Placental abruption, also known as abruption placentae, occurs when bleeding at the decidual-placental interface leads to partial or complete detachment of the placenta before the fetus is delivered. This condition is typically diagnosed in pregnancies beyond 28 weeks of gestation. Major clinical symptoms include vaginal bleeding and abdominal pain, which are often accompanied by hypertonic uterine contractions, uterine tenderness, and a non-reassuring fetal heart rate pattern (1). Abruption occurs in approximately 0.6 to 1.2% of all pregnancies, with nearly half of cases happening at term gestations (1).
Abruption is a serious obstetric complication that poses significant risks for maternal and perinatal morbidity as well as perinatal mortality. The perinatal mortality rate is about 20 times higher in pregnancies with abruption compared to those without (12% versus 0.6%, respectively) (2). In Japan, placental abruption accounted for 9% of all stillbirths (3). It also led to preterm birth, perinatal asphyxia, intrauterine growth restriction, and low birth weight (1). The woman is at risk of severe hemorrhage, necessitating blood transfusions and potentially leading to complications such as hysterectomy, bleeding disorders like disseminated intravascular coagulopathy, and renal failure. These complications can lead to Sheehan syndrome or postpartum pituitary gland necrosis (4). In numerous countries, the occurrence of placental abruption has been on the rise despite advancements in obstetrical care and monitoring methods. This trend highlights a multifactorial etiology that remains poorly understood (5, 6). However, placental abruption is believed to result from a disruption of the maternal-fetal interface, specifically the decidual-placental junction. This disruption can be caused by various factors, including uteroplacental under perfusion, placental inflammation, or mechanical forces, leading to bleeding and subsequent separation of the placenta from the uterine wall (1). Identifying and managing the risk factors for placental abruption is crucial in mitigating these adverse outcomes.
The precise cause of placental abruption is unknown; however, various factors are associated with its occurrence. Placental abruption occurs in 40% of smokers, 14.1% of women with vasculoplacental disorders, and 42.2% of women with pre-eclampsia (7). Maternal age over 35 years, short umbilical cord, sudden decompression of the uterus, previous abruption, and trauma are also the strongest risk factors for abruption (4). Additionally, significant risk factors for placental abruption include frequent motorbike transportation, a history of infertility, and marginal cord insertion (8).
In Ethiopia, 30% of women with antepartum hemorrhage experienced adverse perinatal outcomes. Factors significantly associated with adverse maternal and perinatal outcomes include hemodynamic status, parity, antenatal care, duration of bleeding, gestational age, and the amount of vaginal bleeding (9). Uterine malformations, preterm premature rupture of membranes, and oligohydramnios significantly increased the risk of adverse perinatal outcomes of placental abruption (10). Additionally, maternal age of 20 years or younger, preeclampsia/eclampsia, and chronic hypertensive disorders during pregnancy were associated with adverse perinatal outcomes (11).
In Ethiopia, perinatal mortality rates remain high, and complications related to placental abruption contribute substantially to these adverse outcomes. Despite its severity, there is a lack of comprehensive data on the prevalence, risk factors, and outcomes associated with placental abruption in the Ethiopian healthcare settings. Thus, the study aims to examine the adverse perinatal outcomes associated with placental abruption and identify the factors contributing to these outcomes among pregnant women. Understanding these factors can inform clinical practices and policy interventions to improve maternal and neonatal health in Ethiopia.
Method Study design, area, and periodFrom January 1, 2021, to January 1, 2023, an institution-based retrospective cross-sectional study was conducted at the University of Gondar Comprehensive Specialized Hospital (UOGCSH). UOGCSH is situated in Gondar, 741 kilometers away from Ethiopia’s capital, Addis Ababa. The hospital is a tertiary-level teaching and referral hospital in the Amhara region, which is one of the biggest and oldest medical schools in Ethiopia established as the Public Health College in 1954. UOGCSH provides various health services, including surgery, internal medicine, pathology, dermatology, obstetrics and gynecology, pediatric care, laboratory services, pharmacy, and physiotherapy. The labor and maternity ward is staffed by 8 gynecologic oncology subspecialists, 5 maternal-fetal medicine subspecialists, 4 urogynecology subspecialists, 7 subspecialist fellows, 5 obstetrics and gynecology specialists, 199 midwives, and 71 residents (20 first-year, 19 s-year, 23 third-year, and 13 fourth-year residents).
Population and eligibility criteriaAll randomly selected pregnant women admitted and managed for the diagnosis of placental abruption were the study population. Pregnant women with multiple gestation and lethal fetal congenital anomaly were excluded.
Sample size and sampling techniquesThe required sample size was determined using single population proportion formula for the first and second objective. Taking an assumption of power 80%, margin of error 5, 95% two-sided confidence level, and stillbirth in abruption 66% (12). After adding 10% missing records the final sample size was 380.
Computer-generated simple random sampling was used based on a sampling frame prepared by arranging medical record numbers in order from the maternity triage registration book. A total of 517 pregnant women were admitted with the diagnosis of placental abruption at UOGCSH from January 1, 2021, to January 1, 2023. Using simple random sampling, 380 pregnant women were selected, but 13 charts were missing, incomplete, or damaged.
CovariatesAdverse perinatal outcome was the dependent variable. Socio demographic status included age, residency, marital status, and distance traveled to arrive the study hospital. Details of past and present obstetric factors included prior abortion, prior stillbirth, prior cesarean section, parity, gravidity, interpregnancy interval, ANC follow up, gestation age at diagnosis, polyhydramnios, oligohydramnios, preeclampsia, premature rupture of membrane, and gestational diabetes mellitus. Maternal characteristics included duration of bleeding, degree of placental abruption, blood pressure, anemia, onset of labor, and mode of delivery.
Definition of terms Placental abruptionPlacental abruption was identified from participant medical records if there was a physician documented diagnosis of antepartum or intrapartum placental abruption, if it was noted as the indication for cesarean delivery, or if there was a discharge code indicating abruption.
Mild placental abruptionClinically asymptomatic before delivery and typically detected by the existence of a retro-placental clotting and characterized by no vaginal bleeding to mild vaginal bleeding, slightly tender uterus, normal maternal BP and heart rate, no coagulopathy, and reassuring fetal heart rate (9).
Severe placental abruptionThe presence of one or more of the following maternal or fetal complication in a patient diagnosed with placental abruption. Maternal: disseminated intravascular coagulation, hypovolemic shock, need for blood transfusion, hysterectomy, renal failure, and death. Fetal: non-reassuring fetal heart rate, intrauterine growth restriction, need for preterm birth, and death (13, 14).
Adverse perinatal outcomeThe presence of at least one or more of the following: preterm birth (delivery before 37 completed weeks, but after 28 or more weeks of gestation), stillbirth (death of a fetus after 28 weeks of gestation, but before or during birth), low APGAR score (5th minute APGAR is less than 7), low birth weight (<2,500 gm), IUGR (a birth weight of below 10th percentile for gestational age and fetal sex), NICU admission, and need for resuscitation (15, 16).
Interpregnancy interval (IPI)IPI is the period between the end of one pregnancy and the beginning of the next pregnancy. The IPI was categorized as short if <24 months, Optimal if 24 to <60 months, and long if more than or equal to 60 months (17).
AnemiaAnemia is a decrease in the concentration of erythrocytes or hemoglobin less than 11.0 g/dL / Hct 33%. Categorized into; Mild (10 to 10.9 g/dL), Moderate (7 to 9.9 g/dL), and Severe (7 g/dL) (18).
Data collection tool and quality assuranceData was collected using a structured checklist prepared with the KOBO collect tool. The checklist included demographic information (age, residence), obstetric history (parity, previous abruption), clinical presentation (gestational age at diagnosis, degree of abruption), and perinatal outcomes (birth weight, APGAR scores, NICU admission). A data extraction tool was developed after reviewing previous literature on the subject and validated by a reproductive health expert. A pre-test was conducted with 5% of the total sample size at Debre Berhan Comprehensive Specialized hospital and required modifications were considered. Three research midwife data collectors and two obstetrics and gynecology resident supervisors were included in the data collection process. Data collectors received 1 day of training on the study’s objectives, using the KOBO collect digital data extraction tool, accessing records, data handling, and maintaining participant confidentiality. The principal investigator checked the extracted data for completeness on a daily basis.
Data processing and analysisThe data collected using KOBO collect was exported to SPSS version 25.0. Descriptive statistics were presented through frequency tables, graphs, and text. Binary logistic regression was employed to examine the association between dependent and independent variables. Multivariable logistic regression analysis was performed to identify independent predictors of adverse perinatal outcomes. Variables with p < 0.25 in the bivariate analysis were included in the multivariable model. The Hosmer-Lemeshow test was used to assess the goodness-of-fit of the model. Adjusted odds ratios (AOR) with 95% confidence intervals (CI) were calculated, and variables with p < 0.05 in the final model were considered statistically significant.
Results Baseline characteristicsA total of 367 pregnant women diagnosed with placental abruption were included, representing 96.6% of the final sample size. The participants’ ages ranged from 17 to 40 years, with a mean age of 27.91 ± 5.5 years. Most participants (73.3%) were aged between 20 and 34, and 60% were urban residents (Table 1).
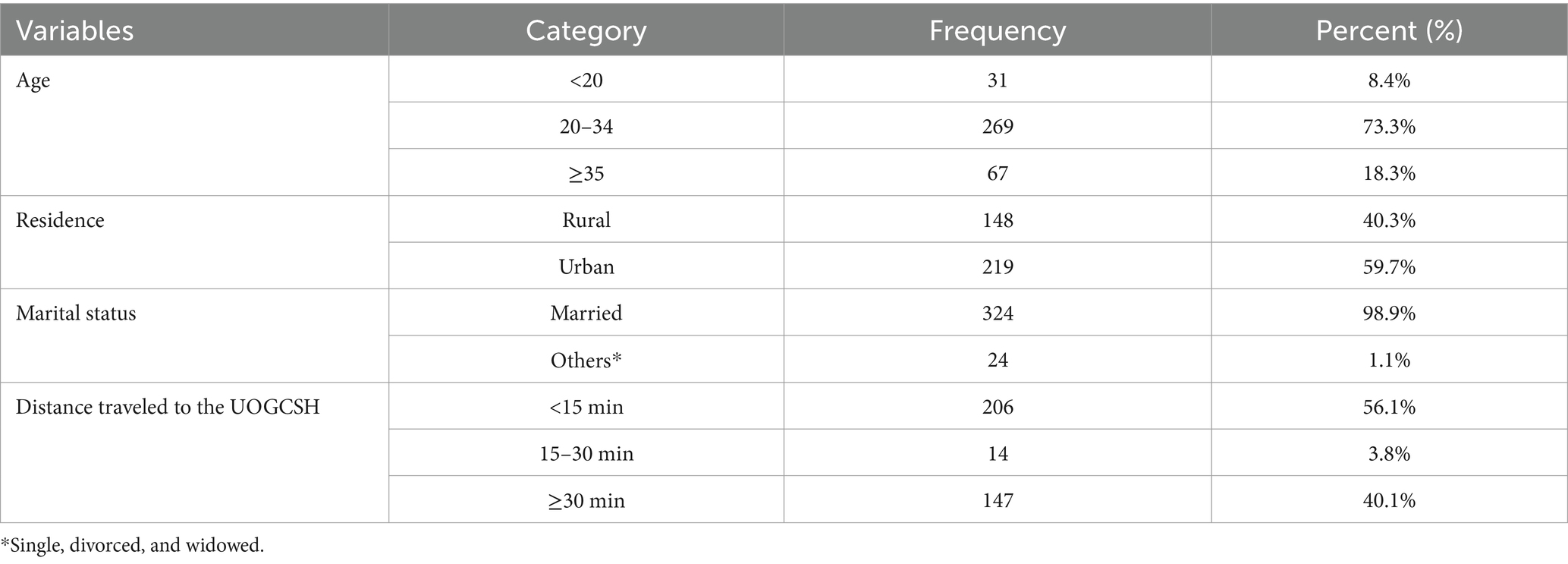
Table 1. Baseline characteristics of pregnant women admitted and managed for the diagnosis of placental abruption at University of Gondar Comprehensive Specialized Hospital (UOGCSH), Ethiopia, 2023.
Reproductive and obstetric characteristicsMore than half (72%) of the women were multigravida, and 56.7% had a short interpregnancy interval. Additionally, 21.5% had previously experienced an abortion, and 4.4% had a history of stillbirth. The majority (93.7%) of participants had antenatal care (ANC) contact for the index pregnancy. Preeclampsia (6.8%), oligohydramnios (5.7%), and premature rupture of membrane (4.9%) were the commonest obstetrics complication identified during the index pregnancy (Table 2).
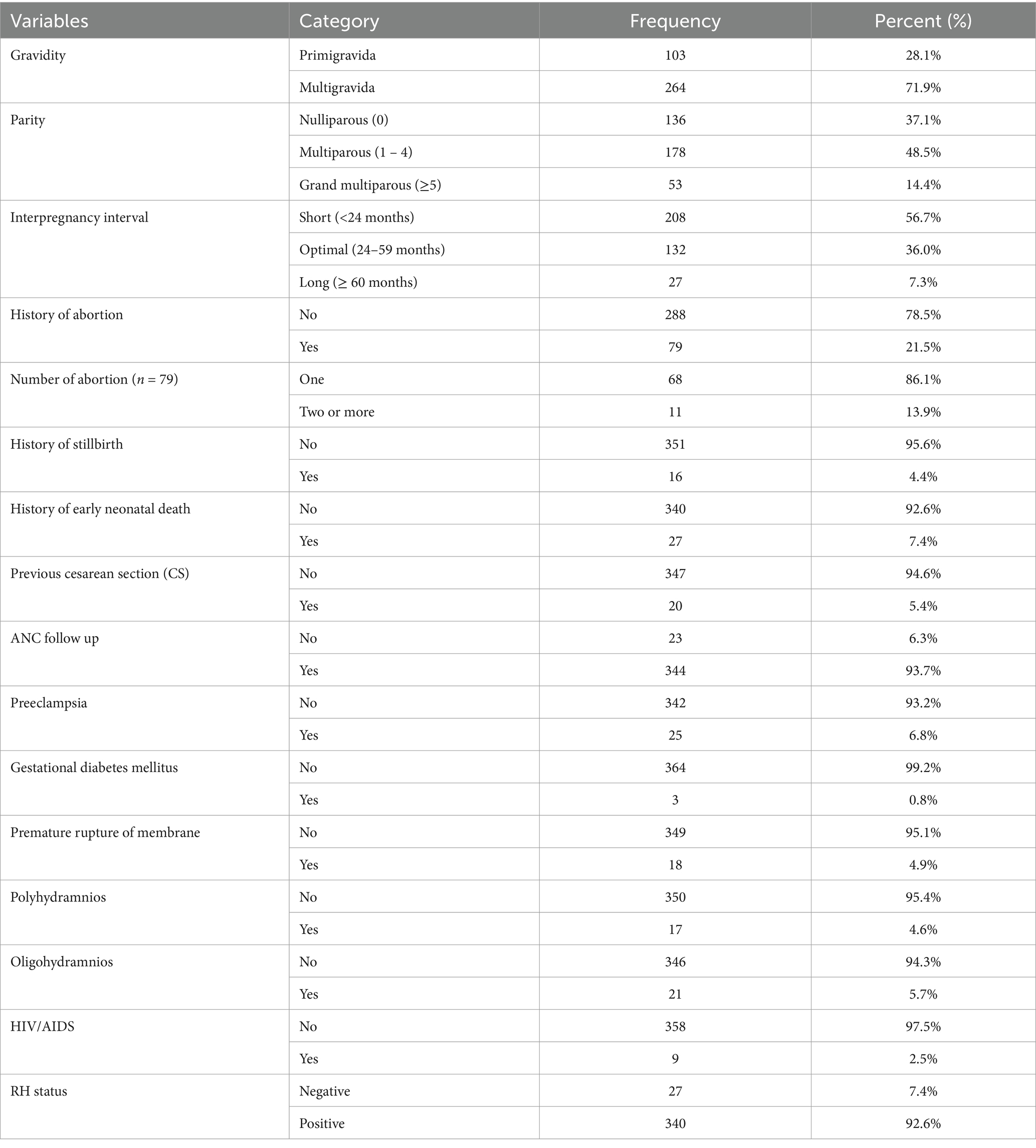
Table 2. Reproductive and obstetric characteristics of pregnant women admitted and managed for the diagnosis of placental abruption at UOGCSH, Ethiopia, 2023.
Maternal condition at admission and deliveryUpon admission, 61% of the women reported bleeding for a duration of 12 h or less, and 58.6% presented at term. More than one-third (70.6%) did not have anemia at the time of admission. Additionally, among the mothers with placental abruption, 25.6% delivered preterm, and 34.1% underwent an emergency cesarean section (Table 3).
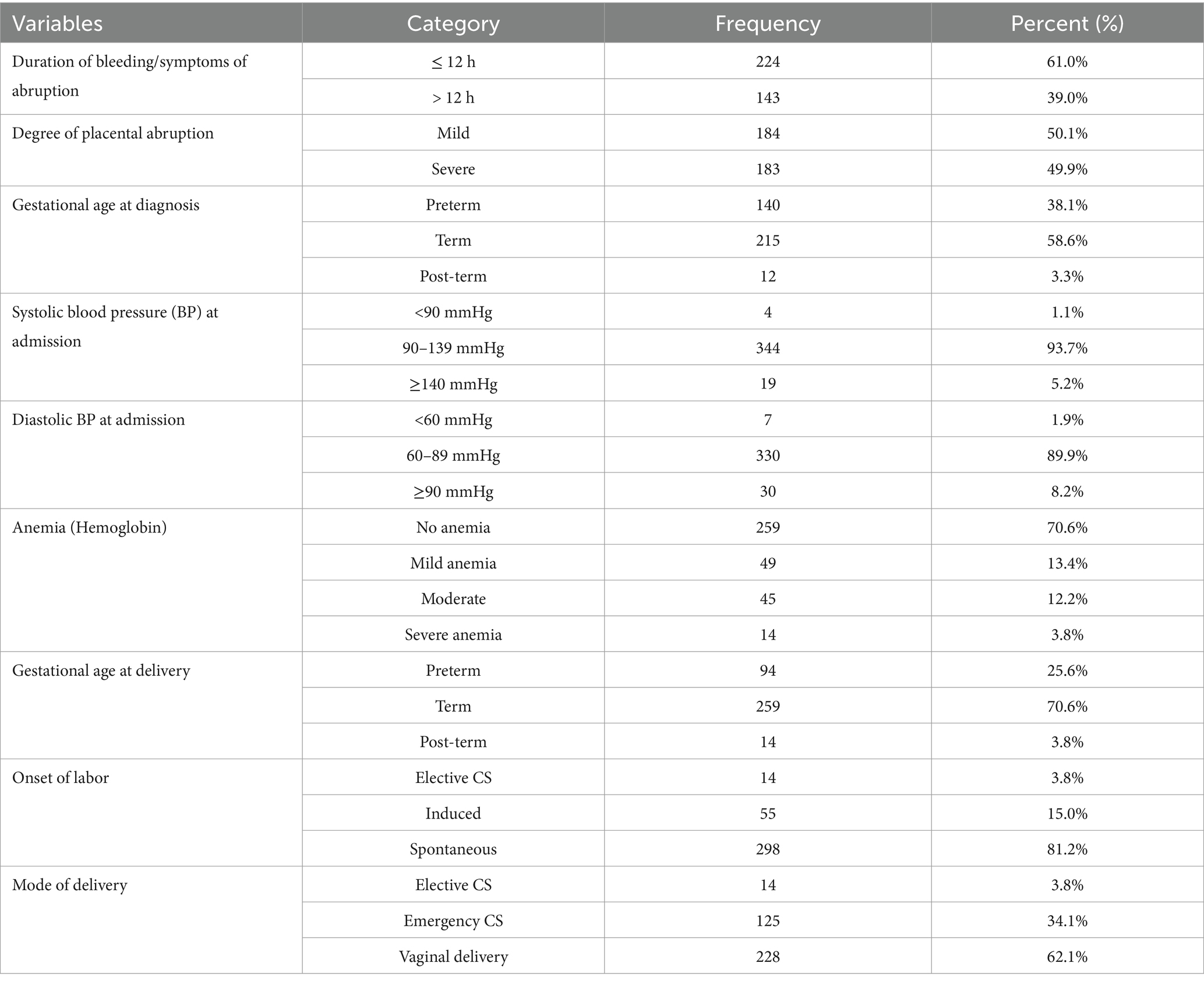
Table 3. Maternal conditions of pregnant women admitted and managed for the diagnosis of placental abruption at UOGCSH, Ethiopia, 2023.
Prevalence of adverse perinatal outcomeIn this study, 39.2% (N = 144) (95% CI: 34.3–44.1) of participants had one or more adverse perinatal outcome of placental abruption. As illustrated in Figure 1, the adverse perinatal outcomes varied in frequency. Prematurity and low birth weight were equally prevalent, each affecting 25.6% of cases. NICU admission was necessary for 13.9% of newborns, while 5.4% exhibited a low APGAR score at 5 min. Stillbirth occurred in 4.9% of cases, and 3.5% required neonatal resuscitation. These findings underscore the significant impact of placental abruption on neonatal health (Figure 1).
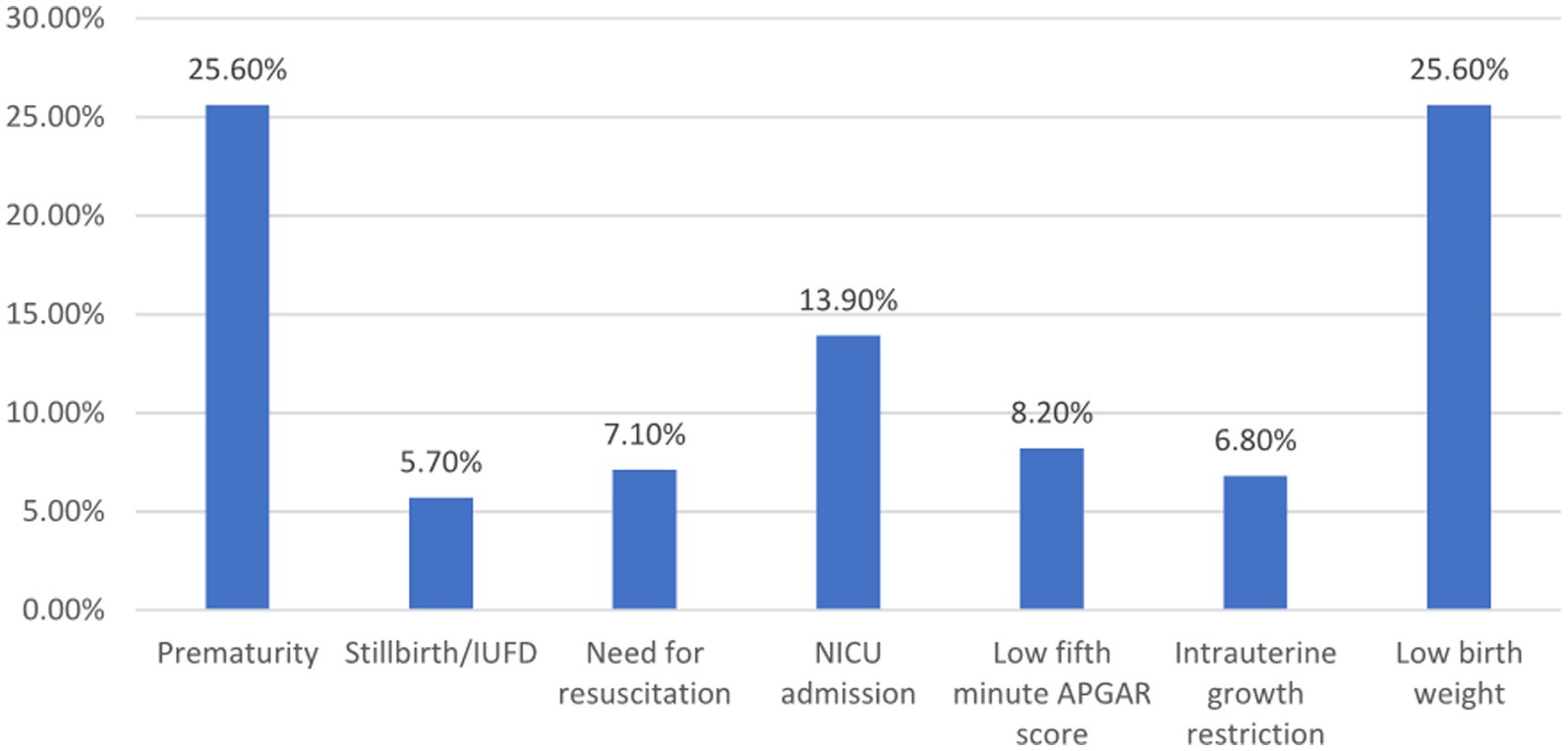
Figure 1. Adverse perinatal outcome among women admitted and managed for diagnosis of placental abruption at UOGCSII, Ethiopia, 2023. APGAR, Appearance, Pulse, Grimace, Activity, and Respiration; NICU, Neonatal intensive care unit; IUFD, Intrauterine fetal death.
Factors of adverse perinatal outcomeBivariable and multivariable logistic regression analysis were conducted to identify factors associated with adverse perinatal outcomes. In the bivariable analysis, variables such as age, residence, parity, ANC follow-up, distance traveled, premature rupture of membranes, duration of bleeding, degree of placental abruption, systolic blood pressure, anemia, and gestational age at diagnosis had p-values <0.25 and were included in the multivariable model. The multivariable logistic regression analysis revealed that the degree of placental abruption and gestational age at diagnosis were significantly associated with adverse perinatal outcomes.
Women who developed severe placental abruption were eight times more likely to experience adverse perinatal outcomes compared to those with mild placental abruption [AOR (CI) = 8.82 (4.48–17.31)]. Additionally, women with placental abruption during preterm pregnancy were 18 times more likely to face adverse perinatal outcomes compared to those whose placental abruption occurred at term [AOR (CI) = 18.71 (9.59–36.42)] (Table 4).
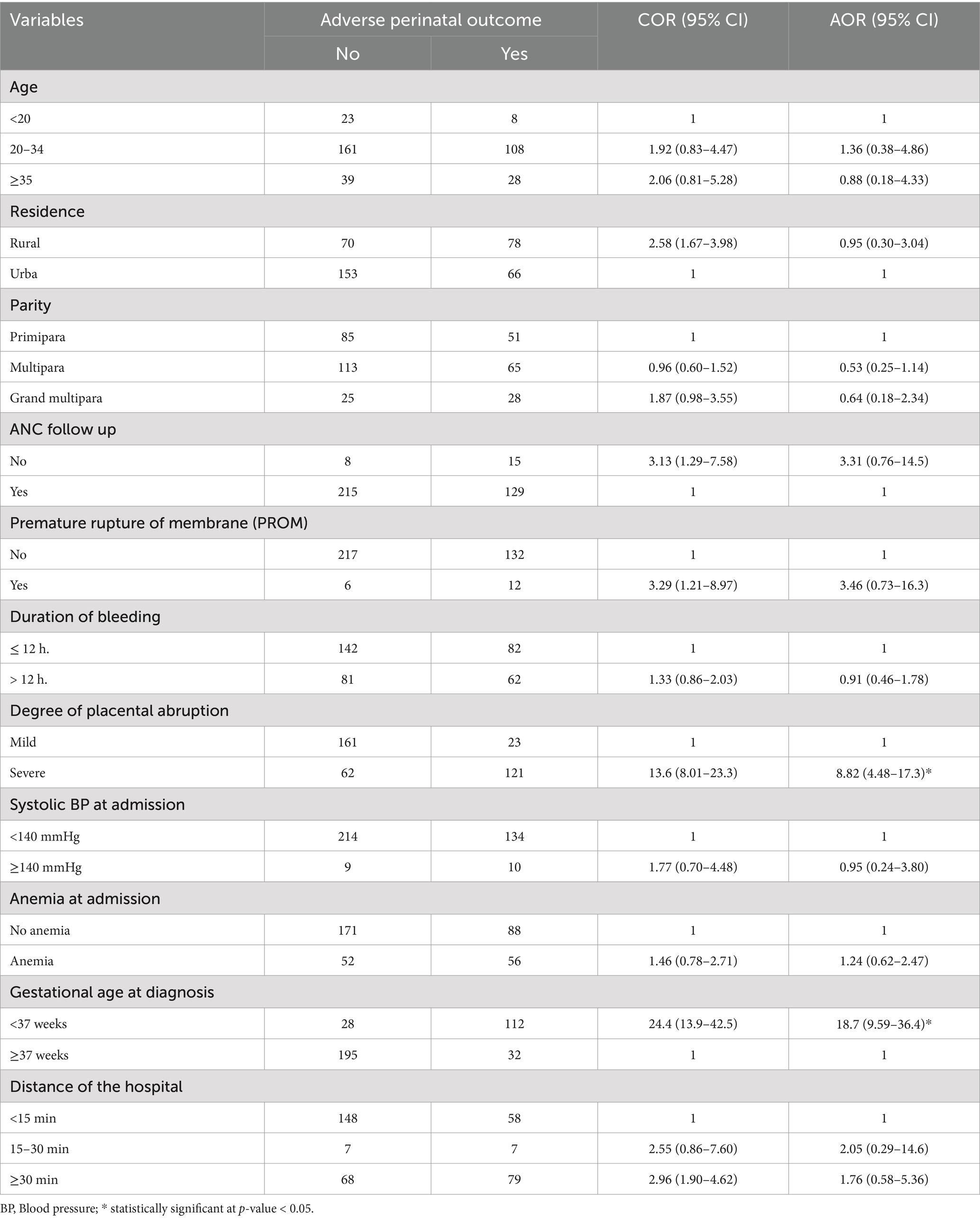
Table 4. Factors associated with adverse perinatal outcome among women admitted and managed for diagnosis of placental abruption at UOGCSH, Ethiopia, 2023.
DiscussionIn this study, 39.2, 95% CI: 34.3–44.1 of pregnant women had developed at least one adverse perinatal outcome. The degree of placental abruption and gestational age at diagnosis were significantly associated with adverse perinatal outcomes.
The prevalence of adverse perinatal outcome among women admitted and managed for placental abruption was 39.2%. From these, 25.6% are premature, 25.6% are low birth weight (LBW), and 14% are referred to NICU. Similarly, in the United States, abruption was associated with an elevated risk of newborn resuscitation, asphyxia, respiratory distress syndrome, NICU admission, and stillbirth (19). It is also consistent with the study conducted in Nepal, where placental abruption is associated with preterm labor, low birth weight, and NICU admission (20). Chronic conditions such as thrombosis, inflammation, infection, and uteroplacental and decidual vasculopathy predispose to placental abruption. Placental hypoperfusion, impaired spiral artery remodeling, placental infarction, and shallow trophoblast invasion are the outcomes of these processes. These long-term changes heighten the risk of abruption and other placental-related complications, such as low birth weight (LBW), preterm birth, and fetal growth restriction (1).
However, this study has shown a higher prevalence of adverse perinatal outcomes than the study conducted at Southwest Ethiopia, where 30% of women with antepartum hemorrhage experienced adverse perinatal outcomes (9). This may be due to the ongoing conflict in the study area throughout the study period. This conflict could have caused transportation problems, preventing women from accessing maternity care when experiencing signs of vaginal bleeding. Consequently, women might present with major placental abruption and severe complications. Similarly, a systematic review and meta-analysis of studies in 12 conflict-zone countries reported an increased risk of small-for-gestational-age births, low 5th-minute APGAR scores, stillbirth, and perinatal mortality (21).
Additionally, our findings indicated a higher prevalence of placental abruption compared to a study conducted in Addis Ababa, where the prevalence was reported at 2.3% (22). This discrepancy may be attributed to the lower institutional delivery rate in the Amhara region, which stands at 54.2%, compared to 94.8% in Addis Ababa, according to the 2019 EDHS report. Similarly, 81.8% of pregnant women in Addis Ababa had four or more ANC visits, whereas only 50.8% in the Amhara region attended that many visits (23).
Preterm placental abruption at the time of admission increased the likelihood of adverse outcomes by 18 times compared to term abruption. Additionally, in this study, 38.1% of pregnant women presented before 37 weeks of gestation, and 25.6% delivered preterm. Patients with preterm abruption are typically managed expectantly if the fetomaternal condition is stable, with delivery planned for 37 to 38 weeks (13). However, these patients remain at risk of maternal or fetal complications due to progressive or recurrent placental separation, leading to relatively high neonatal morbidity and mortality. Partial abruption can suddenly and unpredictably progress to total abruption. Preventing preterm birth in pregnancies complicated by abruption is challenging. Consequently, urgent preterm operative delivery may be required without adequate preparation for neonatal care. Therefore, to reduce adverse perinatal outcome, it is recommended that most patients with acute abruption be delivered at 34 to 36 weeks of gestation, with continuous monitoring, optimal care, and thorough preparation.
Compared to women with mild placental abruption, those with severe abruption had an eight-fold increased risk of adverse perinatal outcomes. Similarly, a retrospective cohort research conducted in the United States found that women who experienced severe placental abruption had a 4.29-fold increased risk of serious maternal and neonatal complications, while women who experienced mild abruption had a 1.52-fold increased risk (14). Severe placental abruption might be characterized by heavy vaginal bleeding, total placental separation, tetanic uterus/ board-like consistency, hypofibrinogenemia and coagulopathy (4). This may aggravate the adverse obstetric and perinatal outcomes.
Our findings emphasize the critical need for vigilant monitoring of pregnancies complicated by placental abruption, particularly those presenting before term. The high rates of prematurity and low birth weight suggest that early detection and management of abruption may be crucial in improving perinatal outcomes. Furthermore, the increased risk associated with severe abruption highlights the importance of prompt recognition and intervention in these cases. These results may inform clinical protocols for the management of placental abruption, potentially including more frequent fetal monitoring and earlier consideration of delivery in cases of severe abruption.
LimitationThis study has several limitations that should be considered when interpreting the results. First, as a retrospective study, it is subject to inherent biases, including potential inaccuracies in medical records and missing data. Second, the single-center design may limit the generalizability of our findings to other settings, particularly those with different resources or patient populations. Third, we were unable to assess long-term neonatal outcomes beyond the immediate perinatal period, which may underestimate the full impact of placental abruption. Finally, while we identified significant predictors of adverse outcomes, the observational nature of our study precludes causal inferences. Furthermore, the limited number of similar studies makes it challenging to engage in a comprehensive discussion.
Conclusion and implicationThe prevalence of adverse perinatal outcome of pregnancy complicated with placental abruption is this study was high compared to the study done in similar setting. The degree of placental abruption and gestational age at diagnosis were significant associates of adverse perinatal outcomes. The study highlights the critical need for patient-centered counseling on antenatal bleeding to encourage early healthcare-seeking behavior, close follow up for those undergoing expectant management and the early detection and management of placental abruption to improve perinatal outcomes.
Our research indicates that placental abruption is associated with a higher risk of low birth weight, preterm delivery, neonatal resuscitation, and NICU admission. This suggests a possible link between abruption and physiological underdevelopment. Additionally, patients under expectant management may experience hypoxia and a gradual worsening of the condition. It is important to have the recommended antenatal care contact for improving the overall maternal and fetal outcomes.
Data availability statementThe raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statementEthical clearance was obtained from the Ethical Review Committee of the University of Gondar, College of Medicine and Health Sciences with reference number SOM 702/2023. Patient confidentiality and privacy were maintained by excluding names from the data extraction forms. Written informed consent from the [patients/participants OR patients/participants legal guardian/next of kin] was not required to participate in this study in accordance with the national legislation and the institutional requirements.
Author contributionsMT: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. GG: Conceptualization, Data curation, Investigation, Methodology, Project administration, Resources, Supervision, Visualization, Writing – original draft. TK: Data curation, Methodology, Project administration, Resources, Supervision, Validation, Visualization, Writing – review & editing. TY: Conceptualization, Data curation, Methodology, Project administration, Resources, Supervision, Validation, Visualization, Writing – review & editing. ST: Conceptualization, Data curation, Methodology, Project administration, Resources, Validation, Visualization, Writing – review & editing. WD: Resources, Software, Validation, Visualization, Writing – review & editing, Conceptualization, Data curation, Investigation, Methodology, Project administration. GS: Conceptualization, Data curation, Investigation, Methodology, Project administration, Resources, Validation, Visualization, Writing – review & editing.
FundingThe author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
AcknowledgmentsThe authors would like to thank the data collectors, supervisors, and the study participants for providing genuine information. We also extend our thanks to University of Gondar, College of Medicine and Health Sciences for approving the study.
Conflict of interestThe authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s noteAll claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
AbbreviationsAPGAR, Appearance, Pulse, Grimace, Activity, and Respiration; BP, Blood pressure; CS, Cesarean section; LBW, Low birth weight; NICU, Neonatal Intensive Care Unit; UOGCSH, University of Gondar Comprehensive Specialized Hospital.
References1. Brandt, JS, and Ananth, CV. Placental abruption at near-term and term gestations: pathophysiology, epidemiology, diagnosis, and management. Am J Obstet Gynecol. (2023) 228:S1313–29. doi: 10.1016/j.ajog.2022.06.059
PubMed Abstract | Crossref Full Text | Google Scholar
2. Tikkanen, M, Luukkaala, T, Gissler, M, Ritvanen, A, Ylikorkala, O, Paavonen, J, et al. Decreasing perinatal mortality in placental abruption. Acta Obstet Gynecol Scand. (2013) 92:298–305. doi: 10.1111/aogs.12030
PubMed Abstract | Crossref Full Text | Google Scholar
3. Kasahara, M, Koshida, S, Tokoro, S, Katsura, D, Tsuji, S, Murakami, T, et al. Potential prevention of stillbirth caused by placental abruption: a regional population-based study in Japan. J Matern Fetal Neonatal Med. (2024) 37:2321485. doi: 10.1080/14767058.2024.2321485
PubMed Abstract | Crossref Full Text | Google Scholar
4. Schmidt, P, Skelly, CL, and Raines, DA. Placental abruption. Treasure Island, FL: StatPearls Publishing (2022).
5. Miller, C, Grynspan, D, Gaudet, L, Ferretti, E, Lawrence, S, Moretti, F, et al. Maternal and neonatal characteristics of a Canadian urban cohort receiving treatment for opioid use disorder during pregnancy. J Dev Orig Health Dis. (2019) 10:132–7. doi: 10.1017/S2040174418000478
PubMed Abstract | Crossref Full Text | Google Scholar
6. Workalemahu, T, Enquobahrie, DA, Gelaye, B, Sanchez, SE, Garcia, PJ, Tekola-Ayele, F, et al. Genetic variations and risk of placental abruption: a genome-wide association study and meta-analysis of genome-wide association studies. Placenta. (2018) 66:8–16. doi: 10.1016/j.placenta.2018.04.008
PubMed Abstract | Crossref Full Text | Google Scholar
7. de Moreuil, C, Hannigsberg, J, Chauvet, J, Remoue, A, Tremouilhac, C, Merviel, P, et al. Factors associated with poor fetal outcome in placental abruption. Pregnancy Hypertens. (2021) 23:59–65. doi: 10.1016/j.preghy.2020.11.004
PubMed Abstract | Crossref Full Text | Google Scholar
9. Gelan, M, Bekela, T, Angasu, K, and Ebisa, M. Adverse perinatal and maternal outcomes and associated factors among women with antepartum hemorrhage in Jimma University medical center, Southwest Ethiopia, 2020. Obstet Gynecol Int. (2022) 2022:1–7. doi: 10.1155/2022/4594136
PubMed Abstract | Crossref Full Text | Google Scholar
10. Bączkowska, M, Kosińska-Kaczyńska, K, Zgliczyńska, M, Brawura-Biskupski-Samaha, R, Rebizant, B, and Ciebiera, M. Epidemiology, risk factors, and perinatal outcomes of placental abruption—detailed annual data and clinical perspectives from polish tertiary center. Int J Environ Res Public Health. (2022) 19:5148. doi: 10.3390/ijerph19095148
PubMed Abstract | Crossref Full Text | Google Scholar
11. Anyanwu, M, Amuzu, C, and Bittaye, M. A longitudinal study of incidence and pregnancy outcome of abruption placenta at the–tertiary hospital in Gambia. Int J Pregn Child Birth. (2019) 5:57–61. doi: 10.15406/ipcb.2019.05.00147
Crossref Full Text | Google Scholar
12. Nethaji, DT, Hurakadli, KM, and Duggavathi, SP. Maternal and perinatal outcome in abruption placenta in tertiary care center: a record based case series study. Int J Reprod Contracept Obstet Gynecol. (2023) 12:1270–4. doi: 10.18203/2320-1770.ijrcog20231208
Crossref Full Text | Google Scholar
14. Ananth, CV, Lavery, JA, Vintzileos, AM, Skupski, DW, Varner, M, Saade, G, et al. Severe placental abruption: clinical definition and associations with maternal complications. Am J Obstet Gynecol. (2016) 214:272.e1–9. doi: 10.1016/j.ajog.2015.09.069
PubMed Abstract | Crossref Full Text | Google Scholar
15. Jaleta, DD, and Abdisa, DK. Predictors of adverse perinatal outcome among women who gave birth at medical Center of Southwest Ethiopia: a retrospective cohort study. BMJ Open. (2022) 12:e053881. doi: 10.1136/bmjopen-2021-053881
PubMed Abstract | Crossref Full Text | Google Scholar
16. Tadese, M, Damesa, WA, Solomon, GS, Fitie, GW, Mitiku, YM, Tessema, SD, et al. Prevalence and determinants of adverse perinatal outcomes of preeclampsia with severe features at two selected public hospitals in Addis Ababa, Ethiopia. Front Pediatr. (2024) 12:12. doi: 10.3389/fped.2024.1345055
Crossref Full Text | Google Scholar
17. Agrawal, S, Chaudhary, M, Das, V, Agarwal, A, Pandey, A, Kumar, N, et al. Association of long and short interpregnancy intervals with maternal outcomes. J Fam Med Prim Care. (2022) 11:2917–22. doi: 10.4103/jfmpc.jfmpc_2231_21
PubMed Abstract | Crossref Full Text | Google Scholar
20. Maharjan, S, Thapa, M, Chaudhary, B, and Shakya, S. Abruptio placenta among pregnant women admitted to the Department of Obstetrics and Gynaecology in a tertiary care Centre: a descriptive cross-sectional study. JNMA J Nepal Med Assoc. (2022) 60:918–21. doi: 10.31729/jnma.7796
PubMed Abstract | Crossref Full Text | Google Scholar
21. Behboudi-Gandevani, S, Bidhendi-Yarandi, R, Panahi, MH, Mardani, A, Prinds, C, and Vaismoradi, M. Perinatal and neonatal outcomes in immigrants from conflict-zone countries: a systematic review and meta-analysis of observational studies. Front Public Health. (2022) 10:766943. doi: 10.3389/fpubh.2022.766943
PubMed Abstract | Crossref Full Text | Google Scholar
22. Assefa, A, Fantahun, Y, and Mesfin, E. Maternal and perinatal outcome of antepartum hemorrhage at three teaching hospitals in Addis Ababa, Ethiopia. Ethiopia J Reprod Health. (2020) 12:12–9. doi: 10.69614/ejrh.v12i3.395
Crossref Full Text | Google Scholar
23. ICF EPHI. Ethiopia mini demographic and health survey 2019: key indicators. Maryland, USA: EPHI ICF (2019).
留言 (0)