The skin, being the largest organ of the human body, covers the entire surface of the body and functions as a crucial protective barrier (Wong et al., 2023). However, the skin is susceptible to microbial, chemical, physical, and immunologic factors that can cause dermatologic diseases (Mummed et al., 2018). Dermatologic disease is a general term for diseases that occur in the skin and its accessory organs (Lim et al., 2022). Interestingly, a variety of diseases occurring in internal organs can also manifest in the skin (Valdez et al., 2015). At present, there are over 2,000 recognized dermatologic diseases with unique clinical characteristics. This extensive variety is indicative of the diverse nature of these conditions and the challenges in their treatment and diagnoses. The incidence of dermatologic diseases has surged due to a confluence of various factors. Social progress, changes in the living environment, increasingly serious air pollution, and poor living and eating habits collectively contribute to this increase. According to a report by the World Health Organization (WHO), approximately 20%–30% of the global population suffers from some form of dermatologic disease (Gu et al., 2024). This means that over 1.5 to 2 billion people worldwide are currently dealing with skin health issues. These conditions can affect individuals at any age, from birth to death, and can involve any part of the body. Some dermatologic diseases are difficult to cure and are prone to recurrence even after treatment. The health burden caused by dermatologic diseases ranks fourth among all diseases in terms of disability-adjusted life years. This not only significantly impacts patients’ quality of life but also places a substantial strain on global healthcare systems (Adly et al., 2021; Donetti et al., 2023).
Clinical manifestations associated with dermatosis often involve sensations of itchiness, pain, and numbness because the skin tissue is very rich in nerves. Beyond physical symptoms, dermatologic injuries can lead to secondary psychological problems such as anxiety, anger, the contagious nature of certain dermatologic diseases poses not only a threat to individual wellbeing but also causes widespread panic and social discrimination, causing great physical and psychological pain to patients (Feldo et al., 2022). Clinically, antibiotics, antihistamines, painkillers, or hormonal drugs are commonly used to treat dermatologic diseases (Fattah and Darwish, 2013). However, these therapeutic measures are associated with certain limitations. The efficacy of these medications is short-lived, and the dermatologic disease is prone to relapse after cure (Xie Y. X. et al., 2023). A major issue is the emergence of drug resistance resulting from prolonged usage of these medications. Additionally, researches have noted potential negative impacts of these medications on liver and kidney functionality, as well as the nervous system. Therefore, the use of these medications is restricted in certain populations, such as pregnant women, the elderly, and individuals in specific high-risk occupations, including pilots, athletes, law enforcement officers, and military personnel, due to their potential adverse effects.
Recently, important insights have emerged about the crucial function of natural phytochemicals in the clinical management of dermatologic disease, such as green tea polyphenols, apigenin, shikonin and others (Fowler et al., 2010; Ranneh et al., 2016; Pourang et al., 2020; Xie W. et al., 2023). These natural medicines present distinct advantages, being less irritating to the skin, less prone to inducing resistance, and exhibiting a good protective effect on the microbiome balance of the skin (Cintosun et al., 2020; Patel et al., 2023). Zhu et al. utilized Epigallocatechin-3-gallate (EGCG), the main chemical metabolite of green tea polyphenols, to treat vitiligo induced by topical application of monobenzone in a mouse model. Histological examination suggested a reduction in CD8+ T cell infiltration at the lesions, and it was hypothesized that EGCG might have an inhibitory effect on vitiligo autoimmunity (Zhu et al., 2014). Zhang et al. conducted research wherein PIG3V cells, stimulated with H2O2, were treated with apigenin (a plant-derived aglycone). Subsequently, they assessed the activity and oxidative stress-related parameters by enzyme-linked immunosorbent assay. Their findings indicated that apigenin enhanced the expression of cellular antioxidants superoxide dismutase (SOD), catalase (CAT), and glutathione peroxidase (GSH-Px) and shielded melanocytes from oxidative damage through activation of the Nrf2 pathway (Zhang et al., 2020). Wu et al. found that the activity of the human oncogene p53 was enhanced in a cycle-blocking assay involving shikonin, a naphthoquinone metabolite extracted from Arnebiae Radix, on human malignant melanoma cells. This study demonstrated that shikonin inhibited the growth of A375-S2 cells in a time- and concentration-dependent manner, with a 48-hour IC50 value of 7.1 ± 0.9 μmol/L (Wu et al., 2004). Another study reported that shikonin derivatives induced apoptosis in mouse melanoma high metastatic cells B16F10 by activating caspase3, a protease involved in apoptosis execution. Furthermore, these derivatives induced sub-G1 cell cycle arrest, showing anticancer activity (Rajasekar et al., 2012). In addition, a significant finding was made about the effects of SSA (saikosaponin A) and SSC (saikosaponin C) obtained from Radix bupleuri. It was discovered that these substances inhibited TNF-α-induced thymic stromal lymphopoietin (TSLP) expression at the transcriptional level through the suppression of mitogen-activated protein kinase (MAPK) signaling, which mediates the transcriptional activation of early growth response factor-1 (EGR-1) in human immortalized epidermal cells (HaCaT) keratinocytes. The topical use of SSA or SSC was shown to enhance the condition of atopic dermatitis-like skin lesions in 2, 4- dinitrochlorobenzene (DNCB) mice (Ahn et al., 2022). These studies indicate that natural phytochemicals significantly affect the clinical management of dermatologic conditions, highlighting their potential, research importance, and practical value in treating dermatologic diseases.
Among the natural phytochemicals, the total glucosides of paeony (TGP), extracted from Paeonia lactiflora Pall., have been widely applied in clinical research for the treatment of dermatologic diseases and have garnered significant attention from scholars. To further elucidate the mechanisms of action and potential applications of TGP in dermatologic diseases, this article provides an overview of the botany and traditional uses of Paeonia lactiflora Pall.. Additionally, it reviews the extraction and purification processes of TGP, its main active metabolites, quality control measures, as well as clinical research and pharmacological mechanisms related to its use in dermatologic diseases. Furthermore, the article identifies gaps in current research and suggests directions for future studies. By reviewing these aspects, the article seeks to provide a valuable and comprehensive reference for future in-depth clinical investigations.
2 Materials and methodsThe literature review was performed using various databases, including Web of Science (https://webofscience.clarivate.cn), Baidu Scholar (http://xueshu.baidu.com/), ScienceDirect (www.sciencedirect.com), PubMed (https://www.ncbi.nlm.nih.gov/), CNKI (https://www.cnki.net/), and WanFang DATA (http://www.wanfangdata.com.cn/index.html). Additional resources were obtained from library searches encompassing classical texts on Chinese herbal medicine, peer-reviewed journals, local periodicals, and master’s and doctoral dissertations. The search utilized keywords such as “Total glucosides of paeony,” “Paeonia lactiflora Pall.,” “Paeonia veitchii Lynch,” “Paeoniae Radix Alba or white peony,” “Paeoniflorin,” “Albiflorin,” “TGP,”“dermatologic disease,”“skin disease,” and their combinations. Duplicate articles identified during the search process were excluded. The Plant List database (http://www.plantsoftheworldonline.org/) was consulted to confirm scientific names and provide details on subspecies and varieties of Paeonia plants.
3 Botanical description and distribution of Paeonia lactiflora Pall.Paeonia lactiflora Pall. is a perennial herbaceous plant belonging to the Ranunculaceae family and the Paeoniaceae subfamily. There are approximately 30 species of plants in the Paeonia genus, which are mainly divided into two categories: herbaceous peonies and tree peonies. Native to China, it is widely distributed across the temperate regions of Asia, North America and Europe. The plant features robust rhizomes and compound leaves with a deep green coloration, and the leaf margins often exhibit wavy or serrated edges. Paeonia lactiflora Pall. is renowned for its large, vibrant flowers, which come in a variety of colors, including white, pink, red and purple, making it highly valued for ornamental purposes (Figures 1A). The rhizome of Paeonia lactiflora Pall. holds significant medicinal value in traditional Chinese medicine (Xie et al., 2017; Zhang et al., 2022a). According to the 2020 edition of the Pharmacopoeia of China, the roots of Paeonia lactiflora Pall. are harvested in the summer and autumn seasons. After harvesting, the roots are cleaned, boiled, peeled and then sun-dried. The dried root of the plant Paeonia lactiflora Pall (Figures 1B, C). has been used in traditional Chinese medicine for many centuries.

Figure 1. Photographs of above-ground parts (A), medicinal portion (B) and commercially applied botanical drugs of Paeonia lactiflora Pall. (C).
4 Traditional uses of Paeonia lactiflora Pall.The medicinal history of Paeonia lactiflora Pall. (Shao Yao) can be traced back to the ''Shen Nong Ben Cao Jing'', the earliest extant pharmacopeia in China, written during the Dong Han dynasty (approximately A.D. 25–220). In the ''Shen Nong Ben Cao Jing'', Shao Yao classified as a top-grade medicinal botanical drug with nourishing properties. The book provides a detailed description of its medicinal properties and therapeutic effects, stating that it can nourish the blood, soothe the liver and alleviate pain and muscle spasms. In China, hundreds of ancient formulas have used Paeonia lactiflora Pall. as the main drug, such as Danggui Shaoyao Powder, Zhishi Shaoyao Powder, Baizhu Shaoyao Powder, Shaoyao Decoction, and Shaoyao Gancao Decoction (Tian et al., 2022). Therefore, Paeonia lactiflora Pall. is one of the most important botanical drugs in traditional medicine.
5 The extraction and purification process of TGPThe dried roots of Paeonia lactiflora Pall., conforming to pharmacopoeial standards, are selected as raw materials and pulverized to a fine particle size. Water and ethanol are commonly used as extraction solvents, employing techniques such as maceration, reflux extraction, and ultrasound-assisted extraction (Wu et al., 2019). However, the crude extract of TGP typically contains a significant number of impurities. Therefore, purification is necessary to obtain high-purity TGP. The primary methods for the isolation and purification of TGP currently include filtration and rotary evaporation, organic solvent extraction, column chromatography, and recrystallization (Deng et al., 2010; Peng et al., 2023).
6 Main active metabolites of TGPTGP primarily contains various monoterpene glycosides. (Chen et al., 2020; Xu et al., 2023). The key metabolites include paeoniflorin (PF), albiflorin, oxypaeoniflorin, benzoylpaeoniflorin, benzoyloxypaeoniflorin, lactiflorin, albiflorin R1, and benzoylpaeoniflorin lactone. Notably, PF accounts for more than 90% of the TGP (Santana et al., 2016; Tu et al., 2019; Xu et al., 2023). As the primary active metabolite of Paeonia lactiflora Pall., PF essentially represents the main efficacy of TGP (Zhang et al., 2008; Jin and Zhang, 2022; Liu et al., 2023). The main monoterpene glycosides metabolites of TGP are shown in Table 1.
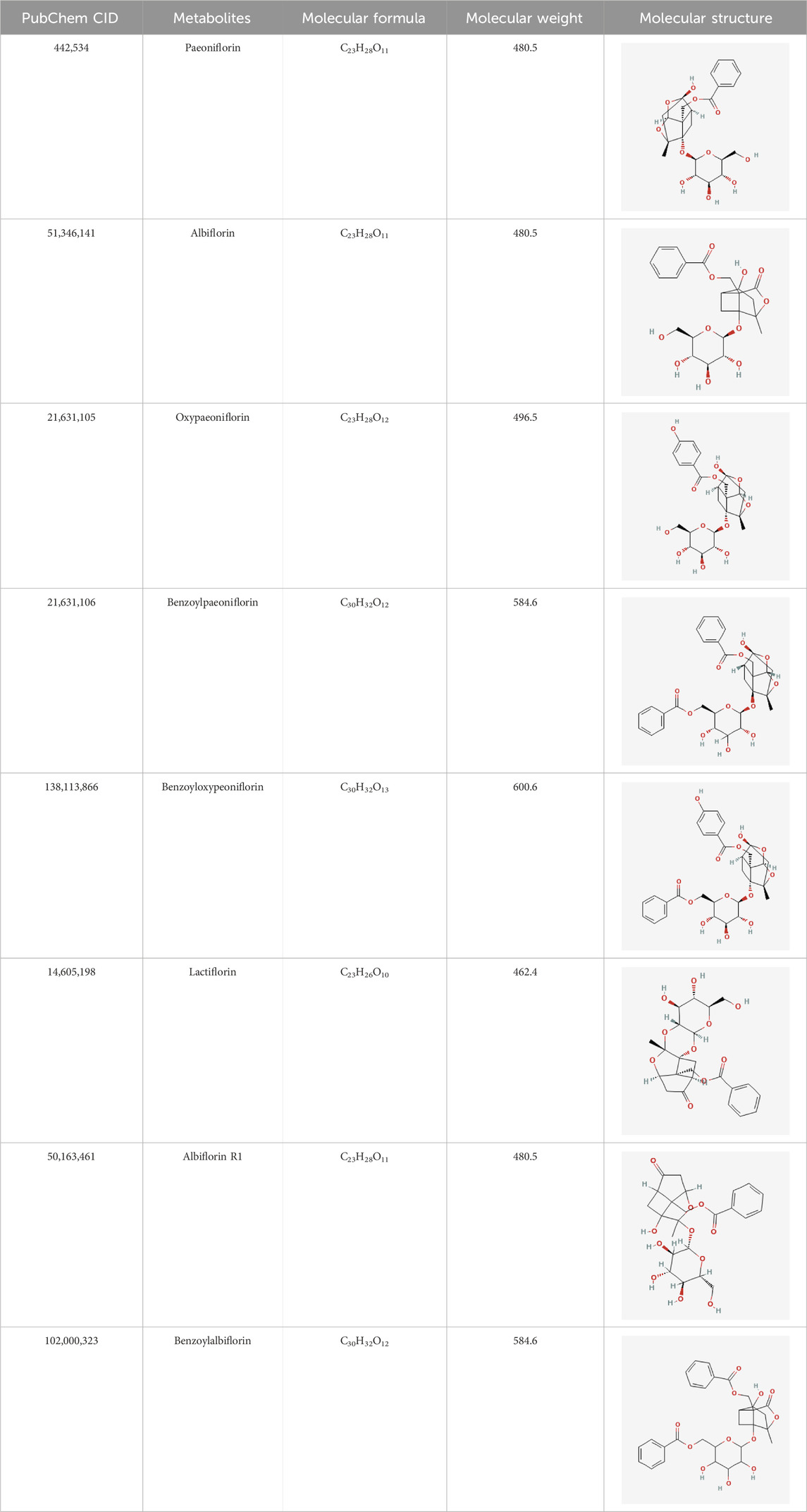
Table 1. The main chemical metabolites of the monoterpene glycosides in TGP.
7 Phytochemical quality control of TGPThe quality of herbal products used by humans is of paramount importance due to their direct impact on human health. Moreover, with the rapidly increasing demand for traditional herbal products in recent years, it is essential to implement stringent quality control measures (Jakimiuk and Tomczyk, 2024). The WHO established guidelines for quality control of medicinal plant materials, focusing on factors such as organoleptic properties, ash content, moisture levels, microbial contamination, and chromatographic and spectroscopic analysis (Chandel et al., 2011). The qualitative assessment of herbal products is primarily conducted using UV, IR and TLC techniques, while quantitative analysis is typically performed using methods such as HP-TLC, HPLC, SFC, LC-MS or GC-MS (Ramaswamy et al., 2014). From a pharmacological perspective, Paeonia lactiflora Pall. seems to be the most valuable. The quality control of TGP also includes the determination of the contents of paeoniflorin, albiflorin, benzoylpaeoniflorin, and other related metabolites (Table 2). According to the Chinese Pharmacopoeia, the paeoniflorin content in Paeonia lactiflora Pall. botanical drugs should be standardized to not less than 1.6% (dry substance). Pu et al. used HPLC to determine the content of ethanol-water extracts of Paeonia lactiflora Pall (Pu et al., 2011). Furthermore, Ren et al. used ultra-high-performance liquid chromatography to determine the content of paeoniflorin in Paeonia lactiflora Pall (Ren et al., 2015).
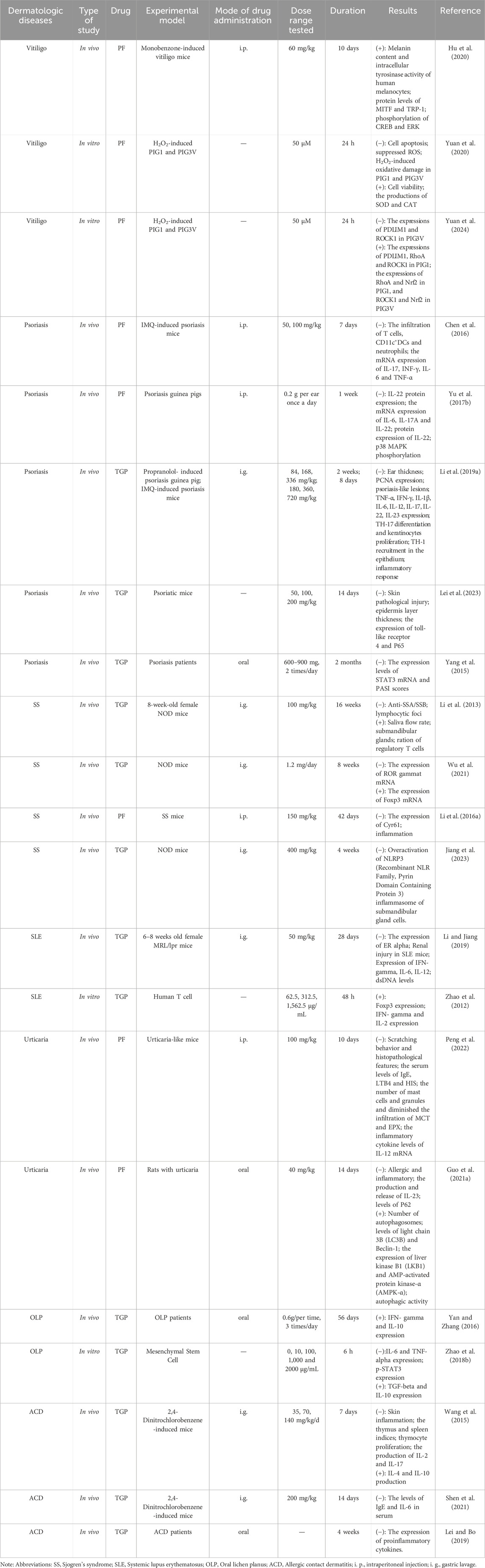
Table 2. The pharmacologic study of TGP and its main active metabolites in dermatologic diseases.
8 The pharmacological and clinical research progress of TGP and its main metabolites in the treatment of dermatologic diseasesModern pharmacological studies have shown the diverse effects of TGP, such as anti-inflammatory, antioxidant, immunomodulatory, hepatoprotective, and analgesic, accompanied by mild adverse effects (Feng et al., 2019). Currently, Paeonia lactiflora Pall. is commonly utilized in managing rheumatoid arthritis (Lin et al., 2012; Wei et al., 2013), diabetic nephropathy (Xu et al., 2014; Shao et al., 2016), ankylosing spondylitis (Huang et al., 2019; Yang et al., 2023), hepatitis B (Zuo et al., 2017), obesity (Fang et al., 2023; Yao et al., 2024), and other diseases. In recent times, using TGP has been extended to the management of a wide range of dermatologic diseases encompassing vitiligo, psoriasis, contact dermatitis, lichen planus, sjogren’s syndrome and systemic lupus erythematosus. The diversity of pharmacological effects and low processing cost of peony make it ideal for treating dermatologic diseases (Gong et al., 2022) (Tables 2, 3). Despite the rich and well-established pharmacological effects of TGP, its utilization in dermatologic disease treatments is still in its infancy. Consequently, researchers globally are delving into the mechanisms underlying the action of TGP (Jiang et al., 2020).
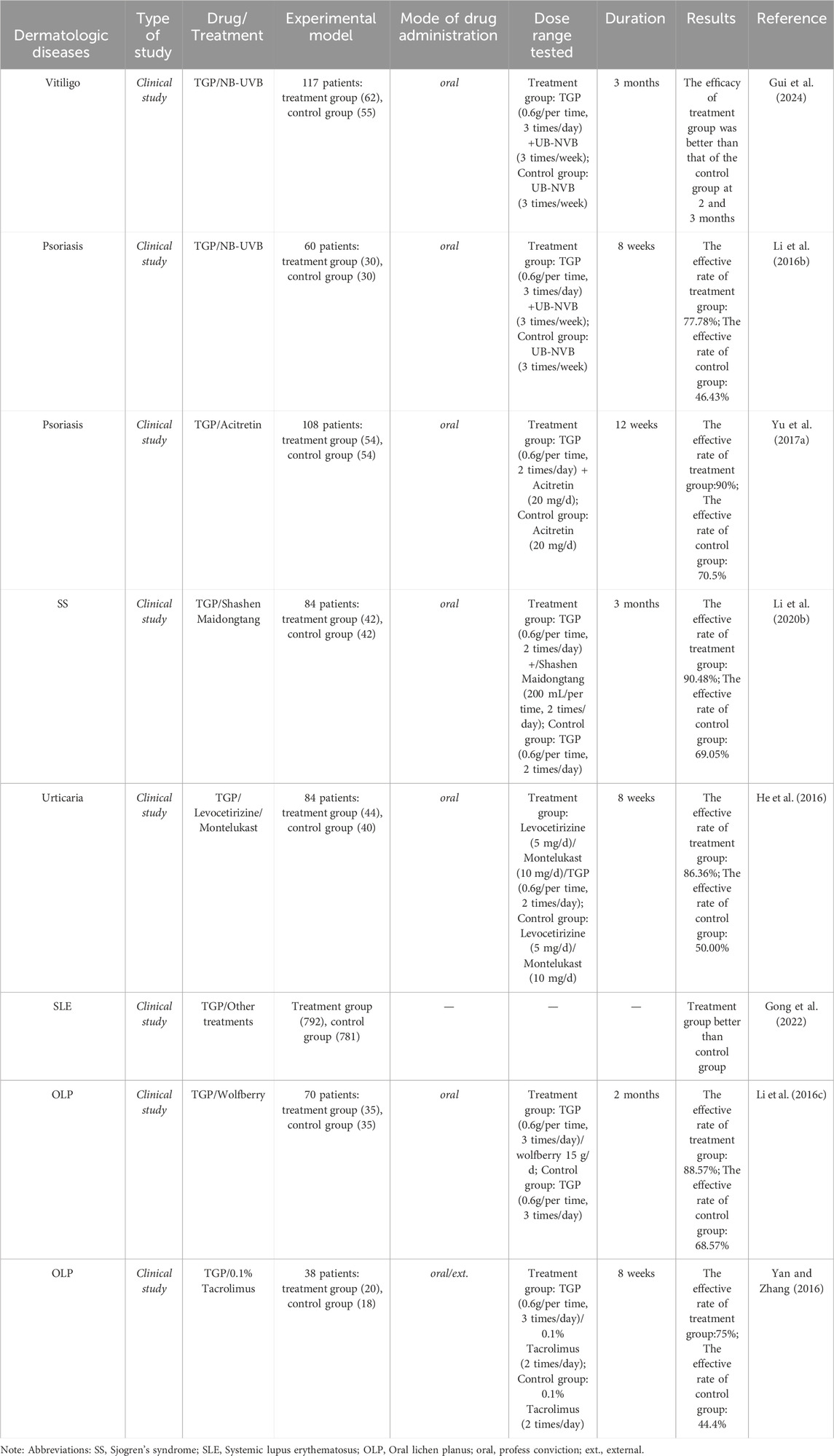
Table 3. The clinical studies of TGP and its main active metabolites in dermatologic diseases.
8.1 Application of TGP in vitiligoVitiligo is a clinically common, limited pigment loss dermatologic disease that is not limited by age or location. The prevalence of vitiligo is about 0.5%–2% worldwide (Hu et al., 2023). The clinical features of vitiligo mainly include skin discoloration, showing well-defined ivory or chalky white patches. These patches can appear on the skin of various parts of the body, including the face, genitals, areola, and areas of repeated trauma, and can also involve mucous membranes (Ezzedine et al., 2015). Vitiligo can be classified into three types based on clinical characteristics: segmental vitiligo, non-segmental vitiligo, and mixed vitiligo (Du et al., 2023). Various external factors such as mental stress, trauma, and exposure to sunlight are closely related to the onset of vitiligo. The condition typically emerges when an individual, influenced by certain genetic factors, experiences the destruction of melanocytes due to various internal and external factors. This disruption impairs the production or melanization process ultimately leading to melanin loss. The mechanism of action of traditional Chinese medicine for vitiligo mainly involves tyrosinase, cholinesterase, melanocytes, immune function, cytokines, inflammatory mediators, and oxidative stress (Xie Y. H. et al., 2023).
Microphthalmia-associated transcription factors (MITF) is significantly involved in the regulation of melanogenesis and it can activate and regulate the expression of several key genes involved in melanin production, including tyrosinase and tyrosinase-related proteins (TRP-1 and TRP-2), thereby promoting melanin synthesis. Tyrosinase, recognized as the rate-limiting enzyme in melanogenesis, catalyzes tyrosine hydroxylation and oxidizes downstream DOPA to DOPAquinone for the spontaneous synthesis of melanin. In addition, TRP-1 and TRP-2 are involved in melanin biosynthesis downstream of tyrosinase (Liu et al., 2017; Pang et al., 2021; Di Bartolomeo et al., 2023). In a study by Hu et al., a mouse model of vitiligo was treated using PF at a level of 10 μg per milliliter to assess its impact and mechanism of action. The acquired data showed that the addition of PF significantly elevated melanin content and intracellular tyrosinase activity. This process upregulated the expression of MITF and TRP-1 through the ERK/CREB pathway (Hu et al., 2020). These processes positively affect the proliferation of human melanocytes, increase melanin biosynthesis, and attenuate the pathological changes in vitiligo mice. The resulting data affirmed the potential of PF for the treatment of vitiligo.
Oxidative stress plays a key role in the development and advancement of vitiligo. Imbalances in oxidative stress pathways such as decreased catalase levels, increased malondialdehyde, increased cell membrane lipid peroxidation, and dysregulation of superoxide dismutase have been observed in vitiligo patients. These events lead to elevated levels of overall oxidative/antioxidant balance in vitiligo patients, which manifests as increased levels of oxidative stress and decreased antioxidant capacity, ultimately leading to vitiligo onset and lesion progression (Jalel and Hamdaoui, 2009; Qiao et al., 2016). Notably, H2O2-induced oxidative stress is a key factor in the pathogenesis and progression of vitiligo. Yuan et al. demonstrated that PF activates the JNK/Nrf2/HO-1 signaling pathway and interacts with the PDLIM1/RhoA/ROCK1 pathway to protect SOD (PIG1, PIG3V) from oxidative damage. In addition, PF reduced the buildup of reactive oxygen species (ROS) and apoptosis by enhancing the levels of the antioxidant enzymes superoxide dismutase (SOD), catalase (CAT), MITF, and TRP-1. In addition, PF upregulated the expression of myoglobin, prostaglandin reductase 1 (PTGR1), LIM structural domain protein 1 (PDLIM1), Ras homologous gene family member A (RhoA), Rho-associated protein kinase (ROCK1), and F-actin in PIG1 cells. Conversely, in PIG3V cells, PF induced a downregulation of the expression of PTGR1, PDLIM1, ROCK1, and F-actin. Notably, these molecular changes were associated with an increase in melanin synthesis. The above studies demonstrated the potential therapeutic effects of PF in vitiligo, revealing that PF may inhibit oxidative stress through the Nrf2-ARE/HO-1 signaling pathway, thereby protecting melanocytes from oxidative damage (Yuan et al., 2020). In a separate investigation, Xiao found that PF promoted the proliferation of PIG3V cells. Subsequently, this process inhibited H2O2-induced expression of chemokine G protein-coupled receptor 17 (GPR17) and expression of IL-1β and IL-6 in PIG3V cells. These regulatory actions were achieved through the nuclear factor (NF)-κB pathway. Notably, this modulation of molecular pathways resulted in attenuating GPR17-mediated cytotoxic T-cell (CD8+T) migration to the site of inflammatory injury and reducing the inflammatory cascade of melanocyte response (Xiao, 2019).
The clinical treatment of vitiligo using TGP and its main active metabolites has also made certain research progress in addition to pharmacological studies. Gui et al. conducted a study to evaluate the efficacy of TGP combined with oral mini-pulse therapy (OMP) and narrow-band ultraviolet B (NB-UVB) in the treatment of active nonsegmental vitiligo (NSV). Data from 62 patients who received the TGP combination therapy and 55 patients who did not receive the TGP combination therapy were analyzed over a 3-month period. The results demonstrated that the combination of TGP with OMP and NB-UVB achieved superior therapeutic outcomes compared to OMP + NB-UVB alone. In the TGP group, the majority of patients experienced improvements in skin white patches during the course of treatment. Notably, there was no increase in side effects or recurrence rates in this group (Gui et al., 2024). Ye et al. utilized TGP combined with pimecrolimus ointment to treat patients with disseminated vitiligo. Clinical findings indicate that TGP may moderately elevate the CD4+/CD8+ T cell ratio and the level of CD4+CD25+ Treg cells in patients’ peripheral blood. These immunological adjustments are crucial for preserving the body’s immune balance. Therefore, TGP is implicated as one of the processes contributing to the healing of skin lesions in patients and in reducing the recurrence of skin lesions in vitiligo. However, this effect diminishes with increasing disease duration. In addition, TGP may provide a new option for patients with combined hepatic insufficiency due to its blood- and liver-nourishing effects (Ye et al., 2013).
In summary, TGP demonstrates positive effects on the clinical treatment of vitiligo and their adverse reactions are few. However, there are fewer current studies utilizing TGP in the treatment of vitiligo. This study presents a safe and promising new approach for treating refractory vitiligo clinically.
8.2 Application of TGP in psoriasisPsoriasis, a chronic inflammatory dermatologic disease, is marked by abnormal T-cell activation, infiltration, and excessive growth of skin keratinocytes (Kim et al., 2017). The disease’s development is associated with immune inflammation, oxidative stress, and imbalances in cell growth and apoptosis (Chimenti et al., 2018). Currently, traditional retinoids, vitamin D3 derivatives, glucocorticoids, immunosuppressants, and biologics are still the mainstay of treatment for psoriasis (Zhang L. H. et al., 2024). Despite being the primary approach, these treatments come with limitations, including significant adverse effects, high relapse rate, long duration of disease, and high price. Hence, the exploration of new methods that offer a high cure rate, are cost-effective, and exhibit lower adverse reactions is extremely important. In recent years, traditional Chinese medicine’s active metabolites have become prominent in psoriasis treatment by virtue of their high cure rate, affordability, and low incidence of adverse effects (Wang and Liu, 2004; Guo et al., 2024; He et al., 2024). TGP has emerged as one such metabolite, exhibiting promising research outcomes and gaining widespread utilization in the treatment of psoriasis.
The main pathological changes in psoriasis include excessive keratinocyte proliferation, epidermal hyperplasia, and increased neoangiogenesis, accompanied by inflammatory cell infiltration (Luo et al., 2020; Orsmond et al., 2021; Pandey et al., 2021). Vascular endothelial growth factor (VEGF), a strong promoter of angiogenesis, is rarely expressed or even absent in normal epidermal cells. However, it is abundantly expressed in keratinocytes of patients with psoriasis, especially in lesions. Therefore, VEGF has the potential to produce psoriasis-like lesions and typical Koebner-like psoriasis damage by inducing a vascular inflammatory response (Voiculescu et al., 2014; Chen et al., 2023). Yu’s study showed that PF had an inhibitory effect on keratinocyte growth factor (KGF)-induced HaCaT cell proliferation. This effect is thought to be achieved by PF through the regulation of the p38 MAPK/NF-κB p65 signaling pathway, which downregulates IL-22 and VEGF mRNA expression (Yu et al., 2007). Zhang’s study found that TGP reduced VEGF mRNA expression in the skin of psoriatic mice. Therefore, TGP may treat psoriasis by inhibiting keratinocyte proliferation and neoangiogenesis (Zhang, 2015).
The onset and progression of psoriasis are closely related to immune dysfunction. In addition to keratinocytes in the skin, immune cells, and their cytokines are crucial in the pathogenesis of psoriasis (Zhao X. T. et al., 2018; Shin et al., 2020). Dendritic cells (DCs) and macrophages in the dermis of psoriasis produce IL-23, which induces the activation of immune cells such as Th1 cells and the release of inflammatory cytokines like IL-22, IL-6, and tumor necrosis factor-α (TNF-α). These inflammatory cytokines act on keratin-forming cells, leading to typical pathological changes associated with psoriasis such as hyperkeratosis of the epidermis and hypertrophy of the stratum spinosum (Zhao et al., 2022). Paeoniflorin, the main metabolite of TGP, can inhibit the differentiation, maturation, and function of DCs, which provides a theoretical basis for the treatment of autoimmune diseases with Paeonia lactiflora Pall (Zhou et al., 2012; Sun et al., 2015). Li et al. found that TGP could reduce psoriasis-like damage, such as erythema, scaling, and inflammatory cell infiltration of the auricular skin of mice induced by imiquimod. Furthermore, TGP exhibited the potential to decrease the proliferation of keratin-forming cells. Mechanistically, TGP exerts antiproliferative effects by blocking the phosphorylation of total or phosphorylated signal transducers and activators of transcription (STAT1 and STAT3) in skin lesions, inhibiting their mRNA expression and T-helper 17 (Th17) cell differentiation, and downregulating IL-17A and IL-22 levels (Li B. B. et al., 2019). According to Zhao’s study, PF inhibited imiquimod-induced psoriasis. They noted that PF reduced keratinocyte proliferation and inflammatory cell infiltration, and decreased Th17 cytokine mRNA expression. In addition, PF did not affect splenocyte viability but decreased IL-17 secretion under Th17-polarized conditions. PF inhibited Th17 cytokine mRNA expression and STAT3 phosphorylation in splenocytes under Th17-polarized conditions. These findings suggest that PF can inhibit imiquimod-induced psoriasis by modulating Th17 cell responses and cytokine secretion through STAT3 phosphorylation (Zhao et al., 2016).
As a chronic inflammatory dermatologic disease, psoriasis is closely linked to chronic inflammatory responses mediated by T cells and epidermal cells (Reich et al., 2001; Homey and Bunemann, 2004; Liao et al., 2023). Reducing the level of pro-inflammatory cytokines can attenuate the inflammatory response at the skin lesions of psoriasis mice, which in turn improves the symptoms of skin lesions (Wang et al., 2023). Sun et al. established a mouse model of psoriasis. In addition, they observed that PF improved psoriasis lesions and decreased the number of F4/80+CD68+ macrophages, CD11b+Gr-1+ neutrophils, and their secretion of cytokines (TNF-α, IL-1β, IL-6, IL-12, and IL-23), inducible nitric oxide synthase (iNOS), and macrophage inflammatory protein-2 (MIP-2) in the lesions. In addition, PF downregulated the expression of Th1/Th17-related cytokines (Sun et al., 2015). In another study, Yu et al. found that TGP-treated psoriasis mice exhibited a reduction in IL-22 and p38 proteins, as well as a decrease in the mRNA levels of IL-6, IL-17A, and IL-22. Therefore, TGP can inhibit the phosphorylation of the p38 MAPK pathway, downregulate the expression of VEGF, IL-23, and its proteins in HaCaT cells, and reduce the expression levels of IL-6, IL-17A, IL-22, IL-23, thus exerting anti-inflammatory and antiproliferative effects (Yu et al., 2007).
Research indicated that the application of TGP in conjunction with other drugs or physiotherapy was effective in treating psoriasis. In addition, this treatment can reduce adverse effects in individuals and improve their adherence to the treatment (Tang et al., 2014). Currently, psoriasis is usually treated with TGP in combination with retinoids or narrow-spectrum medium-wave ultraviolet light (Li T. T. et al., 2019). In a search for alternative treatments, Yu et al. found that the combination of TGP and acitretin not only improved the therapeutic efficacy of psoriasis, but also reduced the liver damage caused by acitretin (Yu C. et al., 2017). A multicenter, double-blind, placebo-controlled, randomized clinical trial on the efficacy and safety of acitretin combined with TGP in the treatment of moderate-to-severe plaque psoriasis (108 cases) was conducted. The resulting data showed that the body surface area (BSA) and psoriasis area and severity index (PASI) values of the experimental group (20–30 mg/d of acitretin +1.8 g/d of TGP for 12 consecutive weeks) were markedly reduced in contrast to the control group (20–30 mg/day of acitretin + placebo for 12 consecutive weeks). In addition, the serum aminotransferase levels of the former group were lower than those of the latter group, suggesting that the combination of TGP treatment can increase the clinical efficacy and reduce the hepatotoxicity of acitretin (Yu et al., 2015). Thus, this implies that the combination therapy not only decreased PASI scores, but also reduced hepatotoxicity of chemical drugs and disease recurrence.
In conclusion, TGP can treat psoriasis by inhibiting keratinocyte hyperproliferation and neoangiogenesis, as well as by immunomodulation and anti-inflammatory responses. However, its specific mechanism of action still needs further study. TGP can be used alone as a therapeutic drug for psoriasis, or combined with other drugs and physical therapy to treat psoriasis. These findings have important reference value for the future clinical treatment of psoriasis.
8.3 Application of TGP in Sjogren’s syndromeSjogren’s syndrome (SS) is a chronic inflammatory autoimmune disease characterized by lymphocyte infiltration of exocrine glands (Alexopoulou, 2022). Clinical manifestations of the syndrome include dryness of the mouth and eyes and the presence of autoantibodies and hyperimmunoglobulins in the serum of the patient (Ren et al., 2023; Zhang A. P. et al., 2023). However, the exact etiology and pathogenesis of desiccation syndrome remain to be further elucidated.
Animal model studies have found that TGP can reduce the concentration of cytokines and autoantigens in the serum of experimental mice, and attenuate the inflammatory response in SS mice (Li B. B. et al., 2020). Using a non-obese diabetic mouse model (NOD), Li et al. found that salivary gland flow rate and the ratio of splenic regulatory T-cells were elevated in the PF-treated group of mice in contrast to the saline control group. However, serum anti-SSA/SSB levels, and lymphocytes were significantly reduced. Furthermore, SS treatment with PF was found to increase the expression of aquaporin (AQP)-5, alleviate inflammation, and restore autoantibody levels in the NOD mouse model. Therefore, PF can restore the function of salivary glands and delay the appearance of early clinical symptoms of Sjogren syndrome (Li et al., 2013).
Cysteine-rich (Cyr61) has been gradually acknowledged as a new inflammatory factor involved in diverse autoimmune and inflammatory diseases (Kular et al., 2011). Li et al. found that Cyr61 expression was upregulated in the salivary glands of SS patients, and also in the submandibular glands of experimental mice. In addition, inhibition of Cyr61 expression in experimental mice alleviated the production of inflammatory cytokines and improved salivary gland secretion. These results suggested that Cyr61 is involved in the progression of SS. PF treatment significantly downregulated the expression of Cyr61 and alleviated the symptoms of SS mice, indicating that PF alleviates SS by inhibiting the expression of Cyr61 (Li H. et al., 2016).
Regarding relevant clinical research, a clinical trial showed that TGP (1.8/d × 24 w) significantly improved dry mouth and eyes, and reduced disease activity and erythrocyte sedimentation rate (ESR) levels in primary SS patients. Additionally, the treatment was well tolerated with mild adverse effects (Liu et al., 2019). Another meta-analysis of the application of TGP in the treatment of SS also showed that TGP significantly improved the function of lacrimal secretion, and its efficacy was better than that of the placebo group. In addition, TGP combined with immunosuppressive drugs (e.g., hydroxychloroquine) not only promotes gland secretion and relieves the dry mouth and eyes of patients, but also reduces inflammatory indices (e.g., ESR, serum C-reactive protein (CPR)) and plasma immunoglobulin (e.g., gamma globulin, IgG, IgA, and IgM) levels. In addition, this combination therapy was safe and well tolerated, demonstrating the advantages of combining TGP in the treatment of Sjogren syndrome (Feng et al., 2019).
In summary, TGP exhibited good therapeutic effects on SS and is free of hepatotoxicity and ocular toxicity. However, the onset of action of TGP is slow, indicating that therapeutic effects might take some time to become evident. Therefore, the development and application of TGP still need to be further explored.
8.4 Application of TGP in lupus erythematosusLupus erythematosus is a chronic autoimmune disease in which the production of autoantibodies causes damage to tissues and organs of the body, resulting in severe systemic damage, i.e., systemic lupus erythematosus (SLE) (Guo X. R. et al., 2021; Qiu et al., 2022; Viatchenko-Karpinski et al., 2023). The pathogenesis of SLE is complex, with the involvement of a multitude of factors in different genetic backgrounds. In SLE, the number and function of T lymphocytes, B lymphocytes, and many cytokines (IL-1, IL-10, TNF-α) are significantly abnormal, and there is obvious heterogeneity in the pathogenesis (Perl, 2012). SLE is characterized by an abnormality in multiple immunomodulatory functions. Therefore, immunomodulation becomes an important tool in the clinical treatment of SLE. Currently, the conventional treatment of SLE mainly involves glucocorticoids and immunosuppressants (Ugarte-Gil and Alarcón, 2014; Zhang L. C. et al., 2024). However, the adverse effects of these drugs limit their clinical use.
Animal experiments on the early application of TGP in SLE showed that TGP exhibited a bidirectional regulatory effect (promoting effect at low concentrations and inhibitory effect at high concentrations) on the proliferative response of splenic lymphocytes induced by sabinin A in mice. In addition, TGP can promote phagocytosis of peritoneal macrophages and partially or completely antagonize the increase of serum IgG-type autoantibody levels in mice, which is protective against SLE-like changes in mice (Zhang and Wei, 2020). In the active phase of SLE, the decrease of Treg cells in the blood correlates with the severity of the disease. Zhao et al. gave different concentrations of TGP to Lupus patients and controls (healthy individuals). The resulting data indicated a significant increase in CD4+CD25+ Treg cells in Lupus patients, whereas no significant change was observed in the controls (Zhao et al., 2012). Forkhead/winged helix transcription factor (Foxp3), the forkhead gene for transcription factors, primarily regulates Treg cell progression (Fontenot et al., 2003). Expression of the Foxp3 gene induces the conversion of CD4+CD25− T cells to CD4+CD25+ Treg cells (Hori et al., 2003). In addition, the use of TGP resulted in upregulating the expression level of Foxp3, causing Foxp3 demethylation and increasing the production of IFN-γ and IL-2, thereby promoting the expression of Treg cells in CD4+ T cells. Another study showed that the expression level of CD4+CD25+ T cells in patients with active SLE was notably lower than that in healthy controls. In addition, TGP combined with immunosuppressive agents (e.g., tacrolimus) significantly increased CD4+CD25+ T-cell expression in patients, decreased DAI, increased clinical efficacy, and reduced the incidence of disease recurrence (Li et al., 2018).
From clinical research perspective, Zhang et al. conducted a clinical study on the therapeutic effects of combining glucocorticoids, mycophenolate mofetil, and total paeoniflorin capsules in the treatment of SLE. They selected 84 patients with SLE and randomly divided them into a control group and a treatment group, with 42 patients in each group. The control group was treated with prednisone and mycophenolate mofetil, while the treatment group received additional TGP capsules based on the control group’s regimen and the treatment lasted for 6 months. The study results showed that the combination of glucocorticoids, mycophenolate mofetil, and TGP capsules was more effective in treating SLE than the combination of glucocorticoids and mycophenolate mofetil alone (Zhang Y. et al., 2023). Chen et al. conducted a meta-analysis on the treatment of SLE with TGP to assess the efficacy of TGP. The study included data from 14 RCTs with 978 patients (492 in the trial group and 486 in the control group). Their results showed a significant improvement in the SLE disease activity index (DAI), an elevation in serum complement levels, and a decrease in blood sedimentation in the test group (TGP combined with glucocorticoids or cyclophosphamide) in contrast to the control group (not combined with TGP). In addition, the average daily dose of glucocorticoid and the cumulative dose of cyclophosphamide were reduced in the experimental group, and the rate of disease recurrence and adverse effects were also reduced. The acquired data demonstrate the effective role of TGP in treating SLE (Chen et al., 2022).
In conclusion, TGP, either alone or in combination, is effective in the treatment of SLE. In addition, TGP exhibited a good hepatoprotective effect, and the adverse effects were only slight gastrointestinal reactions (Wang et al., 2022). These findings provide new ideas for the clinical treatment of SLE.
8.5 Application of TGP in urticariaUrticaria is a limited edematous reaction, characterized by wheals and erythema, caused by dilation and increased permeability of small blood vessels in the skin and mucous membranes (Deng et al., 2020). Chronic urticaria (CU) occurs in about 0.1% of the general population and is increasing (Xiao et al., 2023). CU is categorized into two main types: spontaneous and induced (Bartko et al., 2023). The etiology of the former is complex and involves both immune and non-immune pathogenesis. Of these, type I hypersensitivity is the most common mechanism of reaction. Immunoglobulin IgE mediates mast cell degranulation, releasing a variety of inflammatory chemical mediators, mainly histamine, to induce urticaria (Bracken et al., 2019). The clinical management of CU is complex. Despite the effectiveness of histamines, recurrence may occur after discontinuation (Claveau et al., 1993). In addition, immunosuppressants (e.g., cyclosporine A, Tripterygium wilfordii) are effective in only some patients and have significant toxic side effects (Khan, 2013; Kitsioulis et al., 2017). These limitations greatly restrict the use of these drugs. In recent years, TGP has been applied to the treatment of urticaria.
Peng et al. (2022) applied PF to an animal model of urticaria. The experimental results showed that PF significantly alleviated scratching behavior and histopathological features. In addition, PF reduced the cellular levels of immunoglobulin E (IgE), leukotriene B4 (LTB4), and histamine (HIS) in serum, markedly reduced the number of mast cells and granules in the skin tissues of urticaria mice, and decreased the infiltration of inflammatory cells (MCT and EPX). Oral administration of PF was effective in reducing inflammatory cytokine IL-12 mRNA levels. Another animal model study exhibited that PF inhibited allergic and inflammatory responses and ameliorated urticarial lesions by mitigating pathologic abnormalities, mast cell infiltration, and attenuating histamine secretion. Mechanistically, the application of PF significantly limited the production and release of the inflammatory cytokine interleukin (IL-23), whereas levels of IL-17 remained unchanged. In addition, PF intervention increased the number of autophagosomes, suggesting that PF enhances autophagic activity in urticarial lesions. Additionally, PF treatment elevated the expression of liver kinase B1 (LKB1) and AMP-activated protein kinase α (AMPKα), which promoted PF-enhanced autophagic activity. Thus, PF inhibits the inflammatory cytokine IL-23 via the LKB1/AMPK-α pathway, enhances autophagic activity, and effectively ameliorates urticarial lesions (Guo J. et al., 2021).
In the area of clinical research, He et al. investigated the efficacy and safety of combining levocetirizine dihydrochloride with montelukast sodium and total glucosides of paeony in the treatment of chronic spontaneous urticaria. A total of 84 patients were randomly assigned to two groups: the treatment group (44 patients) and the control group (40 patients). The treatment group received oral levocetirizine dihydrochloride 5 mg once daily, montelukast sodium 10 mg once daily, and total glucosides of paeony 0.6 g twice daily. The control group received only oral levocetirizine dihydrochloride 5 mg once daily and montelukast sodium 10 mg once daily. Both groups underwent an 8-week treatment course, with disease severity scores recorded before treatment and at weeks 2, 4, 6, and 8 during treatment. Clinical efficacy and adverse reactions were assessed at the end of the treatment period. The results showed that after 8 weeks, the reduction in disease severity scores was greater in the treatment group than in the control group, with an efficacy rate of 86.36% in the treatment group compared to 50.00% in the control group (He et al., 2016). Another clinical observational study evaluating the combination of TGP with cetirizine in the treatment of urticaria exhibited favorable results within the treatment group (Long et al., 2010). That study showed that during the initial two-week dosing period, the treatment group with the combination of TGP and cetirizine showed significantly better effects on symptom improvement than the control group with cetirizine alone. The serum IL-4 and IgE levels were significantly lower in the treatment group, and the 1-month relapse rate was also low. Therefore, TGP exhibited the potential to improve the condition of CU patients and reduce the relapse rate by regulating the immune function.
In summary, TGP as a supplementary treatment can provide positive outcomes for CU in adolescents and adults, with minimal and manageable side effects (Li et al., 2022). However, future studies require more high-quality, long-term clinical trials to provide more robust evidence for clinical practice.
8.6 Application of TGP in oral lichen planusOral lichen planus (OLP) is a non-infectious superficial inflammatory disease of the oral mucosa (Didona and Hertl, 2022). The recurrent and cancerous potential of OLP is detrimental to the quality of life and psychological wellbeing of the afflicted individuals. The pathology of OLP is characterized by a dense subepithelial lymphocytic infiltrate, destruction of the basement membrane, and apoptosis of keratinocytes (Deng et al., 2022; Geng et al., 2022; Qing et al., 2023). Currently, the etiology of OLP is unclear and requires further elucidation, although research has implied the involvement of a variety of factors in this disease. The pathogenesis of OLP is closely related to immune factors, and it is a chronic disease characterized by T-lymphocyte-mediated immune response (Liu et al., 2014). Antigen-specific and non-specific mechanisms may be involved in the immune process of OLP. Specifically, the uptake and processing of unknown antigens by DCs triggers an immune response. This response results in the accumulation of T lymphocytes and other immune cells at the lesion site and the secretion of a large number of cytokines. Subsequently, these events contribute to the degradation of the basement membrane and the destruction of keratinocytes, resulting in inflammatory lesions of the oral mucosa (Gobbo et al., 2017; Park et al., 2018).
Wang et al. found high expression of Toll-like receptor 4 (TLR4) and activation of the NF-κB signaling pathway in tissues of patients with oral lichen planus. In addition, keratinocytes from OLP patients showed higher levels of mRNA expression of inflammatory factors interleukin-6 (IL-6) and TNF-α than normal oral epithelial keratinocytes. The local inflammation model of OLP constructed by lipopolysaccharide (LPS)-stimulated keratinocyte HaCaT cells was assessed concerning the impact of TGP application. The data indicated that TGP could dose-dependently suppress the LPS-induced expression of IL-6 and TNF-α in these cells. In addition, TGP treatment reduced the phosphorylation of NF-κB inhibitory protein α and NF-κB p65 protein, causing a reduction in NF-κB p65 nuclear transcription in HaCaT cells (Wang et al., 2016). A clinical observation of TGP combined with glucocorticoids in the treatment of OLP showed that TGP is a safe and effective drug with few side effects in the treatment of OLP. In addition, TGP combined with glucocorticoids exhibits a definite therapeutic effect (Zhou L. L. et al., 2016).
Mesenchymal stem cells (MSCs) have been employed in both clinical and experimental settings to mitigate severe immune-linked diseases utilizing their immunomodulatory properties. However, these beneficial properties are impaired in the presence of inflammation (Park et al., 2018). Zhang et al. explored the immunomodulatory effects of PF on MSCs and the potential role of Th1/Th2 cytokines in OLP. They observed that PF promoted the proliferation, migration, and multilineage differentiation of MSCs in OLP lesions by regulating the Th1/Th2 balance. This resulted in prolonging graft survival time and improving inflammatory infiltration. It was observed that PF enhances MSC immunomodulation and regulates the inflammatory microenvironment via T lymphocytes. Furthermore, it offers a promising therapeutic measure for OLP treatment by improving MSC function (Zhang et al., 2022b). In another study, MSCs from OLP showed elevated levels of IL-6, TNF-α, transforming growth factor β (TGF-β), and IL-10 in comparison to the control group. TGP significantly improved the immunomodulatory function of MSCs via the inhibition of IL-6 and TNF-α expression and elevation of the expression of TGF-β and IL-10. In addition, TGP could inhibit the expression of p-STAT3 by upregulating miR-124. Alterations in IL-6, TGF-β, and p-STAT3 were mediated by overexpression and knockdown of miR-124 in MSCs. Thus, TGP may significantly decrease pro-inflammatory cytokines and increase anti-inflammatory mediators through the miR-124/STAT3 pathway (Zhao Z. F. et al., 2018).
From a clinical research perspective, Wu et al. studied the short-term effects of combined treatment with compound phellodendron gargle and TGP capsules on OLP. Sixty-two patients were randomly divided into a control group and an observation group. Both groups received oral TGP capsules, while the observation group additionally used compound phellodendron gargle as a mouth rinse. The treatment effects were evaluated after 30 days based on the Visual Analog Scale (VAS) for pain and clinical signs assessment. The results showed significant improvement in the VAS and clinical signs scores in the observation group, as well as an improvement in the VAS scores in the control group, compared to pre-treatment levels (Wu and Ma, 2023).
8.7 Application of TGP in allergic contact dermatitisAllergic contact dermatitis (ACD) is a delayed-type hypersensitivity reaction (type IV hypersensitivity reaction) mediated by semi-antigen-specific T cells, which is produced by T cells after allergen contact with the skin and is stimulated by antigen-presenting cells (Scheau et al., 2020). It occurs in two phases: the sensitization and stimulation phases (Weidinger and Novak, 2016). Notably, a small portion of ACD may also present as the delayed phase of type I hypersensitivity reaction. Allergic contact dermatitis is mainly characterized by a series of skin inflammatory cell infiltration and the release of inflammatory mediators in the local skin after antigenic stimulation (Zhou P. et al., 2016). The main clinical manifestations of allergic contact dermatitis are redness, edema, and itching. Repeated exposure to allergens at the lesion site can lead to recurrent episodes of ACD and even trigger atopic dermatitis and disability, seriously affecting the quality of life of the patients (Wong et al., 2015). ACD affects a wide range of populations, with a global prevalence rate of up to 15%–20%, across all age groups. Specifically, ACD exhibits a prevalence of 15.2% in adolescents and 18.6% in adults (Aloui et al., 2022). Exposure to the work environment, age, gender, dietary habits, and genetic predisposition are considered to be the most important risk factors. Currently, western medical treatment of ACD mainly involves the use of topical corticosteroids and antihistamines, which are highly effective (Ta et al., 2021). However, these drugs may trigger serious adverse reactions such as hypercorticism, muscle atrophy of hands and feet, liver function damage, central inhibition, and cardiotoxicity. In recent years, researchers have applied TGP for the treatment of ACD, yielding promising research results.
Dendritic cells (DC) are important antigen-presenting cells, and there are at least two subpopulations of DCs in steady state: intraepidermal Langerhans cells and intradermal DCs (Ouchi et al., 2016). DCs carrying allergens migrate to local draining lymph nodes, where they selectively induce the activation of antigen-specific T cells through the presentation of MHC-II molecules. This results in initiating the development of a type IV allergic response. In addition, DCs also produce various cytokines that regulate the differentiation of T lymphocytes. Therefore, inhibiting the migration of LCs/DCs and the activation of T cells is considered to be an important method to control ACD (Kimber et al., 2012). Using an ACD mouse model, Shi et al. observed that PF increased the expression of a cytokine signaling suppressor (Socs3), which is highly expressed by DCs at both the protein and gene levels. In addition, high expression of Socs3 inhibited IL-6/STAT3 signaling and activation of downstream signaling pathways, thereby suppressing IL-6 levels and TH17 cell proliferation (Shi et al., 2016).
In addition, T-lymphocytes are the main effector cells in the disease progression of ACD. Activation of T lymphocytes by antigen-presenting cells results in the production of a large number of cytokines: IL-2, IL-4, and IL-1. Among these, an imbalance of IL-2 and IL-4 cytokines promotes the progression of hypersensitivity (Popov et al., 2011). Overproduction of IL-17 and underproduction of IL-10 have also been found in ACD patients. It was noted that IL-17 exacerbates the degree of hypersensitivity and increases the production of inflammatory cytokines. However, IL-10 inhibits antigen-presenting cells to relieve the inflammatory response and even improves immune tolerance, reducing IL-17 levels (Harrington et al., 2005).
Wang et al. observed that PF significantly alleviated the degree of ear swelling and infiltration of inflammatory cells and inhibited the proliferation of thymocytes through the establishment of a mouse model of DNCB-induced ACD (Wang et al., 2013). Assessment of the cytokines in serum, thymus, and spleen supernatants revealed elevated levels of IL-4 and IL-10, and decreased levels of IL-2 and IL-17. These findings indicate that PF has a regulatory function in ACD, regulating the balance between inflammatory and anti-inflammatory cytokines. In addition, PF inhibited the production of the inflammatory stimulating factor IL-12 and increased the expression of IL-10 and TGF-β. This may be another mechanism by which PF modulates DC-induced T-cell immune tolerance for the effective treatment of inflammatory and immune-related diseases such as allergic contact dermatitis. It is well known that cytokines IL-2, IL-4, IL-10, and IL-17 represent Th1, Th2, Treg, and Th17 cells, respectively. The differentiation of T lymphocytes is primarily governed by the signaling pathway JAK-STAT transcription factors (Jin et al., 2012). In addition, the expression of aberrant transcription factors (T-box family of novel transcription factors, zinc finger transcription factors, forkhead family of transcription factors, and tretinoin-associated orphan nuclear receptor) causes an imbalance in the ACD cytokine network (Yin et al., 2011). Therefore, PF has the potential to regulate the janus kinase/signal transducers and activators of transcription (JAK/STAT) transcription factor pathway to correct the aberrant differentiation of T cells and ultimately restore the balance of the cytokine network (Gangwar et al., 2021). However, this hypothesis needs to be further verified. Shi et al. established a mouse model of allergic contact dermatitis, which further demonstrated that PF could inhibit the
留言 (0)