Biliary atresia represents a rare infancy ailment, with a global pediatric incidence estimated at approximately 1 per 10,000 births (1, 2). Treatment options for pediatric biliary atresia typically encompass the Kasai procedure, liver transplantation, and adjunctive pharmacotherapies. Among these treatments, the most effective therapy is pediatric liver transplantation. Recent advancements in surgical techniques have significantly improved the overall survival rates post-surgery. The 1-year survival rate among patients of living-donor liver transplantation could exceed 95%, marking a considerable improvement over historical benchmarks. Nevertheless, despite these advancements, about 20% of patients still experience unfavorable prognoses and contend with serious postoperative complications (3).
Neurological impairment emerged as one of the foremost postoperative complications. Previous study found that about 46% of patients may have experienced neurological damage, potentially progressing to cognitive dysfunction and long-term neurodevelopmental delays following pediatric liver transplantation (4). Cerebral oxygen metabolism imbalance has been identified as a contributing factor to such neurological complications (5). Monitoring SctO2 utilizing near-infrared spectroscopy (NIRS) facilitated real-time assessment of oxygen saturation levels within localized brain regions, offering insights into cerebral oxygen metabolism dynamics (6). Previous study (7) had linked decreased SctO2 levels with neuro-dysplasia in children undergoing cardiac surgery. However, the precise relationship between intraoperative SctO2 levels and long-term neurodevelopmental outcomes subsequent to pediatric liver transplantation remained unclear.
In our study, we hypothesize that diminished intraoperative average SctO2 levels might have associated with postoperative neurodevelopmental delays among patients undergoing pediatric living-donor liver transplantation.
Materials and methods Study designThis prospective observational study adhered to the principles outlined in the Declaration of Helsinki and obtained approval from the ethics committee of Renji Hospital (SK2020-059). It was registered at Clinical Trail.gov (NCT04518332). All patients enrolled in this study signed the informed consent form prior to their participation.
Study populationEligible participants were patients aged 3 months to 3 years diagnosed with congenital biliary atresia and scheduled for living-donor liver transplantation. Exclusion criteria encompassed: (1) combined organ transplantation; (2) cutaneous infection or head trauma; and (3) concurrent participation in other clinical trials.
Perioperative managementThe standardized anesthesia protocol was performed in all patients. Prior to anesthesia induction, patients adhered to fasting guidelines (8). Pre-anesthesia assessments included peripheral oxygen saturation (SpO2) and electrocardiography monitoring. Anesthesia induction entailed inhalation of 8 vol.% sevoflurane with 100% oxygen at 8 L/min. Following loss of consciousness, intravenous induction was initiated via peripheral intravenous route, primarily comprising midazolam 0.1 mg/kg, sufentanil 0.5 µg/kg, and rocuronium 1 mg/kg. Tracheal intubation, facilitated by video laryngoscopy, allowed for intermittent positive-pressure ventilation. The oxygen concentration was set at 60%, and the ventilator mode was adjusted to pressure-controlled ventilation. Airway pressure was regulated within the range of 12–25 cmH2O (1 cmH2O = 0.098 kPa) to maintain the end-expiratory carbon dioxide partial pressure at 35–45 mmHg, while the respiratory rate was set between 16 and 20 breaths per minute. Continuous monitoring of arterial blood pressure and central venous pressure was achieved through punctures of the arterial line and right internal jugular vein, respectively. Body temperature was maintained above 36°C using insulation blankets and a fluid warmer (Smith Medical, MN, USA).
Intraoperative monitoring included cerebral tissue oxygen saturation, with NIRS sensors placed on the forehead of patients after proper cleaning. Simultaneous NIRS data were collected and analyzed using the specific brain/body oximeter system (INVOS 5100C, MN, USA) throughout the procedure.
Sevoflurane, rocuronium, and sufentanil were used for anesthesia maintenance. Routine analysis of blood gas was performed at specific time points: at the beginning of surgery, during the anhepatic phase (30 min after clamping), and 10 min and 2 h after re-perfusion. Electrolyte, glucose level, and acid-base balance were adjusted to normal level based on blood gas results. Only crystalloids, 5% glucose, and 20% albumin were used. Red blood cells transfusion was administered only if hemoglobin levels fell below 7 g/dl. Vasoconstrictors, including epinephrine and norepinephrine, were used to maintain blood pressure. Following surgery, patients were transferred to the ICU for further treatment.
Assessment of neurodevelopment and follow-upThe neurodevelopment status of patients was assessed using the Ages Stages Questionnaires (ASQ-3) scale by the same trained evaluator at five time points: baseline (one day before surgery), and at one, three, six, and twelve months after surgery, respectively. The ASQ scale, designed by Professors Squires and Bricker of the University of Oregon, comprised five sub-scales including communication, gross motor, fine motor, problem-solving, and social skills, completed by parents (9). As mentioned in the ASQ User' s Guide (10), the cutoff point was defined as 2 standard deviation below the mean of normal sample. The scores for each sub-scale were compared with cutoff point to identify the possible neurodevelopmental delays.
Outcomes and variablesThe primary outcome assessed in this study was the incidence of neurodevelopmental delay among patients at different intervals following pediatric liver transplantation. Secondary outcomes included the duration of mechanical ventilation, the occurrence of re-intubation, length of ICU stay, postoperative hospitalization duration, and comparisons of intraoperative mean arterial pressure (MAP), arterial partial pressure of oxygen (PaO2), arterial partial pressure of carbon dioxide (PaCO2) and hemoglobin (Hb) concentration. Other perioperative variables included sex, age, body mass index (BMI), pediatric end-stage liver disease score (PELD), average SctO2, surgical duration, anhepatic phase duration, cold ischemia time, intraoperative blood transfusion, and laboratory test results including albumin, alanine transaminase (ALT), total bilirubin (TBIL), Hb, international normalized ratio (INR), and creatinine.
Statistical analysesStatistical analyses were conducted using R software (version 4.0.2). The distribution of data was assessed initially using the Shapiro-Wilk test., with a P > 0.05 indicating conformity to a normal distribution. Categorical variables were reported as numbers (percentages), while continuous variables were expressed as the mean (standard deviation) or median [interquartile range] depending on the normality of the data. Student t test and Mann–Whitney U test were used to analyze continuous variables, whereas the chi-squared test was used to compare categorical variables. A value of P < 0.05 was considered as statistical significant.
Results ParticipantsBetween September 2020 to September 2021, 125 patients undergoing pediatric living-donor liver transplantation were screened for eligibility, This sample size is relative small but conforming to the requirements of neurodevelopmental measurement (11). A total of 123 patients consented and were recruited into the study after excluding 2 patients due to head cutaneous damage. Following assignment was made after the surgery, based on intraoperative average SctO2. 62 patients were assigned to the low saturation (LS) group (SctO2 < 50%), while 61 patients were assigned to the high saturation (HS) group (SctO2 ≥ 50%). 4 patients (1 in LS group and 3 in HS group) were dropped out due to loss of follow-up. One year follow-up for the last patient ended in July 2022. Finally, 119 patients were included in the statistical analysis (Figure 1).
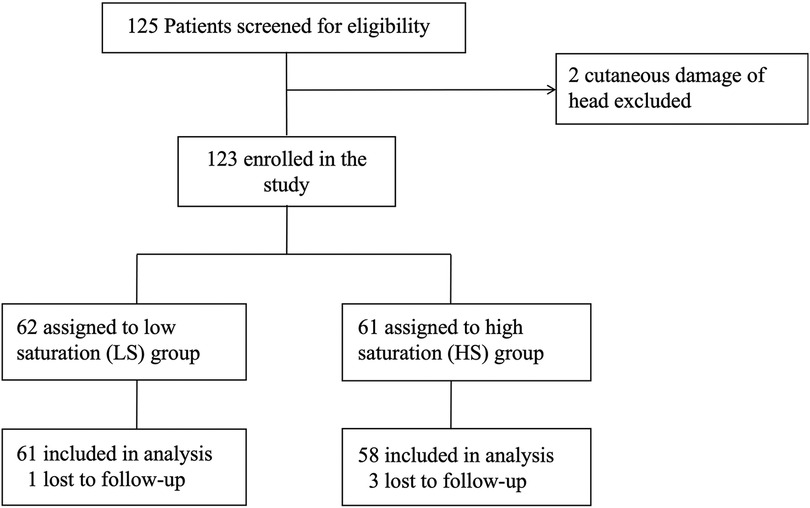
Figure 1. Flowchart of the study.
Characteristics and preoperative variablesThe characteristics and peri-operative variables of patients, including preoperative ASQ assessment results, are shown in Table 1. Most peri-operative variables were well balanced between the two groups. However, significant differences were observed in age [5.0 [5.0, 7.0] vs. 7.5 [6.0, 12.3]; p < 0.001], PELD score [16.6 [11.5, 21.6] vs. 10.1 [−2.0, 15.8]; p < 0.001], INR [1.4 [1.1, 1.8] vs. 1.1 [1.0, 1.4]; p = 0.001], TBIL [240.1 [179.0, 346.2] vs. 109.2 [23.9, 238.1]; p < 0.001], and RBC transfusion [200.0 [200.0–250.0] vs. 200.0 [100.0–200.0]; p = 0.002].
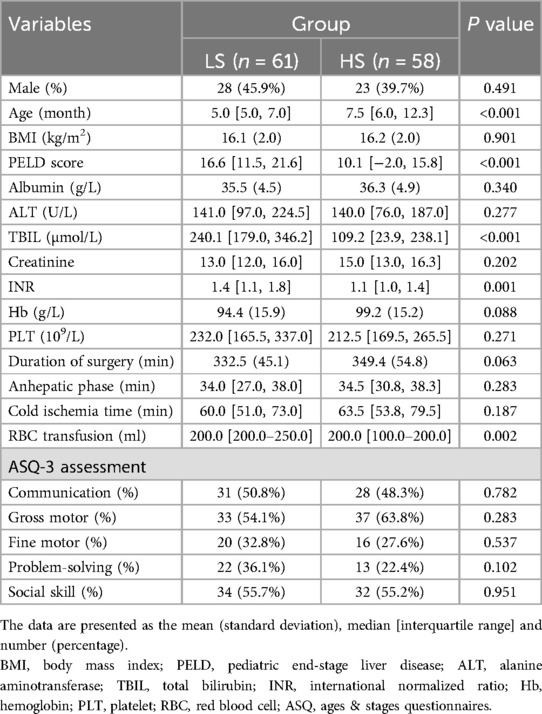
Table 1. Comparisons of patient characteristics and perioperative variables between the two groups.
Primary outcomeThe incidence of neurodevelopmental delay after surgery in the two patient groups is demonstrated in Figure 2. At the 1-month postoperative follow-up, communication [52.5% vs. 27.6%; unadjusted RR, 1.90 (95%CI, 1.20–3.11)] and social skill [50.8% vs. 32.8%; unadjusted RR, 1.55 (95%CI, 1.01–2.44)] were significantly better in the HS group compared to the LS group. At the 3-month postoperative follow-up, communication [36.1% vs. 19.0%; unadjusted RR, 2.16 (95%CI, 1.20–4.01)] and gross motor skills [68.9% vs. 50.0%; unadjusted RR, 1.38 (95%CI, 1.02–1.90)] in the LS group were far worse compared to that in the HS group. Similarly, at the 6-month follow-up, gross motor [47.5% vs. 29.3%; unadjusted RR, 1.62 (95%CI, 1.02–2.64)] and fine motor skills [23.0% vs. 5.2%; unadjusted RR, 4.44 (95%CI, 1.47–13.95)] in the LS group were significant worse. At the 1-year follow-up, patients in the LS group showed poorer problem-solving abilities [37.7% vs. 19.0%; unadjusted RR 1.99 (95%CI 1.09–3.72)].
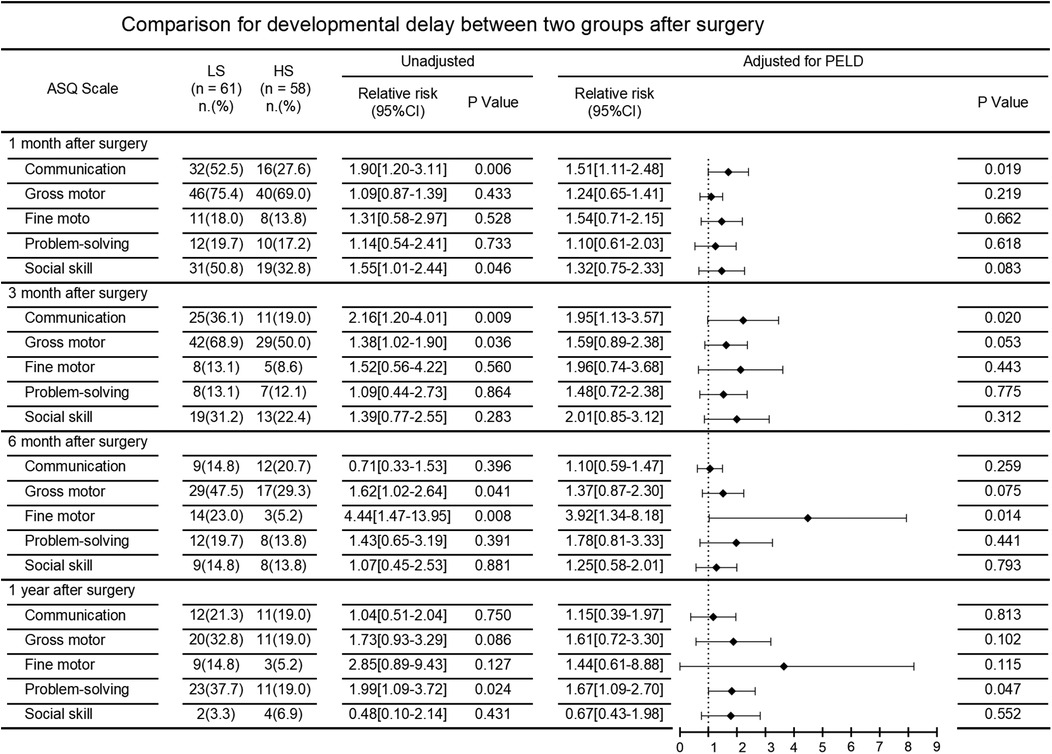
Figure 2. Comparison for developmental delay between two groups after surgery.
After adjusting for PELD score, comparisons between the LS group and HS group still showed significant differences in the incidence of neurodevelopmental delay in communication at 1 and 3 months follow-up [adjusted RR 1.51 [95%CI 1.11–2.48] and adjusted RR 1.95 [95%CI 1.13–3.57], respectively], in fine motor skills at six months follow-up [adjusted RR 3.92 (95%CI 1.34–8.18)], and in problem-solving at 1 year follow-up [adjusted RR 1.67 (95%CI 1.09–2.70)].
Secondary outcomesComparisons of postoperative variables between the LS group and HS group are shown in Table 2. There were no significant differences in mechanical ventilation time [20.4 h IQR [19.2–38.5] vs. 19.9 h IQR[18.5–42.6]; P = 0.343] and the incidence of re-intubation (1.6% vs. 1.7%; P = 0.971). However, the length of ICU stay in the LS group was 6 days [IQR(5.0–7.0)] compared to 5 days [IQR(5.0–6.0)] in the HS group, showing significant difference (P = 0.009). There was a significant difference in the length of postoperative hospitalization between the two groups [35.0 days IQR [28.5–43.0] vs. 31.0 days IQR[23.0–37.0]; P = 0.029].
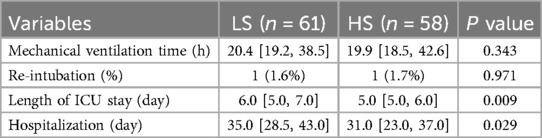
Table 2. Comparisons of postoperative variables between two groups.
The intra-operative vital signs and blood gas results are shown in Table 3. The MAP differed significantly between the LS group and HS groups at the beginning of surgery (P = 0.001) and 2 h after reperfusion (P = 0.011). The Hb concentration in the LS group was significantly lower than that in the HS group at the beginning of surgery (P = 0.015), 10 min after reperfusion (P = 0.001), and 2 h after reperfusion (P = 0.001). No difference was observed in PaCO2 and PaO2 at different periods during surgery.

Table 3. Comparisons of vital signs and blood gas between two groups.
DiscussionThis prospective observational study revealed that patients with low cerebral tissue oxygen saturation (LS group, SctO2 < 50%) during pediatric living-donor liver transplantation exhibited delayed neurodevelopment in terms of communication, fine motor, and problem-solving skills within 1 year follow-up, compared to those with high cerebral tissue oxygen saturation (HS group, SctO2 ≥ 50%). Moreover, the length of ICU stay and postoperative hospitalization were also significantly prolonged in the LS group.
Neurodevelopment outcomes following pediatric transplantation have long been a concern for clinicians and family members of patients. Previous study (12) found that patients undergoing pediatric liver transplantation would experience deficits in various congnitive dunctions, such as visual-spatial abilities, reasoning abilities, and verbal and operational intelligence. A multicenter observational study (13) also revealed that about 60% of parents perceived a decline in their children's cognition and performance after pediatric liver transplantation. Another study (14) found that out of 46 patients who underwent liver transplantation for biliary atresia, 12 required special education, significantly higher than the societal average of 2.4%. These findings aligned with our results, suggesting that a proportion of patients experience a certain degree of neurodevelopment delay in communication skills, motor skills, ability of problem-solving and social interaction following pediatric liver transplantation.
The mechanisms underlying neurodevelopment delay post-transplantation primarily include intraoperative cerebral hypoperfusion (15) and reperfusion injury (16). These changes would lead to short-term neurological damage, which may progress to neurodevelopmental delay detectable by SctO2 monitoring. SctO2 monitor has been proved to reduce the incidence of neurological damage and complications (17). Hyttel-Sorensen et al. discovered that SctO2 monitoring significantly decreased the incidence of cerebral hypoxia in premature infants (18). Despite this, the optimal SctO2 value during surgery remains unclear. According to 2019 edition of Chinese consensus guideline for cerebral protection in the perioperative period of cardiac surgery (19), it was recommended to maintain intraoperative SctO2 above 50% and closely monitor values below this threshold. Based on this guideline, the cutoff point of SctO2 in our study was set at 50%. In our study, we found that patients with SctO2 below 50% were more likely to experience postoperative neurodevelopment delay. However, further research is needed to determine the optimal range of SctO2 for pediatric liver transplantation patients.
Several factors can influence the value of SctO2, with serum total bilirubin level being a significant contributor. SctO2 values are decreased in case of increased serum bilirubin concentration (20, 21). Song et al. found an independent relationship between elevated bilirubin levels and SctO2 < 50% (22). In addition, the value of SctO2 were also influenced by vital signs and blood gas, especially MAP, PaO2, PaCO2 and Hb concentrations (23, 24). Ballard et al. (25) revealed that the value of SctO2 can be elevated by increasing blood pressure, improving hemoglobin level, adjusting respiratory parameters, and oxygen concentration. Our study observed similar results, with patients in the LS group exhibiting significantly lower MAP and Hb concentrations compared to the HS group. These findings suggested that interventions aimed at optimizing these factors may help prevent neurodevelopmental delay in pediatric liver transplantation patients.
Continuous monitoring of SctO2 intraoperatively is essential, particularly in pediatric liver transplantation, which is characterized by its complexity and a higher likelihood of lower SctO2 levels compared to other surgical procedures (26). Natalia et al. reported that the values of SctO2 during pediatric liver transplantation can exhibit considerable variability, ranging from 20% to 90% throughout the procedure (27). Several factors might lead to decreased SctO2 levels during pediatric living-donor liver transplantation. First, the preoperative condition of patients is often compromised, and intraoperative hemorrhage is relatively common in this complicated surgery, leading to hypotension and reduced hemoglobin levels, consequently lowering SctO2 levels. Second, during the anahepatic phase of liver transplantation, clamping of the portal vein and inferior vena cava significantly reduced cardiac output, resulting in decreased cerebral blood flow (28) and SctO2 (26). Third, cerebral autoregulation in young children was less efficient, especially under sevoflurane anesthesia (29). Pether et al. revealed that cerebral perfusion was pressure-dependent in young pediatric patients during sevoflurane anesthesia, suggesting the limited efficiency of the cerebral blood flow autoregulation (30). This made the nerve system more susceptible to hypovolemia or hypoperfusion.
As mentioned above, this is an observational study. No additional intervention were taken and all patients received standardized anesthesia management during the surgery. Great effort has been made to ensure patient stability, such as blood transfusion, usage of vasoactive agents and changes in ventilation strategies. However, due to the complexity of liver transplantation and different preoperative condition of patients, fluctuations in vital signs during the surgery are still inevitable, the value of SctO2 also changed accordingly. Though this study, we considered that more attention should be paid on SctO2 monitor to optimize anesthesia management.
LimitationsThis study has several limitations that need to be acknowledged. First, the total sample size was small, which might have introduced statistical bias. Second, the follow-up duration was only 1 year, potentially insufficient to fully capture the long-term neurological developmental status of patients after surgery. Moreover, the neurological developmental status during the year following surgery might be influenced by various factors such as nutritional intake (31) and parental care (32), which were not accounted for in our study. Third, due to the unique preoperative conditions of patients, there were significant differences in baseline characteristics such as age, total bilirubin, INR, and PELD score between the two groups. To address this bias, the result was adjusted by the PELD score, which mainly includes age, total bilirubin, and INR. Fourth, the ASQ scale used to evaluate neurodevelopment status in this study was testified to be consistent with other measurements (33). However, it was still a subjective evaluation scale that relied on parental attention and cognitive abilities, which may introduce some degree of deviation in the results. Despite these limitations, our study provides valuable isights that can inform the design of future prospective multicenter studies aimed at addressing these challenges.
ConclusionsThis prospective observational study found that decreased average SctO2 may be associated with neurodevelopmental delay following pediatric living-donor liver transplantation. The occurrence of neurodevelopmental delay can be partially prevented by maintaining SctO2 over 50%. Monitoring SctO2 levels can help clinicians to detect potential neurological damage such as cerebral hypoxia. We also recommend the utilization of SctO2 monitoring routinely during the perioperative period of pediatric liver transplantation.
Data availability statementThe raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statementThe studies involving humans were approved by Ethics Committee of Renji Hospital, School of Medicine, Shanghai Jiaotong University. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants' legal guardians/next of kin.
Author contributionsYF: Software, Writing – original draft. QP: Investigation, Writing – original draft. HS: Investigation, Writing – original draft. ZP: Data curation, Formal Analysis, Investigation, Writing – original draft. LZ: Software, Writing – review & editing. BQ: Conceptualization, Writing – review & editing. DS: Investigation, Writing – review & editing. LY: Conceptualization, Writing – review & editing. DH: Formal Analysis, Funding acquisition, Investigation, Project administration, Writing – original draft. WY: Conceptualization, Funding acquisition, Supervision, Writing – review & editing.
FundingThe author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was supported by the Cultivating Fund of Renji Hospital, Shanghai Jiaotong University, School of Medicine (No. PYII-17-009) and Shanghai Municipal Hospital Anesthesiology Specialty Alliance (No. SHDC22023301-A).
Conflict of interestThe authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's noteAll claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
AbbreviationsSctO2, cerebral tissue oxygen saturation; SpO2, peripheral oxygen saturation; ICU, intensive care unit; ASQ, ages stages questionnaires; BIS, bispectral index.
References1. Wildhaber BE, Majno J, Fau-Mayr P, Mayr Z, Fau-Zachariou J, Zachariou J, et al. Biliary atresia: Swiss national study, 1994–2004. J Pediatr Gastroenterol Nutr. (2008) 46:299–307. doi: 10.1097/MPG.0b013e3181633562
PubMed Abstract | Crossref Full Text | Google Scholar
2. Yoon PW, Bresee RS, Fau-Olney JS, Olney LM, Fau-James RS, James MJ, et al. Epidemiology of biliary atresia: a population-based study. Pediatrics. (1997) 99:376–82. doi: 10.1542/peds.99.3.376
PubMed Abstract | Crossref Full Text | Google Scholar
3. Pan ZY, Fan YC, Wang XQ, Chen LK, Zou QQ, Zhou T, et al. Pediatric living donor liver transplantation decade progress in Shanghai: characteristics and risks factors of mortality. World J Gastroenterol. (2020) 26:1352–64. doi: 10.3748/wjg.v26.i12.1352
PubMed Abstract | Crossref Full Text | Google Scholar
4. Couper MR, Shun A, Siew S, O’Loughlin E, Thomas G, Andersen B, et al. Pediatric third liver transplantation-a single-center experience. Pediatr Transplant. (2021) 25:e14092. doi: 10.1111/petr.14092
PubMed Abstract | Crossref Full Text | Google Scholar
5. Meng L, Xiao J, Gudelunas K, Yu Z, Zhong Z, Hu X. Association of intraoperative cerebral and muscular tissue oxygen saturation with postoperative complications and length of hospital stay after major spine surgery: an observational study. Br J Anaesth. (2017) 118:551–62. doi: 10.1093/bja/aex008
PubMed Abstract | Crossref Full Text | Google Scholar
6. Mathieu F, Khellaf A, Ku JC, Donnelly J, Thelin EP, Zeiler FA. Continuous near-infrared spectroscopy monitoring in adult traumatic brain injury: a systematic review. J Neurosurg Anesthesiol. (2020) 32:288–99. doi: 10.1097/ANA.0000000000000620
PubMed Abstract | Crossref Full Text | Google Scholar
7. Finucane E, Jooste E, Machovec KA. Neuromonitoring modalities in pediatric cardiac anesthesia: a review of the literature. J Cardiothorac Vasc Anesth. (2020) 34:3420–8. doi: 10.1053/j.jvca.2020.02.054
PubMed Abstract | Crossref Full Text | Google Scholar
8. American Society of Anesthesiologists Committee on Standards and Practice Parameters. Practice guidelines for preoperative fasting and the use of pharmacologic agents to reduce the risk of pulmonary aspiration: application to healthy patients undergoing elective procedures: an updated report by the American Society of Anesthesiologists Task Force on preoperative fasting and the use of pharmacologic agents to reduce the risk of pulmonary aspiration. Anesthesiology. (2017) 126:376–93. doi: 10.1097/ALN.0000000000001452
PubMed Abstract | Crossref Full Text | Google Scholar
10. Squires J, Potter L, Bricker D. The ASQ User's Guide. 3rd ed. Baltimore, MD: Brookes (2009).
11. American Educational Research Association, American Psychological Association, National Council on Measurement in Education. Standards for Educational and Psychological Testing. Washington, DC: American Educational Research Association (2014).
12. Stewart SM, Silver J, Fau-Nici CH, Nici D, Fau-Waller J, Waller R, et al. Neuropsychological function in young children who have undergone liver transplantation. J Pediatr Psychol. (1991) 16:569–83. doi: 10.1093/jpepsy/16.5.569
PubMed Abstract | Crossref Full Text | Google Scholar
13. Ohnemus D, Neighbors K, Rychlik K, Venick RS, Bucuvalas JC, Sundaram SS, et al. Health-related quality of life and cognitive functioning in pediatric liver transplant recipients. Liver Transpl. (2020) 26:45–56. doi: 10.1002/lt.25634
PubMed Abstract | Crossref Full Text | Google Scholar
14. Rodijk LH, den Heijer AE, Hulscher JBF, Alizadeh BZ, de Kleine RHJ, Verkade HJ, et al. Long-term neurodevelopmental outcomes in children with biliary atresia. J Pediatr. (2020) 217:118–24.e3. doi: 10.1016/j.jpeds.2019.10.054
PubMed Abstract | Crossref Full Text | Google Scholar
15. Dunn MA, Rogal SS, Duarte-Rojo A, Lai JC. Physical function, physical activity, and quality of life after liver transplantation. Liver Transpl. (2020) 26:702–8. doi: 10.1002/lt.25742
PubMed Abstract | Crossref Full Text | Google Scholar
16. Tan W-F, Steadman RH, Farmer DG, Hong JC, Busuttil RW, Apinyachon W, et al. Pretransplant neurological presentation and severe posttransplant brain injury in patients with acute liver failure. Transplantation. (2012) 94:768–74. doi: 10.1097/TP.0b013e3182620596
PubMed Abstract | Crossref Full Text | Google Scholar
17. Julien-Marsollier FA-O, Cholet C, Coeffic A, Dupont T, Gauthier T, Loiselle M, et al. Intraoperative cerebral oxygen saturation and neurological outcomes following surgical management of necrotizing enterocolitis: predictive factors of neurological complications following neonatal necrotizing enterocolitis: predictive factors of neurological complications following neonatal necrotizing enterocolitis. Pediatric Anesthesia. (2022) 32:421–8. doi: 10.1111/pan.14392
PubMed Abstract | Crossref Full Text | Google Scholar
18. Hyttel-Sorensen S, Pellicer A, Alderliesten T, Austin T, van Bel F, Benders M, et al. Cerebral near infrared spectroscopy oximetry in extremely preterm infants: phase II randomised clinical trial. Br Med J. (2015) 350:g7635. doi: 10.1136/bmj.g7635
PubMed Abstract | Crossref Full Text | Google Scholar
19. R. Cerebral Protection in Cardiac Intensive Care Group Neural and A, Repair Committee Chinese Research Hospital. [Chinese consensus guideline for cerebral protection in the perioperative period of cardiac surgery (2019)]. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue. (2019) 31:129–34 doi: 10.3760/cma.j.issn.2095-4352.2019.02.001
PubMed Abstract | Crossref Full Text | Google Scholar
20. Rodríguez MJ, Corredera A, Martínez-Orgado J, Arruza L. Interference between cerebral NIRS and conjugated bilirubin in extremely low birth weight neonates. An Pediatr (Engl Ed). (2021) 95:371–3. doi: 10.1016/j.anpedi.2020.12.017
Crossref Full Text | Google Scholar
21. Madsen PL, Skak C, Rasmussen A, Secher NH. Interference of cerebral near-infrared oximetry in patients with icterus. Anesth Analg. (2000) 90:489–93. doi: 10.1213/00000539-200002000-00046
PubMed Abstract | Crossref Full Text | Google Scholar
22. Song JG, Jeong SM, Shin WJ, Jun IG, Shin K, Huh IY, et al. Laboratory variables associated with low near-infrared cerebral oxygen saturation in icteric patients before liver transplantation surgery. Anesth Analg. (2011) 112:1347–52. doi: 10.1213/ANE.0b013e318214b2b0
PubMed Abstract | Crossref Full Text | Google Scholar
24. Robu CB, Koninckx A, Docquier MA, Grosu I, De Kerchove L, Mastrobuoni S, et al. Advanced age and sex influence baseline regional cerebral oxygen saturation as measured by near-infrared spectroscopy: subanalysis of a prospective study. J Cardiothorac Vasc Anesth. (2020) 34:3282–9. doi: 10.1053/j.jvca.2020.06.025
PubMed Abstract | Crossref Full Text | Google Scholar
25. Ballard C, Jones E, Gauge N, Aarsland D, Nilsen OB, Saxby BK, et al. Optimised anaesthesia to reduce post operative cognitive decline (POCD) in older patients undergoing elective surgery, a randomised controlled trial. PLoS One. (2012) 7:e37410. doi: 10.1371/journal.pone.0037410
PubMed Abstract | Crossref Full Text | Google Scholar
26. Plachky J, Hofer S, Volkmann M, Martin E, Bardenheuer HJ, Weigand MA. Regional cerebral oxygen saturation is a sensitive marker of cerebral hypoperfusion during orthotopic liver transplantation. Anesth Analg. (2004) 99:344–9. doi: 10.1213/01.ANE.0000124032.31843.61
PubMed Abstract | Crossref Full Text | Google Scholar
27. Magasich-Airola NP, Momeni M, Sanchez Torres C, De Magnée C, Tambucci R, Reding R, et al. Regional oxygen saturation measured by two different oximetry monitors in infants and children undergoing living donor liver transplantation with bilirubin measurements: a prospective observational study. Paediatr Anaesth. (2023) 33:201–10. doi: 10.1111/pan.14597
PubMed Abstract | Crossref Full Text | Google Scholar
28. Shih TH, Huang CE, Chen CL, Wang CH, Huang CJ, Cheng KW, et al. Correlation between changes in end-tidal carbon dioxide concentration and cardiac output during inferior vena cava clamping and unclamping in living-donor liver transplantation. Transplant Proc. (2016) 48:1077–9. doi: 10.1016/j.transproceed.2015.10.061
PubMed Abstract | Crossref Full Text | Google Scholar
29. Rhondali O, Mahr A, Simonin-Lansiaux S, De Queiroz M, Rhzioual-Berrada K, Combet S, et al. Impact of sevoflurane anesthesia on cerebral blood flow in children younger than 2 years. Paediatr Anaesth. (2013) 23:946–51. doi: 10.1111/pan.12166
PubMed Abstract | Crossref Full Text | Google Scholar
30. Jildenstål P, Widarsson Norbeck D, Snygg J, Ricksten SE, Lannemyr L, Vutskits L. Cerebral autoregulation in infants during sevoflurane anesthesia for craniofacial surgery. Pediatric Anesthesia. (2021) 31:563–9. doi: 10.1111/pan.14146
PubMed Abstract | Crossref Full Text | Google Scholar
32. Lewis H, Trowbridge A, Jonas D, Rosenberg AR, Bogetz JF. A qualitative study of clinicians and parents of children with severe neurological impairment on tools to support family-centered care. J Palliat Med. (2022) 25:1338–44. doi: 10.1089/jpm.2021.0579
PubMed Abstract | Crossref Full Text | Google Scholar
33. Flamant C, Branger S, Fau-Nguyen B, Tich T, Tich ENT, Fau-de la Rochebrochard S, et al. Parent-completed developmental screening in premature children: a valid tool for follow-up programs. PLoS One. 6:e20004. doi: 10.1371/journal.pone.0020004
留言 (0)