Autoimmune disorders in childhood encompass a diverse group of conditions where the immune system, which is designed to protect the body, mistakenly targets and attacks its own tissues. These disorders can affect various organs and systems, leading to a range of symptoms. The most common autoimmune disorders in childhood include: juvenile idiopathic arthritis (JIA), type 1 diabetes (T1D), coeliac disease, systemic lupus erythematosus, autoimmune thyroid disorders, inflammatory bowel disease, psoriasis, etc. (1, 2).
On the other hand, autoinflammatory disorders in childhood are a group of rare conditions characterized by recurrent episodes of systemic inflammation that are not driven by the immune system’s typical mechanisms of antigen recognition, as seen in autoimmune disorders. These disorders result from dysregulation of the innate immune system, leading to excessive inflammation and symptoms such as fever, joint pain, skin rashes, and inflammation of various organs. Some notable autoinflammatory disorders in childhood include: familial mediterranean fever, tumor necrosis factor receptor-associated periodic syndrome, cryopyrin-associated periodic syndrome, hyperimmunoglobulin D syndrome, chronic nonbacterial osteomyelitis, etc. (3).
Finding potential causes of inflammation and diagnosing autoimmune and autoinflammatory disorders in children often involves extensive examinations, imaging studies, and sometimes biopsy or genetic testing. Treatment strategies vary depending on the specific disorder and may involve medications to modulate the immune response, manage symptoms, and sometimes lifestyle modifications such as dietary changes. Given the complexity of these disorders, their management often involves a multidisciplinary approach. Collaboration between different pediatric specialists is crucial for effective management, although, it is sometimes challenging to achieve good clinical outcomes.
Case presentationIn February 2017, an 11-year-old female of Caucasian ethnic background was referred to a pediatric rheumatologist due to persistent right hip pain, radiating to the knee and foot. Additionally, she reported painful leg movements accompanied by intermittent leg stiffness. Her medical history revealed no serious injuries, chronic illnesses, or surgeries. The patient had normal body composition. There were no instances of consanguinity or chronic diseases in her family history.
Upon physical examination, she was afebrile with a generally good overall condition, albeit displaying a slight limp. Painful right hip adduction and rotation in all directions, limited range of right hip joint movements, along with tenderness at sacroiliac joints projections, were noted. No pathological findings were observed in any other joints. The assessment of her skin and neurological status was normal.
Haematological parameters, including complete blood count (CBC) and C-reactive protein (CRP) level, were within normal limits. However, a slightly elevated erythrocyte sedimentation rate (ESR) of 14 mm/h (normal range: 0-11 mm/h) was identified. Furthermore, the patient exhibited low blood calcium and vitamin D levels. Initial radiological investigations, comprising X-rays and ultrasounds of the spine, pelvis, and lower extremities, revealed no noticeable pathology. Based on these findings, she was prescribed vitamin D, calcium supplements and nonsteroidal anti-inflammatory drugs (NSAIDs) as needed.
During the next two years patient started feeling thoracic and lumbar pain each day, despite frequent use of NSAIDs. The physical examination revealed painful paravertebral palpation of the thoracic, lumbar, and sacral areas. On spine computed tomography (CT) there were signs of old compression fractures of the Th7, Th8, Th11 vertebrae and juvenile osteochondrosis. Subsequently, magnetic resonance imaging (MRI) of the spine revealed the same compression fractures in thorasic region with perifocal bone marrow edema, and additional stress fractures in the S2-S5 vertebrae (Figure 1). However, no radiological evidence suggestive of tumor presence was observed. Bone density evaluated by Dual Energy X-ray Absorptiometry (DXA), was normal according to reference values for gender and age, thus, osteopenia and osteoporosis were excluded. As well as, lung and skeletal tuberculosis (TB) was ruled out with negative serology.
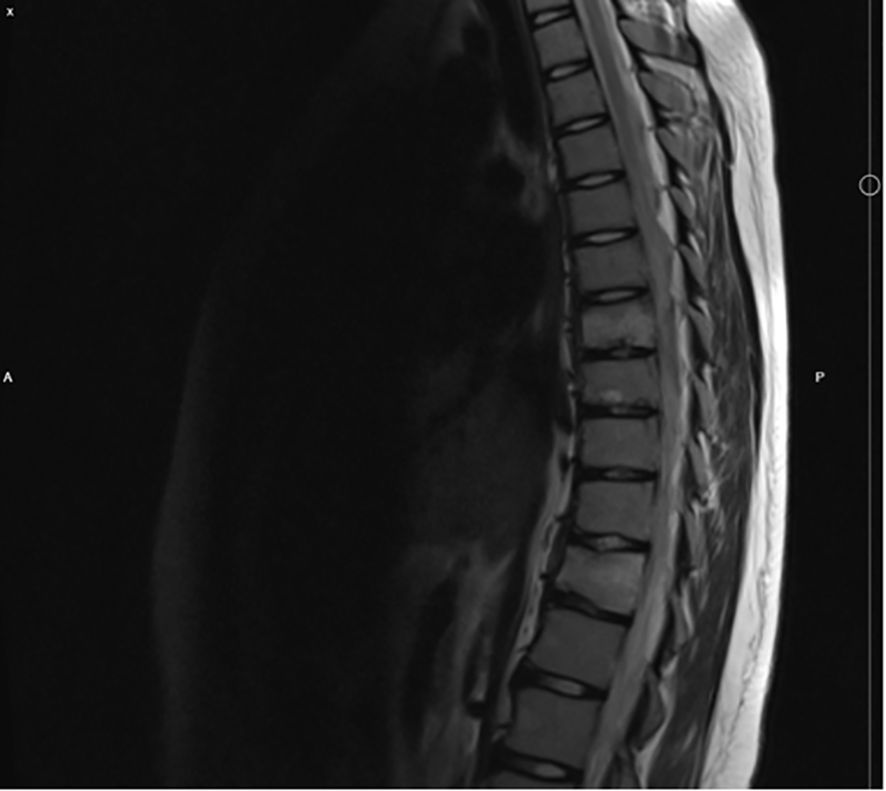
Figure 1. MRI of the spine, showing compression fractures of the Th7, Th8 and Th11.
For differential rheumatological diagnosis the patient underwent a large panel of immunological tests, presented in Table 1. However, despite a slightly elevated ESR, all tests (CBC, hepatic enzymes, kidney function, lactate dehydrogenase), were within normal ranges.
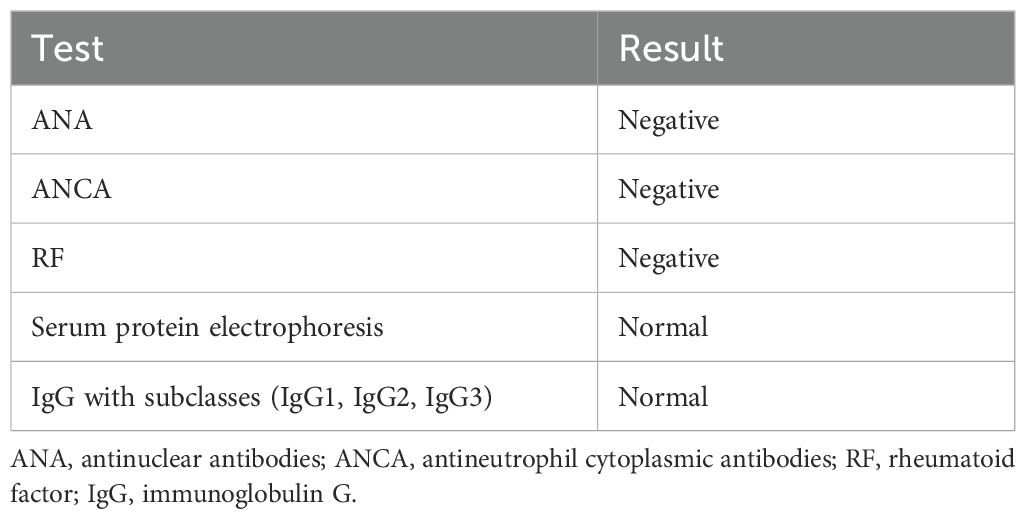
Table 1. Immunological examinations.
Because of lumbar pain it was decided to perform pelvic MRI. It revealed lesions with bone marrow edema in both femoral heads (Figure 2). After evaluating all the data and radiological images, the multidisciplinary team agreed that patient’s signs and symptoms were characteristic for CRMO.
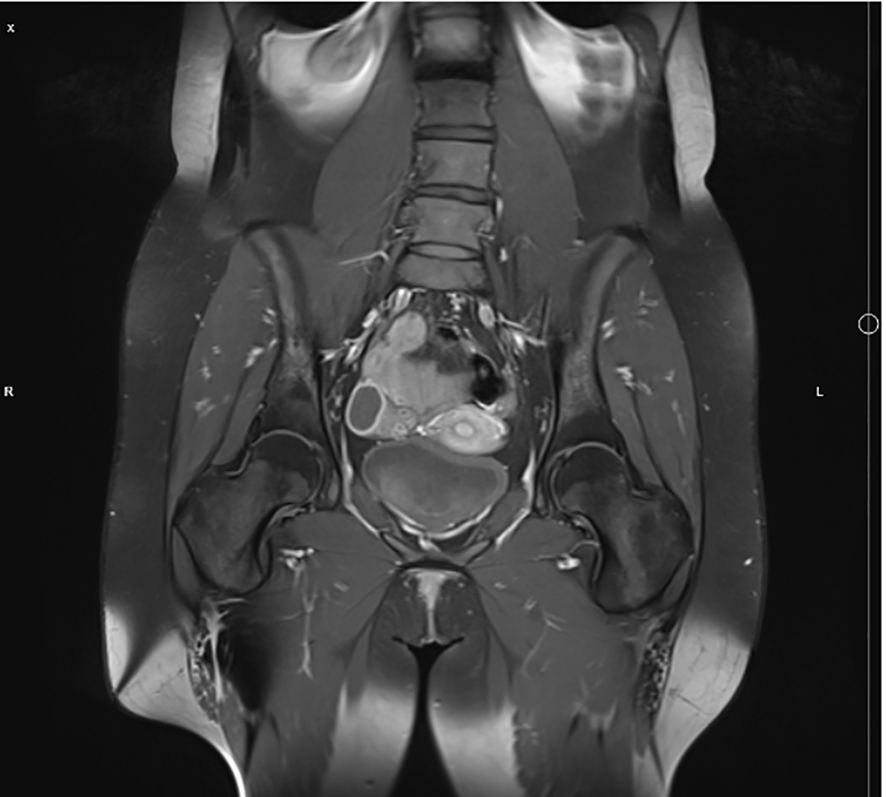
Figure 2. Pelvic MRI showing signs of bone marrow edema in both femoral heads.
Given these findings, treatment with NSAIDs was prescribed and a proactive follow-up involved pelvic and spine MRI after six months. Despite sustained NSAIDs use, the patient presented with increased back pain and severe pain in her right knee six months later. An MRI of the right knee unveiled a tibial metaphyseal lytic lesion accompanied by bone marrow edema, while the spine MRI revealed additional sites of bone marrow edema. Considering the entire medical history and scientific literature, in June 2020 bisphosphonate therapy (pamidronate at 1 mg/kg per month) was initiated. Three doses were administered at monthly intervals, which resulted in significant clinical improvement and resolution of edema signs in femoral heads in repeated MRI.
However, by December 2020, the patient’s back and hip pain intensified, and a new lesion in the ischial bone was identified in MRI. Consequently, a second course of pamidronate at 1 mg/kg (three doses, one month apart) was deemed necessary. Just before the 4th dose of pamidronate, a urine test revealed glucosuria, and a blood test indicated hyperglycemia (14.13 mmol/l), without exhibiting signs or symptoms characteristic of diabetic ketoacidosis. Consequently, the patient was screened for pancreatic antibodies, they confirmed diagnosis of T1D (Glutamic acid decarboxylase (GAD) autoantibodies 611.73 kU/l, highly elevated; insulin autoantibodies – negative; tyrosine phosphatase-related islet antigen 2 (IA-2) antibodies – negative). Glycosylated haemoglobin (HbA1c) was 11.6% (103 mmol/mol), prompting initiation of multiple daily insulin injections and diabetes education.
Additionally, thyroid function and celiac disease were screened according to the International Society for Pediatric and Adolescent Diabetes (ISPAD) guidelines. Tests revealed normal thyroid function with negative thyroid antibodies, however, tissue transglutaminase imunoglobulin (Ig) A level was elevated at 200 U/ml. Subsequent consultation with a pediatric gastroenterologist and screening for IgA and IgG class anti-endomysial antibodies and antibodies against deaminated gliadin (GAF3X) confirmed definite celiac disease, prompting recommendations for a gluten-free diet.
Till May of 2021 the patient was treated with maximum doses of NSAIDs (mainly diclofenac), however, follow-up MRI showed remaining lessions in the sacrum region. Reevaluation of the patients condition and imaging studies dynamics, biopsy of the sacrum lession was done. Hystological examination showed only sclerotic bone changes, no signs of malignancy including Langerhans hystiocytosis were seen.
Taking into account the worsening clinical symptoms, previous improvement after biphosphonate therapy, and disrupted pamidronate supply, due to the Covid-19 pandemic, it was decided to initiate treatment with zolendronic acid (ZA) in May 2022. However, if the 1st dose of ZA seemed promising, the 2nd dose brought no clinical improvement. Therefore, it was decided to start with methotrexate (MTX) 20 mg subcutaneously once per week in November 2022.
In addition, after consultation with genetician, whole exome sequencing (WES) and primary and secondary bioinformatics analyses were carried out by CeGaT GmbH, using their proprietary exome design to cover coding and disease-relevant regions. The Illumina NovaSeq6000 system was employed to sequence enriched regions, with an average coverage of 110x, and reads were processed and aligned to the hg19-cegat reference genome. Tertiary analysis, focused on skeletal and connective tissue disorders, was conducted, however, no pathogenic or likely pathogenic variants related to the clinical situation were found.
Up to the age of 18 (July 2024) the clinical symptoms were stable, dynamics in MRI showed at least some improvements, and no additional lesions, the pain was not worsening. Therefore, it was decided to continue with MTX, as recommended by Childhood Arthritis and Rheumatology Research Alliance (CARRA) guidelines. Her glycemic control has been consistently well-managed, with HbA1c levels typically under 7% and minimal glycemic fluctuations. She has used insulin pump therapy with the Medtronic 780 and ultra-fast-acting insulin (Fiasp). Adhering strictly to a celiac disease diet, she reported no gastrointestinal or related symptoms. At the age of 18 she was successfully transitioned to adult care, provided mainly by rheumatologist and endocrinologist.
Just after turning 18, in summer of 2024, she had a relapse of CRMO, and experienced a CRMO relapse, prompting the adult rheumatologist to initiate treatment with a TNF-α inhibitor, Adalimumab. Her follow up plan consists of regular visits to rheumatologist and endocrinologist, in order, to track her health alterations and reduce the risk of disease relapse. The main timepoints of clinical course, diagnostics and treatment are presented in Figure 3.
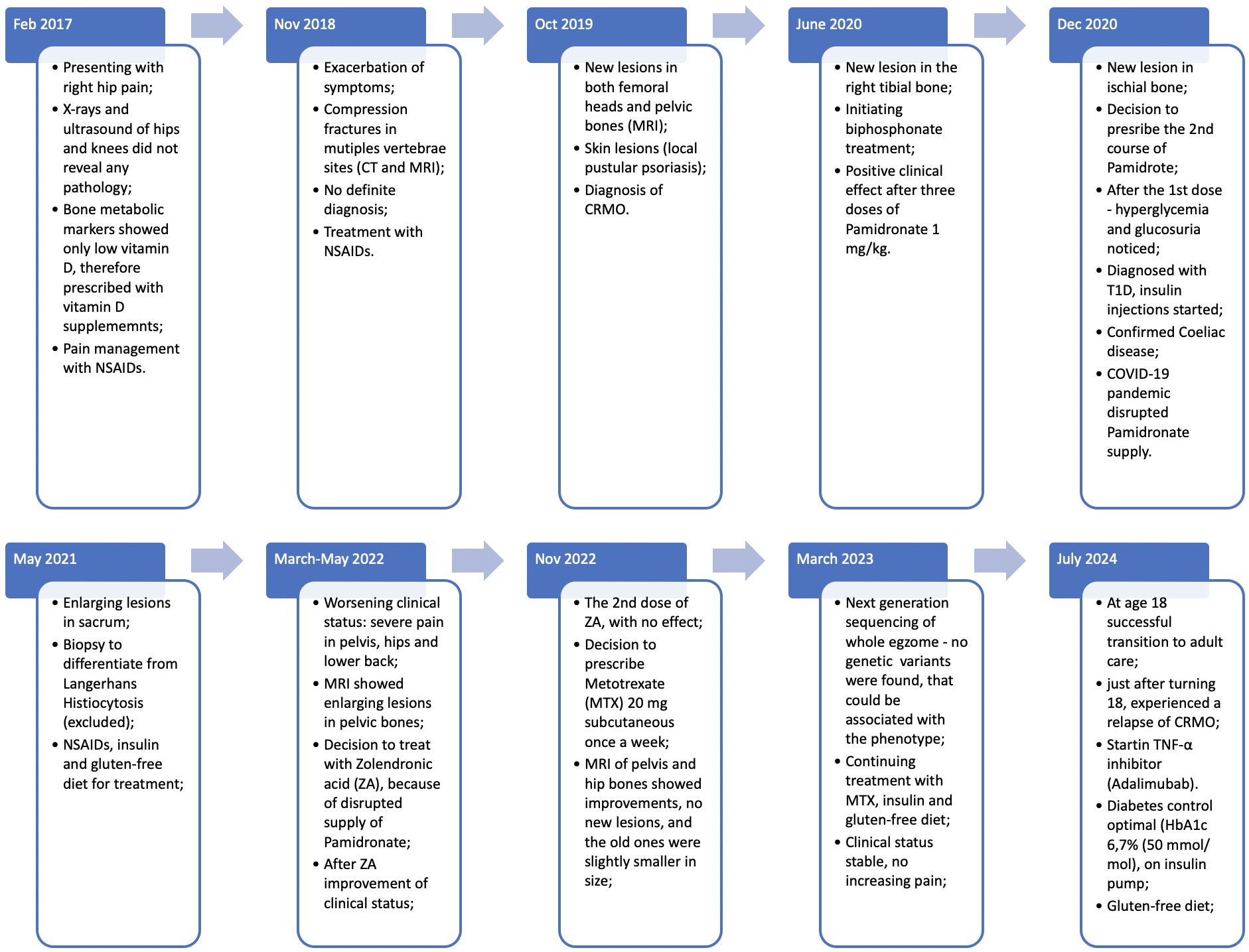
Figure 3. Main timepoints and clinical presentation of the case.
DiscussionWe present a unique case of combined autoimmune and autoinflammatory diseases in a young teenager. To our knowledge, this is the first case report of a child with CRMO, T1D and celiac disease. Presently, there is an escalating incidence of CRMO cases concomitant with additional inflammatory or autoimmune conditions such as Crohn’s disease, ulcerative colitis, and skin diseases (4). However, there is a notable absence of documented case reports concerning the coexistence of CRMO and T1D. Consequently, the objective of this case report is to explain shared processes in the etiology and pathogenesis of these disorders.
CRMO sometimes called chronic non-bacterial osteomyelitis (CNO) is an autoinflammatory bone disorder predominantly affecting children and adolescents. Usually typical lesions occur in the metaphysis of long bones, pelvic bones, vertebrae, and other skeletal regions (5). Despite its rarity, with approximately 500 documented cases worldwide, awareness of CRMO is expanding, and the number of reported patients is increasing (6). The clinical presentation lacks specific features; however, pain and swelling in the affected bone regions are the most common (5). In the largest study involving 486 CNO patients from the Eurofever registry, Girschick et al. highlighted diverse clinical manifestations, potentially involving additional inflammatory disorders impacting the skin (psoriasis, acne), digestive system (Crohn’s disease, ulcerative colitis), lymphoid tissue (hepatosplenomegaly, lymphadenopathy), or heart (pericarditis) (7). Typically, CRMO diagnosis is based on the exclusion of other rheumatological diseases. Since 2016 Bristol’s diagnostic criteria have facilitated diagnostics of CRMO. Notably, the authors advocate against bone biopsy in typical pediatric cases (8).
The pathogenesis of CRMO is usually explained by altered functions of innate immune cells. In this autoinflammatory disorder monocytes exhibit a diminished expression of key regulatory cytokines, namely interleukin (IL)-10 and IL-19, which play crucial roles in immune system modulation. Concurrently, there is an upregulation in the production of pro-inflammatory cytokines (IL-1ß, IL-6, tumor necrosis factor (TNF)-α) and various chemokines. The dysregulated cytokine milieu induces osteoclast differentiation and activation, ultimately contributing to the development of bone lesions (3, 6).
In presented case, the definitive diagnosis of CRMO was reached after intensive investigations that took almost 2 years. The progression of clinical symptoms, and multiple lesions with perifocal bone marrow edema in MRI scans guided to the final diagnosis. However, the diagnostic process necessitated the systematic exclusion of alternative etiologies, including malignancy, infection, or other autoimmune conditions. Considering the data available now, we may hypothesize that earlier utilization of MRI for the affected sites or whole-body MRI could bring an accurate diagnosis sooner. Moreover, whole-body MRI is the preferred imaging modality due to its enhanced capacity for lesion detection and identification of asymptomatic lesions in comparison to localized MRI (9).
The second pathology that developed in our patient was a well-known endocrinopathy – T1D. Glycosuria and hyperglycemia were noticed during routine laboratory tests before scheduled pamidronate infusion, and our patient avoided diabetic ketoacidosis. It is known that T1D is caused by the autoimmune destruction of pancreatic beta-cells. There are several known pathways for the activation of apoptosis in beta-cells. Firstly, activated T helper lymphocytes activate macrophages via the release of pro-inflammatory cytokines (IL-1ß, TNF-α, interferon (INF)-γ). Secondly, autoantigen-specific T cytotoxic (CD8) cells are activated, also causing destruction of beta-cells. Moreover, activation of B lymphocytes leads to production of autoantibodies against beta-cell structures (10, 11). Due to complete destruction of beta-cells, individuals become insulin dependent and necessitate lifelong treatment.
After screening for specific markers, following international guidelines for diabetes diagnosis and management (12), patient was diagnosed with the most common diabetes associated autoimmune disorder – celiac disease. Both conditions share identical genetic susceptibility markers, specifically the HLA genotypes (HLA DQ2 and HLA DQ8). After the confirmed diagnosis of celiac disease, the patient was recommended to follow gluten-free diet.
Considering all known pathophysiological mechanisms of mentioned disorders, we found out that two possible etiological pathways could lead to this combined pathology. Clearly, this dysregulation of immune system in our patient emerged from interactions between genetic predisposition and various environmental, immunologic factors, like infant nutrition, viral infections, already known to contribute to various immunological disorders (12, 13). First of all, increased released of IL-1ß and TNF-α, as proinflammatory cytokines from macrophages, play an important role in CRMO and T1D pathogenesis. Secondly, we hypothesize that asymptomatic and undiagnosed celiac disease could be the primary disorder in our patient, resulting “leacky gut” syndrome, that is known to predispose the dysregulation of immune system. It is known that due to dysregulation of microbiome and increased intestinal permeability, fragments of gliadin invade the lamina propria, cross the intestinal epithelial barrier, and then they induce immune system response and activation of CD4+ T lymphocytes. The consequence of this is increased levels of pro-inflammatory cytokines, eventually activating B lymphocytes, which differentiate into plasma cells producing autoantibodies (14). Also the activation of CD8 lymphocytes contributes to autoimmune process and destruction of pancreatic insulin producing cells (15). The short scheme of hypothesized etiology and pathogenesis leading to described clinical phenotype is presented in Figure 4.
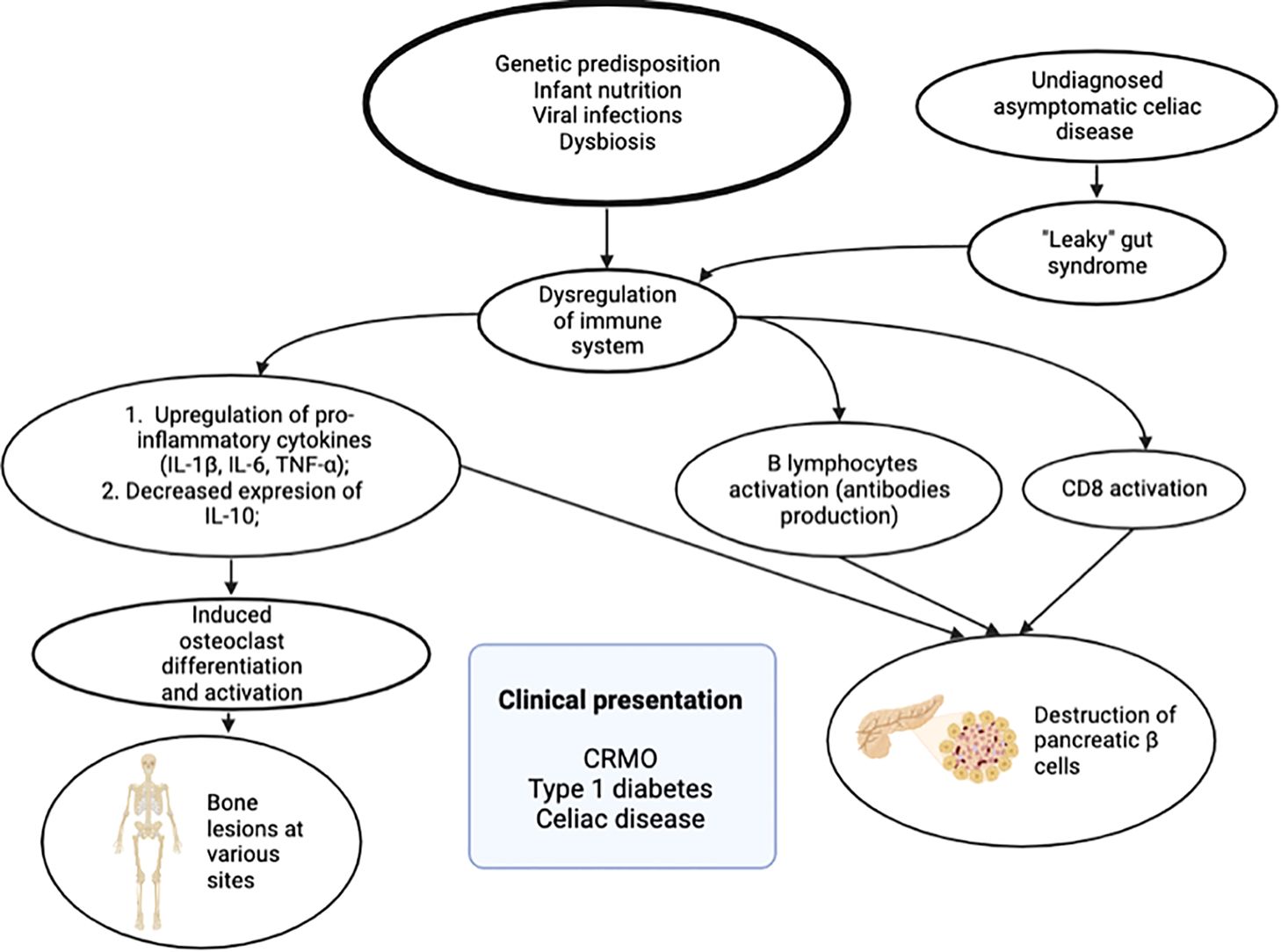
Figure 4. Etiological factors and pathogenesis leading to clinical phenotype: chronic recurrent multifocal osteomyelitis (CRMO), type 1 diabetes, and celiac disease.
Managing autoimmune or autoinflammatory disease is challenging, especially when dealing with the combination of both these pathologies. In 2018, Childhood Arthritis and Rheumatology Research Alliance (CARRA) provided treatment guidelines for CNO/CRMO (16). NSAIDs are recommended as a first line treatment, however, as monotherapy it is not effective in patients with vertebral lesions, as seen also in our case (6, 16). According to the analysis of reported cases, commonly used treatments were MTX, TNF inhibitors or bisphosphonates (16). Regarding T1D treatment it is clearer: insulin is the only one possible treatment choice (17). For coeliac disease, usually it is enough to be complaint with the gluten-free diet (12).
The main limitation that could be highlighted is the lack of cases of this kind in scientific databases, as we report the unique case to our knowledge. Therefore, we referenced studies discussing the pathophysiology of CRMO and T1D separately, and proposed a potential mechanism that could explain the coexistence of these autoinflammatory and autoimmune disorders. To validate our assumption, we plan to collaborate with a medical biologist to conduct laboratory experiments in vitro and animal models. Moreover, it is planned to perform whole genome sequencing, that may help identify genetic variants contributing to this clinical phenotype.
Overall, our case has shown that managing the combination of these disorders was very challenging, with fluctuating clinical course, but finally, we managed to find the most suitable treatment for this individual patient.
ConclusionsTo conclude, as our case report has shown, the combination of autoimmune and autoinflammatory diseases, brings not only a challenging diagnostic process, but also complicated treatment. Therefore, we believe that discussions in multidisciplinary national and international teams could bring precise diagnosis and individualize the treatment quicker for patients with rare or complicated pathologies. Moreover, there is a definite need for the development of new drugs targeting specific pathophysiological mechanisms in autoimmune and autoinflammatory diseases.
Data availability statementThe raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statementThe patient was informed and the written consent was obtained for the usage of anonymized clinical data. Written informed consent was obtained from the participant/patient(s) for the publication of this case report.
Author contributionsIS: Writing – original draft, Writing – review & editing. AS: Writing – original draft, Writing – review & editing. LR: Writing – original draft. RD: Writing – original draft. KA: Writing – review & editing.
FundingThe author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interestThe authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s noteAll claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
GlossaryANA: antinuclear antibodies
ANCA: antineutrophil cytoplasmic antibodies
CARRA: Childhood Arthritis and Rheumatology Research Alliance
CBC: complete blood count
CD4: T (helper) lymphocyte
CD8: cytotoxic T cell
CRMO: chronic recurrent multifocal osteomyelitis
CRP: C-reactive protein
CT: computed tomography
DXA: dual energy X-ray absorptiometry
ESR: erythrocyte sedimentation rate
GAD: glutamic acid decarboxylase
GAF3X: antibodies against deaminated gliadin
HbA1c: glycosylated haemoglobin
HLA: human leucocyte antigen
IA-2: tyrosine phosphatase related islet antigen 2
Ig: immunoglobulin
IL: interleukin
INF: interferon
ISPAD: International Society for Pediatric and Adolescent Diabetes
MRI: magnetic resonance imaging
MTX: methotrexate
NGS: next generation sequencing
NSAID: nonsteroidal anti-inflammatory drug
RF: rheumatoid factor
T1D: type 1 diabetes
TB: tuberculosis
TNF: tumor necrosis factor
ZA: zolendronic acid
References1. Pohl D, Bendeler S. Systematic inflammatory and autoimmune disorders. In: Handbook of Clinical Neurology, Vol. 112 (3rd series). Pediatric Neurology Part II. Netherlands: Elsevier (2013).
2. Diamanti A, Capriati T, Bizzarri C, Panetta F, Ferretti F, Ancinelli M, et al. Celiac disease and endocrine autoimmune disorders in children: an update. Expert Rev Clin Immunol. (2013) 9:1289–301. doi: 10.1586/1744666X.2013.850029
PubMed Abstract | Crossref Full Text | Google Scholar
5. Hofmann SR, Kapplusch F, Girschick HJ, Morbach H, Pablik J, Ferguson PJ, et al. Chronic recurrent multifocal osteomyelitis (CRMO): presentation, pathogenesis, and treatment. Curr Osteoporos Rep. (2017) 15:542–54. doi: 10.1007/s11914-017-0405-9
PubMed Abstract | Crossref Full Text | Google Scholar
6. Zhao DY, McCann L, Hahn G, Hedrich CM. Chronic nonbacterial osteomyelitis (CNO) and chronic recurrent multifocal osteomyelitis (CRMO). J Transl Autoimmun. (2021) 4:100095. doi: 10.1016/j.jtauto.2021.100095
PubMed Abstract | Crossref Full Text | Google Scholar
7. Girschick H, Finetti M, Orlando F, Schalm S, Insalaco A, Ganser G, et al. The multifaceted presentation of chronic recurrent multifocal osteomyelitis: a series of 486 cases from the Eurofever international registry. Rheumatology. (2018) 57:1203–11. doi: 10.1093/rheumatology/key058
PubMed Abstract | Crossref Full Text | Google Scholar
8. Roderick MR, Shah R, Rogers V, Finn A, Ramanan AV. Chronic recurrent multifocal osteomyelitis (CRMO) - advancing the diagnosis. Pediatr Rheumatol. (2016) 14:1–5. doi: 10.1186/s12969-016-0109-1
PubMed Abstract | Crossref Full Text | Google Scholar
9. Schaal MC, Gendler L, Ammann B, Eberhardt N, Janda A, Morbach H, et al. Imaging in non-bacterial osteomyelitis in children and adolescents: diagnosis, differential diagnosis and follow-up—an educational review based on a literature survey and own clinical experiences. Insights Into Imaging. (2021) 12:1–14. doi: 10.1186/s13244-021-01059-6
PubMed Abstract | Crossref Full Text | Google Scholar
11. Giwa AM, Ahmed R, Omidian Z, Majety N, Karakus KE, Omer SM, et al. Current understandings of the pathogenesis of type 1 diabetes: Genetics to environment. World J Diabetes. (2020) 11:13–25. doi: 10.4239/wjd.v11.i1.13
PubMed Abstract | Crossref Full Text | Google Scholar
12. Fröhlich-Reiterer E, Elbarbary NS, Simmons K, Buckingham B, Humayun KN, Johannsen J, et al. ISPAD Clinical Practice Consensus Guidelines 2022: Other complications and associated conditions in children and adolescents with type 1 diabetes. PediatrDiabetes. (2022) 23:1451–67. doi: 10.1111/pedi.13445
PubMed Abstract | Crossref Full Text | Google Scholar
13. Ferguson PJ, Laxer RM. New discoveries in CRMO: IL-1β, the neutrophil, and the microbiome implicated in disease pathogenesis in Pstpip2-deficient mice. Semin Immunopathol. (2015) 37:407–12. doi: 10.1007/s00281-015-0488-2
PubMed Abstract | Crossref Full Text | Google Scholar
14. Parzanese I, Qehajaj D, Patrinicola F, Aralica M, Chiriva-Internati M, Stifter S, et al. Celiac disease: From pathophysiology to treatment. World J Gastrointest Pathophysiol. (2017) 8:27. doi: 10.4291/wjgp.v8.i2.27
PubMed Abstract | Crossref Full Text | Google Scholar
15. Gearty SV, Dündar F, Zumbo P, Espinosa-Carrasco G, Shakiba M, Sanchez-Rivera FJ, et al. An autoimmune stem-like CD8 T cell population drives type 1 diabetes. Nature. (2022) 602:156–61. doi: 10.1038/s41586-021-04248-x
PubMed Abstract | Crossref Full Text | Google Scholar
16. Zhao Y, Wu EY, Oliver MS, Cooper AM, Basiaga ML, Vora SS, et al. Consensus treatment plans for chronic nonbacterial osteomyelitis refractory to nonsteroidal anti-inflammatory drugs and/or with active spinal lesions. Arthritis Care Res (Hoboken). (2018) 70:1228. doi: 10.1002/acr.2018.70.issue-8
Crossref Full Text | Google Scholar
17. Cengiz E, Danne T, Ahmad T, Ayyavoo A, Beran D, Ehtisham S, et al. ISPAD Clinical Practice Consensus Guidelines 2022: Insulin treatment in children and adolescents with diabetes. Pediatr Diabetes. (2022) 23:1277–96. doi: 10.1111/pedi.13442
留言 (0)