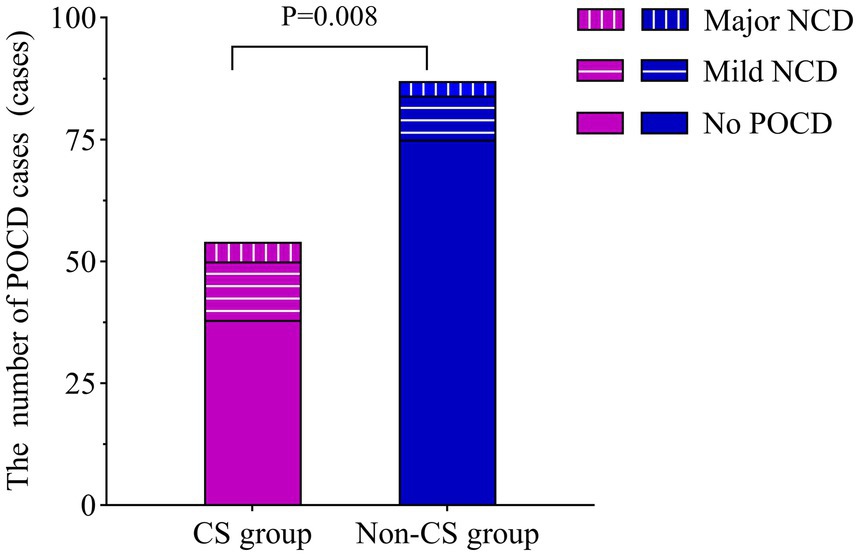
Figure 2. Comparison of POCD cases on postoperative day 30. The incidence of POCD was compared between the CS and non-CS groups using the chi-square test. CS, central sensitization; POCD, postoperative cognitive dysfunction; NCD, neurocognitive disorder.
3.3 Comparison of MMSE scoresRepeated measures ANOVA indicated a significant main effect of group on MMSE scores (F = 45.08, p < 0.001), indicating a notable difference in cognitive recovery between the CS group and the non-CS group. The main effect of time was also significant (F = 60.68, p < 0.001), reflecting a trend of change in MMSE scores over time. Moreover, the interaction between time and group was significant (F = 3.443, p = 0.009), suggesting that the two groups exhibited different trends in postoperative cognitive recovery.
Paired comparisons within each group showed significant differences in MMSE scores for both the CS group and the non-CS group at T2 and T3 compared to T0 (p < 0.05). Between-group comparisons revealed significant differences in MMSE scores at all time points except T0 (p < 0.05). These findings suggest that patients in the CS group exhibited a marked delay in postoperative neurocognitive recovery, particularly during the early recovery phase (Table 2; Figure 3).
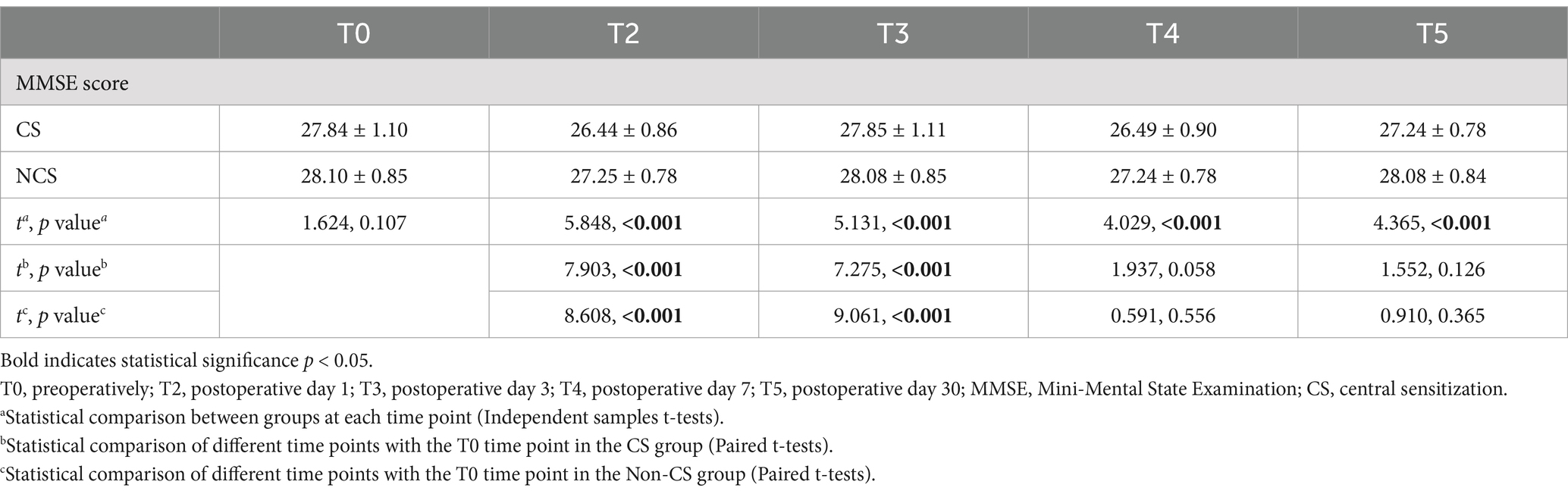
Table 2. Comparison of MMSE scores between two groups.
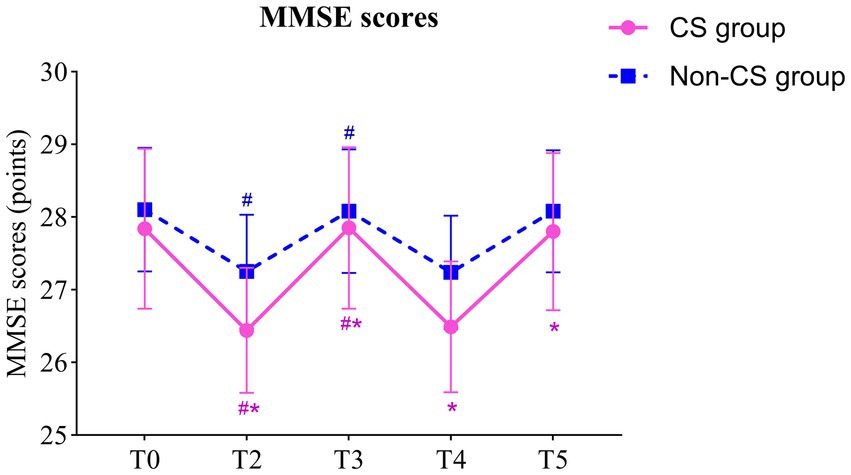
Figure 3. Comparison of MMSE scores between two groups. # indicates that compared with T0 (Paired t-tests), p < 0.05; * indicates comparison with non-CS group (Independent samples t-tests), p < 0.05; T0, preoperatively; T2, postoperative day 1; T3, postoperative day 3; T4, postoperative day 7; T5, postoperative day 30. MMSE, mini-mental state examination.
3.4 Comparison of KOOS scoresRepeated measures ANOVA indicated a significant main effect of group on the pain and QoL subdomains of the KOOS scores (F = 85.26, p < 0.001; F = 85.88, p < 0.001), while no significant main effects were observed for symptoms, ADL, and Sport/Rec subdomains (F = 3.797, p = 0.052; F = 3.294, p = 0.07; F = 0.841, p = 0.360). This suggests that there were significant differences between the CS group and non-CS group in pain perception and quality of life recovery, but no significant differences in symptoms, daily activities, or sports/recreation. The main effect of time was significant across all five KOOS subdomains (F = 209.4, p < 0.001; F = 480.0, p < 0.001; F = 659.3, p < 0.001; F = 911.5, p < 0.001; F = 651.4, p < 0.001), indicating that patients experienced significant improvements in all KOOS subdomains over time. Furthermore, the interactions between time and group were observed for both pain and QoL scores (F = 10.74, p < 0.001; F = 6.201, p < 0.001), suggesting that the two groups exhibited different trends in postoperative pain relief and quality of life recovery.
Paired comparisons within each group showed significant differences in all KOOS subdomains at all postoperative time points compared to T0 in both the CS and non-CS groups (p < 0.05), indicating overall recovery in pain, symptoms, daily activities, sports/recreation, and quality of life following TKA. Between-group comparisons revealed that the CS group had significantly lower pain and quality of life scores at T0, T4, and T5 compared to the non-CS group (p < 0.05), indicating a marked delay in postoperative pain relief and quality of life recovery in the CS group (Table 3; Figure 4).
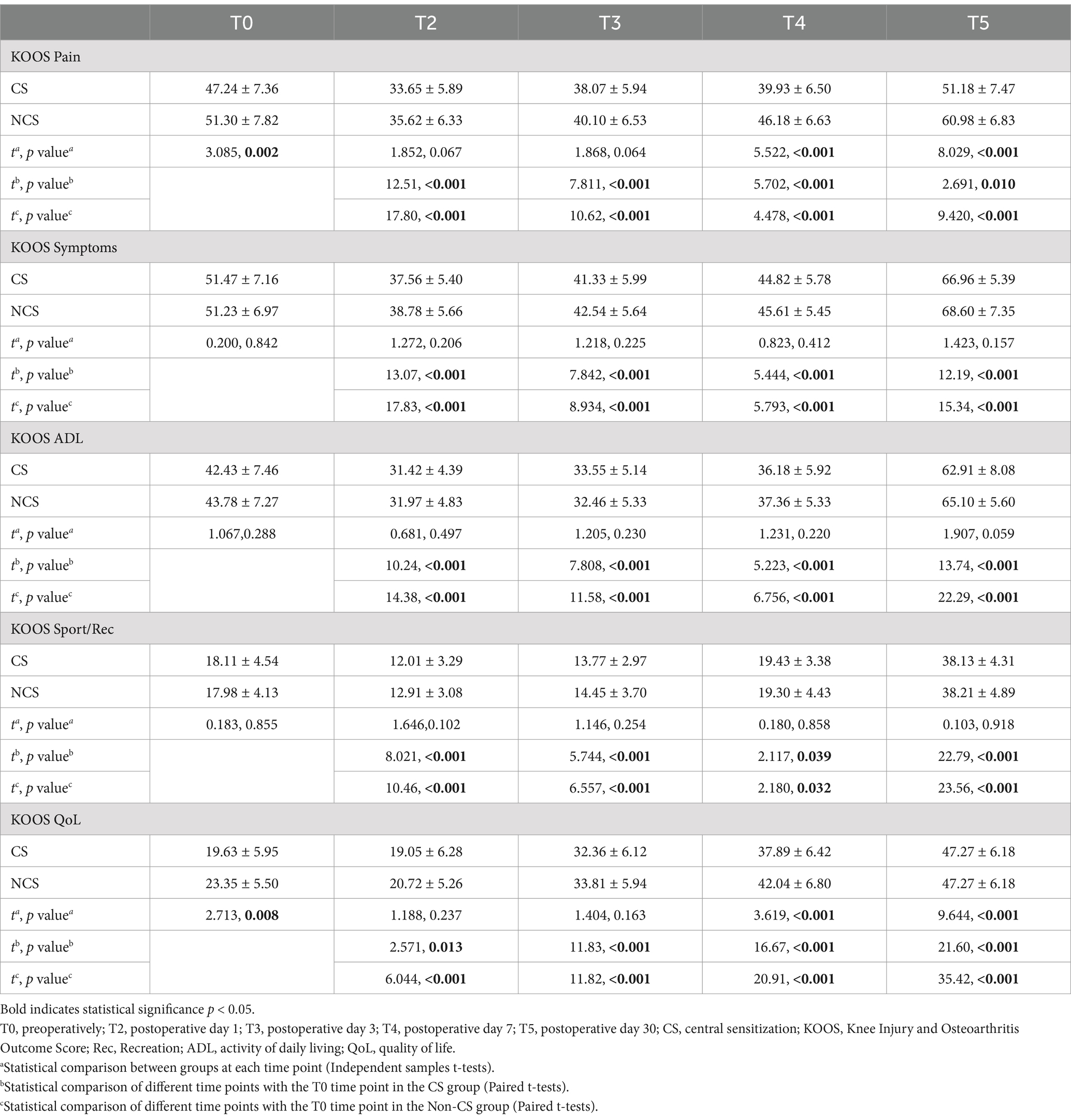
Table 3. Comparison of KOOS scores between two groups.
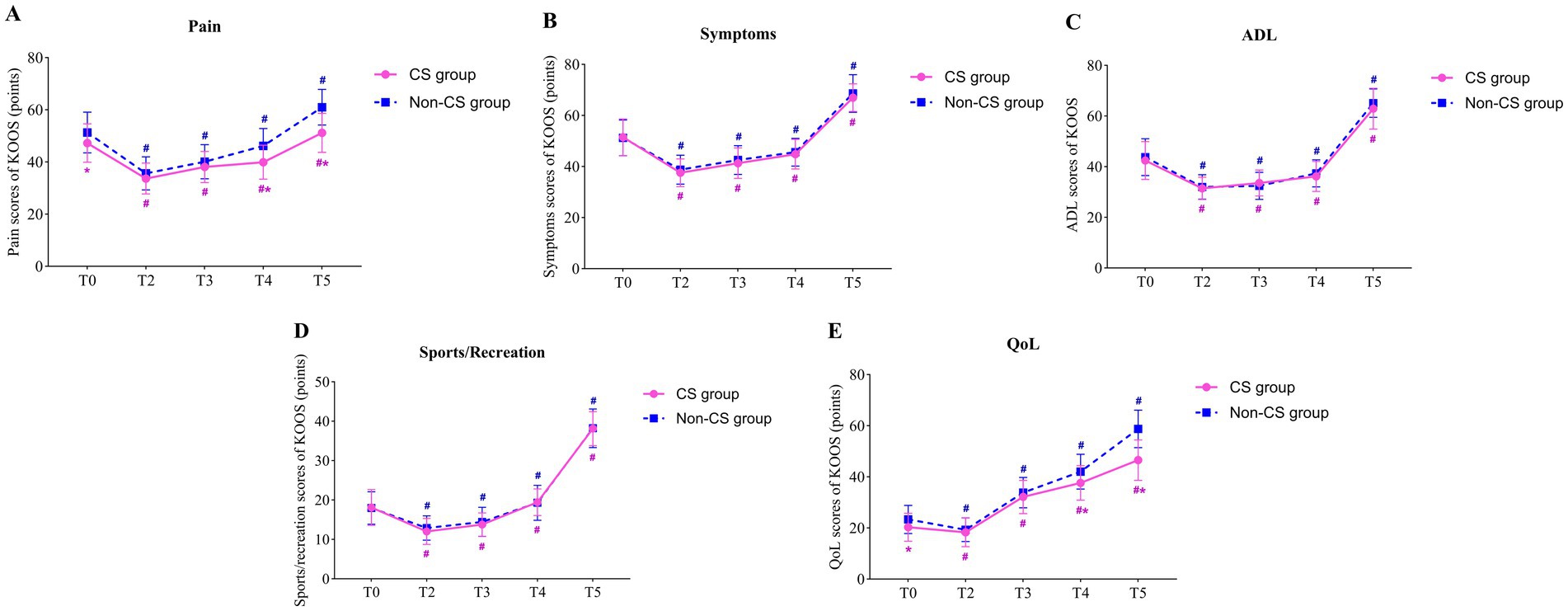
Figure 4. Comparison of KOOS scores between two groups. (A) the pain scores of KOOS; (B) the symptoms scores of KOOS; (C) the ADL scores of KOOS; (D) the sports/recreation scores of KOOS; (E) the QoL scores of KOOS; # indicates that compared with T0 (Paired t-tests), p < 0.05; * indicates comparison with non-CS group (Independent samples t-tests), p < 0.05; T0, preoperatively; T2, postoperative day 1; T3, postoperative day 3; T4, postoperative day 7; T5, postoperative day 30. KOOS, The Knee Injury and Osteoarthritis Outcome Score; ADL, activity of daily living; QoL, quality of life.
3.5 Univariate and multivariate logistic regression analysis of postoperative POCD occurrenceThe univariate logistic regression identified significant associations between POCD and CSI score, coronary heart disease, cerebrovascular disease, preoperative MMSE scores, intraoperative hemorrhage, and anesthesia time (all p < 0.05). To further evaluate the independent impact of central sensitization (CSI score) on the incidence of POCD, a multivariate logistic regression model was constructed. The analysis revealed that a CSI score of ≥40 (OR = 4.05, 95% CI = 1.52–11.54, p = 0.006), cerebrovascular disease (OR = 4.33, 95% CI = 1.09–18.68, p = 0.026), preoperative MMSE score < 27 (OR = 1.81, 95% CI = 1.04–3.26, p = 0.045), and intraoperative hemorrhage ≥200 mL (OR = 5.07, 95% CI = 1.58–20.14, p = 0.011) were significantly associated with a higher risk of POCD in TKA patients after adjusting for confounding factors (Table 4).
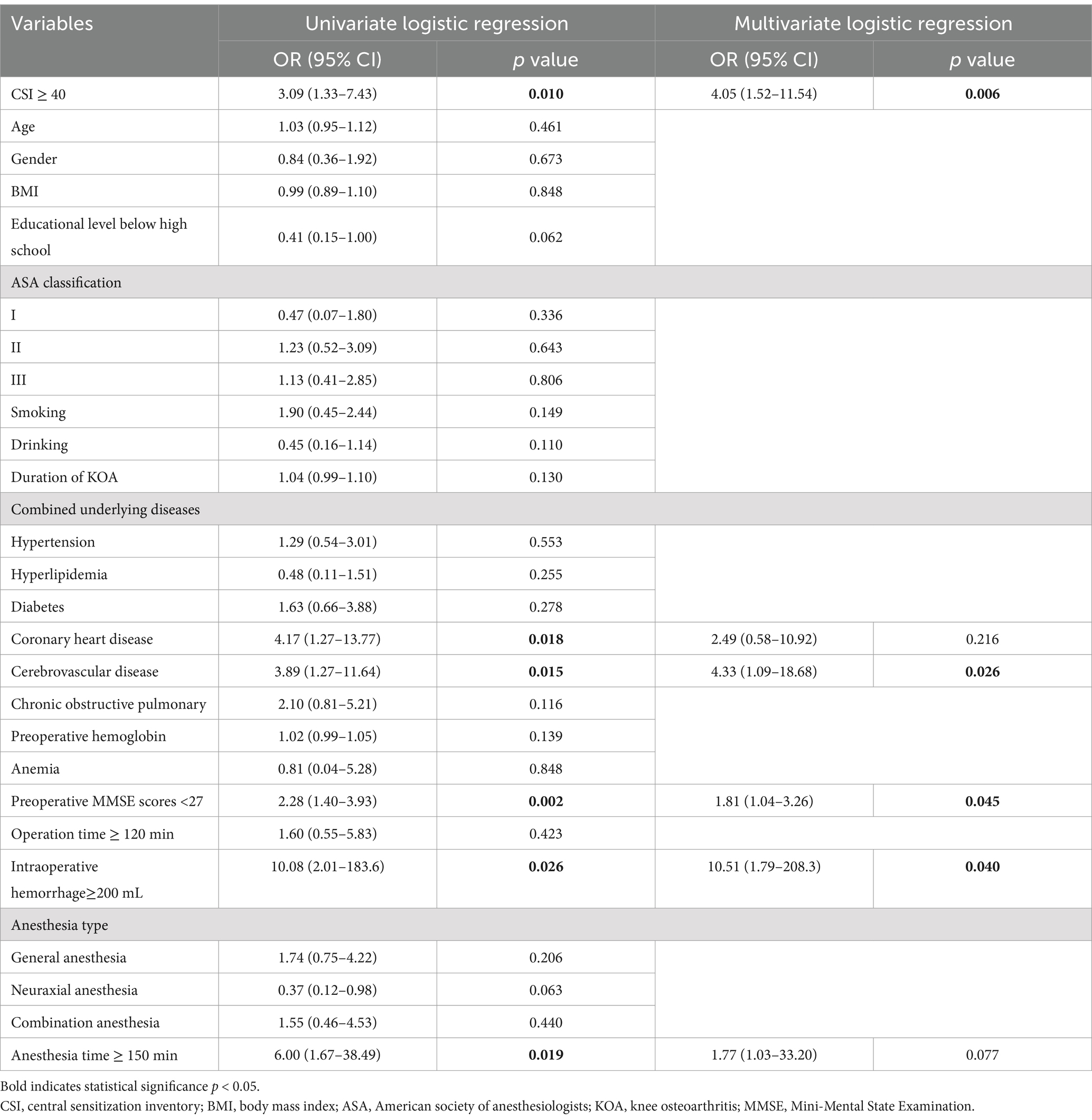
Table 4. Univariate and multivariate logistic regression of postoperative POCD occurrence.
4 DiscussionThis study demonstrates that CS is a significant risk factor for POCD following TKA. A retrospective analysis of 142 patients revealed that 30.91% of the CS group experienced POCD, compared to 12.64% in the non-CS group. Additionally, the CS group exhibited lower MMSE scores at multiple postoperative time points, along with lower pain and quality of life scores in the KOOS subdomains compared to the non-sensitized group. Logistic regression analysis further indicated that even after accounting for potential confounders, a higher CSI score (≥40) remained an independent risk factor for POCD. This finding clearly underscores the critical role of CS in impairing neurocognitive recovery following TKA.
TKA is one of the most cost-effective surgeries in orthopedics. Despite its high success rate, postoperative complications, especially POCD, are increasingly concerning, with rates reported to range from 19.4 to 72.0% 1 week postoperatively (4). Although the precise mechanisms underlying POCD remain unclear, several risk factors are known to be closely associated, including lower educational level (29), older age (30), alcohol use disorder (31, 32), preexisting cognitive impairment (33, 34). This study is the first to reveal a close link between CS and POCD, suggesting that CS not only affects postoperative pain management but may also exacerbate cognitive decline following TKA.
CS refers to a persistent and exaggerated response of the central nervous system to painful stimuli, characterized by a lowered pain threshold and abnormal pain perception. Recent studies have demonstrated that CS not only alters pain perception but may also impair cognitive function through various mechanisms (10). Neuroinflammation is considered a key pathophysiological foundation of CS. In chronic pain conditions, pro-inflammatory signals released from peripheral tissues can penetrate the blood–brain barrier, activating microglia and astrocytes, which in turn heightens neuronal excitability, leading to neuronal damage and apoptosis, potentially affecting cognitive function (35, 36). TKA is a highly invasive procedure often accompanied by severe postoperative pain and inflammation. During this process, the release of pro-inflammatory cytokines, such as IL-6, TNF-α, and IL-1β, exacerbates the effects of CS, potentially contributing to the development of POCD (37, 38). This mechanism may explain the occurrence of POCD in TKA patients, and the impact is likely to be more pronounced in those with CS. The results of this study’s MMSE assessments further support this hypothesis, indicating that CS may negatively affect postoperative cognitive recovery through similar mechanisms. We observed significant declines in MMSE scores on postoperative days 1and 3 compared to preoperative scores in both the CS and control groups, suggesting marked cognitive deterioration in the acute phase of TKA, which aligns with previous research. Additionally, MMSE scores in the CS group were significantly lower than those in the control group on postoperative days 1, 3, and 7, indicating a notable delay in cognitive recovery during the acute postoperative period among CS patients. This trend persisted through postoperative day 30, suggesting that CS has a sustained negative impact on long-term cognitive recovery. However, the potential impact of the learning effect could not be ignored. Conducting the MMSE assessment five times within 1 month might have led to patients becoming familiar with the test, artificially improving scores without truly reflecting cognitive recovery. Although we implemented standardized testing procedures and multidimensional data analyses to minimize the learning effect, it is not entirely possible to rule out its influence on the results. Furthermore, as this is a retrospective study, we were unable to directly verify or quantify the existence of the learning effect through experimental design. Future research should consider longer intervals between assessments or incorporate more complex cognitive tests to reduce the impact of the learning effect.
The KOOS results further demonstrated the negative impact of CS on postoperative recovery following TKA. Repeated measures ANOVA revealed that the CS group had significantly lower pain and quality of life scores compared to the non-sensitized group, while no significant differences were observed in the subdomains of symptoms, daily living activities, or sports/recreation. This suggests that CS primarily affects pain perception and quality of life, rather than other functional domains of the knee. Moreover, time had a significant main effect across all KOOS subdomains, indicating that overall knee function improved postoperatively for all patients. However, the interaction between time and group was significant for pain and quality of life scores, indicating that the trajectories of postoperative pain relief and quality of life recovery differed significantly between the high-sensitization and low-sensitization groups. Notably, by postoperative days 7 and 30, the CS group showed a marked delay in pain perception relief and quality of life improvement. This suggests that CS not only exacerbates postoperative pain perception but may also hinder the recovery of knee function by affecting patients’ psychological states and fear of physical activity, thereby exerting a prolonged negative effect on long-term quality of life. Consequently, during postoperative recovery in CS patients, greater emphasis should be placed on pain management and quality of life enhancement.
Notably, in the univariate and multivariate logistic regression analyses, even after adjusting for multiple confounding factors, central sensitization (CSI score ≥ 40) was identified as a significant risk factor for POCD following TKA, further emphasizing the critical role of CS in the development of POCD. Additionally, patients with cerebrovascular disease, intraoperative hemorrhage exceeding 200 mL and preoperative MMSE scores below 27 were significantly associated with an increased risk of POCD, suggesting that underlying cerebrovascular conditions, physiological stressors (such as intraoperative blood loss) and baseline cognitive function are key contributors to the occurrence of POCD. Consistent with previous research (2, 39), we speculate that patients with central sensitization may experience a heightened stress response during surgery, potentially leading to increased blood loss and a compounded neurocognitive burden. This finding also underscores the necessity of a thorough preoperative assessment, including screening for cognitive impairment, and cerebrovascular health, as well as meticulous intraoperative management for patients at risk of central sensitization to mitigate these adverse outcomes.
Although this study provides new evidence for understanding the association between CS and POCD, several limitations should be acknowledged. First, as a retrospective study, this research can only reveal correlations between patients with more pronounced CS symptoms and POCD, without establishing causality. Second, although multiple potential confounding factors were adjusted for, other factors not accounted for in the study-such as postoperative infections, preoperative and intraoperative drug use, emotional disorders like depression and anxiety, and sleep disturbances-may also influence the occurrence of POCD. Third, conducting the MMSE assessment five times within 1 month may have introduced a learning effect, where patients’ familiarity with the test could artificially improve scores rather than reflect true cognitive recovery. Moreover, the MMSE may lack the sensitivity required to identify nuanced cognitive changes, potentially affecting the accuracy of POCD evaluation. Future studies should consider adopting more detailed neuropsychological assessment tools to comprehensively evaluate specific cognitive domains. Fourth, the sample size of this study was relatively small, and the follow-up points were limited to postoperative days 1, 3, 7, and 30. The short follow-up duration and small sample size may introduce bias, and the findings should therefore be interpreted with caution. Finally, the study did not utilize imaging techniques, such as structural MRI, FDG-PET, or resting-state fMRI, nor were biomarkers employed to explore the impact of CS on patients (40). This limits the understanding of the neural mechanisms underlying POCD and hinders the development of personalized treatment strategies.
5 ConclusionThis study suggests that CS may be a novel risk factor for POCD following TKA, and may adversely affect postoperative recovery in terms of cognitive function, pain, and quality of life. Preoperative assessment of CS, particularly through individualized management of patients with chronic pain, may help reduce the incidence of POCD and improve overall postoperative outcomes after TKA. Future prospective, multicenter studies are needed to validate these findings and explore additional interventions to mitigate the negative impact of CS on postoperative cognitive function.
Data availability statementThe original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Ethics statementThe studies involving humans were approved by the Ethics Committee of the Second Affiliated Hospital of Anhui University of Chinese Medicine (Approval number: 2023-zj-40). The studies were conducted in accordance with the local legislation and institutional requirements. The ethics committee/institutional review board waived the requirement of written informed consent for participation from the participants or the participants' legal guardians/next of kin due to the retrospective nature of the study.
Author contributionsQY: Conceptualization, Data curation, Writing - original draft, Writing – review & editing. CL: Data curation, Formal analysis, Validation, Writing – original draft, Writing – review & editing. MY: Conceptualization, Formal analysis, Software, Writing – original draft. XZ: Data curation, Validation, Writing – original draft. WL: Data curation, Validation, Writing – review & editing. FL: Conceptualization, Funding acquisition, Supervision, Visualization, Writing – review & editing.
FundingThe author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This work is supported by the National Youth Qihuang Scholar Cultivation Program of National Administration of Traditional Chinese Medicine [Document No. 256 (2022)], the Natural Science Foundation of Anhui Province (No.2308085MH297), and the Natural Science Research Project Funding of Higher Education Institutions of Anhui Province (Nos. 2023AH040099, KJ2021A0549).
AcknowledgmentsWe sincerely thank all the participants of this study for their invaluable contributions to its completion.
Conflict of interestThe authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statementThe authors declare that no Gen AI was used in the creation of this manuscript.
Publisher’s noteAll claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
AbbreviationsPOCD, Postoperative cognitive dysfunction; TKA, total knee arthroplasty; CS, Central sensitization; KOA, Knee osteoarthritis; CSI, Central sensitization inventory; MMSE, Mini-Mental State Examination; KOOS, Knee Injury and Osteoarthritis Outcome Score; ASA, American society of anesthesiologists; OR, Odds ratios; ADL, Activities of daily living; QoL, Quality of life.
References1. Cram, P, Lu, X, Kates, SL, Singh, JA, Li, Y, and Wolf, BR. Total knee arthroplasty volume, utilization, and outcomes among Medicare beneficiaries, 1991-2010. JAMA. (2012) 308:1227–36. doi: 10.1001/2012.jama.11153
PubMed Abstract | Crossref Full Text | Google Scholar
2. Zhao, Y, Zang, C, Ren, S, Fu, J, Liu, N, Zhou, Z, et al. Effects of different levels of controlled hypotension on regional cerebral oxygen saturation and postoperative cognitive function in patients undergoing total knee arthroplasty. Front Med (Lausanne). (2022) 9:989341. doi: 10.3389/fmed.2022.989341
PubMed Abstract | Crossref Full Text | Google Scholar
3. Liu, K, Liu, Y, Ma, X, Fu, D, and Fan, Z. Effect of cognitive behavioral therapy on pain, knee function, and psychological status in patients after primary total knee arthroplasty: a systematic review and meta-analysis. BMC Musculoskelet Disord. (2024) 25:280. doi: 10.1186/s12891-024-07413-1
PubMed Abstract | Crossref Full Text | Google Scholar
4. Monk, TG, Weldon, BC, Garvan, CW, Dede, DE, van der Aa, MT, Heilman, KM, et al. Predictors of cognitive dysfunction after major noncardiac surgery. Anesthesiology. (2008) 108:18–30. doi: 10.1097/01.anes.0000296071.19434.1e
PubMed Abstract | Crossref Full Text | Google Scholar
5. Hu, XC, Yang, XM, Li, L, Yu, J, Si, XB, and Niu, ZQ. Effects of dexmedetomidine on renal function, inflammatory markers, and cognitive functioning in elderly patients undergoing hip replacement surgery. Am J Transl Res. (2024) 16:3713–22. doi: 10.62347/YFAI7091
PubMed Abstract | Crossref Full Text | Google Scholar
6. Kitsis, P, Zisimou, T, Gkiatas, I, Kostas-Agnantis, I, Gelalis, I, Korompilias, A, et al. Postoperative delirium and postoperative cognitive dysfunction in patients with elective hip or knee arthroplasty: a narrative review of the literature. Life (Basel). (2022) 12:314. doi: 10.3390/life12020314
Crossref Full Text | Google Scholar
9. Huang, J, Bin, ARH, and Yeo, SJ. Incidence of postoperative delirium in patients undergoing total knee arthroplasty-an Asian perspective. Ann Transl Med. (2017) 5:321. doi: 10.21037/atm.2017.06.40
PubMed Abstract | Crossref Full Text | Google Scholar
10. Yu, L, Yang, D, Zhou, Q, Yin, C, Zhang, Q, Li, W, et al. The effect of central sensitization on postoperative neurocognitive dysfunction in hospitalized elderly patients: a prospective cohort clinical trial. Exp Aging Res. (2024) 50:155–70. doi: 10.1080/0361073X.2023.2182093
PubMed Abstract | Crossref Full Text | Google Scholar
12. Sasaki, E, Kasai, T, Araki, R, Sasaki, T, Wakai, Y, Akaishi, K, et al. Central sensitization and postoperative improvement of quality of life in Total knee and Total hip arthroplasty: a prospective observational study. Prog Rehabil Med. (2022) 7:20220009. doi: 10.2490/prm.20220009
PubMed Abstract | Crossref Full Text | Google Scholar
13. Kim, MS, Koh, IJ, Sohn, S, Kang, BM, Kwak, DH, and In, Y. Central sensitization is a risk factor for persistent postoperative pain and dissatisfaction in patients undergoing revision Total knee arthroplasty. J Arthroplast. (2019) 34:1740–8. doi: 10.1016/j.arth.2019.03.042
PubMed Abstract | Crossref Full Text | Google Scholar
14. Vervullens, S, Meert, L, Smeets, R, Verbrugghe, J, Verdonk, P, and Meeus, M. Does pain intensity after total knee arthroplasty depend on somatosensory functioning in knee osteoarthritis patients? A prospective cohort study. Clin Rheumatol. (2024) 43:2047–59. doi: 10.1007/s10067-024-06976-7
PubMed Abstract | Crossref Full Text | Google Scholar
15. Rajani, AM, Mittal, A, Kulkarni, VU, Desai, MK, Dubey, RR, Rajani, KA, et al. Duloxetine as an analgesic in patients who do not have central sensitivity undergoing single-setting, bilateral Total knee arthroplasty: a prospective, double-blinded, randomized, Placebo-Controlled Trial. J Arthroplasty. (2024) 39:2055–60. doi: 10.1016/j.arth.2024.02.007
PubMed Abstract | Crossref Full Text | Google Scholar
16. Zhou, M, Pang, F, Liao, D, Yang, Y, Wang, Y, Yang, Z, et al. Electroacupuncture improves allodynia and central sensitization via modulation of microglial activation associated P2X4R and inflammation in a rat model of migraine. Mol Pain. (2024) 20:824459393. doi: 10.1177/17448069241258113
PubMed Abstract | Crossref Full Text | Google Scholar
17. Puts, S, Njemini, R, Bilterys, T, Lefeber, N, Scheerlinck, T, Nijs, J, et al. Linking intra-articular inflammatory biomarkers with peripheral and central sensitization in late-stage knee osteoarthritis pain: a pilot study. J Clin Med. (2024) 13:13. doi: 10.3390/jcm13175212
PubMed Abstract | Crossref Full Text | Google Scholar
18. Mokhtari, T, Irandoost, E, and Sheikhbahaei, F. Stress, pain, anxiety, and depression in endometriosis-targeting glial activation and inflammation. Int Immunopharmacol. (2024) 132:111942. doi: 10.1016/j.intimp.2024.111942
PubMed Abstract | Crossref Full Text | Google Scholar
19. Niu, J, Li, Y, Zhou, Q, Liu, X, Yu, P, Gao, F, et al. The association between physical activity and delayed neurocognitive recovery in elderly patients: a mediation analysis of pro-inflammatory cytokines. Aging Clin Exp Res. (2024) 36:192. doi: 10.1007/s40520-024-02846-z
PubMed Abstract | Crossref Full Text | Google Scholar
20. Ji, RR, Nackley, A, Huh, Y, Terrando, N, and Maixner, W. Neuroinflammation and central sensitization in chronic and widespread pain. Anesthesiology. (2018) 129:343–66. doi: 10.1097/ALN.0000000000002130
PubMed Abstract | Crossref Full Text | Google Scholar
21. Campbell, CM, Buenaver, LF, Finan, P, Bounds, SC, Redding, M, McCauley, L, et al. Sleep, pain catastrophizing, and central sensitization in knee osteoarthritis patients with and without insomnia. Arthritis Care Res (Hoboken). (2015) 67:1387–96. doi: 10.1002/acr.22609
PubMed Abstract | Crossref Full Text | Google Scholar
22. Martin, JR, Coronado, RA, Wilson, JM, Polkowski, GG, Shinar, AA, and Bruehl, SP. Central sensitization: the missing link between psychological distress and poor outcome following primary Total knee arthroplasty. J Arthroplast. (2024) 39:1201–6. doi: 10.1016/j.arth.2023.12.026
PubMed Abstract | Crossref Full Text | Google Scholar
23. Scerbo, T, Colasurdo, J, Dunn, S, Unger, J, Nijs, J, and Cook, C. Measurement properties of the central sensitization inventory: a systematic review. Pain Pract. (2018) 18:544–54. doi: 10.1111/papr.12636
PubMed Abstract | Crossref Full Text | Google Scholar
24. Neblett, R, Hartzell, MM, Mayer, TG, Cohen, H, and Gatchel, RJ. Establishing clinically relevant severity levels for the central sensitization inventory. Pain Pract. (2017) 17:166–75. doi: 10.1111/papr.12440
PubMed Abstract | Crossref Full Text | Google Scholar
25. Kaya, MN, Tecer, D, Kilic, O, Ozgunen, MS, and Yilmaz, S. Impact of central sensitization on clinical and functional aspects of psoriatic arthritis. Medicina (Kaunas). (2024) 60:60. doi: 10.3390/medicina60091449
留言 (0)