Bladder cancer is one of the most common malignant tumors in the urinary system globally, and its incidence is gradually increasing (1). Approximately 75% of the bladder cancers cases were classified as pure urothelial carcinoma (PUC), while the remaining 25% present histological variants (2, 3), such as squamous differentiation, glandular differentiation, Micropapillary urothelial carcinoma. As manifested by the WHO 2022 classification of urothelial tumors of the urinary tract, this classification presented a subtype of invasive urothelial carcinoma with histological variants and divergent differentiation encompassing tumors that exhibit a mix of “typical type” urothelial carcinoma and other morphologies (4, 5). Correct understanding of bladder cancer’s pathological types and appropriate treatment is crucial for improving the prognosis of patients (6, 7). For the vast majority of patients with bladder cancer (pure urothelial carcinoma, PUC) scheduled for radical cystectomy, cisplatin-based neoadjuvant therapy has proven significantly effective in enhancing patients’ postoperative prognosis (8, 9). However, in the minority of patients with bladder cancer in the presence of histological variants, they are more prone to early lymph node metastasis and generally have a poorer prognosis (4, 10). Additionally, they often respond poorly to cisplatin-based neoadjuvant chemotherapy (11). Therefore, early identification and tailored treatment adjustments for these patients are extremely vital to improve their prognosis.
The current gold standard for diagnosing the type of pathology in bladder cancer patients relies on the pathologist’s correct identification of surgical or biopsy specimens (12, 13). However, the whole process suffers from the following challenges: (1) All tumor tissue after radical cystectomy can be accurately identified by the pathologist and evaluated for pathological types associated with histological variants (since the pathological specimen is the entire bladder), meanwhile, the vast majority of pathological specimens are obtained by transurethral cystoscopic biopsy or transurethral resection of bladder tumor (TURBT); This often results in residual tumor tissue remaining in the patient’s bladder. If there is histologically variant tumor tissue remaining in the patient that was not retrieved in the biopsy, the pathologist will make an incorrect initial determination of the pathologic type of bladder cancer. This misclassification can subsequently impact the urologist’s treatment decisions (14, 15). (2) Considering that survival rate and prognosis of bladder cancer patients with histological variants are worse, delays in obtaining biopsy or TURBT results and subsequent pathology analysis can lead to disease progression and worsen the patient’s condition. (3) Meanwhile, both biopsy and TURBT are invasive operations, posing additional risks and discomfort for the patient. Based on this, there is an urgent need to develop a simple, efficient, and noninvasive method for early identification of patients with bladder cancer combined with histologic variants.
Artificial intelligence has made major advances in the field of medicine in recent years and is poised to revolutionize the practice of medicine (16, 17). The most significant advantage of AI over clinicians is the ability to effectively utilize various medical data to assist clinicians in disease diagnosis, prognosis (18–21). In the field of bladder cancer, previous researches have indicated that it is feasible to apply radiomics and machine learning to access pathological grade and muscle invasiveness in bladder carcinoma (22–25). However, few studies have been conducted to identify histological variants of bladder cancer by radiomics, especially CT. And we posit that the application of radiomics combined with machine learning could also effectively predict histological variants in bladder cancer.
Therefore, we created a system to enable the identification of histological variants of bladder cancer in a simple, efficient, and noninvasive manner (the dependent variable (histological variant) of the classification task is a binary categorical variable, which can be divided into non-histological variant and histological variant). This will be used to guide the clinical practice of urologists, facilitating the selection of the most suitable treatment for patients.
Materials and methodsPatientsWe retrospectively gathered basic information and CT images of patients who underwent radical cystectomy between 2013 and 2023 from the databases of three completely independent healthcare institutions, the First Affiliated Hospital of Chongqing Medical University, the Second Affiliated Hospital of Chongqing Medical University, and Chongqing University Fuling Hospital. The exclusion criteria for this study were: (1) absence of CT images or availability of only plain CT images; (2) poor quality of CT images; and (3) patients whose final pathology reports did not reveal significant tumor signs due to neoadjuvant therapy. From the database of the First Affiliated Hospital of Chongqing Medical University, A total of 410 patients were finally included, including 55 patients with non-pure urothelial carcinoma (NPUC). From the databases of the Second Affiliated Hospital of Chongqing Medical University and Chongqing University Fuling Hospital, a total of 60 patients were finally included, including 14 patients with NPUC. A detailed distribution of basic information and pathology type of the included patient cohort across these hospitals is provided in Table 1. The postoperative pathological results of the participants will be used as the gold standard to assess whether the bladder cancer is associated with histological variation. The detailed data collection and screening process is shown in Figure 1. Data from the First Affiliated Hospital of Chongqing Medical University will be used for the model construction, while data from the Second Affiliated Hospital of Chongqing Medical University and Chongqing University Fuling Hospital will be used for the external validation which effectively avoided potential statistical dependence.
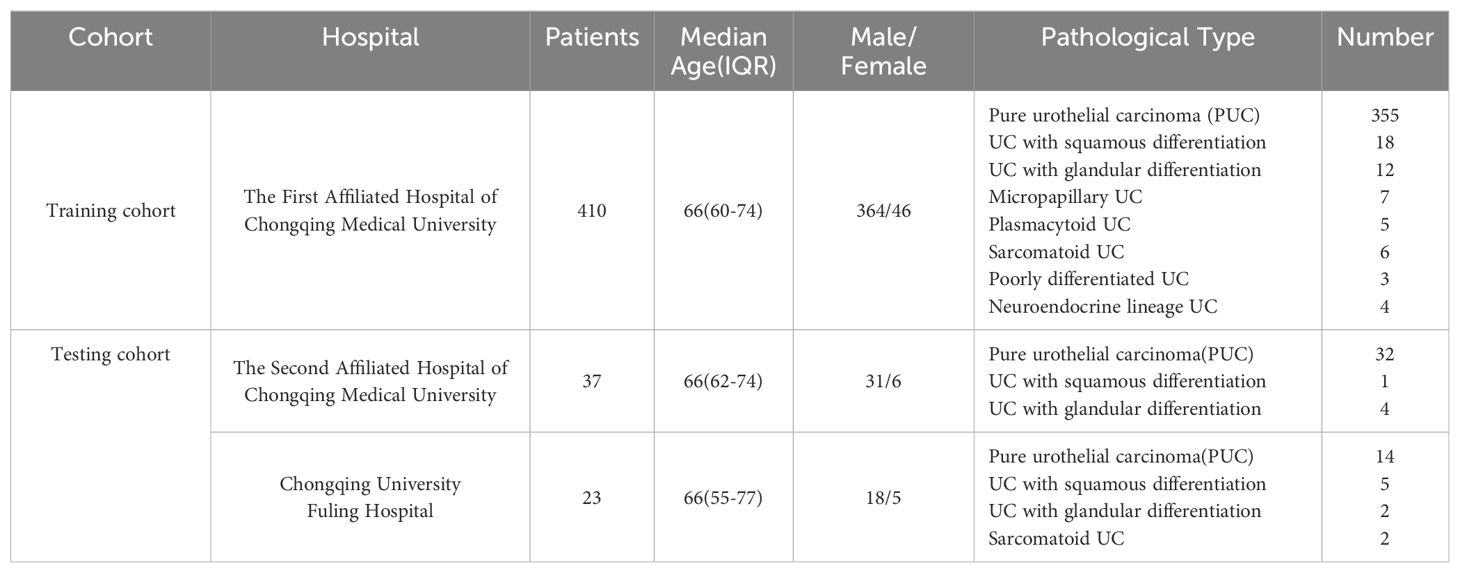
Table 1. A detailed distribution of basic information and pathology type of the included patient cohort across these hospitals.
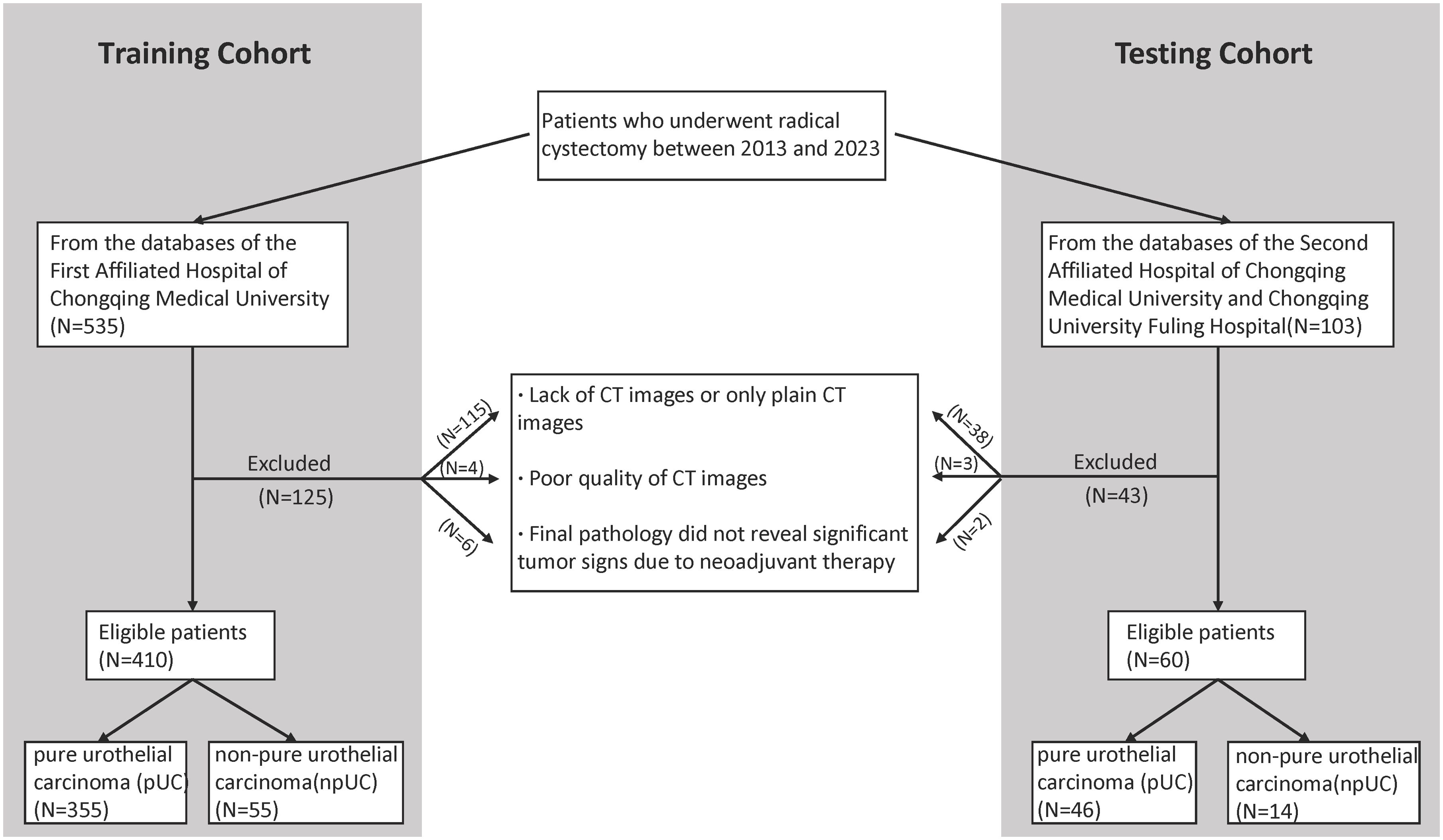
Figure 1. Flowchart shows patient selection.
Interactive framework and features extractionDistinguishing from the traditional manual sketching of regions of interest (ROI) markers to extract features, we constructed an interactive deep learning-based bladder cancer image segmentation framework. The segmentation framework is built on the Swin UNETR algorithm, a novel 3D transformer-based architecture specifically designed for medical image segmentation. Additional Swin UNETR description can be found in Supplementary Materials. In our proposed framework, the Swin UNETR model is used to process raw image information and refine the results through user interaction. These user’ interactions are translated into geodesic distance maps, which are then incorporated into the Swin UNETR’s input (26). Figure 2 provides a comprehensive illustration of the Swin UNETR model’s structure. For this interactive segmentation model’s development, two experienced radiologists (more than 5 years working experience) annotated ROI in bladder cancer images using ITK-SNAP software. They incorporated image data from three different planes—axial, sagittal, and coronal—when delineating tumor margins. In instances of ambiguous sketching, an additional senior radiologist (Lv Fajin, more than 20 years working experience) is consulted to discuss and finalize the results collaboratively. This interactive segmentation model will be used for subsequent extraction of both radiomic features and deep learning features.
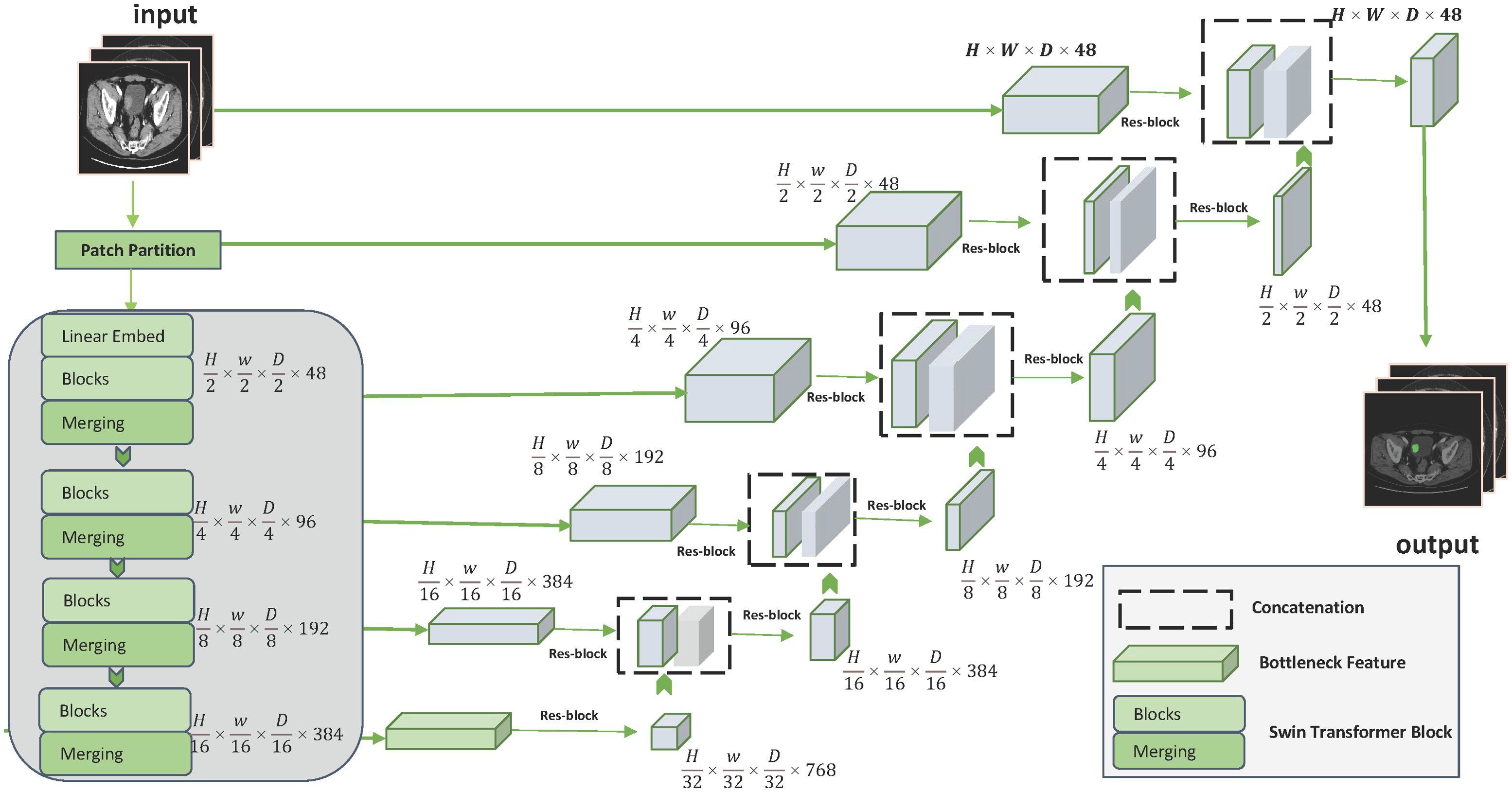
Figure 2. Detailed structure of the Swin UNETR model.
Radiomic features are categorized into three types: (1) first-order statistics, (2) shape features, and (3) second-order features. Image types for radiomic features fall into three distinct categories: (1) Original, (2) Log, and (3) Wavelet. Utilizing the default parameters set from the official Pyradiomics YAML file, we successfully extracted 1231 radiomic features for each subject. For the extraction of deep learning features, we employed a 3D-cropbox designed to encapsulate the bladder cancer region. In terms of width and length, the 3D-cropbox correspond to the maximum cross-sectional area of the bladder cancer, while in terms of height, it aligns with the Z-axis dimension that encompass the bladder cancer region. Within the 3D-cropbox, any NumPy array values outside the ROI are set to zero. The 3D-cropbox region is then input into a 3DResnet50 model equipped with weights that have been pretrained. Through removing the pre-trained model’s last layer, disabling gradient updates and adding a 3D maximum pooling layer, we successfully extract 2048 deep learning features per subject. Further details on the 3DResnet50 structure are available in Supplementary Table S1. Additionally, Supplementary Figure S1 displayed the feature screening related processes and Supplementary Figure S2 provides an in-depth view of the 3D-cropbox workflow. Radiomic features and deep learning features (without clinically relevant data) will be used to build subsequent predictive models.
Reliability assessment of selected featuresTo assess the reproducibility and reliability of the selected features, we used the intra-class correlation coefficients and inter-class correlation coefficients (ICC). The inter-class correlation coefficients were calculated based on the re-labeling of 25% participants’ ROIs in both the training and testing cohorts. This re-labeling was performed with the interactive segmentation model by two independent readers. Additionally, these participants were randomly selected by another independent radiologist. As for the intra-class correlation coefficients, they were determined by a single reader who randomly plotted the ROIs of the same participants using the interactive segmentation model in the enrolled datasets twice, with a one-month interval between sessions (27).
Quality control proceduresThe process of quality control for fusion feature extraction and model construction in our study is methodically organized into five distinct sections: (1) quality control of images: ensuring the integrity and appropriateness of images used; (2) quality control of ROI: verifying the accuracy and relevance of the selected ROI; (3) quality control of feature extraction: monitoring the precision and consistency in extracting features; (4) quality control of feature selection: evaluating the criteria and methods used for selecting features; and (5) quality control of machine learning algorithms: reviewing the application and effectiveness of machine learning techniques. In accordance with the Image Biomarker Standardization Initiative (IBSI), we complied with its recommendations (28). To assess the reliability of our research, we adopted the Radiomics quality score (RQS) (29). For more detailed information on our quality control procedures and the results of the RQS calculation, please refer to the Supplementary Material.
Stacking ensemble learning approachIn our study, we employed a stacking ensemble learning approach. Specifically, we used XGBoost, Random Forest (RF), and Decision Tree (DT) as the base models. The predictions from these base models were then used as inputs (meta-features) for the LightGBM model, which served as the meta-learner in our stacking framework. LightGBM was selected for its efficiency and scalability, particularly in handling large datasets and complex feature interactions. By leveraging this hierarchical structure, our stacking ensemble method could capture and combine different levels of information from each base model, leading to more accurate stratification.
Statistical analysisWe utilized ITK-SNAP (version 3.6.0) to generate ROIs. Pyradiomics package (version 3.0.1) was used to extract radiomic features. The pretrained weights of 3DResnet50 model derived from 23 medical datasets, which included images of various parts of the human body. The pretrained weights file, along with the related codes, are openly available in the Tencent Medicalnet project (https://github.com/Tencent/MedicalNet). The originally chosen radiomics and deep learning features were analyzed for redundancy using Pearson correlation coefficients for normal distributions and Spearman’s rank correlation coefficients for non-normal distributions. Figure 3 illustrates the entire model construction procedure. To assess the model’s performance, we used the Receiver Operating Characteristics (ROC) curve and the Accuracy Score (ACC). The DeLong test was employed to evaluate if there was significant heterogeneity in the area under the ROC curve (AUC). Additionally, a calibration curve was used to assess the consistency of the final model’s performance in the external validation dataset. Two-sided p values less than 0.05 were considered statistically significant.
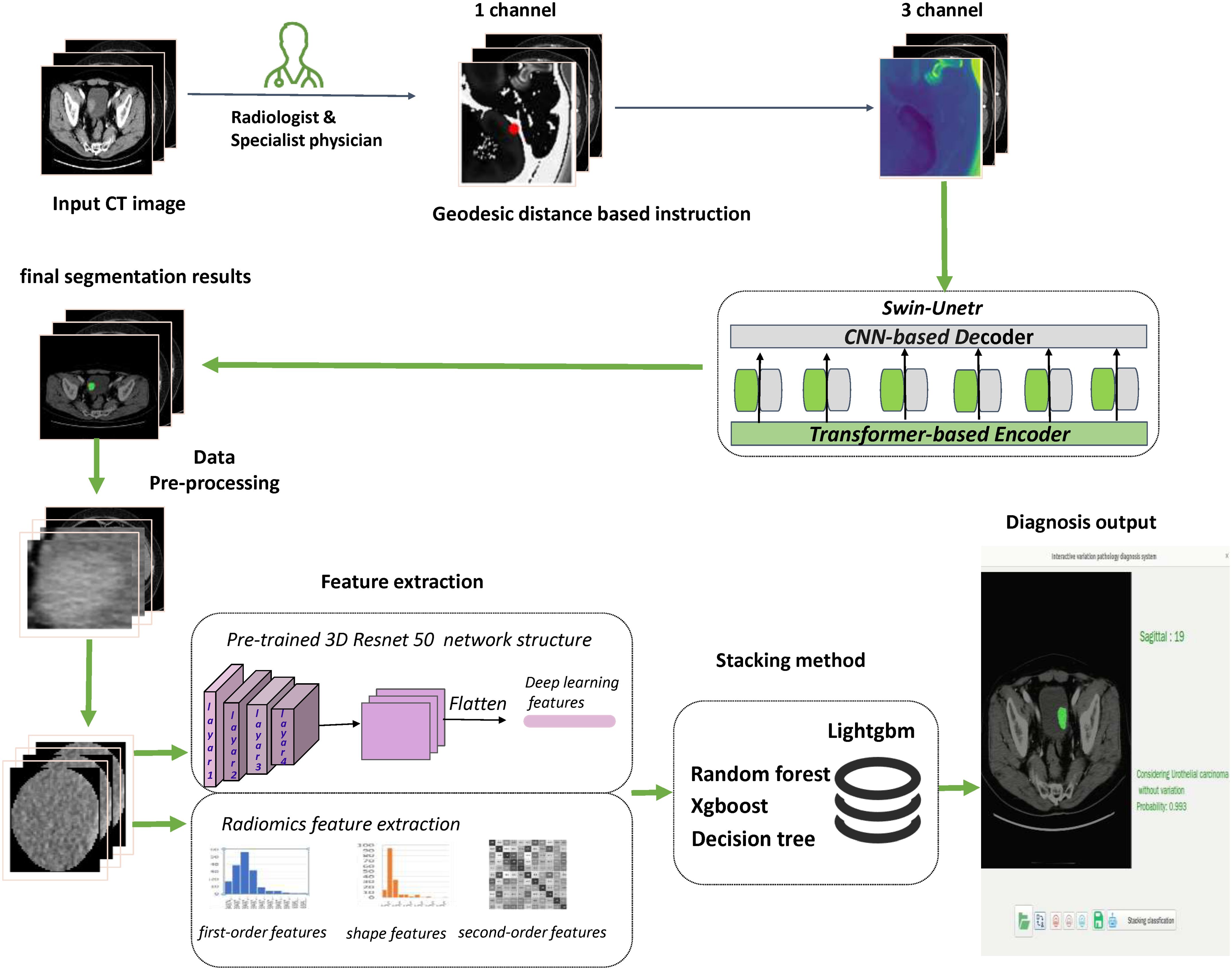
Figure 3. Flowchart presented the step-by-step procedures in machine learning model construction.
The scikit-learn package and “Pycaret” package are used to develop the final machine learning model. All model building processes and plot drawings were conducted in Python (version 3.9) environment and R software (version 4.0.5).
Ethical approval and consent to participateWe confirm that all methods were carried out in accordance with the relevant guidelines and regulations. This retrospective analysis received approval from the ethics committees of each participating hospital. The written informed consents were waived. All patient information enrolled in this research was anonymized.
ResultsThe interactive segmentation model exhibited excellent performance in both training (Dice = 0.78, IOU = 0.65, SEN = 0.83, SPE = 1.00) and testing datasets (Dice = 0.80, IOU = 0.67, SEN = 0.89, SPE = 1.00).
In the validation set, the Dice of the segmentation model reaches 0.78; in the test set, the Dice of the segmentation model reaches 0.80. These results indicate that our interactive segmentation model exhibits excellent performance in segmentation tasks. Supplementary Table S2 shows the performance of interactive segmentation model in training and testing datasets in detail. Figure 4 demonstrates in detail how this segmentation model works.
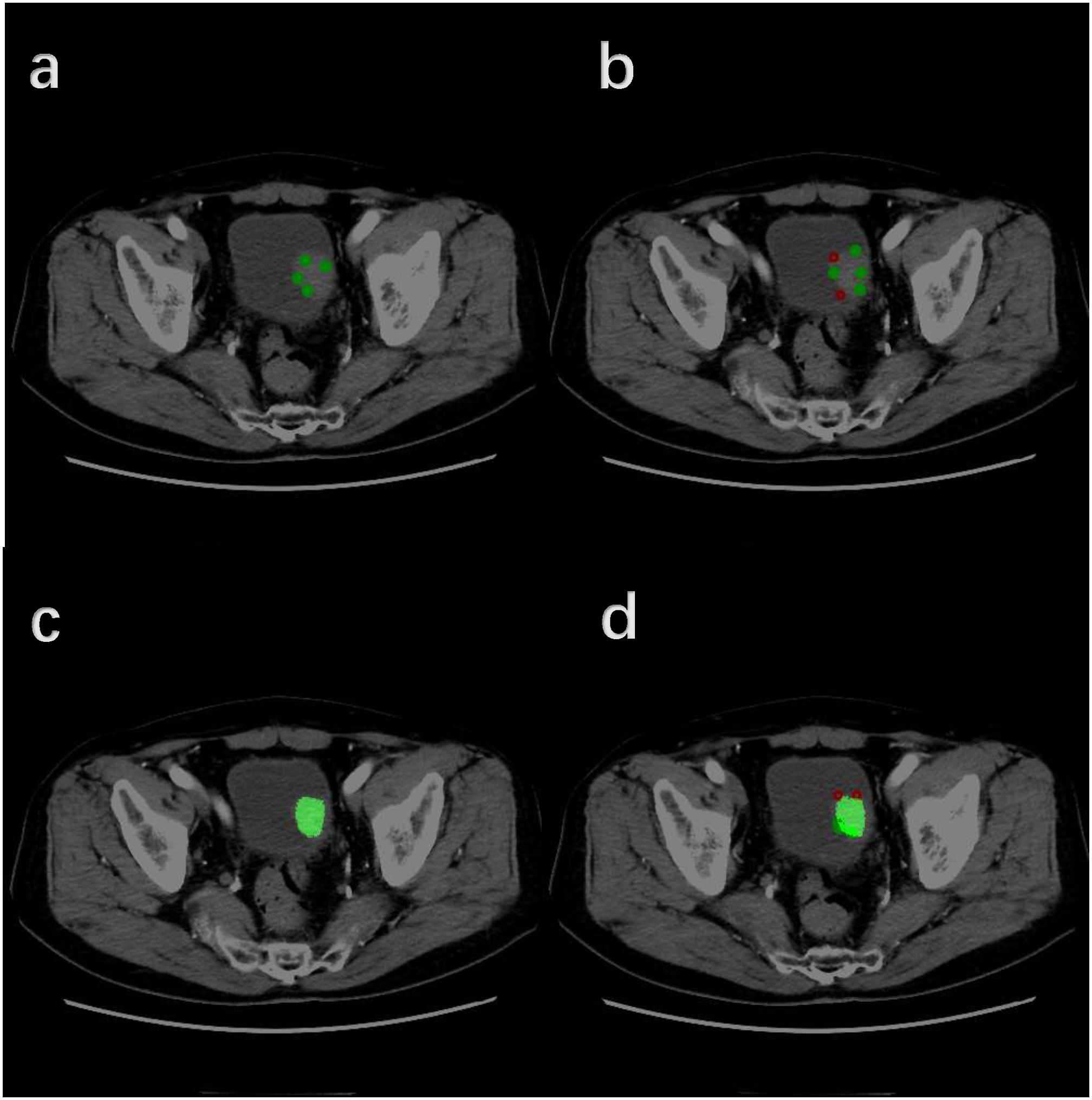
Figure 4. Detailed workflow of the Interactive Segmentation Framework (Operate in order of A–D). (A) Initial interactive segmentation process combined with positive indicator points (green dots). (B) Initial interactive segmentation process combined with negative indicator points (red dots). (C) Initial segmentation results. (D) Further segmentation refinement.
We initially extracted a total of 3279 features, comprising 1231 radiomic features and 2048 deep learning features. Utilizing the Least Absolute Shrinkage and Selection Operator (LASSO) method for feature screening, we narrowed this down to 64 features. By using Pearson’s correlation coefficient and Spearman’s rank correlation coefficients to check whether there is redundancy in the initial selection of radiomic features and deep learning features, as well as by using intraclass correlation coefficient and interclass correlation coefficient to inspect the reproducibility and reliability of the selected features, 50 features were finally retained, including 46 radiomic features and 4 deep learning features. Supplementary Table S3 displayed the selected features weights after lasso selection. Supplementary Table S4 displayed the ICC values in the final selected 46 radiomic features and 4 deep learning features.
Figure 5 demonstrates a detailed analysis of the performances of the fusion-features based machine learning algorithm. The AUC value for the training cohort is 0.940 and the AUC value for the testing cohort is 0.905. These values indicate that our model not only demonstrates excellent performance in standard evaluations but also maintains robust predictive ability during external validation. Besides, an ACC value of 0.900 was measured in the stacking ensemble model, indicating a high level of discrimination in the diagnosis of urothelial carcinoma with and without histological variants in this study. A comprehensive comparison of the final model’s performance against other common machine learning algorithms is presented in Table 2. Moreover, the decision curve analysis applied to external validation datasets reveals that our fusion-feature machine learning model outperforms the “none” and “all” treatment strategies across various threshold probabilities, offering a higher net benefit as shown in Supplementary Figure S8. Supplementary Figures S3, S4 displayed the calibration curve in training and external validation dataset.
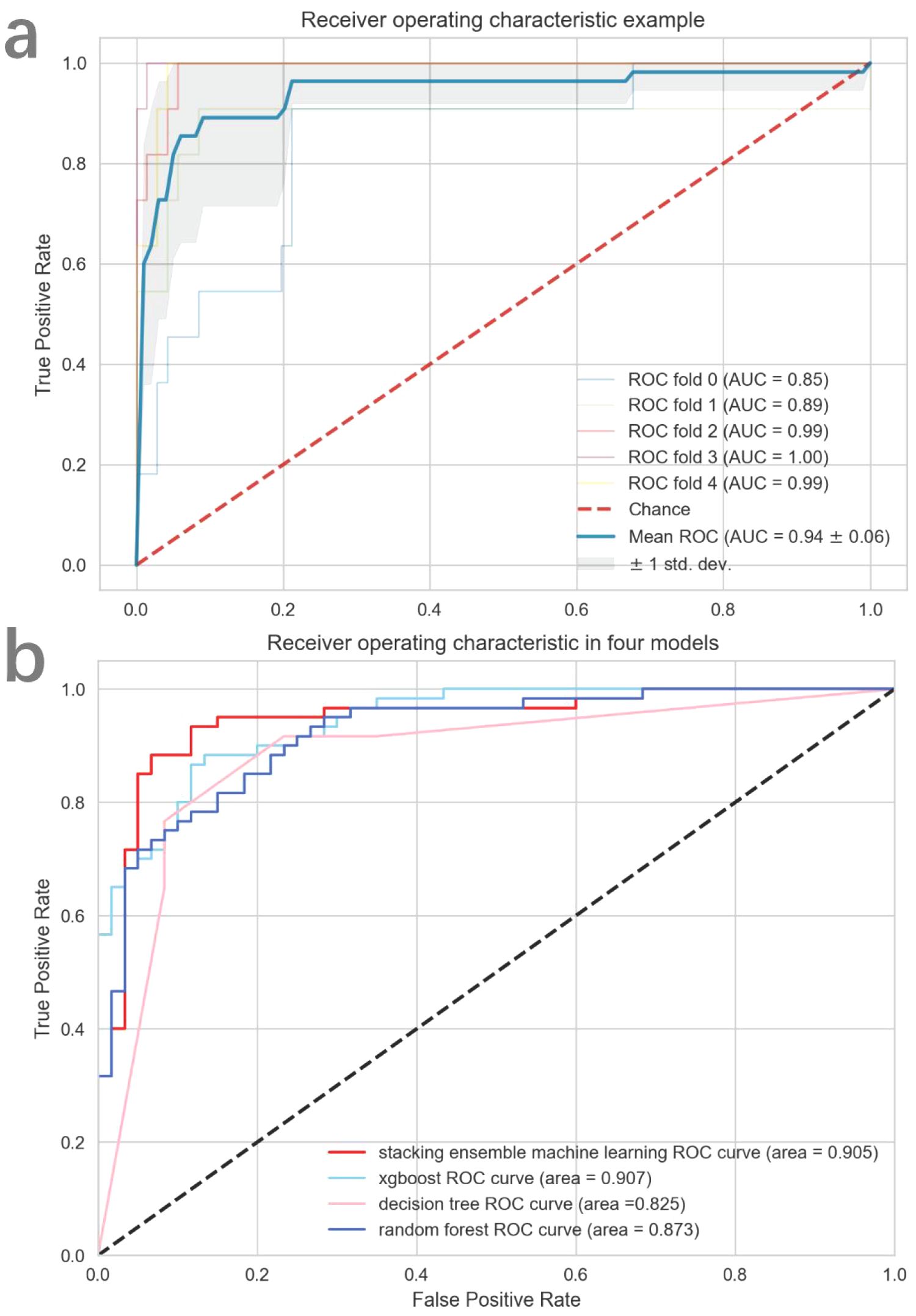
Figure 5. The diagnostic efficacy of each model assessed by ROC curve. In (A), the mean cross-validated ROC of stacking ensemble model was 0.94. In (B), all four models performed excellently in external validation dataset.
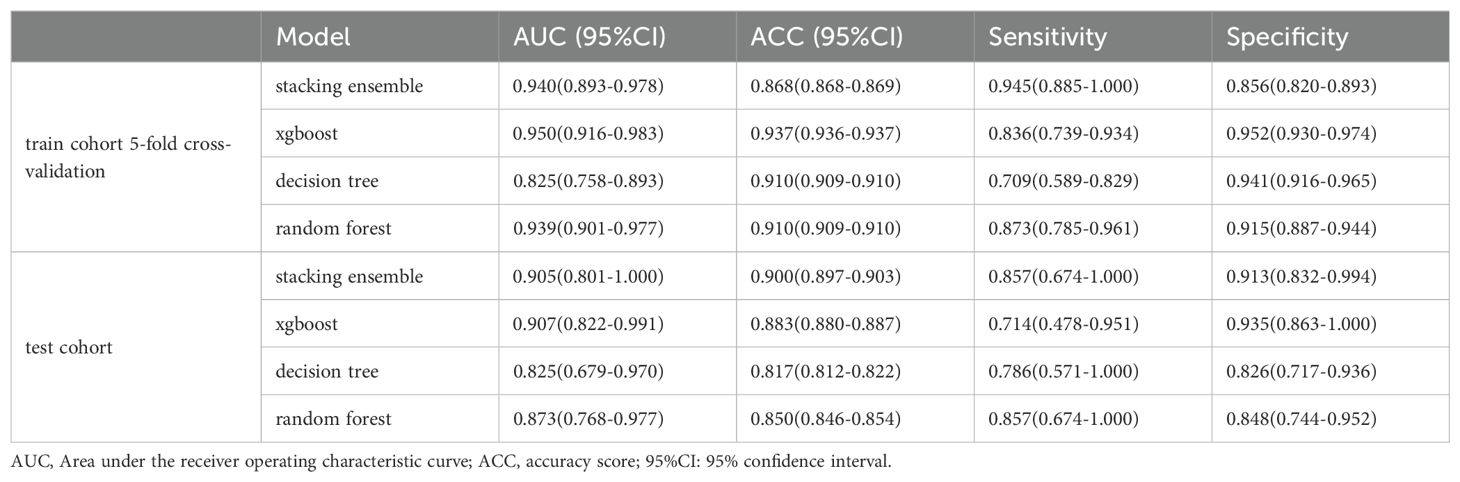
. TABLE 2 The performance of four classifier models.
DiscussionIn our study, we developed and validated a system that integrates both radiomic and deep learning features extracted from CT images. This system is designed to efficiently and non-invasively identify histological variants of bladder cancer at an early stage. The system incorporates a total of 46 radiometric features and 4 deep learning features, achieving an AUC value of 0.940 for the training cohort, which demonstrates that the model we constructed has excellent classification performance. To mitigate the risk of overfitting, we employed a stacking ensemble machine learning approach coupled with cross-validation methods. This strategy proved effective in maintaining the model’s robustness during the training phase. In addition, we performed external validation of the model and achieved an AUC value of 0.905 and an ACC value of 0.900 in the test cohort, which indicates that our model has strong predictive performance beyond the initial training cohort as well. Not only that, the RQS analysis result of this study yielded a result of 15, which attests to the study’s high quality, trustworthiness, and reproducibility.
To enhance the efficiency of identifying ROIs in bladder tumor-enhanced CT scans, we have employed a deep learning framework designed for interactive segmentation of tumor areas. The results of the interactive framework’s segmentation of bladder tumor regions contain a variety of data such as tumor size, and participate in the construction of subsequent stacking ensemble system. Meanwhile, the segmentation model not only delivers superior segmentation performance, but also achieves a Dice of 0.80 in external validation, which indicates that our constructed segmentation framework has robust generalizability. Obviously, traditional manual segmentation is time-consuming, labor-intensive and often yields unsatisfactory results; Fully automated segmentation frequently falls short in accuracy due to poor image quality and the variability among patients (30). In contrast, our interactive segmentation approach substantially reduces the time required for manual segmentation by radiologists while delivering exceptional results. An efficient interactive segmentation tool is of great importance for practical applications. Not only that, we used a more powerful deep learning algorithm, Swin UNETR, in building this segmentation model, which makes it possible to achieve strong segmentation results with less user interaction and less user time.
Bladder cancer with histological variants tends to be more aggressive and responds less effectively to intravesical and cisplatin-based systemic therapies (11, 31, 32). In non-muscle-invasive bladder cancer (NMIBC), the standard treatment typically involves transurethral resection of the bladder tumor (TURBT) with the subsequent adjuvant intravesical instillation of Bacillus Calmette-Guérin (BCG) or chemotherapeutic agents, as needed (33). However, certain bladder cancer with histological variants has a lower response rate to intravesical bladder therapy compared to pure urothelial carcinoma (32, 34, 35). Adhering to standard treatments in such cases may lead to a poorer prognosis. Therefore, for these patients, early radical cystectomy might be a more effective treatment option (31, 36, 37). In muscle-invasive bladder cancer (MIBC), cisplatin-based combination neoadjuvant chemotherapy followed by radical cystectomy is its main treatment modality (8). However, for certain histological variants of bladder cancer, cisplatin-based combination neoadjuvant chemotherapy is less effective, potentially leading to disease progression if used as the initial treatment (11, 34, 38). In these situations, incorporating immune checkpoint inhibitors or other targeted agents into the neoadjuvant therapy, or opting for direct radical cystectomy, might be more beneficial therapeutic approaches (39, 40). Consequently, the early and accurate identification of histological variants in bladder cancer is crucial for urologist’s clinical decision-making, which significantly impact the prognosis of patients with bladder cancer.
The gold standard for identifying histological variants in bladder cancer relies on the pathologist’s judgment of the pathology specimen (12, 13). However, most of these specimens are obtained through transurethral cystoscopic biopsy or TURBT. This leads to the possibility that the pathology specimens obtained are not all bladder tumor, which in turn affects the pathologist’s ability to accurately identify the presence or absence of histological variants. Furthermore, considering that bladder cancer with histological variants tends to be more aggressive, and the long process of conducting the preoperative examination and waiting for pathological results may lead to tumor progression, which in turn affects patient’s prognosis. In addition, transurethral cystoscopic biopsy or TURBT is an invasive procedure, which in itself is also a harm to the patient. Clearly, the conventional method of determining histological variants of bladder cancer by means of routine pathological biopsy is flawed. There is a pressing need for a simpler, more efficient, and noninvasive method to identify these histological variants of bladder cancer. And the classification model we constructed in this study is just enough to fulfill the existing needs. Moreover, because the AI algorithm can identify a lot of information about the tumor area on CT images, the percentage of histological variants in bladder cancer does not affect the accuracy of the model.
Currently, in the realm of bladder cancer research, there have been a large number of studies evaluating the pathological grading, muscle invasiveness, efficacy and prognosis of bladder cancer through models constructed by machine learning algorithms (22–25, 41, 42), but fewer studies have been conducted in the field of histological variants. Jingwen Huang et al. (43) constructed a machine-learning model based on radiomic features of MRI to predict whether urothelial carcinoma is accompanied by squamous differentiation. Despite the model ultimately achieved excellent predictive performance, only being able to differentiate between urothelial carcinoma with or without squamous differentiation was the primary limitation of the study, as the histological variants of bladder cancer are diverse. For example, when the model is confronted with an MRI image of a urothelial carcinoma with sarcomatoid differentiation, it is bound to give the wrong answer. Moreover, the study only included a sample of 119 cases from a single institution, so the effect of external validation of the model’s performance is uncertain. Sehnaz Evrimler et al. (44) developed a machine learning model using Computed Tomography (CT) to predict histological variants in bladder cancer. However, only a sample of 37 cases of radical cystectomy was included in this study. The authors indicated that the small sample size was attributed to the exclusive focus on patients who underwent radical cystectomy and the absence of preoperative CT-enhanced abdominal scans in some patients. Obviously, our study bridges the gaps existing in previous studies as much as possible. We focus not only on maintaining model performance but also on expanding the sample size and diversifying the sources of our samples, thus enhancing the robustness and applicability of our findings in this critical area of bladder cancer research.
Despite our constructed model demonstrates strong diagnostic performance, the following restrictions exist throughout the study. Firstly, while this study achieved external validation as well as secured an adequate sample size, it’s evident that larger sample sizes could further enhance the construction and generalizability of machine learning models. Secondly, our study solely relied on imaging data for model construction. Therefore, additional research is necessary to determine if integrating clinical and genetic data into the model could enhance its diagnostic performance. Thirdly, our study incorporated data from multiple institutions, where variations in Computed Tomography specifications across these organizations may have influenced the model’s construction. Fourthly, due to the sample size of various types of histological variants, our study was temporarily unable to identify a specific type of histological variants. This is also a key direction of our follow-up research.
ConclusionIn summary, we constructed a stacking ensemble system for early, non-invasive identification of histological variants in bladder cancer and its model performance achieved excellent results in an external validation set, which will help urologists make clinical decisions.
Data availability statementThe data that support the findings of this study are available on request from the corresponding author upon reasonable request.
Ethics statementThe studies involving humans were approved by The Institutional Review Board of the First Affiliated Hospital of Chongqing Medical University The Institutional Review Board of the Second Affiliated Hospital of Chongqing Medical University The Institutional Review Board of Chongqing University Fuling Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and institutional requirements.
Author contributionsCP: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Writing – original draft, Writing – review & editing. QH: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Writing – original draft, Writing – review & editing. FJL: Conceptualization, Data curation, Investigation, Methodology, Project administration, Resources, Writing – review & editing. QJ: Conceptualization, Data curation, Investigation, Methodology, Project administration, Resources, Writing – review & editing. YC: Conceptualization, Data curation, Investigation, Methodology, Project administration, Resources, Writing – review & editing. ZW: Conceptualization, Data curation, Investigation, Methodology, Project administration, Resources, Writing – review & editing. YX: Conceptualization, Methodology, Project administration, Writing – review & editing. FTL: Conceptualization, Methodology, Writing – review & editing. MX: Conceptualization, Data curation, Investigation, Methodology, Project administration, Resources, Supervision, Writing – review & editing.
FundingThe author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by the Medical Health Care Ecosystem Innovation Team of the First Affiliated Hospital of Chongqing Medical University (CYYY-DSTDXM-202409).
AcknowledgmentsThe authors acknowledge the staff of the Department of Radiology of the First Affiliated Hospital of Chongqing Medical University for their contribution to this study.
Conflict of interestThe authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s noteAll claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary materialThe Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2024.1469427/full#supplementary-material
References1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2021) 71:209. doi: 10.3322/caac.21660
PubMed Abstract | Crossref Full Text | Google Scholar
2. Wasco MJ, Daignault S, Zhang Y, Kunju LP, Kinnaman M, Braun T, et al. Urothelial carcinoma with divergent histologic differentiation (mixed histologic features) predicts the presence of locally advanced bladder cancer when detected at transurethral resection. UROLOGY. (2007) 70:69. doi: 10.1016/j.urology.2007.03.033
PubMed Abstract | Crossref Full Text | Google Scholar
3. Cai T, Tiscione D, Verze P, Pomara G, Racioppi M, Nesi G, et al. Concordance and clinical significance of uncommon variants of bladder urothelial carcinoma in transurethral resection and radical cystectomy specimens. UROLOGY. (2014) 84:1141. doi: 10.1016/j.urology.2014.06.032
PubMed Abstract | Crossref Full Text | Google Scholar
4. Moschini M, D'Andrea D, Korn S, Irmak Y, Soria F, Compérat E, et al. Characteristics and clinical significance of histological variants of bladder cancer. Nat Rev UROL. (2017) 14:651. doi: 10.1038/nrurol.2017.125
PubMed Abstract | Crossref Full Text | Google Scholar
5. Netto GJ, Amin MB, Berney DM, Compérat EM, Gill AJ, Hartmann A, et al. The 2022 world health organization classification of tumors of the urinary system and male genital organs-part B: prostate and urinary tract tumors. Eur UROL. (2022) 82:469. doi: 10.1016/j.eururo.2022.07.002
PubMed Abstract | Crossref Full Text | Google Scholar
7. Compérat E, Amin MB, Cathomas R, Choudhury A, De Santis M, Kamat A, et al. Current best practice for bladder cancer: a narrative review of diagnostics and treatments. LANCET. (2022) 400:1712. doi: 10.1016/S0140-6736(22)01188-6
PubMed Abstract | Crossref Full Text | Google Scholar
8. Alfred WJ, Max BH, Carrión A, Cathomas R, Compérat E, Efstathiou JA, et al. European association of urology guidelines on muscle-invasive and metastatic bladder cancer: summary of the 2023 guidelines. Eur UROL. (2023) 85:17–31. doi: 10.1016/j.eururo.2023.08.016
PubMed Abstract | Crossref Full Text | Google Scholar
9. Advanced Bladder Cancer (ABC) Meta-analysis Collaboration. Neoadjuvant chemotherapy in invasive bladder cancer: update of a systematic review and meta-analysis of individual patient data advanced bladder cancer (ABC) meta-analysis collaboration. Eur UROL. (2005) 48:202, 205. doi: 10.1016/j.eururo.2005.04.006
PubMed Abstract | Crossref Full Text | Google Scholar
10. Fu M, Klose C, Sparks A, Whalen M. Impact of variant histology on occult nodal metastasis after neoadjuvant chemotherapy for muscle-invasive bladder cancer: A review of the national cancer database. Clin Genitourin Cancer. (2022) 20:e135. doi: 10.1016/j.clgc.2021.11.011
PubMed Abstract | Crossref Full Text | Google Scholar
11. Vetterlein MW, Wankowicz S, Seisen T, Lander R, Löppenberg B, Chun FK, et al. Neoadjuvant chemotherapy prior to radical cystectomy for muscle-invasive bladder cancer with variant histology. CANCER-AM Cancer Soc. (2017) 123:4346. doi: 10.1002/cncr.30907
PubMed Abstract | Crossref Full Text | Google Scholar
12. Powles T, Bellmunt J, Comperat E, De Santis M, Huddart R, Loriot Y, et al. Bladder cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann Oncol. (2022) 33:244. doi: 10.1016/j.annonc.2021.11.012
PubMed Abstract | Crossref Full Text | Google Scholar
14. Mantica G, Tappero S, Parodi S, Piol N, Spina B, Malinaric R, et al. Bladder cancer histological variants: which parameters could predict the concordance between transurethral resection of bladder tumor and radical cystectomy specimens? Cent Eur J Urol. (2021) 74:355. doi: 10.5173/ceju.2021.140.R1
PubMed Abstract | Crossref Full Text | Google Scholar
15. La Croce G, Naspro R, Finati M, Pellucchi F, Sodano M, Manica M, et al. The accuracy of transurethral bladder resection in detecting bladder cancer histological variants and their prognostic value at radical cystectomy. J Clin Med. (2022) 11:550. doi: 10.3390/jcm11030550
PubMed Abstract | Crossref Full Text | Google Scholar
18. Loftus TJ, Tighe PJ, Filiberto AC, Efron PA, Brakenridge SC, Mohr AM, et al. Artificial intelligence and surgical decision-making. JAMA Surg. (2020) 155:148. doi: 10.1001/jamasurg.2019.4917
PubMed Abstract | Crossref Full Text | Google Scholar
19. Bhinder B, Gilvary C, Madhukar NS, Elemento O. Artificial intelligence in cancer research and precision medicine. Cancer Discovery. (2021) 11:900. doi: 10.1158/2159-8290.CD-21-0090
PubMed Abstract | Crossref Full Text | Google Scholar
21. Zhu XH, Wu CL, Zu XB, Lu J. Editorial: The application of artificial intelligence in diagnosis, treatment and prognosis in urologic oncology. Front Oncol. (2022) 12:1118442. doi: 10.3389/fonc.2022.1118442
PubMed Abstract | Crossref Full Text | Google Scholar
22. Wang H, Hu D, Yao H, Chen M, Li S, Chen H, et al. Radiomics analysis of multiparametric MRI for the preoperative evaluation of pathological grade in bladder cancer tumors. Eur Radiol. (2019) 29:6182. doi: 10.1007/s00330-019-06222-8
PubMed Abstract | Crossref Full Text | Google Scholar
23. Zheng J, Kong J, Wu S, Li Y, Cai J, Yu H, et al. Development of a noninvasive tool to preoperatively evaluate the muscular invasiveness of bladder cancer using a radiomics approach. CANCER-AM Cancer Soc. (2019) 125:4388. doi: 10.1002/cncr.v125.24
PubMed Abstract | Crossref Full Text | Google Scholar
24. Wang H, Xu X, Zhang X, Liu Y, Ouyang L, Du P, et al. Elaboration of a multisequence MRI-based radiomics signature for the preoperative prediction of the muscle-invasive status of bladder cancer: a double-center study. Eur Radiol. (2020) 30:4816. doi: 10.1007/s00330-020-06796-8
PubMed Abstract | Crossref Full Text | Google Scholar
25. Song H, Yang S, Yu B, Li N, Huang Y, Sun R, et al. CT-based deep learning radiomics nomogram for the prediction of pathological grade in bladder cancer: a multicenter study. Cancer Imaging. (2023) 23:89. doi: 10.1186/s40644-023-00609-z
PubMed Abstract | Crossref Full Text | Google Scholar
26. Wang G, Zuluaga MA, Li W, Pratt R, Patel PA, Aertsen M, et al. DeepIGeoS: A deep interactive geodesic framework for medical image segmentation. IEEE Trans Pattern Anal Mach Intell. (2019) 41:1559. doi: 10.1109/TPAMI.34
PubMed Abstract | Crossref Full Text | Google Scholar
27. Pleil JD, Wallace M, Stiegel MA, Funk WE. Human biomarker interpretation: the importance of intra-class correlation coefficients (ICC) and their calculations based on mixed models, ANOVA, and variance estimates. J Toxicol Environ Health B Crit Rev. (2018) 21:161. doi: 10.1080/10937404.2018.1490128
PubMed Abstract | Crossref Full Text | Google Scholar
28. Zwanenburg A, Vallières M, Abdalah MA, Aerts H, Andrearczyk V, Apte A, et al. The image biomarker standardization initiative: standardized quantitative radiomics for high-throughput image-bas
留言 (0)