Acute acquired concomitant esotropia (AACE) is a subtype of esotropia characterized by diplopia and a sudden onset of esotropia (Clark et al., 1989). AACE predominantly affects adults or older adolescents, presenting with normal eye movements and equal deviation angles in all direction. Recently, the incidence of AACE has increased, especially during and after the COVID-19 lockdown (Vagge et al., 2020; Neena et al., 2022; Mohan et al., 2021).
The pathogenesis of AACE is multifaceted and not fully understood, with hypotheses suggesting links to accommodation and convergence-divergence dysfunctions (Shanker and Nigam, 2015). Myopic overcorrection and presbyopia-related accommodation decline are implicated, with the latter showing higher AC/A ratios (Fresina et al., 2016; Tong et al., 2020). AACE’s occurrence in aphakic eyes indicates that non-accommodative factors (Ruatta and Schiavi, 2020), such as extraocular muscle abnormalities, may play a role. Our research reveals significant changes in the size and volume of dominant eye muscles, possibly as a compensatory response to binocular diplopia, with the LR muscle potentially enlarging to counteract increased convergence (Chen et al., 2024). Additionally, the anterior positioning of the medial rectus muscle in AACE patients may cause over-concentration, disrupting the balance between convergence and divergence and leading to esotropia (Cai et al., 2019).
Conversely, the emergence of AACE may also be linked to complex neural network within the visual system. Advances in functional magnetic resonance imaging (fMRI) enable precise functional localization of the visual cortex, allowing for the assessment of central visual functions in AACE patients without intracranial lesions. Previous studies have identified functional deficits in the visual cortex associated with various types of strabismus (Guo et al., 2022; Yan et al., 2019). These abnormalities in spontaneous brain activity, which can be attributed to visual compensation, can also be considered as one of the causative factors of such disorders. Notably, using the amplitude of low-frequency fluctuation (ALFF) in resting-state fMRI, one study found deficits in the primary visual cortex and dorsal pathways in AACE patients, with alterations in the fusiform gyrus correlating with deviation angles, suggesting a connection between AACE and visual processing centers (Hu et al., 2023).
Synchronization between cerebral hemispheres is crucial for visual experience. Voxel-mirrored homotopic connectivity (VMHC) accurately and efficiently assesses changes in functional connectivity between hemispheres related to a patient’s behavior and cognition by measuring correlations between hemispheric blood oxygen level-dependent time series that reflect the pattern of information exchange and integration between hemispheres (Mancuso et al., 2019). Recent studies have increasingly focused on VMHC, establishing its associations with a variety of diseases and functional states. It has been applied to analyze numerous ocular conditions, including primary open-angle glaucoma (Wang et al., 2018), monocular blindness (Shao et al., 2018), strabismic amblyopia (Peng et al., 2021; Zhang et al., 2021; Liang et al., 2017), high myopia (Cheng et al., 2022), blepharospasm (Wei et al., 2018), unilateral acute open globe injury (Ye et al., 2018), optic neuritis (Song et al., 2023), concomitant exotropia (Zhang et al., 2018), and congenital nystagmus (Wen et al., 2023).
The objective of our study is to utilize VMHC analysis to detect functional brain changes in patients with AACE. We aim to identify whether these alterations in brain functional connection impact visual quality and overall quality of life, thereby offering novel insights into the neural underpinnings of AACE. This approach is pivotal for advancing our understanding of the condition.
2 Materials and methods 2.1 ParticipantsThe study population consisted of patients diagnosed with AACE at Beijing Tongren Hospital (Beijing, China) from January 2021 to December 2022, along with healthy subjects recruited from the local community. A total of 32 patients with AACE (15 males and 17 females) and 31 HC (10 males and 21 females) were included in this study. This study adhered to the principles outlined in the Declaration of Helsinki and received approval from the Ethics Committee of Beijing Tongren Hospital (TRECKY2021-228), and registered with the China Clinical Trials Registry (ChiCTR2100053717).
The inclusion criteria for both the AACE group and the HC group were as follows: (1) Subjects in both groups exhibited symptoms of diplopia or acute episodes of esotropia and were diagnosed with AACE by an experienced clinician for the AACE group, while the HC group was required to match the AACE patients in terms of gender, age, years of education, handedness, height, and weight; (2) All subjects cooperated with the study examinations; (3) Informed consent was provided by the subjects themselves or through a legal guardian.
The exclusion criteria for both groups were as follows: (1) Presence of developmental abnormalities, cranial or neurological diseases, or a history of head trauma; (2) History of neuropsychiatric disorders or use of psychotropic medications in the past month; (3) History of drug or alcohol addiction or abuse; (4) other organic eye diseases; (5) Inability to cooperate with all required examinations.
2.2 Ophthalmic examinationAll participants underwent comprehensive ophthalmological examinations, including assessment of best-corrected visual acuity (BCVA), refractive status, fundus examination, synoptophore testing for binocular vision and stereoacuity evaluation, as well as alternate cover test to assess eye alignment. The spherical equivalent (SE) was calculated by summing the sphere power with half of the cylinder power (sphere +0.5 × cylinder). The angles of deviation were measured at near fixation (1/3 m) and distance fixation (6 m) using a prism in combination with alternative cover testing.
Binocular vision at distance fixation, encompassing simultaneous vision, fusion, and distance stereopsis, was evaluated using a synoptophore device (CLEMENT-CLARKE, UK; type 2001), with the normal simultaneous vision range is defined as −3° ~ +3°. Fusion and distance stereopsis were qualitative measured. Near stereopsis was assessed using a Random Dot Stereogram (RDS) at an optimal viewing distance of 40 cm, with patients achieving results within or below 60 s classified as having good near stereopsis.
2.3 Questionnaire surveyThe age of onset and duration of the AACE in all patients was determined based on self-reported information. Patients were extensively questioned about their diplopia symptoms, which were categorized as follow: distance only, near only, or both distance and near. Additionally, patients were queried as about any history of near vision work prior to the onset of the disease, and if applicable, the daily duration of near vision work was recorded.
All patients completed the Beck Depression Inventory-II (BDI-II) (Wang and Gorenstein, 2013), the Montreal Cognitive Assessment (MoCA) (Kang et al., 2018), and the State–Trait Anxiety Inventory (STAI) (Marteau and Bekker, 1992). The STAI consists of two scales: the State Anxiety Inventory (S-AI) scale, which measures the severity of current anxiety symptoms, and the Trait Anxiety Inventory (T-AI) scale, which assesses the subject’s typical or general anxiety levels.
2.4 Resting-state fMRI parametersAll the subjects underwent scanning using a 3-Tesla MRI scanner (MAGNETOM Prisma, Siemens Healthcare, Erlangen, Germany) equipped with a standard 64-channel head coil. Foam pads were strategically placed around the subjects’ heads to minimize any potential head motion artifacts during the scan, while earplugs were provided to attenuate scanner noise.
Three-dimensional T1-weighted anatomical images were acquired using a 3D magnetization-prepared rapid gradient-echo imaging sequence, with the following scan parameters: repetition time = 2000 ms; echo time = 2.25 ms; flip angle = 8°; field of view = 256 mm × 256 mm; in-plane image resolution = 1 mm × 1 mm; slice thickness = 1 mm, no gap; and 192 continuous sagittal slices.
Functional images were obtained with the following parameters: repetition time = 1,000 ms; echo time = 33 ms; flip angle = 64°; field of view = 208 mm × 180 mm; in-plane image resolution = 2 mm × 2 mm; matrix size = 104 × 90; slice thickness = 2 mm; slice gap = 0.4 mm; and 60 slices.
2.5 VMHC statistical analysisFunctional data were analyzed with the Data Processing Assistant for Resting-State fMRI Advanced Edition (DPARSFA) and Statistical Parametric Mapping (SPM12) based on MATLAB R2016a (Mathworks, Natick, MA).
To enhance normality, the individual VMHC maps were transformed into z values using a Fisher z-transformation with REST software. Subsequently, the global VMHC was computed and included as a covariate in the subsequent statistical analysis. The Anatomical Automatic Labeling (AAL) 3 version 1 was used to extract the average value of the quantitative indicators of all brain regions, and the graph was drawn using the XjView toolbox.
2.6 Statistical analysisThe difference in the z-maps VMHC between the AACE groups and the health controls was examined with two-sample t-tests in the SPM8 toolkit (p < 0.001 for multiple comparisons using Gaussian Random Field theory). SPSS software (version 27.0; IBM, IL, United States). The chi-square test was used for categorical variable differences (p < 0.05), and two-sample independent test or non-parametric test was used for continuous variable differences (p < 0.05). Spearman’s correlation analysis evaluated the relationship between fMRI indicators and clinical features in abnormal areas (p < 0.05).
3 Results 3.1 Demographics and visual measurementsNo significant differences were observed between the two groups regarding gender, age, height, and weight (Table 1). Patients in the AACE group had a mean age of onset of 33.28 ± 9.15 years and a mean disease duration of 2.99 ± 2.26 years. Their mean angle of deviation was 29.22 ± 20.44Δ at near and 29.78 ± 20.44Δ at distance. The AACE group had worse binocular visual function than the normal group, but there was no significant difference in spherical equivalent and time of near vision work (Table 1). The psychological scale scores for both groups are presented in Table 2.
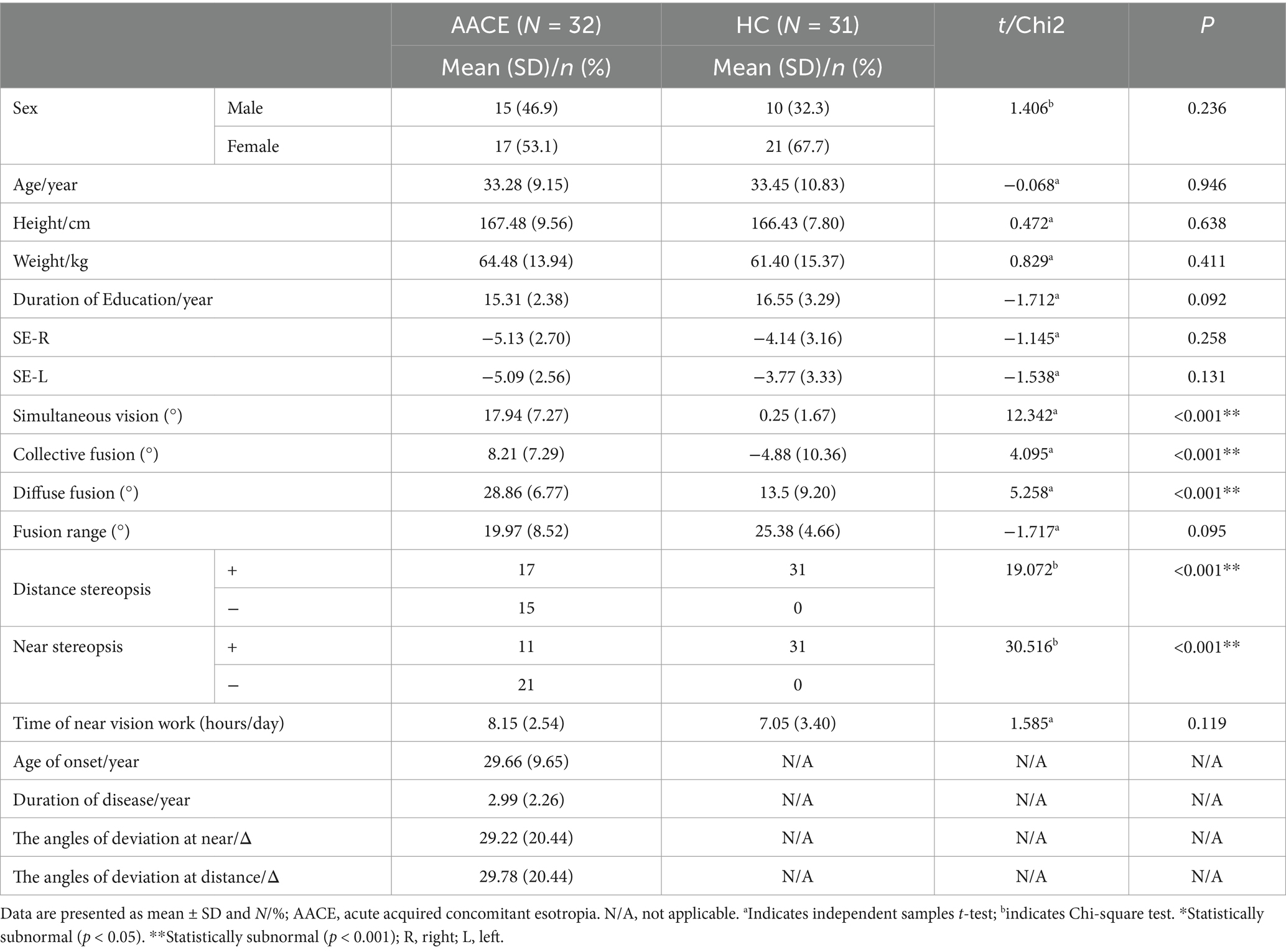
Table 1. Demographic and clinical characteristics of patients in AACE and HC groups.
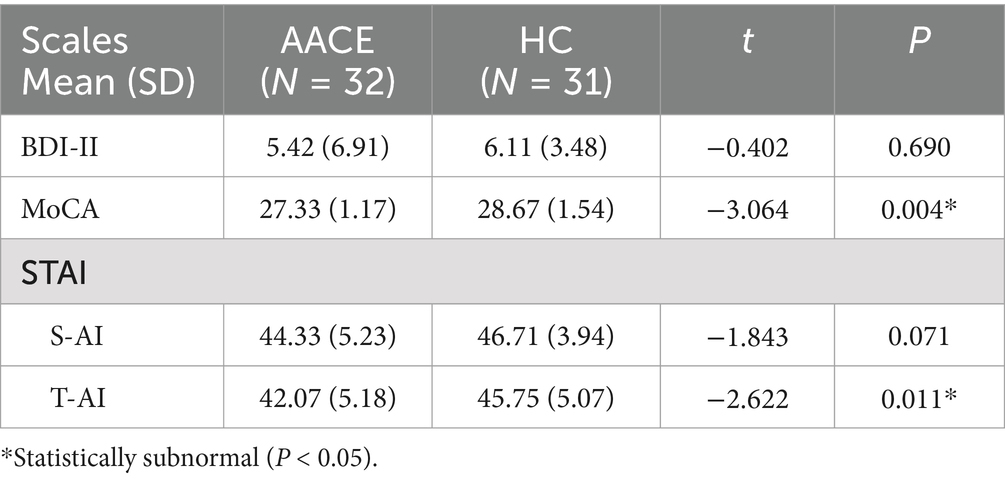
Table 2. Scores of physical scales of patients in AACE and HC groups.
3.2 VMHC differencesCompared with HC, AACE patients had significantly lower VMHC values in the superior frontal gyrus (SFG) and anterior cingulate gyrus (ACG) (Figure 1 and Table 3). Differences between the two groups appeared in Brodmann area 11 and Brodmann area 24. Figure 2 shows the significantly different VMHC values between the two groups.
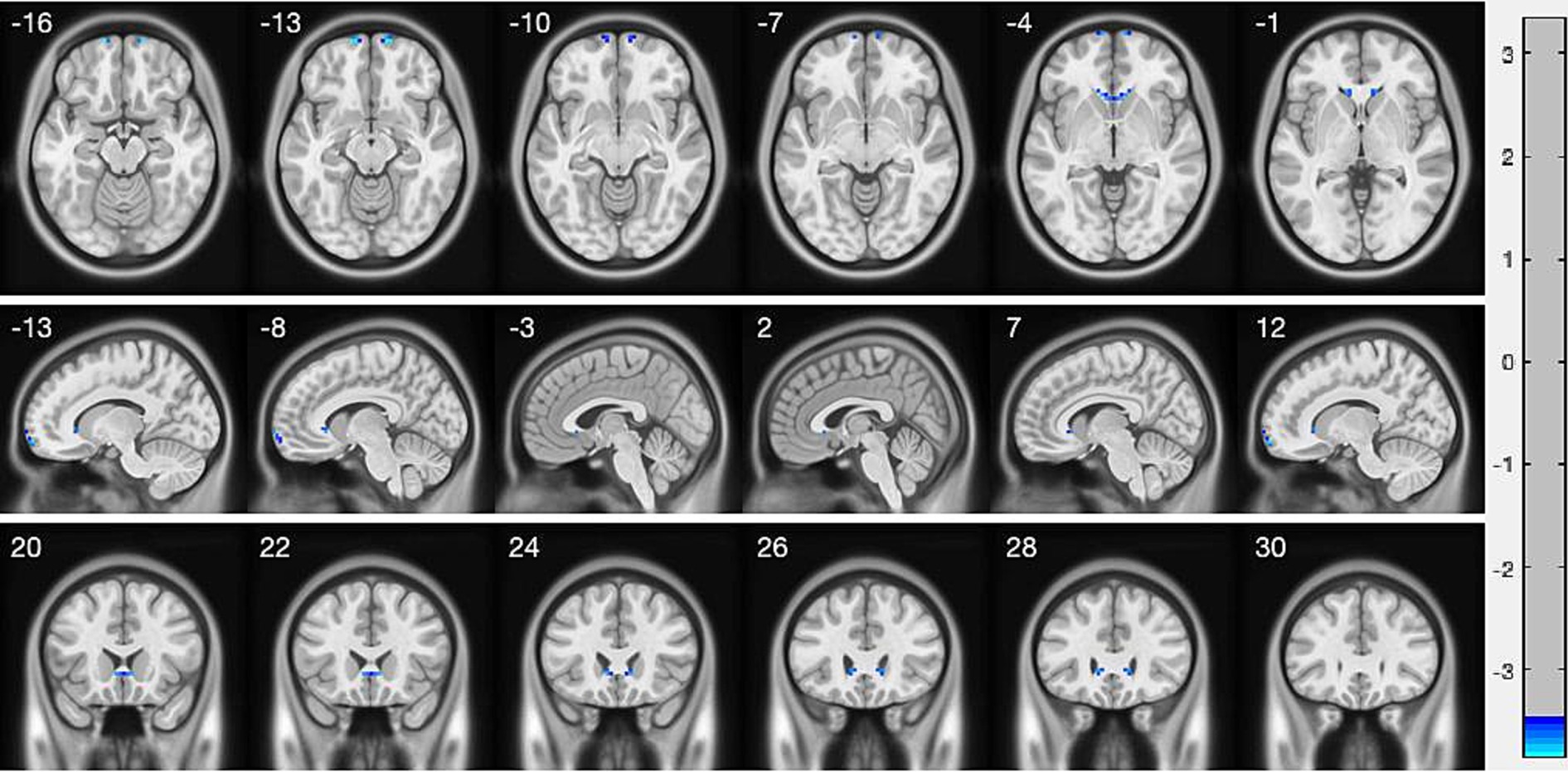
Figure 1. Interhemispheric connectivity in the CSA versus NCs. Blue areas indicated the lower VMHC values showed p < 0.001.
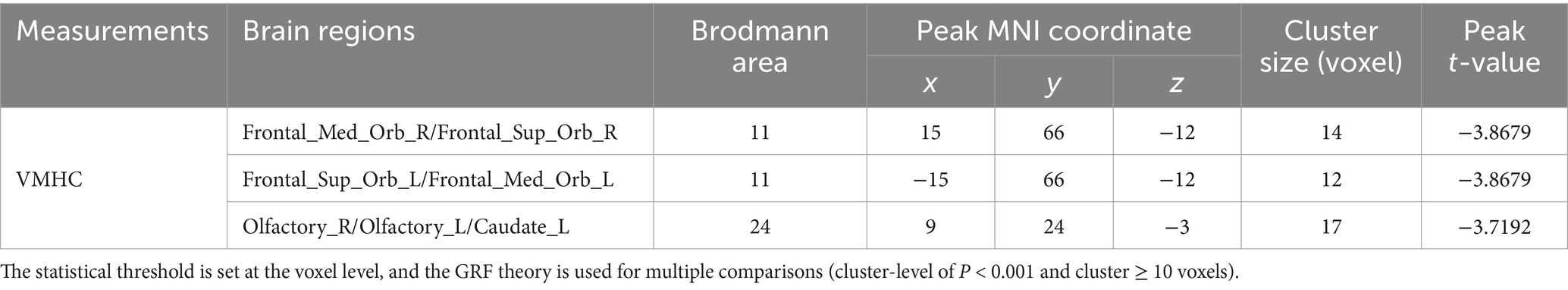
Table 3. Brain regions with significant changes in regional fMRI (VMHC) between patients with AACE and HC groups.
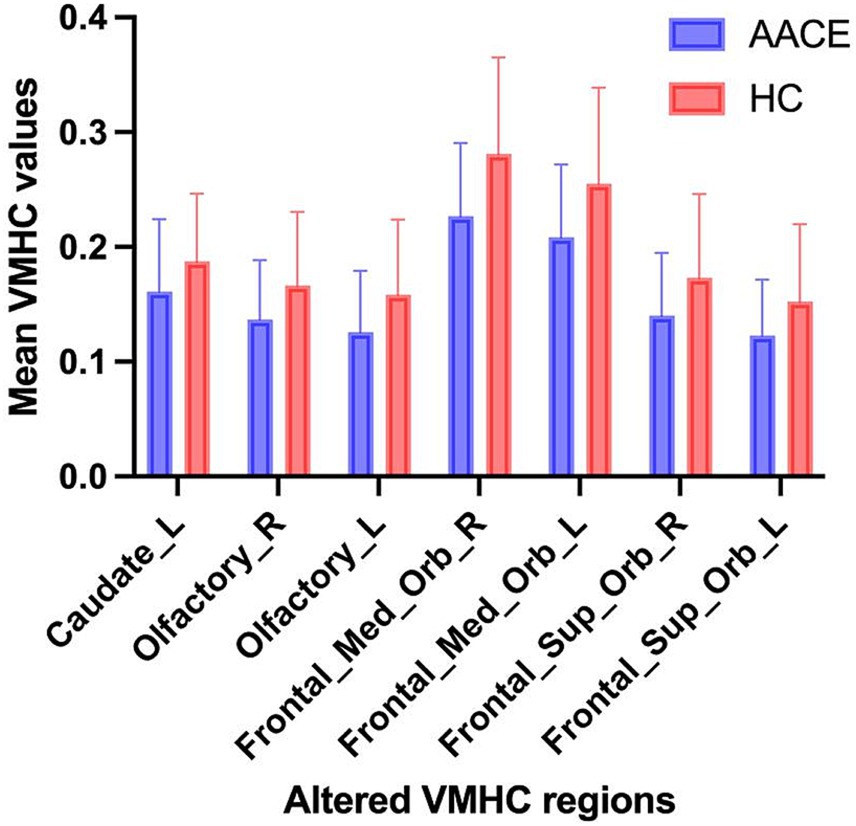
Figure 2. The mean values of altered VMHC values between the AACE and HC groups. Data presented as mean ± standard deviation.
3.3 Correlation analysisIn the AACE group, the VMHC values of the left caudate positively correlated with the time of near vision work (r = 0.3813, p = 0.0343), the angles of deviation at near (r = 0.4276, p = 0.0146) and at distance (r = 0.4158, p = 0.0179). The VMHC values of the bilateral olfactory cortex positively correlated with the duration of near vision work (Right: r = 0.3888, p = 0.0306; Left: r = 0.3718, p = 0.0394) while the BDI scores showed a negative correlation with the VMHC values of the left olfactory cortex (r = −0.3588, p = 0.0475) (Figure 3).
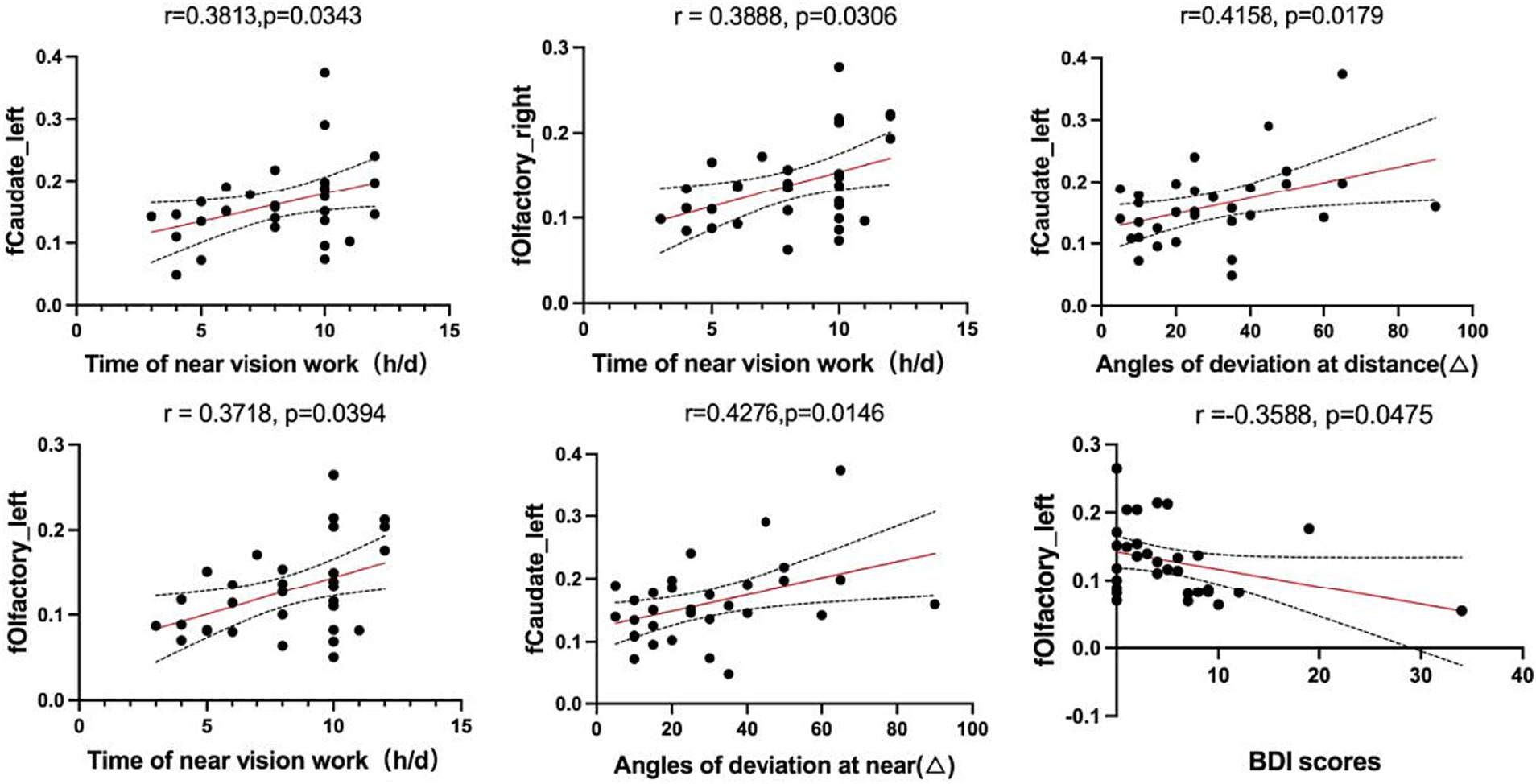
Figure 3. Correlations between the values of fMRI in abnormal brain regions and clinical features.
4 DiscussionThis study first utilized the VMHC method to survey functional network brain activity changes in AACE patients. We observed reduced VMHC values in the SFG and ACG in patients with AACE compared to HC, and these reduced values correlated with time of near vision work, angles of deviation at near and distance and BDI scores.
The frontal lobe is located at the front of the cerebral hemispheres and is the most evolved part of the developing brain (Neulinger et al., 2016). Damage to this area can lead to impairments in voluntary movement, language expression and memory. Among these, supplementary eye field (SEF), located in the medial frontal cortex, is involved in the control of random eye movements and are particularly associated with eye tracking (Purcell et al., 2012). Activity in the frontal eye fields (FEF) was also observed during visual search (Lowe et al., 2022). The superior frontal gyrus (SFG) is located superiorly in the prefrontal cortex and is thought to consist of several cytoarchitecturally distinct subregions, including Brodmann’s zones 6, 8, 9, and 32 (Petrides and Pandya, 1999; Petrides and Pandya, 2002). Functional impairment was found in the above areas in all types of strabismus (Huang et al., 2016a; Tan et al., 2016; Tan et al., 2018; Huang et al., 2018).
One study found significantly higher levels of VMHC in some brain regions in the middle frontal lobe of patients with concomitant exotropia, possibly reflecting abnormal activity in the kinetic eye area due to a compensatory mechanism that may not be the cause of strabismus, but rather a consequence of strabismus (Yan et al., 2019). Besides, another study found that patients with strabismus and amblyopia had significantly lower VMHC values in the bilateral frontal super orbits and bilateral frontal gyrus, which is similar to our findings (Peng et al., 2021). A previous study from our team revealed fusional vergence dysfunction is present in adult AACE cases (Zhao et al., 2022). All of the patients in this study had varying degrees of diplopia symptoms that did not resolve on their own after rest. They had significant abnormalities in fusion function. Since it has been found that the bilateral frontal gyrus regulates normal fusion function in human eyes (Xubo et al., 2014), we hypothesized that a decrease in frontal VMHC, resulting in fusion dysfunction, contributes to the development of AACE. This may be the difference between AACE and other types of strabismus, especially concomitant exotropia.
The cingulate gyrus, situated within the limbic system, has been linked to the formation of mood, the experience of depression and the perception of pain (Ebert and Ebmeier, 1996). The limbic system is intimately associated with memory and emotion. The anterior cingulate gyrus plays a role in a number of established functions, including emotion, cognition, locomotion, visceral movement, maternal behavior and social interaction (Apps et al., 2016; Bliss et al., 2016; Zou et al., 2021). A study utilizing the DTI technique to investigate brain-wide microstructural alterations in individuals with common strabismus revealed that patients with this condition exhibited markedly elevated mean diffusivity values in the left anterior cingulate gyrus, indicating microstructural modifications in this region (Huang et al., 2016a). Patients with common strabismus were also observed to have higher regional homogeneity (ReHo) values were observed in the bilateral cingulate gyrus in the study by Huang et al. (2016b). Additionally, some researchers have discovered that the diffusion coefficient (DC) values in the anterior cingulate cortex (ACC) were markedly elevated in adult patients with common external strabismus in comparison to controls, indicating that it may be a contributing factor to anterior cingulate gyrus dysfunction (Tan et al., 2018). Meanwhile, some researchers have found that optic neuritis patients had reduced VMHC values in the left middle cingulate gyrus, suggesting that functional abnormalities in the cingulate gyrus may lead to cognitive decline or loss in patients (Song et al., 2023).
Numerous studies have found increased signal levels of various indicators in the cingulate gyrus in patients with exotropia, abnormal activation of the cingulate gyrus to compensate for the impairment of fusional function due to exotropia. One study found higher bilateral cingulate VMHC values in strabismic amblyopia than in the HC group, due to a compensatory increase in visual input deficits caused by strabismic amblyopia (Zhang et al., 2021). However, a study of functional brain connectivity in patients with exotropia found no changes in the cingulate gyrus. In the present study, the VMHC values of the cingulate gyrus in AACE patients were significantly lower than those of the normal group, suggesting that our dysfunctional functional connectivity between the bilateral cingulate gyrus may be an important factor affecting the control of the direction and eye position of strabismus.
In our study, the VMHC signal values in the anterior cingulate gyrus were significantly lower in the AACE group compared to the control group and exhibited a negative correlation with BDI depression scores. Prior research has indicated a correlation between olfactory bulb volume and depression (Sabiniewicz et al., 2022). Concurrently, numerous studies have indicated that individuals with strabismus frequently exhibit psychological irregularities, including depressive symptoms (Lin et al., 2014; Lee et al., 2022). Consequently, we postulate that strabismus may be linked to anterior cingulate gyrus dysfunction, which could elucidate the prevalence of depressive symptoms in patients with strabismus. This underscores the necessity for greater emphasis on the psychological well-being and quality of life of patients, in addition to conventional diagnosis and treatment in the clinic.
It is noteworthy that the VMHC values of the left caudate in the AACE group exhibited a positive correlation with the duration of near vision work, the angles of deviation at near and at distance. Additionally, the VMHC values of the bilateral olfactory cortex demonstrated a positive correlation with the duration of near vision work. Excessive near vision work may be a significant contributing factor to the development of AACE (Topcu Yilmaz et al., 2020). It is possible that excessive tension in the medial rectus muscle, caused by near vision work, may result in esotropia if the fusion force is insufficient to overcome this tension. Alternatively, prolonged use of smartphones may stimulate the ciliary muscle, leading to convergence spasms and the development of AACE (Kaur et al., 2019).
Although the present study revealed functional connectivity differences between AACE patients and healthy controls in the resting state by means of fMRI, it remains unclear what association exists between this and the visual impairment associated with strabismus. A more in-depth longitudinal study of brain changes after strabismus correction will be conducted in future investigations.
The VMHC values of the frontal and marginal lobes of AACE patients were changed, suggesting that dysfunction of the medial frontal gyrus and anterior cingulate gyrus may lead to fusion dysfunction, thereby contributing to the development of AACE. This impairment this is related to poor eye habits and the severity of strabismus.
Data availability statementThe raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statementThe studies involving humans were approved by Ethics Committee of Beijing Tongren Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributionsJC: Data curation, Formal analysis, Investigation, Writing – original draft. JH: Methodology, Project administration, Writing – review & editing, Funding acquisition. JL: Writing – review & editing. HL: Data curation, Investigation, Writing – review & editing. ZM: Methodology, Project administration, Writing – review & editing. JF: Data curation, Funding acquisition, Methodology, Project administration, Resources, Supervision, Validation, Writing – review & editing.
FundingThe author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This study was supported by National Natural Science Foundation of China (82070998), Key research projects in the capital’s health development scientific research (first launch 2022–1-2053), Program of Beijing Hospitals Authority (XMLX202103), Program of Beijing Municipal Science & Technology Commission (Z201100005520044) from Jing Fu, Youth Scientific Foundation of National Natural Science Foundation of China (82101174), and Beijing Hospitals Authority Youth Programme (QML20230205) from Jie Hao.
AcknowledgmentsWe are grateful for the support from the Beijing Tongren Hospital and all the subjects for helping complete the study, especially Dr. Zhaohui Liu and Dr. Lirong Zhang of the Department of Radiology.
Conflict of interestThe authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statementThe authors declare that no Generative AI was used in the creation of this manuscript.
Publisher’s noteAll claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Footnotes ReferencesApps, M. A., Rushworth, M. F., and Chang, S. W. (2016). The anterior cingulate gyrus and social cognition: tracking the motivation of others. Neuron 90, 692–707. doi: 10.1016/j.neuron.2016.04.018
PubMed Abstract | Crossref Full Text | Google Scholar
Bliss, T. V., Collingridge, G. L., Kaang, B. K., and Zhuo, M. (2016). Synaptic plasticity in the anterior cingulate cortex in acute and chronic pain. Nat. Rev. Neurosci. 17, 485–496. doi: 10.1038/nrn.2016.68
PubMed Abstract | Crossref Full Text | Google Scholar
Chen, J. Y., Zhang, L. R., Liu, J. W., Hao, J., Li, H. X., Zhang, Q. Y., et al. (2024). Magnetic resonance imaging of extraocular rectus muscles abnormalities in acute acquired concomitant esotropia. Int. J. Ophthalmol. 17, 119–125. doi: 10.18240/ijo.2024.01.16
PubMed Abstract | Crossref Full Text | Google Scholar
Cheng, Y., Chen, X. L., Shi, L., Li, S. Y., Huang, H., Zhong, P. P., et al. (2022). Abnormal functional connectivity between cerebral hemispheres in patients with high myopia: a resting FMRI study based on voxel-mirrored homotopic connectivity. Front. Hum. Neurosci. 16:910846. doi: 10.3389/fnhum.2022.910846
PubMed Abstract | Crossref Full Text | Google Scholar
Clark, A. C., Nelson, L. B., Simon, J. W., Wagner, R., and Rubin, S. E. (1989). Acute acquired comitant esotropia. Br. J. Ophthalmol. 73, 636–638. doi: 10.1136/bjo.73.8.636
PubMed Abstract | Crossref Full Text | Google Scholar
Ebert, D., and Ebmeier, K. P. (1996). The role of the cingulate gyrus in depression: from functional anatomy to neurochemistry. Biol. Psychiatry 39, 1044–1050. doi: 10.1016/0006-3223(95)00320-7
PubMed Abstract | Crossref Full Text | Google Scholar
Fresina, M., Giannaccare, G., Versura, P., and Campos, E. C. (2016). Accommodative spasm might influence surgical planning and outcomes in acute acquired distance esotropia in myopia. Med. Hypotheses 94, 66–67. doi: 10.1016/j.mehy.2016.06.023
PubMed Abstract | Crossref Full Text | Google Scholar
Guo, J., Chen, Y., Liu, W., Huang, L., Hu, D., Lv, Y., et al. (2022). Abnormal developmental trends of functional connectivity in young children with infantile esotropia. Front. Neurosci. 16:972882. doi: 10.3389/fnins.2022.972882
PubMed Abstract | Crossref Full Text | Google Scholar
Hu, Y., Wang, S., Wu, L., Xi, S., Wen, W., and Zhao, C. (2023). Deficits of visual cortex function in acute acquired concomitant Esotropia patients. Invest. Ophthalmol. Vis. Sci. 64:46. doi: 10.1167/iovs.64.13.46
PubMed Abstract | Crossref Full Text | Google Scholar
Huang, X., Hai-Jun, L., Zhang, Y., De-chang, P., Peihong, H., Yu-Lin, Z., et al. (2016a). Microstructural changes of the whole brain in patients with comitant strabismus: evidence from a diffusion tensor imaging study. Neuropsychiatr. Dis. Treat. 12, 2007–2014. doi: 10.2147/NDT.S108834
PubMed Abstract | Crossref Full Text | Google Scholar
Huang, X., Li, S. H., Zhou, F. Q., Zhang, Y., Zhong, Y. L., Cai, F. Q., et al. (2016b). Altered intrinsic regional brain spontaneous activity in patients with comitant strabismus: a resting-state functional MRI study. Neuropsychiatr. Dis. Treat. 12, 1303–1308. doi: 10.2147/NDT.S105478
PubMed Abstract | Crossref Full Text | Google Scholar
Huang, X., Zhou, S., Su, T., Ye, L., Zhu, P. W., Shi, W. Q., et al. (2018). Resting cerebral blood flow alterations specific to the comitant exophoria patients revealed by arterial spin labeling perfusion magnetic resonance imaging. Microvasc. Res. 120, 67–73. doi: 10.1016/j.mvr.2018.06.007
PubMed Abstract | Crossref Full Text | Google Scholar
Kang, J. M., Cho, Y. S., Park, S., Lee, B. H., Sohn, B. K., Choi, C. H., et al. (2018). Montreal cognitive assessment reflects cognitive reserve. BMC Geriatr. 18:261. doi: 10.1186/s12877-018-0951-8
PubMed Abstract | Crossref Full Text | Google Scholar
Kaur, S., Sukhija, J., Khanna, R., Takkar, A., and Singh, M. (2019). Diplopia after excessive smart phone usage. Neuroophthalmology 43, 323–326. doi: 10.1080/01658107.2018.1518988
PubMed Abstract | Crossref Full Text | Google Scholar
Lee, Y. H., Repka, M. X., Borlik, M. F., Velez, F. G., Perez, C., Yu, F., et al. (2022). Association of Strabismus with Mood Disorders, schizophrenia, and anxiety disorders among children. JAMA Ophthalmol. 140, 373–381. doi: 10.1001/jamaophthalmol.2022.0137
PubMed Abstract | Crossref Full Text | Google Scholar
Liang, M., Xie, B., Yang, H., Yin, X., Wang, H., Yu, L., et al. (2017). Altered interhemispheric functional connectivity in patients with anisometropic and strabismic amblyopia: a resting-state fMRI study. Neuroradiology 59, 517–524. doi: 10.1007/s00234-017-1824-0
PubMed Abstract | Crossref Full Text | Google Scholar
Lin, S., Congdon, N., Yam, J. C., Huang, Y., Qiu, K., Ma, D., et al. (2014). Alcohol use and positive screening results for depression and anxiety are highly prevalent among Chinese children with strabismus. Am. J. Ophthalmol. 157, 894–900.e1. doi: 10.1016/j.ajo.2014.01.012
PubMed Abstract | Crossref Full Text | Google Scholar
Lowe, K. A., Zinke, W., Cosman, J. D., and Schall, J. D. (2022). Frontal eye fields in macaque monkeys: prefrontal and premotor contributions to visually guided saccades. Cereb. Cortex 32, 5083–5107. doi: 10.1093/cercor/bhab533
PubMed Abstract | Crossref Full Text | Google Scholar
Mancuso, L., Costa, T., Nani, A., Manuello, J., Liloia, D., Gelmini, G., et al. (2019). The homotopic connectivity of the functional brain: a meta-analytic approach. Sci. Rep. 9:3346. doi: 10.1038/s41598-019-40188-3
PubMed Abstract | Crossref Full Text | Google Scholar
Marteau, T. M., and Bekker, H. (1992). The development of a six-item short-form of the state scale of the Spielberger state-trait anxiety inventory (STAI). Br. J. Clin. Psychol. 31, 301–306. doi: 10.1111/j.2044-8260.1992.tb00997.x
Crossref Full Text | Google Scholar
Mohan, A., Sen, P., Mujumdar, D., Shah, C., and Jain, E. (2021). Series of cases of acute acquired comitant esotropia in children associated with excessive online classes on smartphone during COVID-19 pandemic; digital eye strain among kids (DESK) study-3. Strabismus 29, 163–167. doi: 10.1080/09273972.2021.1948072
PubMed Abstract | Crossref Full Text | Google Scholar
Neena, R., Remya, S., and Anantharaman, G. (2022). Acute acquired comitant esotropia precipitated by excessive near work during the COVID−19-induced home confinement. Indian J. Ophthalmol. 70, 1359–1364. doi: 10.4103/ijo.IJO_2813_21
PubMed Abstract | Crossref Full Text | Google Scholar
Neulinger, K., Oram, J., Tinson, H., O'Gorman, J., and Shum, D. H. (2016). Prospective memory and frontal lobe function. Neuropsychol. Dev. Cogn. B Aging Neuropsychol. Cogn. 23, 171–183. doi: 10.1080/13825585.2015.1069252
PubMed Abstract | Crossref Full Text | Google Scholar
Peng, J., Yao, F., Li, Q., Ge, Q., Shi, W., Su, T., et al. (2021). Alternations of interhemispheric functional connectivity in children with strabismus and amblyopia: a resting-state fMRI study. Sci. Rep. 11:15059. doi: 10.1038/s41598-021-92281-1
PubMed Abstract | Crossref Full Text | Google Scholar
Petrides, M., and Pandya, D. N. (1999). Dorsolateral prefrontal cortex: comparative cytoarchitectonic analysis in the human and the macaque brain and corticocortical connection patterns. Eur. J. Neurosci. 11, 1011–1036. doi: 10.1046/j.1460-9568.1999.00518.x
PubMed Abstract | Crossref Full Text | Google Scholar
Petrides, M., and Pandya, D. N. (2002). Comparative cytoarchitectonic analysis of the human and the macaque ventrolateral prefrontal cortex and corticocortical connection patterns in the monkey. Eur. J. Neurosci. 16, 291–310. doi: 10.1046/j.1460-9568.2001.02090.x
Crossref Full Text | Google Scholar
Purcell, B. A., Weigand, P. K., and Schall, J. D. (2012). Supplementary eye field during visual search: salience, cognitive control, and performance monitoring. J. Neurosci. 32, 10273–10285. doi: 10.1523/JNEUROSCI.6386-11.2012
PubMed Abstract | Crossref Full Text | Google Scholar
Ruatta, C., and Schiavi, C. (2020). Acute acquired concomitant esotropia associated with myopia: is the condition related to any binocular function failure? Graefes Arch. Clin. Exp. Ophthalmol. 258, 2509–2515. doi: 10.1007/s00417-020-04818-1
Crossref Full Text | Google Scholar
Shanker, V., and Nigam, V. (2015). Unusual presentation of spasm of near reflex mimicking large-angle acute acquired Comitant Esotropia. Neuro-Ophthalmology 39, 187–190. doi: 10.3109/01658107.2015.1053619
PubMed Abstract | Crossref Full Text | Google Scholar
Shao, Y., Bao, J., Huang, X., Zhou, F. Q., Ye, L., Min, Y. L., et al. (2018). Comparative study of interhemispheric functional connectivity in left eye monocular blindness versus right eye monocular blindness: a resting-state functional MRI study. Oncotarget 9, 14285–14295. doi: 10.18632/oncotarget.24487
PubMed Abstract | Crossref Full Text | Google Scholar
Song, K., Lv, Y. L., Yang, L. J., Lv, P., Ren, B., Tian, J., et al. (2023). Alternations of interhemispheric functional connectivity in patients with optic neuritis using voxel-mirrored homotopic connectivity: a resting state fMRI study. Brain Imaging Behav. 17, 1–10. doi: 10.1007/s11682-022-00719-5
PubMed Abstract | Crossref Full Text | Google Scholar
Tan, G., Dan, Z., Zhang, Y., Huang, X., Yu-Lin, Z., Lin-Hong, Y., et al. (2018). Altered brain network centrality in patients with adult comitant exotropia strabismus: a resting-state fMRI study. J. Int. Med. Res. 46, 392–402. doi: 10.1177/0300060517715340
PubMed Abstract | Crossref Full Text | Google Scholar
Tan, G., Huang, X., Zhang, Y., Wu, A. H., Zhong, Y. L., Wu, K., et al. (2016). A functional MRI study of altered spontaneous brain activity pattern in patients with congenital comitant strabismus using amplitude of low-frequency fluctuation. Neuropsychiatr. Dis. Treat. 12, 1243–1250. doi: 10.2147/NDT.S104756
留言 (0)