Inflammatory bowel disease (IBD), encompassing ulcerative colitis and Crohn’s disease, represents a group of chronic, idiopathic inflammatory conditions affecting the gastrointestinal tract (Abraham and Cho, 2009). The epidemiologic patterns of IBD are evolving, and at the turn of the 21st century, it has become a major public health challenge worldwide (Buie et al., 2023). The incidence of IBD varies greatly by geographical region, with rapidly increasing rates in newly industrialized countries (Asia, Eastern Europe, and Latin America), ranging from 10 to 80 per 100,000 individuals (Wang et al., 2023). While the incidence appears to be stabilizing in Western countries, the burden on healthcare systems remains high, with prevalence surpasses 0.3% (Ng et al., 2017; Kaplan, 2015). Particularly concerning is the steady rise in the incidence of pediatric IBD over time (Sykora et al., 2018). These trends suggest that the global impact of IBD is increasing and that the healthcare burden of managing this complex disease is substantial.
Patients with IBD often suffer from abdominal pain, diarrhea, rectal bleeding, and possible extraintestinal manifestations due to impaired intestinal mucosal barrier (Turner, 2009; Wehkamp et al., 2016). Diagnosing IBD based solely on a constellation of symptoms and signs can be challenging in clinical practice. Meanwhile, mainstream detection methods such as colonoscopy, capsule endoscopy, computed tomography (CT), and nuclear magnetic resonance imaging (MRI) (Plevris and Lees, 2022; Flynn and Eisenstein, 2019) are not only enormously distressing for patients but also insufficiently sensitive, especially at the early stage of IBD. Currently, there are no definitive interventions for IBD (Fu et al., 2023; Morris and Chu, 2015). Clinical medical management mainly relies on aminosalicylate, corticosteroids, and immunosuppressants (Agrawal et al., 2021; Wright et al., 2018). However, these treatments generally provide symptom relief rather than addressing the underlying causes of IBD. Long-term use of these medications can lead to serious adverse effects, including nausea, vomiting, headache, and liver and kidney toxicity (Stallmach et al., 2010; Rosen et al., 2015). Worst of all, patients with IBD face an elevated risk of developing life-threatening diseases such as colorectal cancer (Porter et al., 2021). Many patients eventually require surgical intervention, and in some cases, the condition can be fatal (Keller et al., 2019). These studies highlight the urgent need for research on IBD to facilitate the improvement of diagnostic sensitivity and the effectiveness of disease treatment.
Nanomedicine, which refers to the applications of nanotechnology in medicine (Kim et al., 2010), is revolutionizing the diagnosis and treatment of various diseases, including IBD, by leveraging the unique structure, as well as physical and chemical characteristics of nanomaterials (Patra et al., 2018; Hu et al., 2023). Firstly, nano-based imaging allows for the early detection of IBD and the monitoring of disease activity. Nanoparticles with different sizes and coatings can specifically accumulate in the colitis areas, providing enhanced visualization of inflamed regions and enabling more accurate and earlier diagnosis (Yue et al., 2023; Elinav and Peer, 2013). Nanomaterial-based probes that target the molecular or cellular processes involved in IBD pathology can detect subtle metabolic changes and inflammation more effectively than traditional methods, allowing for prompt disease assessment and timely treatment adjustments (Wu et al., 2016; Fu et al., 2023). Additionally, the use of nanomaterials in the treatment of IBD is an emerging and promising area of research, offering primary benefits such as improved drug delivery, enhanced stability, and targeted therapy (Zhang Y. et al., 2023). Nanomaterials can improve the chemical and physical properties of drugs, including solubility, stability, and circulating half-life. Besides, nanoparticles can be engineered to deliver drugs specifically to inflamed tissues, reducing systemic exposure and minimizing side effects (Liu et al., 2021; Naeem et al., 2020). This targeted delivery increases the concentration of drugs at the site of inflammation, improving therapeutic outcomes (Yang et al., 2024). Inspiringly, nanomedicine allows for the combination of diagnostic and therapeutic agents, enabling theranostic applications that provide real-time feedback on treatment efficacy (Patra et al., 2018; Zhang Y. et al., 2023; Yan et al., 2022). Naha et al. (2020) developed dextran coated cerium oxide nanoparticles (Dex-CeNP) as a CT contrast agent for IBD. Dex-CeNP not only accumulated in inflamed areas and protected against oxidative damage but are also efficiently cleared from the body within 24 h. This capability is particularly valuable in managing IBD, where monitoring disease progression and response to therapy is critical. In summary, nanomedicine offers significant advancements in the diagnosis and treatment of IBD. By harnessing the unique properties of nanomaterials, it is possible to develop more effective and targeted therapies that improve the outcomes of patients and reduce the burden on healthcare systems. Although the applications of nanomedicine in IBD have been explored from multiple-perspective, a comprehensive and systematic analysis of the field remains lacking.
Bibliometrics refers to an interdisciplinary science that utilizes mathematical and statistical measurement techniques to quantitatively analyze scholar publications (Moed, 2009; Yuan et al., 2023). The holistic and objective bibliometric evaluation of literature on a specific topic can enhance understanding of the current research landscape and assist in selecting research directions (Dong et al., 2023). As far as we are aware, there has not been any bibliometric analysis in this field to data. This study conducted a bibliometric visualization analysis of literature related to nanomedicine applications in IBD, including authors, institutions, countries/regions, journals, co-cited references, and keywords. The aim is to objectively assess the research structure and development trends in the major fields of IBD nanomedicine, offering new insights and clues for the subsequent study in this area.
2 Materials and methods2.1 Data source and search strategyThe Web of Science Core Collection (WoSCC) bibliographic database stands as one of the largest and the most comprehensive electronic scientific literature databases globally (Jin et al., 2023), widely favored by academic researchers. An extensive search was conducted on the database on 15 July 2024, and relevant literature published was downloaded since 2001 for analysis. The search strategy utilized in this study was set as follows: TS = (“Inflammatory bowel diseases” OR “Ulcerative colitis” OR “colitis” OR “Crohn Disease”) AND (“nano*”). The type of publication was limited to Article or Review, and the language was set to English only. Subsequently, the search results were documented with the content of “Full Record and Cited Reference” in the “Plain Text” format. A total of 1,554 papers were included in the analysis.
2.2 Data analysis and visualizationThe downloaded files were imported into CiteSpace (version 6.3.R3), VOSviewer (version 1.6.18), and Microsoft Excel 2021 (version 16.48) for bibliometric visualization analysis. CiteSpace, developed by Prof. Chao-mei Chen, is a Java application for bibliometric analysis and visualization (Chen, 2004). In our study, CiteSpace was applied to analyze and visualize national and institutional contributions, authors and co-cited authors, co-cited reference clusters and timelines, keywords, and keywords burst. The specific settings of CiteSpace were established as follows: time slicing was from January 2001 to July 2024, with each slice representing 1 year. The term sources and links were set to the default parameters. VOSviewer is another bibliometric analysis software developed by Nees Jan van Eck and Ludo Waltman, which constructs visual network maps based on data (van Eck and Waltman, 2010). We used VOSviewer software to generate core authors, countries, research institutions, and keyword co-occurrence networks. For keywords and co-cited journals, the minimum occurrence frequency was set at 100 and 5, respectively. In addition, visualization of the annual publication numbers was performed using Microsoft Excel 2021. The procedure for data collection and bibliometric analysis are shown in Figure 1.
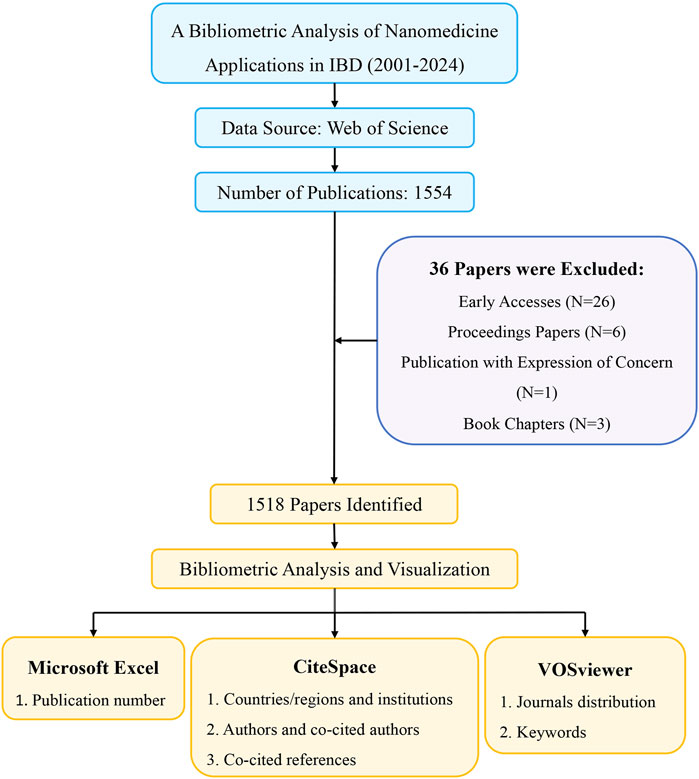
Figure 1. Flowchart for screening publications and conducting bibliometric analysis.
3 Results3.1 Quantitative analysis of annual publication outputAccording to the search criteria, a total of 1,554 pieces of literature were identified from the WoSCC database. 36 irrelevant records were screened out based on the exclusion criteria of document type and language, including 26 early accesses, 6 proceedings papers, 1 publication with expression of concern, and 3 book chapters. Ultimately, 1,518 studies, comprising 1,248 articles and 270 reviews, were collected for the final analysis. Figure 2 shows the annual number of papers published related to nanomedicine applications in IBD from 2001 to 2024. With the exception of 11 articles published in 2005, the annual academic output remained below 10 in the initial phase lasting from 2001 to 2007. During the subsequent phase, the output of publications overall continuously increased in a fluctuating manner. Strikingly, the number of papers rose rapidly from 2019 to 2023, peaking at 291 scholarly articles published in 2023. Based on these results, we anticipate that the number of annual publications will persistently increase, indicating that studies on nanomedicine applications in IBD are gaining interest.
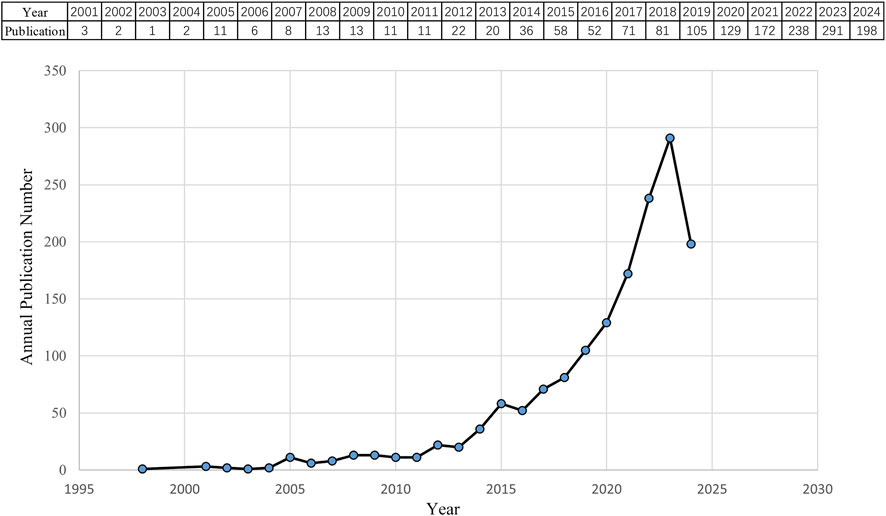
Figure 2. Quantity and trend in publication output for nanomedicine applications in IBD from 2001 to 2024.
3.2 Distribution of countries/regions and institutionsA total of 287 countries/regions and 5,459 institutions have contributed to the field of IBD nanomedicine. The top 10 most productive countries/regions and institutions are summarized in Table 1. The results show that Germany (2001), Japan (2001), Italy (2001), France (2001), the United States (2002) started research in this field earlier, establishing a strong foundation for future advancements. Although China (2007) started relatively late, its development has been rapid, contributing the largest volume of publications (712, 45.8%), significantly higher than other countries/regions, followed by the United States (305, 19.6%), and India (107, 6.9%). The output from other countries/regions in the top 10 list have fewer than 90 publications each. Findings from Figure 3 reveal that China is a leading cooperation center (centrality = 0.33) in this field, with the United States (0.25), Italy (0.16), and France (0.15) ranking second, third, and fourth respectively. The top 10 most productive institutions are distributed across 4 countries, with 4 located in China and 4 in the United States. The three institutions that publishing the most relevant papers are the Chinese Academy of Sciences (70, 4.5%), the University of Georgia (55, 3.5%), and Georgia State University (51, 3.3%). Although the Egyptian Knowledge Bank (EKB) from Egypt ranks fourth (44, 2.8%), it has the highest centrality (0.22), closely followed by the Chinese Academy of Sciences (0.20), indicating that these institutions occupy significant positions in the research of the IBD nanomedicine (Figure 4).
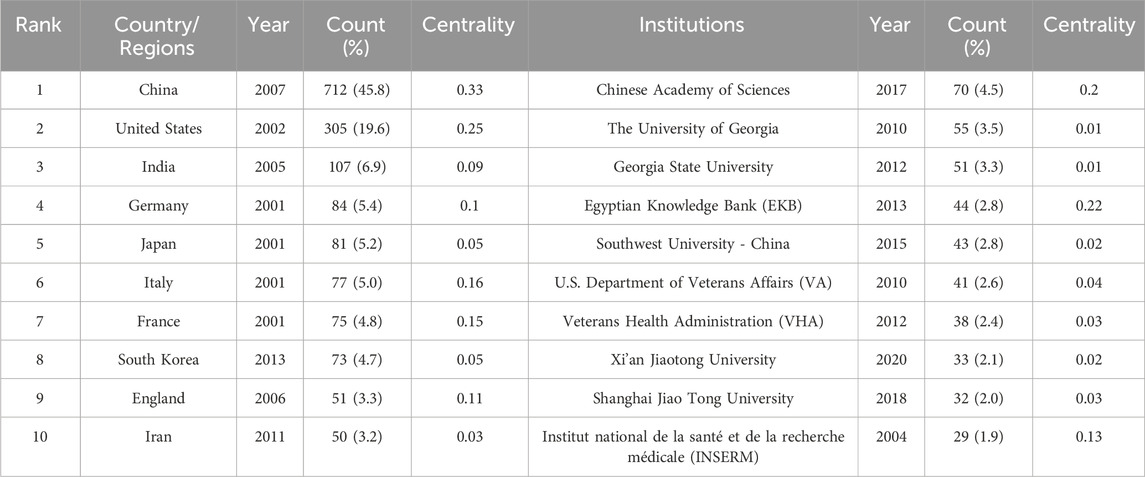
Table 1. Publications in the 10 most productive countries/regions and institutions.
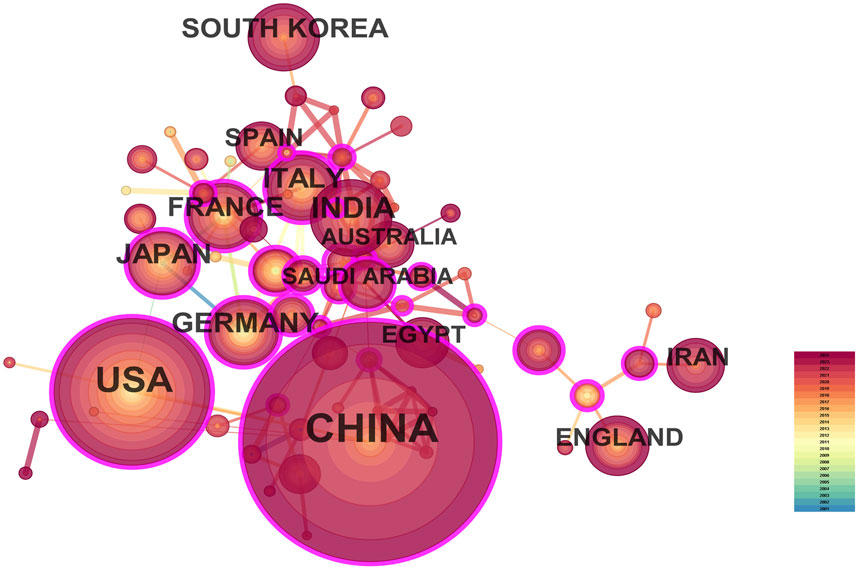
Figure 3. The visualization map of leading countries contributing to research on nanomedicine applications in inflammatory bowel disease (IBD).
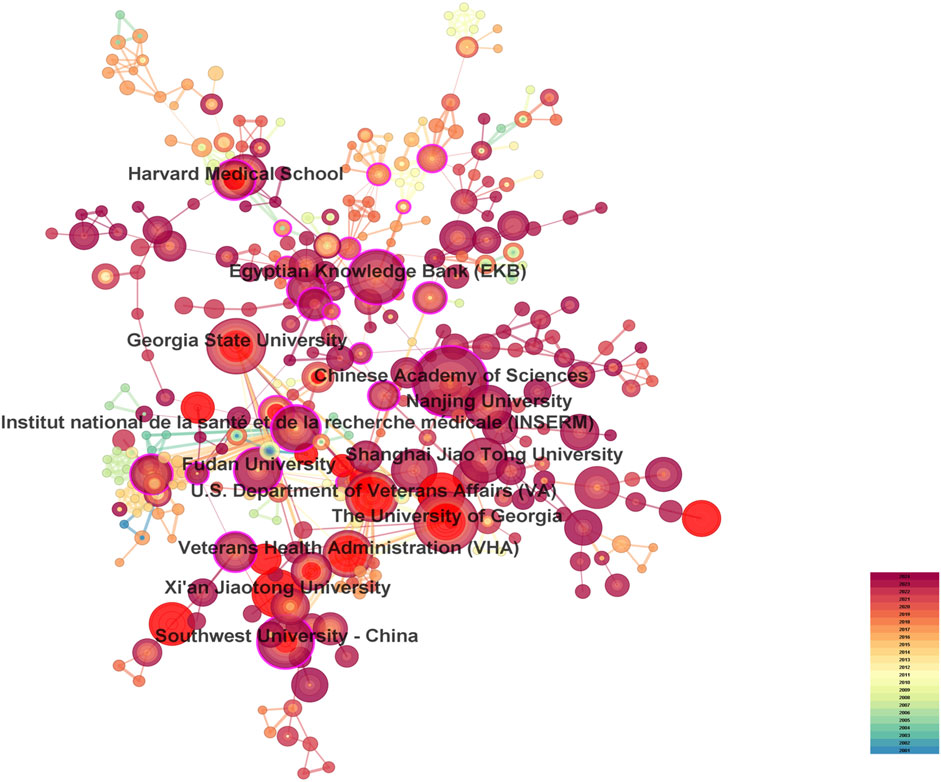
Figure 4. The visualization map of leading institutions contributing to research on nanomedicine applications in IBD.
3.3 Analysis of authors and co-cited authorsResearch on nanomedicine applications in IBD has seen contributions from 9,334 scholars, and the top 10 productive authors and co-cited authors are list in Table 2. Among these top authors with the most published papers, half are from China, two are from the United States, two from South Korea, and one from Germany. In terms of high productivity, Bo Xiao (Southwest University, China) leads with 37 literature, right after whom Didier Merlin (Georgia State University, United States) with 32, and Mingzhen Zhang (Georgia State University, United States) with 28. The other authors in the top 10 list have fewer than 20 documents each (Figure 5A). We cannot ignore that the centralities of all authors are less than 0.01, indicating that these researchers should strengthen cooperation with others. Co-cited author analysis examines instances where the works of two authors are simultaneously cited by a third author. A greater number of co-citations suggests that the authors have similar academic interests and that their research is closely aligned (Cheng et al., 2021). The top 10 co-cited authors were referenced more than 100 times each. The most frequency co-cited author is Bo Xiao, with 260 citations, who is also the most productive author (Figure 5B). Following him are Alf Lamprecht (246 citations; University of Bonn, Germany), Mingzhen Zhang (232 citations), and Hamed Laroui (168 citations; Georgia State University, United States). China has not only produced the largest number of publications but also has the most productive and frequently co-cited authors, highlighting its rapid advancement in this research area despite a relatively late start.
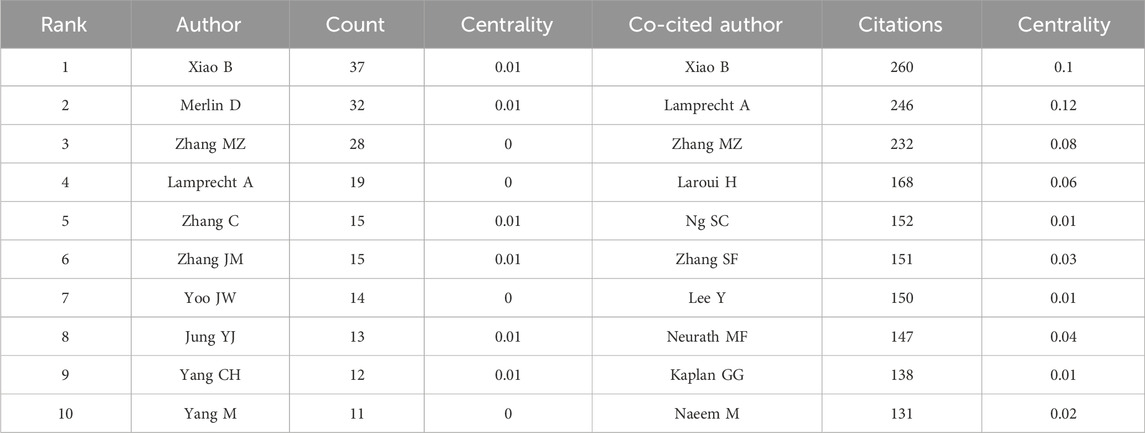
Table 2. Top 10 authors and co-cited authors.
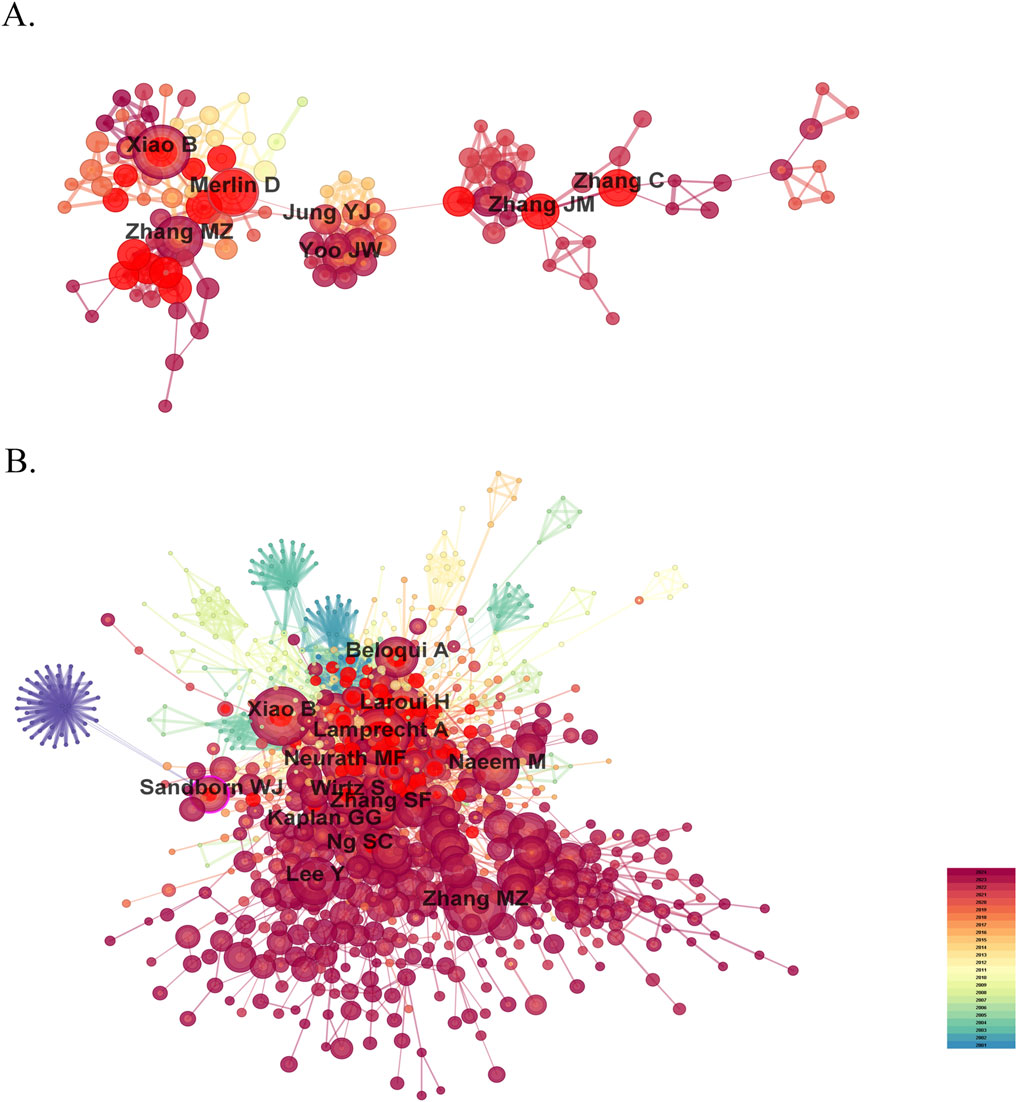
Figure 5. The visualization map of influential authors (A) and co-cited authors (B) in the field of nanomedicine applications in IBD.
3.4 Analysis of journals distributionTo gain a comprehensive understanding of the publication landscape and citation dynamics within the field of nanomedicine applications for IBD, we investigated the distribution of documents across various journals and disciplines. All papers on nanomedicine applications in IBD are sourced from 447 journals. As shown in Table 3, the Journal of Controlled Release (IF 2023 = 10.5), a renowned journal in pharmacy, published the most papers, followed by International Journal of Pharmaceutics (IF 2023 = 5.8), and International Journal of Biological Macromolecules (IF 2023 = 7.7) (Figure 6A). In order to determine the causal relationships between journal citations, we used a dual-map overlay of relevant journals to visualize the citation paths across related fields. Based on Figure 6B, we note that the analyzed papers are predominantly distributed across the fields of Physics, Materials Chemistry, and Molecule, Biology, Immunology, with their cited papers also primarily found in the similar disciplines. Table 4 presents details on the top 10 co-cited journals in the field of nanomedicine applications in IBD. Among these, the United States and the Netherlands contribute four journals each, and the UK has two. Notably, the most co-cited journals are from medical-related fields, Gastroenterology (IF 2023 = 10.5, United States) and Gut (IF 2023 = 10.5, UK), the top two journals in gastroenterology, are ranked first (1,247 citations) and fourth (893 citations), respectively. In contrast, there is only one journal from the materials science field: Biomaterials (IF 2023 = 12.8, United States), which is ranked third with 903 citations.
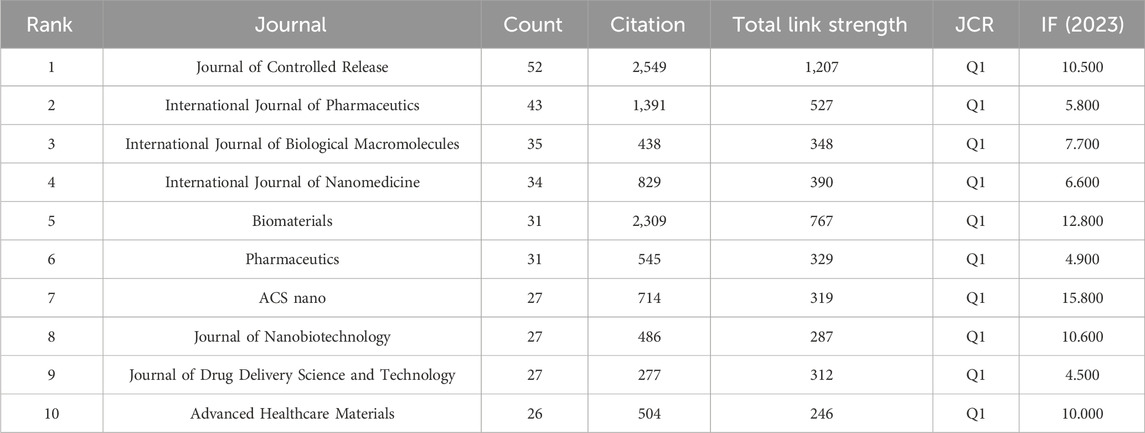
Table 3. The top 10 journals with the most publications.
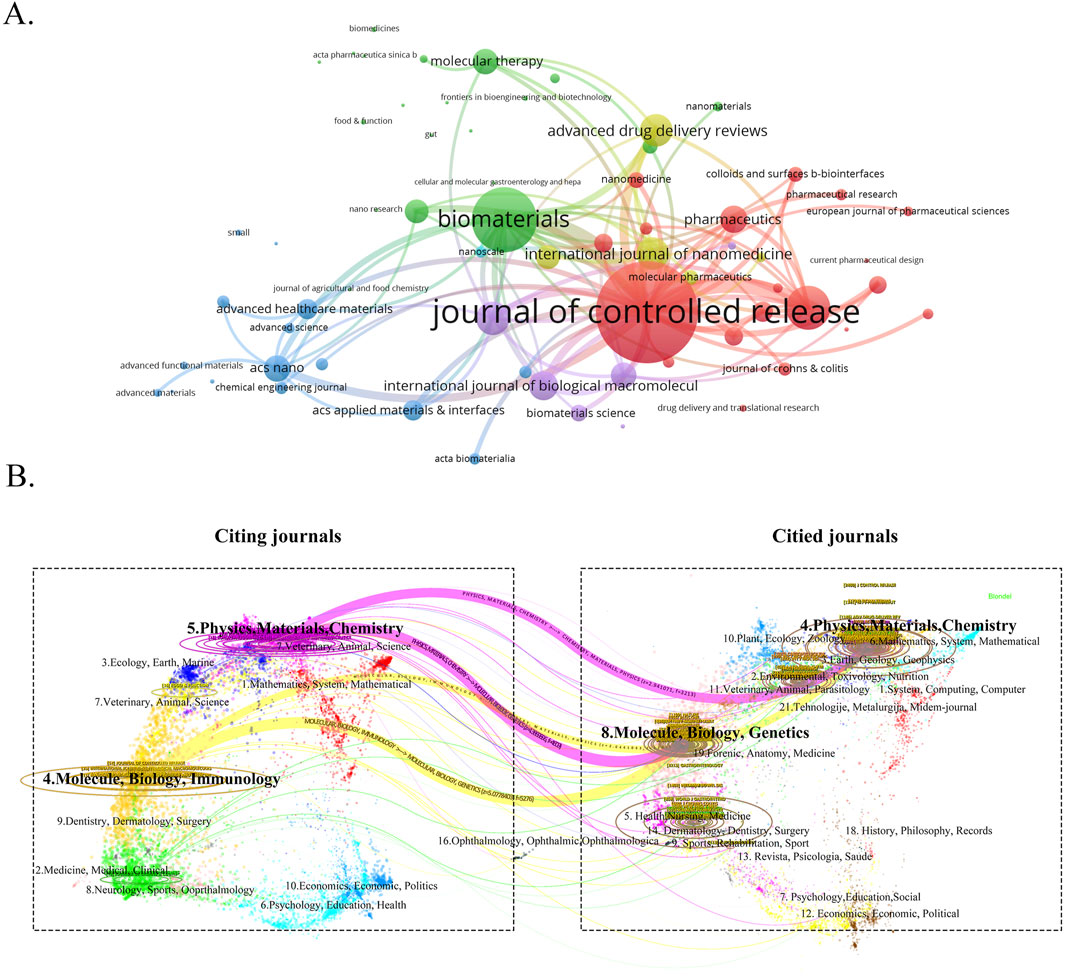
Figure 6. (A) The visualization map of journals publishing papers on nanomedicine applications in IBD. (B) The dual-map overlay of the relevant journals.
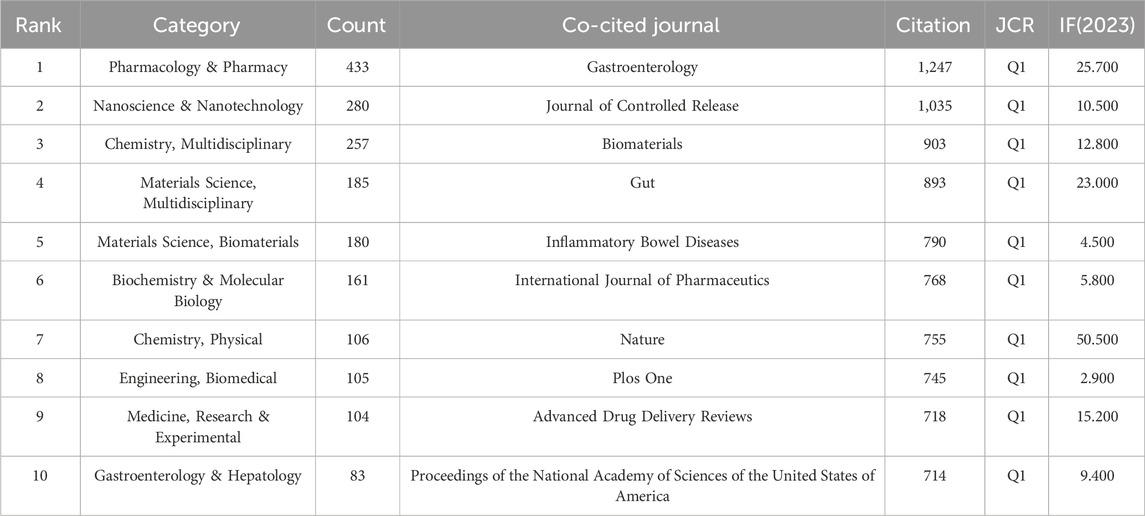
Table 4. Document types of the publications.
3.5 Co-cited references analysis and clustering networkLiterature co-citation analysis reveals the relationship between documents by examining the frequency at which they are cited (Yang et al., 2020). A total of 1,119 co-cited references in the research of nanomedicine applications in IBD were visualized, and the first authors of the top 11 most co-cited references are depicted in Figure 7A. The size of the circles is proportional to the co-citation frequency, and the redder the color, the more co-citations it has within the time zone. Additionally, a complete list of the top 10 co-cited references is present in Table 5. Among these publications, only one study is a systematic review, ranked third, while the other 9 studies are clinical trials. All references were co-cited at least 50 times. The top 2 highly co-cited are as follows: The most co-cited article is ‘Hyaluronic Acid-bilirubin Nanomedicine for Targeted Modulation of Dysregulated Intestinal Barrier, Microbiome and Immune Responses in Colitis’ published in Nature Materials with 125 citations, authored by (Lee et al., 2020). The second article, published in Biomaterials by Gou et al. (2019), is titled “Multi-bioresponsive Silk Fibroin-based Nanoparticles with On-demand Cytoplasmic Drug Release Capacity for CD44-Targeted Alleviation of Ulcerative Colitis.” These two original articles introduce the biocompatibility and targeted delivery ability of nanomaterials, demonstrating that nanomedicine can improve the therapeutic efficiency of IBD by maintaining gut microbiome homeostasis and regulating the innate immune response. The results of these studies lay the foundation for further research on nanomedicine applications in IBD. The third co-cited reference is ‘Worldwide Incidence and Prevalence of Inflammatory Bowel Disease in the 21st Century: A Systematic Review of Population-based Studies’ published in Lancet by (Ng et al., 2017). This systematic review is widely cited because it offers comprehensive data on IBD incidence and prevalence from a global perspective, with significant implications for public health policy and clinical practice.
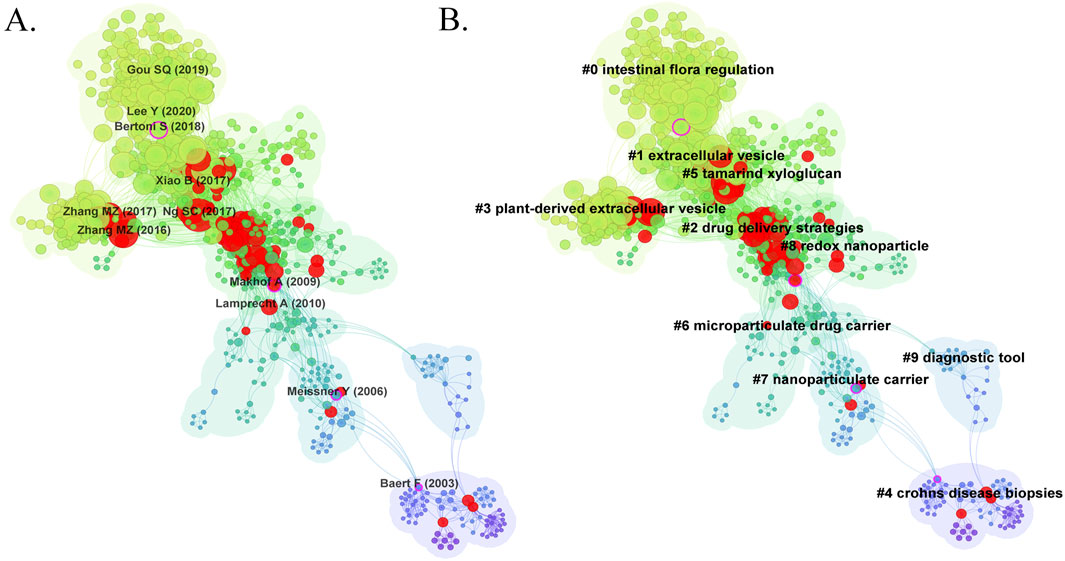
Figure 7. (A) The visualization map of co-cited references on nanomedicine applications in IBD. (B) The clustering network map of co-cited references.
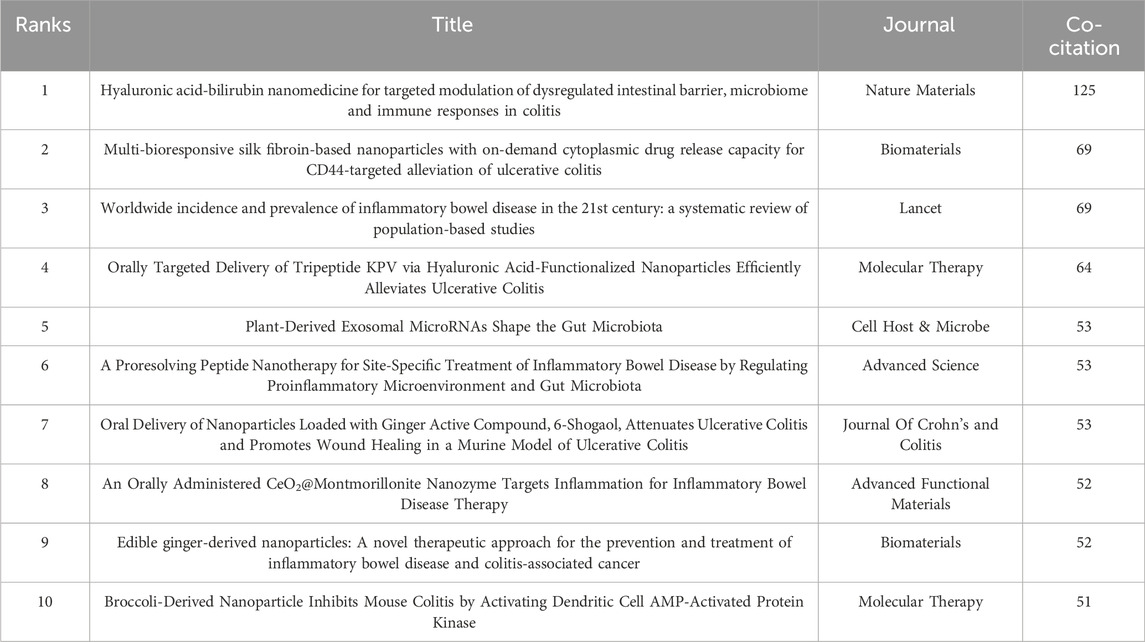
Table 5. Top 10 co-cited references.
Cluster analysis of literature co-citation objectively reveals the knowledge structure of the research field (Yang et al., 2019). We further generated a visual map of the top 10 clusters based on the co-cited references (Figure 7B). Cluster #0, identified as intestinal flora regulation, was the largest cluster, followed by extracellular vesicle (cluster #1), drug delivery strategies (cluster #2), plant-derived extracellular vesicle (cluster #3), Crohn’s disease biopsies (cluster #4), tamarind xyloglucan (cluster #5), microparticulate drug carrier (cluster #6), nanoparticulate carrier (cluster #7), redox nanoparticle (cluster #8), and diagnostic tool (cluster #9). As shown in Figure 8A, we conducted a timeline of co-cited references to visualize the evolution of research trends and hotspots over time. As we have seen, cluster #4 and cluster #9 began study earlier, cluster #2 has a high concentration of nodes with citation bursts in recent years, and cluster #0, #1, #3, and #5 are considered research fronts as they are still ongoing. Figure 8B illustrates the dependency relationships between different research clusters, highlighting the mutual influence and citation relationships among them. Consistent with Figure 8A, cluster #2 serves as a significant reference basis for subsequent research, while cluster #0, #1, and #5 represent the current frontiers of research. This graph indicates that in the study of intestinal flora regulation, research on cluster #2, #3, and #8 serve as important reference foundation; in the study of extracellular vesicles, research on cluster #2, #3, #6, and #8 provide crucial reference foundation; similarly, in the study of tamarind xyloglucan, research on cluster #2 serves as an important reference foundation.

Figure 8. (A) The timeline view of co-citation clusters. (B) The dependency relationships among different co-citation clusters.
3.6 Keywords co-occurrence analysis of research hotspotsThrough the co-occurrence analysis of keywords (Figures 9A, B), we can quickly capture the current hotspots and future research directions in this discipline (Wu et al., 2022). A total of 3,433 keywords were extracted from these papers, with 52 keywords appearing more than 10 times. Table 6 presents the top 20 high-frequency keywords in research on nanomedicine applications in IBD. Among these keywords, “inflammatory bowel disease” (n = 367) and “ulcerative colitis” (n = 294) are the most frequently used terms, followed by “nanoparticles” with 172 occurrences. Additionally, the total link strengths, which refers to the total number of co-occurrences of a keyword with others (Cai et al., 2023), for these three keywords exceed 160. We analyzed the top 20 keywords with the strongest citation bursts from 2001 onwards (Figure 10). As indicated by the red segments, these keywords experienced a blowout in usage at this period. The keyword “immune regulating cells” has the strongest burst (burst strength 7.27). The keywords “colonic mucosa” (burst duration from 2005 to 2018, 13 years) and “immune regulating cells” (2005–2016, 11 years) receive the most sustained attention over time. Furthermore, we also found that “nanovesicles,” “gut microbiota,” and “macrophage polarization” have emerged more recently, revealing that these topics might represent current hotspots in the field of nanomedicine applications in IBD.
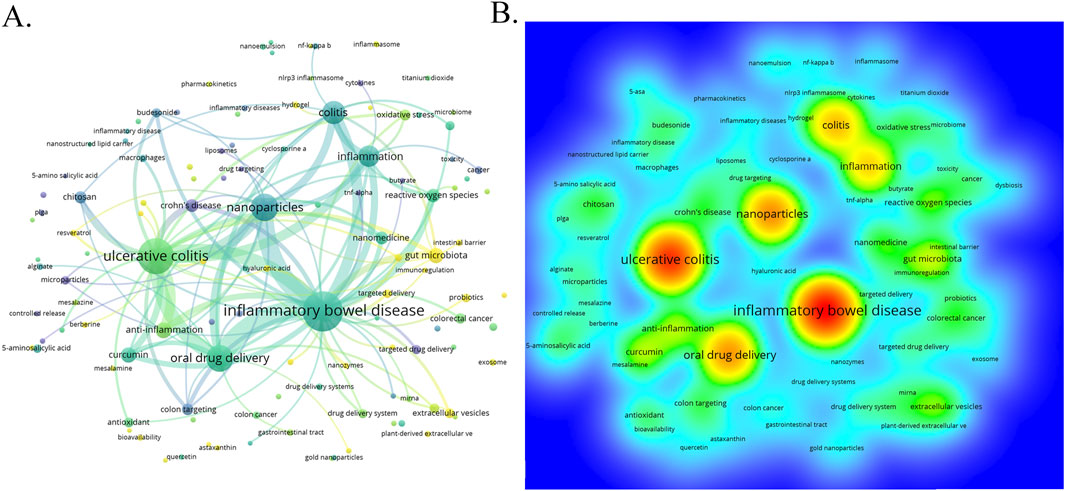
Figure 9. (A) The visualization map of keywords co-occurrence network on nanomedicine applications in IBD. (B) The heatmap of keywords.
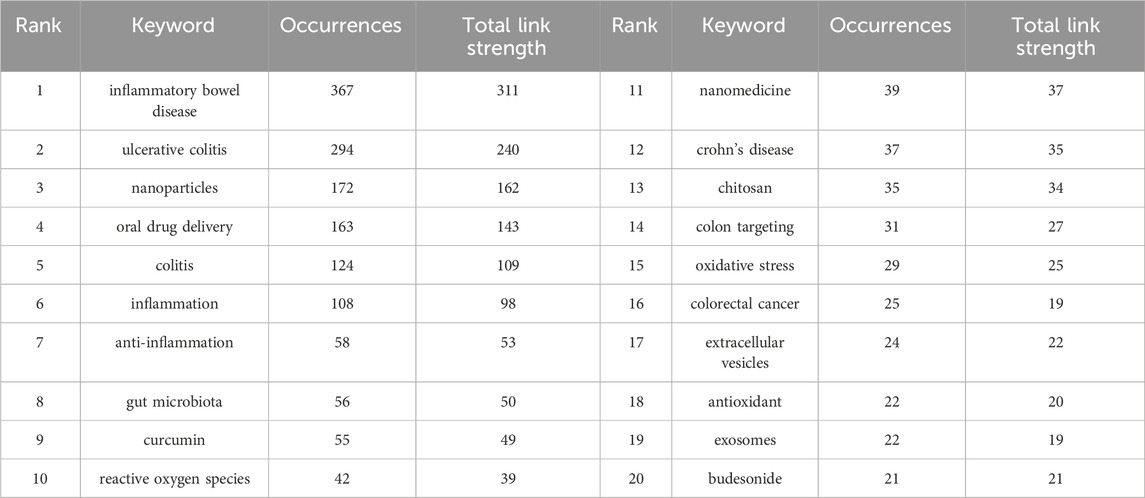
Table 6. The top 20 keywords.
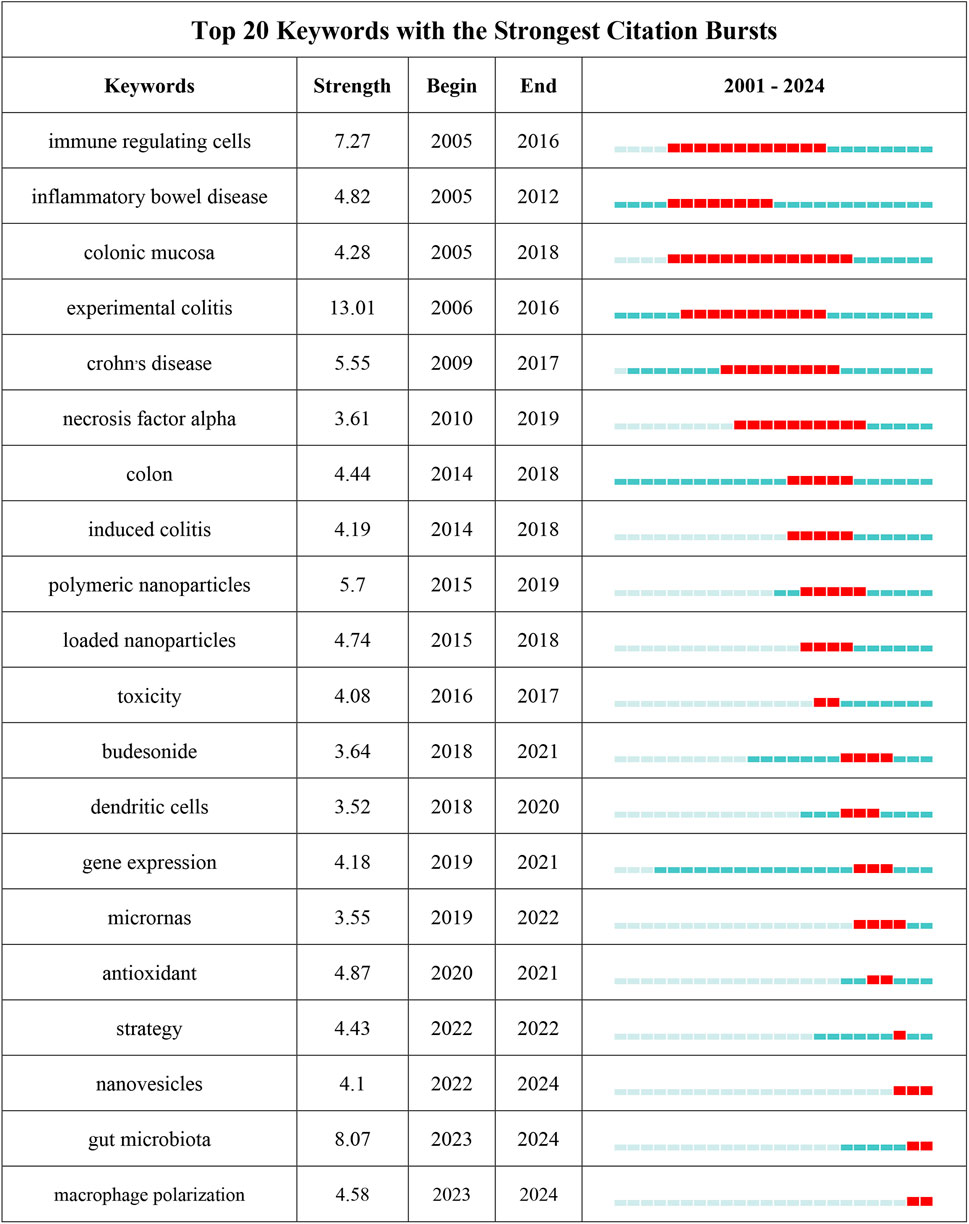
Figure 10. The top 25 keywords with the strongest citation bursts on nanomedicine applications in IBD.
4 DiscussionIn this era of information overload, staying on the top of the industry developments and keeping informed about the latest research findings is increasingly challenging (Liu L. et al., 2022). To present the current global intellectual base and research trends of nanomedicine applications in IBD, we gathered scholarly articles from 2001 to 2024 and conducted a bibliometric analysis to visualize the knowledge structures within this specific field.
4.1 General informationAs of 15 July 2024, a total of 1,518 publications are included, with contributions from 9,334 authors across 447 journals, 5,459 institutions and 287 countries/regions. Alf Lamprecht is considered a pioneer in nanomedicine applications for IBD. His seminal 2001 paper investigated the size-dependent deposition of microparticles and nanoparticles in a rat model of experimental colitis, establishing the foundation that nano-sized carriers could enhance targeted drug delivery to inflamed regions in IBD (Lamprecht et al., 2001a). From 2001 to 2004, research on nanomedicine applications in IBD was in its infancy stage, with less than 3 publications per year. The field experienced a slow growth phase from 2005 to 2011, averaging 10.4 papers annually. However, from 2011 to 2024, the field entered a rapid growth phase, with a particularly notable surge in the past 6 years, where the number of annual publications consistently exceeded 100 each year. This trend suggests that research on nanomedicine applications in IBD is currently in an explosive period and is likely to remain active in the coming years as targeted medicine continue to gain prominence among scholars (Takedatsu et al., 2015).
China and the United States are the leading countries in research on nanomedicine applications in IBD, contributing the greatest output of publications. The United States began research in this field earlier, establishing a leading position and laying a strong foundation for future advancements. In contrast, China entered the field later but has rapidly become a dominant contributor, gradually solidifying its position. China not only produces the highest number of publications but has also emerged as a central hub for international collaborations in recent years, with a centrality score of 0.33. With the exception of one institution from Egypt and one from France, the remaining 8 institutions in the top 10 most productive are all located in China and the United States. The Global Burden of Disease Study 2019 revealed that China and the United States had the highest number of prevalent cases (Wang et al., 2023). Therefore, the incidence of IBD is a contributing factor to their dominance in IBD-related publications, particularly in the United States, where the disease burden is high, and in China, where the incidence has been rapidly rising in recent years. Additionally, the distribution of institutions and collaboration network explain why these two nations involved in the most papers in this field. Their strong academic infrastructures, substantial government funding, and international collaborations contributed to the growth of IBD research and implies that establishing premier research institutions is crucial for elevating the prominence of the academic community (Pei et al., 2022).
Research on nanomedicine applications in IBD was spearheaded by Alf Lamprecht, an influential expert in drug development. Dr. Lamprecht initially focused on the preparation and structural analysis of microparticles and nanoparticles. In 2001, he published 2 trailblazing experimental studies on the applications of nanoparticles in IBD treatment (Lamprecht et al., 2001a; Lamprecht et al., 2001b). His work introduced the potential of nanoparticles for targeted drug delivery to inflamed colonic mucosal areas, demonstrating that this approach is more effective than traditional drug solution, with the added benefit of minimizing systemic side effects and preventing relapse. Alf Lamprecht laid the foundation for nanomedicine applications in IBD. In subsequent studies, Dr. Lamprecht enhanced the therapeutic efficacy of nanocarriers encapsulating the drugs for IBD by incorporating PH-sensitive delivery mechanisms (Meissner et al., 2006) and implementing specific surface modifications (Wachsmann et al., 2013). Bo Xiao has not only published the greatest number of related papers but also ranks as the top co-cited authors, highlighting his prominent contribution to this field. The research of Dr. Xiao primary focused on developing advanced drug delivery systems for IBD. The initial work involved creating foundational nanoparticle systems, including a mannosylated bioreducible cationic polymer that designed to efficiently targets macrophages and inhibit TNF-α expression, thereby effectively treating IBD (Xiao et al., 2013). As the research advanced, the team incorporated more sophisticated materials and methods, such as PH-sensitive materials (Xiao et al., 2015) and functional modifications (Xiao et al., 2014b), to optimize nanoparticle performance, improving the accumulation and action of drugs within specific cell types via enhancing drug release characteristics and cellular uptake efficiency. Dr. Xiao revealed that orally administered nanoparticles, functionalized with surface antibodies against CD98 and carrying CD98 siRNA, could significantly reduce CD98 expression in colonic epithelial cells and macrophage, thereby alleviating colitis severity in mice (Xiao et al., 2014a). They also demonstrated that Hyaluronic Acid-Functionalized nanoparticles (HA-NPs) loaded with Lysine-proline-valine could effectively target colonic cells, promote mucosal healing and reduce inflammation in IBD (Xiao et al., 2017). As their research progressed, they attention gradually shifted toward combination therapies, such as using HA-NPs to co-deliver CD98 siRNA and the anti-inflammatory agent curcumin, thus enhancing therapeutic efficacy through the combined strategy of multiple drugs (Xiao et al., 2016). At the same time, Bo Xiao has cooperated with Didier Merlin (ranks 2ed among the top 10 authors), Mingzhen Zhang (3rd), Jinming Zhang (6th), and Yunjin Jung (8th), resulting in several high-level publications, demonstrating that the development of a team relies heavily on collaborative efforts.
The analysis of journal sources for documents on nanomedicine applications in IBD reveals a distribution across various academic fields. Most of these publications are concentrated in journals within pharmacy, chemistry, and materials science, while medical-related fields ranking lower, occupying the ninth and tenth positions. This indicates that the advancement of nanomedicine for IBD is predominantly driven by progresses in fundamental sciences, particularly in the design, synthesis, and applications of novel materials (Kong et al., 2023; Shahcheraghi et al., 2022). Interestingly, although the majority of these studies are published in materials and pharmaceutical science journals, the most frequently co-cited journals are from the medical fields, particularly those specializing in gastroenterology, such as Gastroenterology and Gut. This indicates the clinical significance of these foundational studies and suggests that while the research originates in material and chemical sciences, its most profound applications and impact are in the medical field (Fu et al., 2023; Yang and Merlin, 2019). Overall, these findings emphasize the interdisciplinary nature of this research area and underscore the critical need for continued collaboration between fundamental sciences and clinical medicine to advance the nanomedicine applications in IBD.
The cluster analysis of literature co-citation provides an insightful perspective on the evolving knowledge structure in the field of nanomedicine applications for IBD (Yuan et al., 2024). Cluster #4 (Crohn’s disease biopsies) and Cluster #9 (diagnostic tools) represent earlier research, highlighting foundational work in nanomedicine at the diagnostic level. In contrast, Cluster #2 (drug delivery strategies) has experienced a recent surge in citations, suggesting an increased emphasis on the development and refinement of therapeutic approaches in recent years. The largest cluster, Cluster #0 (intestinal flora regulation), and the most recent cluster Clusters #1 (extracellular vesicle), signify significant areas of focus within the field. Notably, Clusters #3 (plant-derived extracellular vesicle) has emerged as a new and unique research cluster, reflecting their growing recognition due to their distinctive bioactive properties, biocompatibility, and low immunogenicity (Rome, 2019; Mu et al., 2023). Plant-derived extracellular vesicles have become a promising and rapidly developing area of research in the treatment of IBD. Current research trends are increasingly concentrated on innovative drug delivery systems, extracellular vesicles, and the role of gut microbiota.
Co-cited references are regarded as the foundational research within a particular field (Pei et al., 2022). In this bibliometric study, we identified the 10 most co-cited references in the field of nanomedicine applications in IBD. Except for one review that assessed the changing incidence and prevalence of IBD globally in the 21st century (Ng et al., 2017), the remaining papers, all experimental studies, focus on the development and applications of natural and synthetic nanomedicines and their potential for widespread use in IBD treatment. There are 3 studies explore the potential of plant-derived nanoparticles in treating IBD by targeting specific pathways and mechanisms within the gut (Zhang et al., 2016; Deng et al., 2017; Teng et al., 2018). This novel and natural delivery mechanism open new therapeutic avenues for addressing IBD while overcoming the potential toxicity in traditional nanoparticles. Synthetic nanomedicines, with their multifunctional properties, not only can precisely target inflamed colon tissues but also restore gut barrier integrity through various mechanisms. For instance, hyaluronic acid-bilirubin nanomedicine (HABN) (Lee et al., 2020) and Multi-bioresponsive silk fibroin-loaded nanoparticles (Gou et al., 2019) have shown enhanced therapeutic efficacy by promoting gut microbiome homeostasis and modulating immune responses, making them promising candidates for IBD treatment. Li et al. (2019) developed a smart nanotherapy using oxidation-responsive nanoparticles that release a package proresolving peptide in response to elevated reactive oxygen species (ROS) at diseased sies. This approach effectively reduces inflammation and promotes gut healing while demonstrating safety in a mouse model, paving the way for further development of targeted precision therapies for IBD and other inflammatory diseases. Overall, the co-cited references primarily focus on drug delivery strategies, which are the research basis for nanomedicine treatment in IBD.
4.2 Hotspots and emerging frontiersIn bibliometrics, the occurrence and burst of keywords can reflect the emerging hotspots in a specific field, providing crucial insights into its development and growth (Liu et al., 2014). Excluding keywords such as IBD, colitis, and nanoparticles, the mainly keywords include drug delivery, immune regulating cells, antioxidants, gut microbiota, and nanovesicles. According to keywords clustering analysis and the strongest citation bursts, the hotspots and current frontiers in the field of nanomedicine applications in IBD can be summarized as follows (Figure 11):
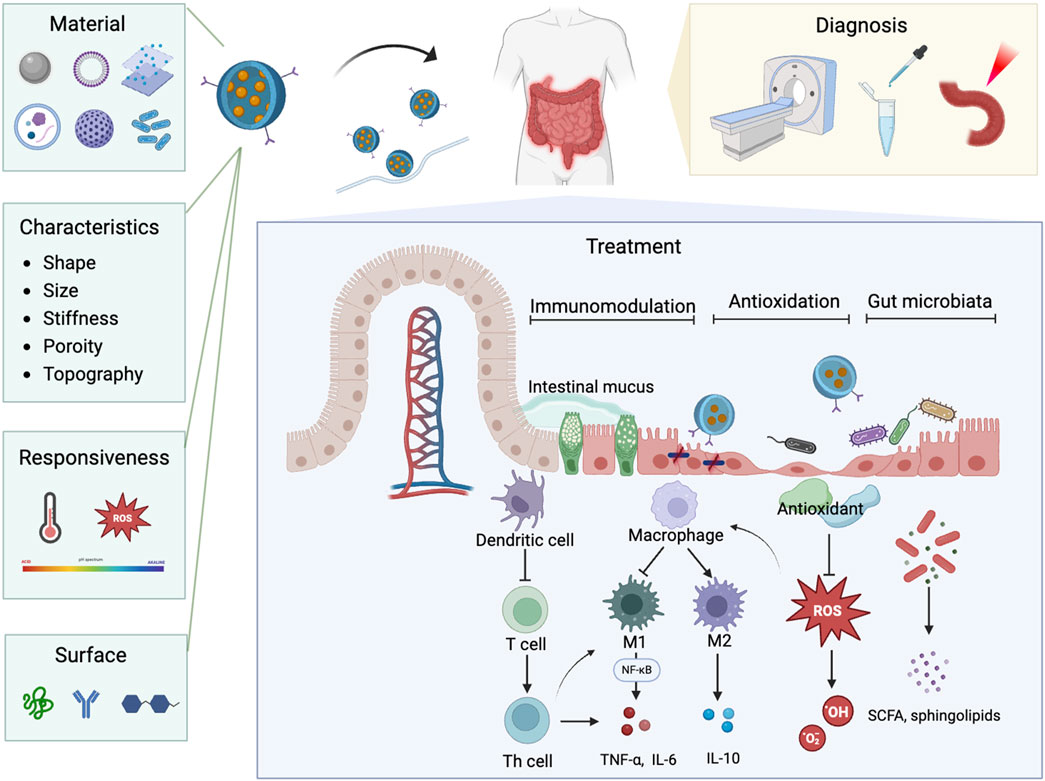
Figure 11. The schematic illustration of nanoparticle properties and their diverse applications in nanomedicine for IBD.
Nanodelivery systems have transformed modern medicine by offering precise and efficient drug delivery to targeted tissues and cells. These systems can encapsulate therapeutic agents like probiotic (Xu C. et al., 2022), protecting them from degradation. Nanoparticles can be engineered to respond to specific stimuli, such as pH changes (Zhang et al., 2017), allowing for the controlled and sustained release of drugs directly at the colonic inflamed areas. Additionally, the surface of nanoparticles can be modified with specific ligands that recognize and bind to receptors on target cells, ensuring precise drug release at the desired locations (Lv et al., 2023). The targeting capabilities of nanotechnology also show great promise in the diagnosis of IBD. Nanoparticles can be engineered to specifically bind to inflamed tissues, allowing for more precise imaging and detection (Yue et al., 2023; Truffi et al., 2017). Integration with imaging modalities such as CT and MRI have enhanced early detection, disease activity monitoring, and assessment of therapeutic response (Expert Panel on Gastrointestinal et al., 2020; Yin et al., 2023). Multifunctional bio-nano platforms, including contrast agents, near-infrared fluorescent probes, and bioactive substance detection agents, have been developed to facilitate more accurate diagnosis and ongoing monitoring of IBD (Fu et al., 2023; Zhou D. et al., 2022; Assadsangabi et al., 2024). This precision in targeting not only improves diagnostic accuracy but also aids in monitoring disease progression and evaluating the effectiveness of treatments at a molecular level. Encouragingly, high specific nanoparticles designed to target molecular and cellular processes associated with IBD can simultaneously treat the disease while tracking its progression (Yin et al., 2023; Yan et al., 2022; Elinav and Peer, 2013). This targeted delivery positions nanodelivery systems as promising tool for advanced medical diagnostics and therapies for IBD.
Nanodelivery systems, with their precision targeting and controlled release capabilities, not only enhance drug delivery but also lay a crucial foundation for advancing immunotherapy approaches in IBD treatment. Nanotechnology can target specific inflamed tissues and immune cells with precision, effectively modulating immune system. One of the key advantages of nanoparticles in IBD therapy is their ability to specifically target immune-regulating cells, which are overactive in IBD and lead to chronic inflammation and tissue damage (Hou et al., 2020; Saez et al., 2023). CD44-targeted nanoparticles can actively target inflammatory colonic epithelial cells and macrophages (Lv et al., 2023; Vafaei et al., 2016), improving the uptake of encapsulated drugs, thereby suppressing macrophage proliferation and downreg
留言 (0)