Spirometry has so far been the most accepted mode of diagnosis of obstructive airway disease (OAD)1. The expiratory flow-volume and the time-volume relationships are critical to appreciate and quantitate airflow limitation in a person2-4. Forced expiratory volume in one second/ forced vital capacity (FEV1/FVC), when <0.7, is regarded as a marker of chronic airflow limitation5,6. The reduction in FEV1 can signify the severity of chronic obstructive pulmonary disease (COPD)2-4,7. Moreover, the quantitative change of FEV1 in terms of acute bronchodilator response to salbutamol not only gives an idea of the prospective therapeutic response to the agent8 but also reflects the response to an inhaled corticosteroid to a good extent9. Such bronchodilator responsiveness assessment has become useful in the diagnosis of asthma10 and obstructive airway diseases (OAD)11.
The observation of deliberate abstinence of bronchodilators is recommended for prescribed periods (varying with the pharmacokinetics of the concerned agents)12. In real-world referral, observation of such abstinence is difficult and often impossible. Hence, physicians tend to adhere to the principles of abstinence as far as practicable. Such exercise on the same day is proposed as ‘pragmatic’ spirometry13 enlightening the need for its recognition in practise and research as well.
Testing of bronchodilator responsiveness with salbutamol (representative of the β2-agonists) inhalation, an integral part of spirometry, can also be included in the pragmatism mentioned above. Salbutamol responsiveness is essential for the syndromic diagnosis of the OADs since the FEV1 reversibility and the post-bronchodilator FEV1/FVC are counted for diagnosis of asthma and COPD10,14.
Of late, inhalation of dry powder of glycopyrronium bromide, an anti-muscarinic agent, is used to see the class-specific changes for anticholinergic bronchodilators15-17. Tested serially after salbutamol, it has been seen to provide information to appreciate a likely scope and the degree of improvement from proactive prescription of the agent as an additional bronchodilator to salbutamol in a patient with obstructive airway disease.
The present study elaborates on the role of such pragmatic spirometry in a real-world effort to appreciate the syndromic diagnosis and bronchodilator responsiveness to the available class-specific bronchodilators (salbutamol and glycopyrronium) in symptomatic patients of suspected obstructive airway diseases.
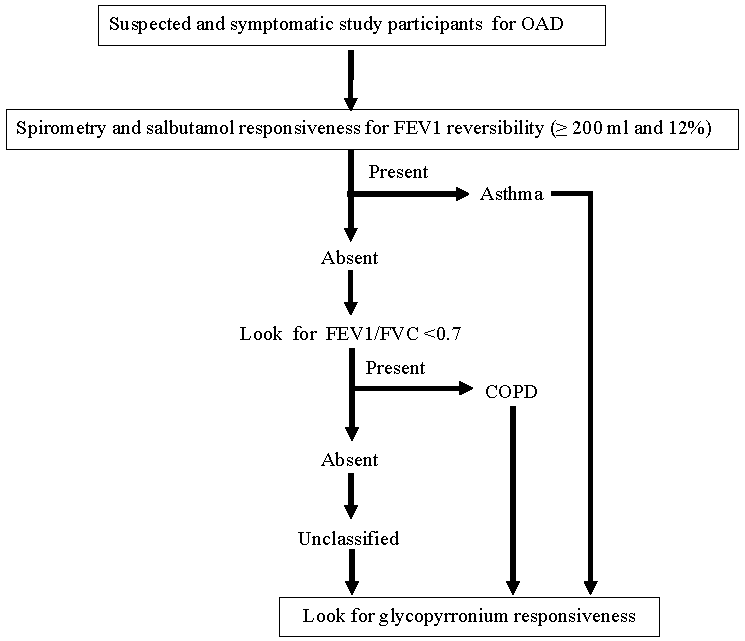
Materials & MethodsThe study was conducted at the Institute of Pulmocare and Research, Kolkata, India from February 2022 to June 2023 after obtaining the ethical clearance and research plan approval from the Institutional Ethics Committee of the institute. The study participants were recruited from the individuals attending the outpatient department of the institute. The workflow with the study participants is presented in the diagram (Fig. 1).
Export to PPT
Study participants and inclusion/exclusion criteriaThe study participants at presentation having any four of the five symptoms: a) cough, b) shortness of breath, c) wheeze, d) expectoration, and e) tightness of chest were considered to have OAD. Study participants with a previous diagnosis of an OAD as per verbal or documented reports with a history of bronchodilator treatment (oral or inhalational) were also included. Spirometry was performed in all such study participants whenever possible during office hours on the same day after the consultation observing the technical recommendations for performance in the American Thoracic Society/European Respiratory Society (ATS/ERS) guidelines18 without considering the prescribed abstinence from any of the drugs used by the study participants except long-acting anti-muscarinic agents (LAMA). However, a gap of at least three hours was maintained after meals and smoking for the test. The affected individuals unwilling to join the research, not capable of performing the test, and those with the presence or history of active exacerbations in the preceding six wk or any other significant pulmonary diseases were excluded. No pregnant or breastfeeding women and none with obvious or suspected contraindications for spirometry12 or the use of the bronchodilators (salbutamol and glycopyrronium) were included.
Study parameter and protocolSpirometry was repeated after 20 min of four puffs of salbutamol (100 µg each) to elaborate the responsiveness12. The newly described glycopyrronium responsiveness was assessed through inhalation of 50 µg of the dry powder of glycopyrronium bromide (AIRZ, Glenmark pharmaceuticals) immediately after completing the salbutamol responsiveness and repeating spirometry after 30 min of inhalation of the drug. At this stage, any affected individuals with normal spirometry and no bronchodilator response were excluded. The spirometric status on FVC, FEV1, FEV1/FVC, Forced Expiratory Flow between 25 and 75 per cent of vital capacity (FEF25-75) were noted at rest and after the individual responsiveness effort (salbutamol and glycopyrronium). Further, the study participants were classified accepting a definition of asthma as salbutamol reversibility of FEV1 ≥12% and 200 ml19, COPD as FEV1/FVC<0.7 and FEV1 reversibility as < 12 per cent and 200 ml)20. The rest of the subjects with variable spirometric defects were grouped as ‘unclassified’.
Statistical analysisThe relative responsiveness status in terms of absolute reversibility (200 ml for Salbutamol, and 100 ml for glycopyrronium) for the three groups: asthma, COPD, and ‘unclassified’ had been examined. Statistical analyses were performed with Graph-pad Prism version-8 software to estimate the relative frequency and response to the two different bronchodilators. The values were compared statistically with paired ‘t-test’ and the significance of the post-salbutamol (compared to pre-bronchodilator resting state) and the post-glycopyrronium (compared to post-salbutamol state) improvements were determined for each diagnosis. The FEV1 reversibility was compared in terms of both absolute and percentage-predicted values. We looked at the relative responsiveness between the two drugs (salbutamol and glycopyrronium) in asthma, COPD, and the ‘unclassified group’.
ResultsFifteen hundred and eighty (1580) study participants were included from February 2022 to June 2023 from our outpatient services consisting of asthma [n=329 (20.8%)], COPD [n=641 (40.56%)], and unclassified [n=610 (38.61%)] (demographic details with the spirometric abnormalities in Table I and distribution in Fig. 2).
Table I: Distribution and demographic details of 1580 obstructive airway disease study participants
Total participants, (n=1580) n (%) Age (yr) Male:Female BMI (kg/m2)Asthma
(n=329)
329
(20.8%)
50.61±17.03 206:123 25.73±4.48COPD
(n=641)
641
(40.56%)
61.51±12.59 496:145 23.97±4.15Unclassified
(n=610)
610
(38.61%)
55.02±15.99 389:221 25.25±4.66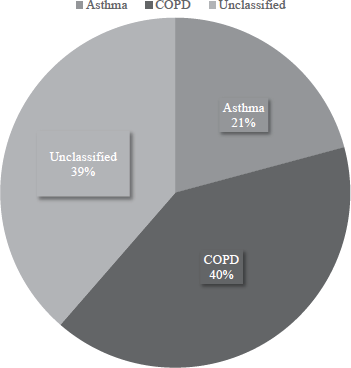
Export to PPT
The pre- and post-bronchodilator spirometric values of FVC, FEV1, FEV1/FVC, FEF25-75, and the bronchodilator responsiveness [in absolute value (ml) and % predicted values] following salbutamol and thereafter glycopyrronium inhalation are displayed for asthma, COPD, and unclassified group (Table II). The same changes in percentage-predicted values are shown in bar charts (Fig. 3).
Table II: A comparison of salbutamol and subsequently glycopyrronium induced changes elaborated in terms of FVC, FEV1, FEV1/FVC, and FEF 25-75
Absolute values % predicted values Pre-BD Post-Salbuta mol Post-GP Pre-BD Post-Salbuta mol Post-GP FVC Overall (n=1580) 2.31±0.78 2.46±0.78* 2.54±0.79* 72.34±16.54 76.97±16.13* 79.54±16.10* Asthma (n=329) 2.37±0.76 2.71±0.75* 2.78±0.78 71.65±15.38 82.26±14.60* 84.46±15.08 COPD (n=641) 2.24±0.72 2.32±0.71 2.42±0.72* 71.29±16.52 73.72±16.49* 77.07±16.67* Unclassified (n=610) 2.36±0.85 2.47±0.83* 2.52±0.82 73.82±17.06 77.54±15.73* 79.38±15.48 FEV1 Overall (n=1580) 1.44±0.67 1.56±0.70* 1.63±0.71* 56.07±19.98 61.19±20.66* 63.86±20.66* Asthma (n=329) 1.47±0.59 1.79±0.63* 1.87±0.64 54.98±16.58 67.75±16.80* 70.38±16.65 COPD (n=641) 1.26±0.53 1.29±0.53 1.38±0.56* 50.75±17.38 52.33±17.85 55.60±18.60* Unclassified (n=610) 1.61±0.78 1.72±0.78* 1.77±0.79 62.23±22.38 66.97±21.92 68.90±21.76 FEV1/FVC Overall (n=1580) 0.61±0.14 0.62± 0.14* 0.63±0.15 73.52±15.76 75.58±16.67* 76.61±16.71 Asthma (n=329) 0.61±0.11 0.66±0.12* 0.67±0.12 73.60±12.80 78.89±13.75* 80.20±12.95 COPD (n=641) 0.55±0.11 0.55±0.16 0.56±0.12 67.83±13.54 67.57±13.91 68.75±14.41 Unclassified (n=610) 0.66±0.15 0.68±0.15* 0.69±0.15 79.45±17.14 82.23±17.24* 82.81±17.45 FEF25-75 Overall (n=1580) 0.86±0.69 1.00±0.84* 1.08±0.89 23.43±17.09 27.37±20.52* 29.40±21.83* Asthma (n=329) 0.86±0.60 1.19±0.80* 1.27±0.86 23.24±15.58 32.03±20.14* 34.28±21.49 COPD (n=641) 0.57±0.36 0.58±0.35 0.64±0.41 16.19±9.01 16.31±8.61 18.26±11.18* Unclassified (n=610) 1.16±0.87 1.35±1.01* 1.43±1.07 31.15±20.65 36.50±23.90* 38.34±25.03 FEV1 Reversibility Overall (n=1580) —– 126.20±142.90 67.00±102.80* —– 10.47±12.40 5.08±7.84* Asthma (n=329) —– 327.10±120.90 73.74±115.80* —– 25.83±14.73 4.55±7.19* COPD (n=641) —– 35.40±70.33 84.07±96.66* —– 3.25±5.22 7.056±8.53* Unclassified (n=610) —– 113.30±96.18 45.43±97.82* —– 9.52±7.91 3.34±6.88*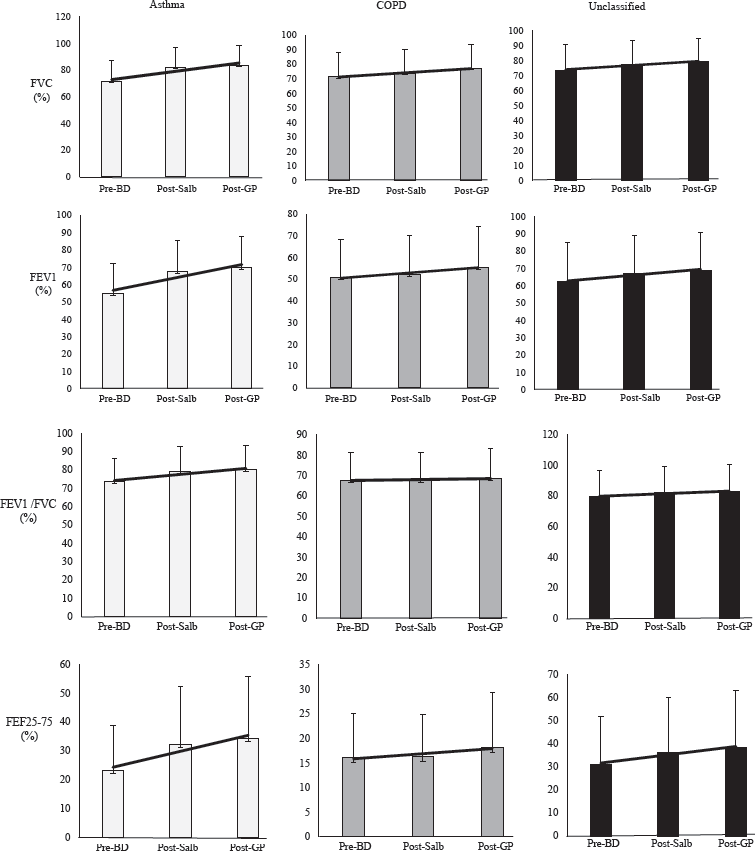
Export to PPT
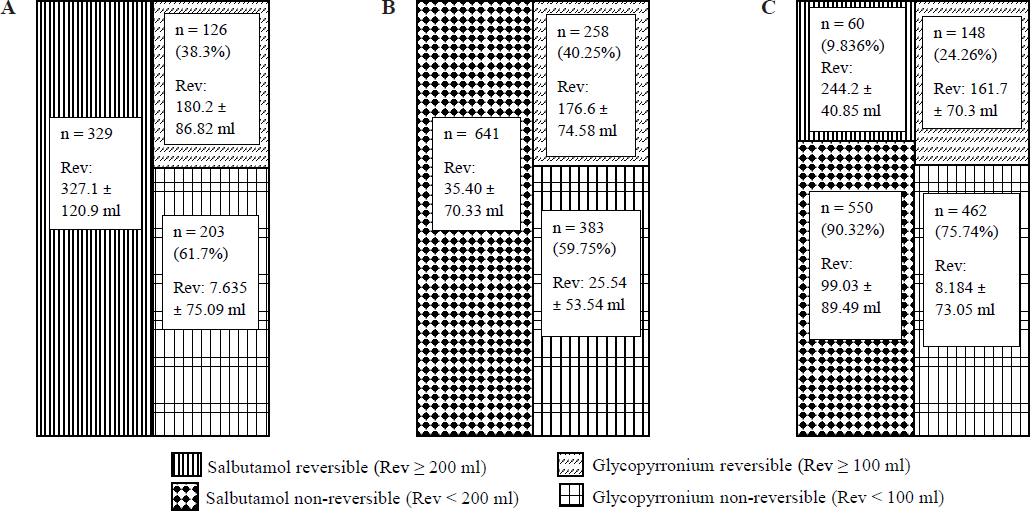
The relative frequency of changes (responsiveness) in terms of improvement (200 ml for Salbutamol, and 100 ml for glycopyrronium) for the three groups: asthma, COPD and ‘unclassified’ have been represented in Fig. 4.
Export to PPT
DiscussionThe real-world ‘pragmatic’ spirometry appears efficient in identifying OAD as a whole and the individual diseases (Table I). Further, the results help to appreciate the responsiveness status of glycopyrronium compared to that of salbutamol in the serial bronchodilator-responsiveness assessment for overall and individual groups of OAD. It also forwards the prospect of selection of a combination of both the classes of agents based on such responsiveness assessment.
Overall, there was male dominance; the age and body mass index (BMI) of the ‘unclassified’ group lies between those of asthma and COPD (Table I). The salbutamol responsiveness in FEV1 showed significant improvement in overall, asthmatics, and ‘unclassified’ group while in COPD there was a significant improvement in FVC alone (Table II). We selected FEV1, FVC, FEV1/FVC, and FEF25-75 in spirometry to demonstrate both direct and indirect impacts on airflow obstruction by the agents.
The serial reversibility testing with glycopyrronium after salbutamol was an interesting adjunct to our exercise. The add-on reversibility (≥100 ml) with glycopyrronium had been present in 38.3, 40.2, and 26.26 per cent, respectively for asthma, COPD, and ‘unclassified’ OAD study participants (Fig. 3). While the asthmatics were universally salbutamol responsive in terms of FEV1 (327.1±120.9 ml), the COPD affected individuals were universally unresponsive (35.40±70.33 ml). Only 9.835 per cent of the ‘unclassified’ group showed salbutamol responsiveness (244.2±40.85 ml). The glycopyrronium responsiveness was most frequent in COPD (40.25%; mean 176.6±74.58 ml) followed by asthma (38.3%; 180.2±86.82) and the ‘unclassified’ (24.26%; 161.7±70.30 ml) group. The combined responsiveness had improved universally compared to that of the salbutamol alone (Table II) and was quantitatively well above the minimum perceptible change in FEV1 value of 100 ml for all three groups of OAD21,22. The translation of the observation meant a likely superior bronchodilatation with combined bronchodilators across OADs. The other distinctiveness of the observation was the pragmatism in performing the bronchodilator responsiveness based on clinical diagnosis without formal preparation for spirometry. This recognized and exemplified the intention to offer the best possible treatment of an airway disease under real-world circumstances. The results elaborate that it is possible to identify the syndromic diagnosis (asthma or COPD) through performing such ‘on-the-day’ pragmatic spirometry whenever a patient seeks consultation with respiratory symptoms and the clinical suspicion favours obstructive airway disease. Moreover, it opens the prospect of treating them more effectively and precisely based on such bronchodilator responsiveness. The very fundamental premise of the pragmatism is that the symptomatic affected individuals of OAD (excluding moderate or severe exacerbations in our observation) remain inadequately treated with the persistence of inflammation. Therefore, these individuals, although not treatment-abstained at the point of evaluation, happen to be equivalent to that. Thus, despite having a dampened response, they probably tend to maintain reasonably good bronchodilator responsiveness as those with proper abstinence from the bronchodilators.
Adherence to the preparation with abstinence of bronchodilators for the respective ‘wash-out periods’ can provide the so-called ‘true’ salbutamol reversibility on spirometry. Such spirometry should be treated as the ‘gold standard’. Hence, the bronchodilator response derived from an ‘on-the-spot’ pragmatic assessment is likely lower than the gold standard’. Despite the limitation, the revelations in our observation make pragmatic spirometry interesting and worthwhile. Ideally, the assessment of the true bronchodilator response is only possible in absolutely treatment naïve patients. In the real world, such a treatment naïve situation is rare, and performing spirometry with selective abstinence from the bronchodilator is inadequate to reveal the ‘true’ bronchodilator response. This is because many of the patients are treated with inhaled corticosteroid (ICS) containing regimens. Most of the asthmatics have type 2 high endotype23 and ICS acts to reduce type-2 bronchial inflammation24 and, thus, indirectly influences broncho-constriction and bronchial hyper-responsiveness. ICS-β2 agonist combinations are used commonly in OADs25-27 as long-acting β2-agonist (LABA) with ICS is found superior to ICS alone in the long term28. The situation of attaining partial stabilization of bronchospasm can influence their salbutamol responsiveness. The same implies to rampantly prescribed montelukast that can reduce bronchial inflammation29 and it is found to attenuate the airway responsiveness to hypertonic saline in COPD subjects30. In the real world, the treatment is started frequently on clinical diagnosis alone for an obstructive airway disease such as asthma or COPD. When these patients turn up to referral centres for inadequate relief, the elimination of the impact of the use of inhaled steroids or montelukast becomes almost impossible. Hence, the ‘gold standard’ assessment falls short in the ‘actual true’ assessment of bronchodilator response.
Pragmatic spirometry performed within the washout periods of bronchodilators is likely to carry the variable residual effect of the immediate previous dose of the medicines been used. Despite that, our study participants reflect a good presence (both in terms of frequency and degree) of bronchodilator response. The usefulness of the information in clinical practice seems significant and it testifies the role of ‘pragmatic’ spirometry in the real world.
The exercise for glycopyrronium reversibility is a novel addition. We had taken 100 ml of reversibility from glycopyrronium as ‘significant’ to bring that into statistical consideration. This figure of 100ml is important as it is thought to be the minimum perceptible change for improvement22,23. A 100 ml or more reversibility has been found in 38.3 per cent of asthmatics identified in our series and the prospect of this additional benefit with add-on glycopyrronium inhalation could have been missed, had we not contemplated the glycopyrronium bronchodilator response test. Again, for COPD, 59.75 per cent of study participants were non-responsive to glycopyrronium. These study participants could have unnecessarily received an AMA as per the guideline recommendation21, had the glycopyrronium responsiveness was not performed. The same argument implies to the unclassified group where the prescription of a combination of optimum bronchodilators is possible since both the salbutamol and glycopyrronium responsiveness are looked for.
The idea behind testing glycopyrronium bronchodilator responsiveness is to identify the proportion of affected individuals displaying this characteristic and the extent of changes to the agent in different subcategories of individuals with OAD in our cohort. This theoretical understanding has applied importance as it allows one to decide pre-emptively an up-front ‘precision’ prescription of bronchodilator therapy. Our previous experience of using glycopyrronium in the serial reversibility after salbutamol helped us decide the same in this pragmatic observation16-18.
Such pragmatism, to our mind, is rational since otherwise we are likely to miss the opportunity to treat our patients on pharmaco responsiveness. The pragmatic approach described above, appears to improve the rate of performance of spirometry in the real-world scenario in major parts of the globe. Of course, the efficacy of such ‘pragmatism’ needs to be compared against ideal or standard approach preached by the guidelines. Our previous experience with protocol-based selection of COPD subjects resulted in similar bronchodilator response by dual agents (glycopyrronium and salbutamol) irrespective of the order of use17. We appreciate that this pragmatic approach of spirometry and bronchodilator responsiveness testing is both convenient and useful in real-world practice16.
As per authors’ knowledge this is the first effort of its kind with dual purpose to impress upon the pragmatic spirometry and the appreciation of the role of performing responsiveness to dual bronchodilators proactively. There may be questions about the definitions used by us. We opted for a simple approach. The possible residual effects of the medications used might have influenced our findings
Pragmatic spirometry on the same day of consultation with serial bronchodilators (salbutamol followed by glycopyrronium) and responsiveness assessment appears universally feasible across all clinically suspected situations of obstructive airway diseases. Such pragmatism should be welcome in the evaluation and treatment of OAD, irrespective of the specific diagnosis in real-world practice where prescribed abstinence of bronchodilators might not be feasible. The glycopyrronium responsive test appears as an adjunct to routine spirometry allowing a useful insight in offering the agent as part of the precision therapy.
留言 (0)