Gestational diabetes mellitus (GDM) is defined as any degree of glucose intolerance with onset or first recognition during pregnancy (1). Studies have reported that GDM prevalence is approximately 14.0% globally and that it is approximately 14.8% in Mainland China (2, 3). GDM is considered to be associated with multiple adverse outcomes during pregnancy and childbirth in pregnant women. Hyperglycemic mothers are more likely to develop polyhydramnios, pre-eclampsia, obstructed labor, cesarean section, uterine prolapse, and infections, among others (4), while their offspring may be more prone to suffer from spontaneous abortion, stillbirth, congenital malformation, shoulder dystocia, birth injuries, infant respiratory distress syndrome, and macrosomia, to name a few (5). Moreover, GDM parturient and their children are both at a high risk of developing type 2 diabetes, obesity, metabolic syndrome, and cardiovascular diseases in later life (6). GDM poses a serious threat to maternal and infant health, but its etiology is still not fully understood.
Considering the adverse effects of GDM on mothers and fetuses, it is important to develop reasonable strategies to identify and intervene high-risk individuals to reduce the incidence rate of GDM. Currently, the well-established risk factors of GDM can be advanced maternal age, pre-pregnancy overweight or obesity, family history of T2DM, history of GDM, parity, polycystic ovary syndrome (PCOS), ethnicity, diet, and physical activity, among others (7). Among these risk factors, heredity plays an indispensable role. A study conducted in southern China reported that the GDM risk of pregnant women with a family history of diabetes in first-degree relatives were at 2.52 times higher than those without the history (8). Furthermore, Wan et al. showed that Chinese women migrating to Australia had an elevated risk at developing GDM compared to Australian-born Caucasian women (9). In addition, Asian women had a higher risk of GDM than Caucasian women. This further emphasized the importance of genetic background in the pathogenesis of GDM (10).
Genetic studies such as candidate gene studies and genome-wide association studies (GWASs) have constantly identified the DNA sequence variant [single-nucleotide polymorphism (SNP)], which might play a role in altering the promoter and enhancer activity, alternative splicing, mRNA conformation and its posttranscription level, protein function, etc., leading to individual differences in disease susceptibility (11–13). To date, studies including newly two large-scale GWASs performed in east Asia and Finland have detected numerous GDM-associated SNPs (14, 15), for instance, MTNR1B gene rs10830963, CDKAL1 rs7766070, TCF7L2 rs34872471, CDKN2B rs1333051, CMIP rs2926003, and CPO rs1597916. In preliminary studies, we also have identified a series of GDM genetic polymorphisms in the Guilin population, such as the OR2D2 gene rs1965211, RXR-γ rs2134095, TSNARE1 rs7814359, XAB2 rs3760675, ERBB4 rs1595066, MTNR1B rs10830963, CDKAL1 (rs7756992 and rs7754840), and ACE2 (rs6632677 and rs2074192) (16–21). These variants were considered to significantly affect individuals′ susceptibility to GDM by influencing gene expression or interacting with age, pre-pregnancy BMI, blood glucose, or lipid levels.
The clinical practice of GDM screening and diagnosis focuses on 24–28 weeks, which is already in the middle and late stages of pregnancy and cannot prevent the pathological and physiological processes of GDM (22). Thus, a rational strategy of GDM prevention in early pregnancy was desired for clinical application. A nomogram model is a method that can predict the probability of disease outcome events that may occur in individuals with specific characteristics in the future (23). Previously predictive models of GDM were constructed based on the maternal demographic and clinical indicators during early pregnancy, such as age, pre-pregnancy BMI, parity, FPG, and other blood test indicators (24–26). Even though the performances of their model were acceptable, these studies did not comprehensively consider genetic background of pregnant women in the model. Therefore, a risk predictive model containing both genetic and environment components was essential to improve clinicians’ decision for individualized early prevention and intervention of GDM.
This case–control study aimed to detect the associations between selected functional variants and clinical traits and GDM risk. Then, a nomogram prediction model based on the GDM positively associated genetic and clinical markers was constructed, and its diagnostic efficacy was evaluated.
2 Materials and methods2.1 SubjectsA total of 1,164 participants in early pregnancy, namely, 538 GDM cases and 626 healthy controls, aged 18–45 years, were recruited in the Affiliated Hospital of Guilin Medical University between September 2014 and April 2016. A standard 75-g oral glucose tolerance test (OGTT) was conducted at 24–28 weeks of gestation, and according to the criteria recommended by the International Association of Diabetes and Pregnancy Study Groups (IADPSG), GDM was diagnosed if any of the three threshold values was reached or exceeded: fasting plasma glucose (FPG) ≥5.1 mmol/L, 1-hour plasma glucose (1hPG) ≥10.0 mmol/L, and 2-hour plasma glucose (2hPG) ≥8.5 mmol/L (27). Moreover, subjects in our study should satisfy the following requirements: singleton pregnancy, local residents, and having no kinship with each other. Pregnant women who were progestationally diagnosed as having endocrine and metabolic diseases such as type 1 or type 2 diabetes, and have used long-term glucose metabolism-affecting drugs before pregnancy were excluded. The present study was approved by the Ethics Committee of Guilin Medical University (number GLMC20131205) and conducted according to the principles of the Declaration of Helsinki. The study design is shown in Figure 1.
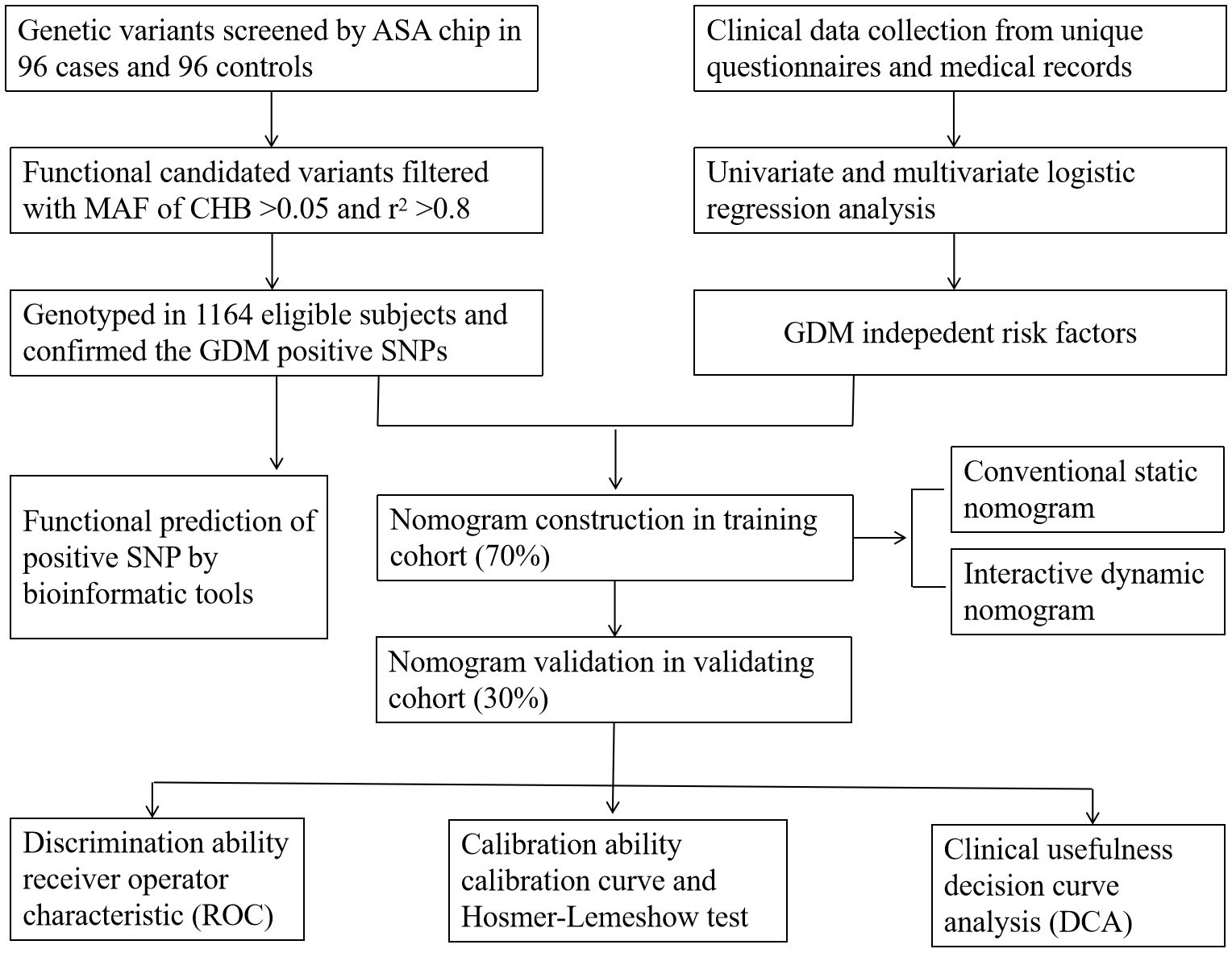
Figure 1. Design process of the study. ASA chip, infinium Asian Screening Array (ASA, illumina) BeadChip; MAF, minimum allele frequency. CHB, the Chinese Han population in Beijing. r2 was the index of linkage disequilibrium.
2.2 Data collectionParticipants′ information such as age, pre-pregnancy weight, height, systolic blood pressure (SBP), diastolic blood pressure (DBP), hemoglobin A1c (HbA1c), blood glucose levels (FPG, 1hPG, and 2hPG) and triglyceride (TG), total cholesterol (TC), low-density lipoprotein cholesterol (LDL-c), and high-density lipoprotein cholesterol (HDL-c) levels were collected from structured questionnaires and hospital medical records. Pre-pregnancy body mass index (BMI) was calculated as pre-pregnancy weight (kg) divided by the square of height (m).
2.3 Genomic DNA extractionThe genomic DNA was extracted from EDTA-treated peripheral whole blood using the Aidlab DNA extraction kit (Aidlab Biotechnologies Co., Ltd, China) and stored at −80°C before polymerase chain reaction (PCR).
2.4 Candidate variants selection and genotypingAfter conducting Infinium Asian Screening Array (ASA, Illumina) BeadChip analysis on 96 cases of GDM and controls, a series of genetic variants were detected at the test level of 10−4 (Figure 2). Candidate genetic variants must meet the following conditions: located in the current functional region of the genome, such as transcription factor binding sites (TFBS), splicing sites (SS), and miRNA binding sites (MBS); the minimum allele frequency (MAF) in the Chinese Han population in Beijing (CHB) is greater than 5%; and the linkage disequilibrium (LD) coefficient r2 between variants is less than 0.8. If r2 > 0.8, only TagSNP is selected. Finally, five functional polymorphisms were selected, of which four (rs4134819, rs720918, rs11109509, and rs12524768) were located at TFBS, and one (rs2034410) was located at miRNA binding sites (Supplementary Table S1).
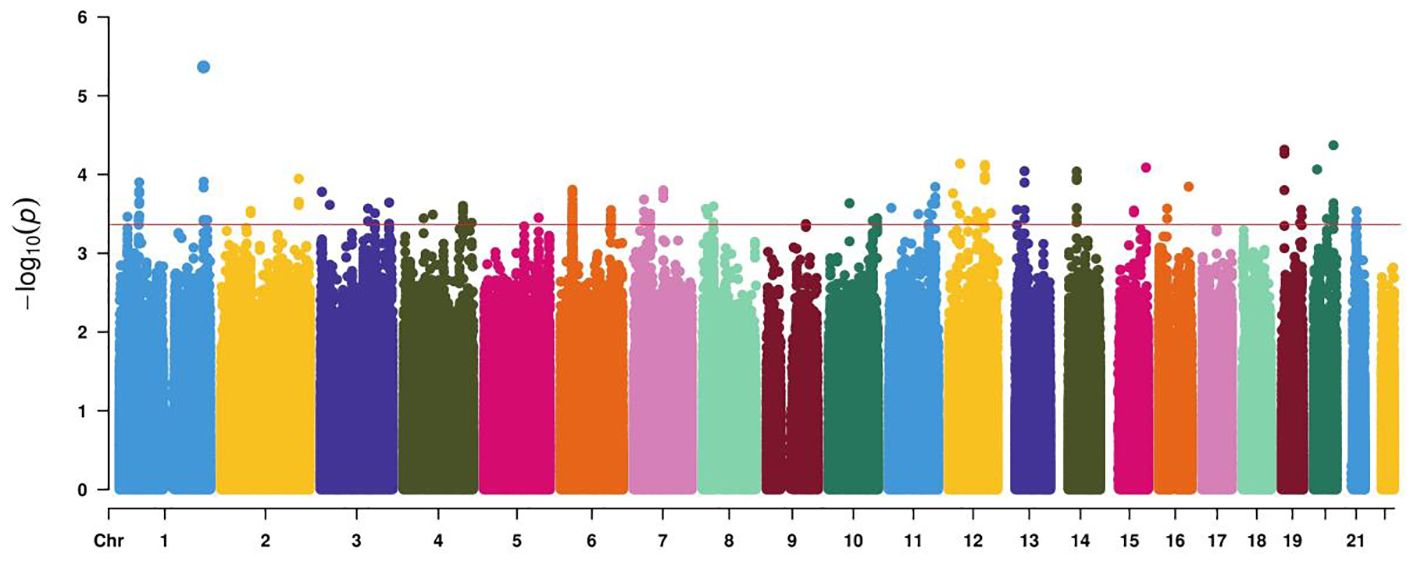
Figure 2. Manhattan plot demonstrating the −log10 p-value for GDM-associated SNPs at the discovery stage. The red line represents a genome-wide significance threshold (p = 5×10−4).
Candidate variants were genotyped by the Sequenom MassARRAY Platform. The PCR master mix was composed of 1 μL of template DNA (20–100 ng/μL), 1.850 μL of ddH2O, 0.625 μL of 1.25×PCR buffer (15 mmol/L MgCl2), 0.325 μL of 25 mmol/L MgCl2, 0.1 μL of 25 mmol/L dNTP mix, 1 μL of 0.5 mmol/L primer mix, and 0.1 μL of 5 U/mL HotStar Taq polymerase. The reaction was conducted at 94°C for 15 min, followed by 45 cycles at 94°C for 20 s, 56°C for 30 s, and 72°C for 1 min, with a final incubation at 72°C for 3 min. The PCR primers are listed in Supplementary Table S2.
2.5 Functional analysisAs predicted by the SNPinfo Web Server (https://manticore.niehs.nih.gov/snpinfo/snpfunc.html) (28), rs4134819 was located at TFBS. We thus used the Alibaba 2.1 tools (http://gene-regulation.com/pub/programs/alibaba2/index.html) to predict the potential functional influence (29). In addition, expression quantitative trait loci (eQTL) analysis was adopted to observe the effect of rs4134819 on the expression regulation of XAB2 gene using VarNote-REG (http://www.mulinlab.org/varnote/application.html#REG) (30) online tools.
2.6 Statistical analysisData were processed using the IBM SPSS Statistics 28 for Windows (IBM Corp., Armonk, NY, USA) and R software (4.3.1). Continuous variables according to normal distribution were described as mean ± standard deviation (mean ± SD) and independent samples Student’ s t-test was used to compare the difference between case and control groups, while variables with non-normal distribution were presented as median (interquartile range) and Mann–Whitney U test was utilized for difference comparison between the two groups. The chi-square (χ2) test was used for categorical variables. The Hardy–Weinberg Equilibrium (HWE) assessed by the χ2 goodness of fit was conducted to determine whether the genotype frequencies are in equilibrium in the control group. Odds ratios (ORs) and their corresponding 95% confidence intervals (CIs) were employed to evaluate the associations between variants and GDM risk. Stratified analysis was carried out to detect the relationship between positive SNP and GDM risk in specific subgroups based on the mean value of clinical variables. A two-tailed test with p < 0.05 indicates that the difference is statistically significant. The false-positive report probability (FPRP) analysis was also performed to assess the significant associations. A cutoff value of 0.2 and a prior probability level of 0.1 were preset to observe an OR of 1.5 for the combined genotypes with an increased risk. Only the pFPRP < 0.2 can be considered as genuine association (31).
Clinical variables with a p < 0.05 in univariate logistics regression can be subsequently incorporated in multifactorial regression analysis to further detect the GDM risk factors. The subjects were randomly split into two groups (training cohort and validating cohort) at a ratio of 7:3, and the nomogram model was constructed to predict GDM occurrence using the “rms” package in the training cohort. The receiver operator characteristic (ROC) curve and the area under the curve (AUC) were produced to evaluate the predictive performance of the nomogram. The calibration curve conducted via a bootstrap method with 1,000 resamples and the Hosmer–Lemeshow test were used to assess the level of consistency between the predicted probabilities and the observed outcomes. Furthermore, the clinical validity and net benefit of the nomogram were appraised by adopting a decision curve analysis (DCA). To facilitate the clinical implementation and application of the risk model, we developed an interactive dynamic nomogram based on the “DynNom” package and a web application “shinyapps” (https://www.shinyapps.io/).
3 Results3.1 Subjects’ characteristicsThe anthropometric and biochemical materials were significantly different between case and control groups. Compared to the control group, age, pre-BMI, blood pressure levels (SBP and DBP), blood glucose, and lipid metabolism levels (FPG, 1hPG, 2hPG, HDL-c, LDL-c, and TG) were higher in the case group (p < 0.05), as shown in Table 1.
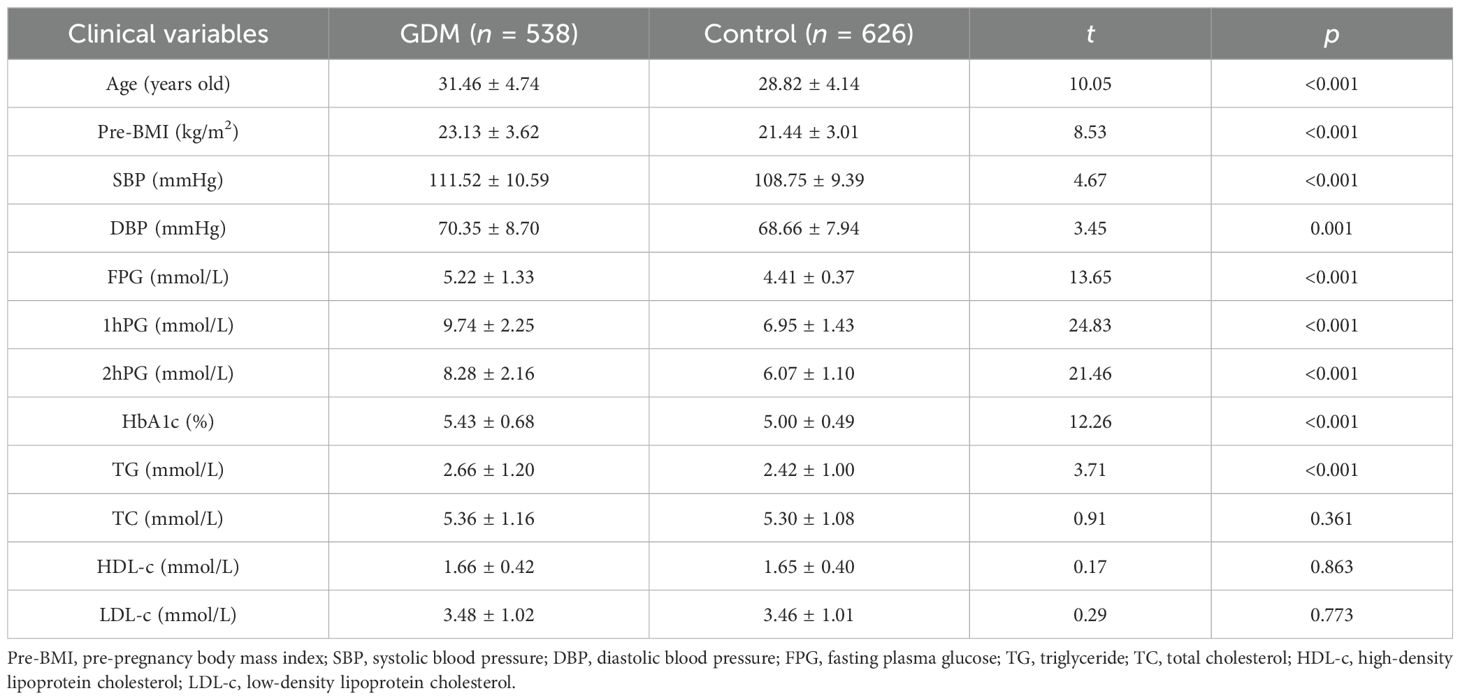
Table 1. The baseline characteristics of subjects (mean ± SD).
3.2 Association between variants and GDM riskGenotype frequencies of variants (rs4134819, rs720918, rs11109509, and rs12524768) followed the principle of HWE (pHWE > 0.05) except for rs2034410 (Table 2). Only the genotype distribution of rs4134819 was different between GDM cases and healthy controls (χ2 = 6.83, p = 0.033). After adjusting age and pre-BMI, the rs4134819 CT genotype could significantly increase GDM risk compared to the CC genotype (adjusted OR = 1.38, 95% CI: 1.01–1.87, p = 0.044). Under the dominant model, CT/TT genotypes could increase the GDM risk by 42% compared with the CC genotype (crude OR = 1.42, 95% CI: 1.08–1.86, p = 0.013). However, after adjusting for age and pre-BMI, this significant association disappeared (Table 2).
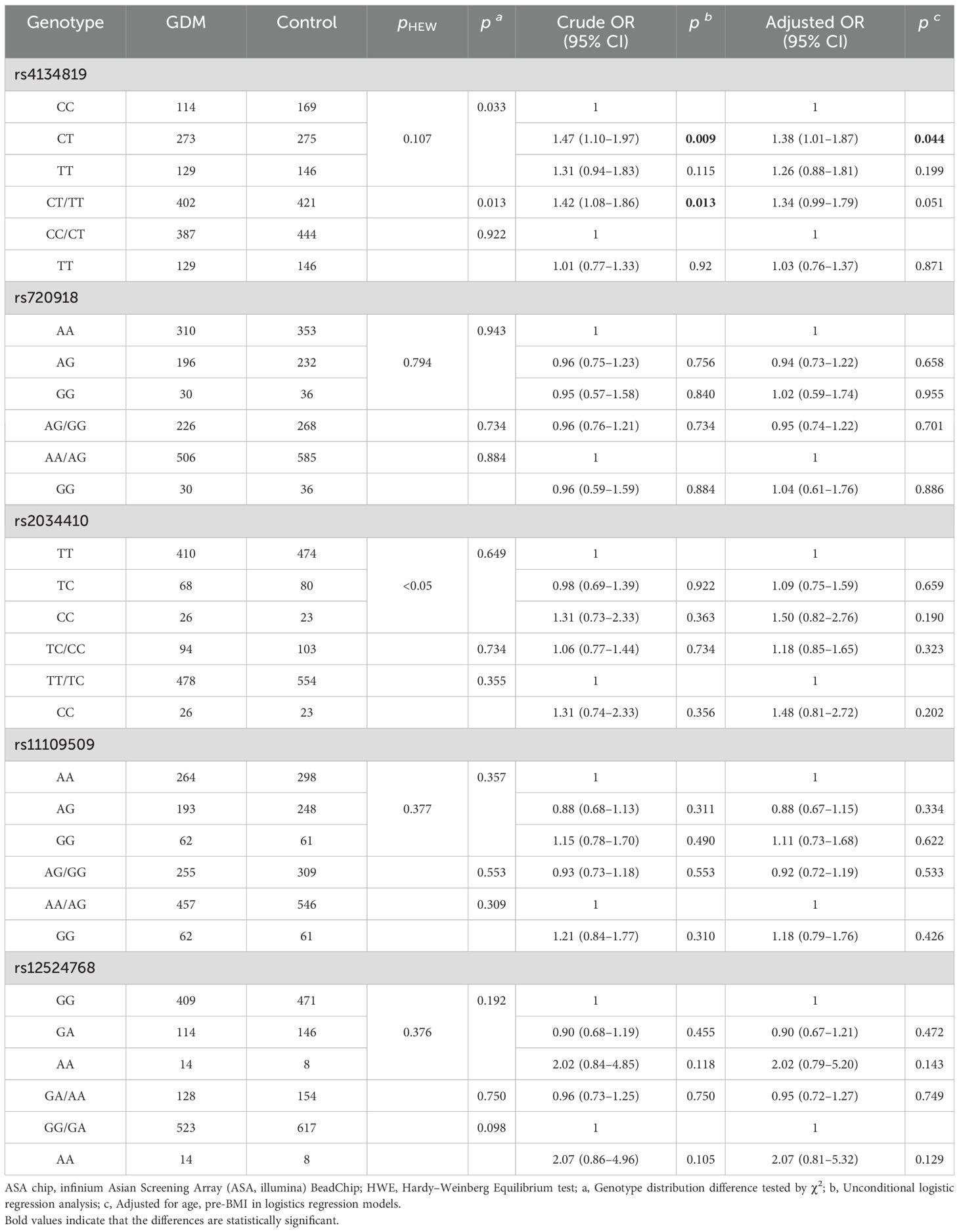
Table 2. The associated analysis of GDM and screened functional variants.
The stratified analysis was performed under the dominant model. Compared to the CC genotype, CT/TT genotypes had a higher GDM risk in subgroups with SBP > 110.03 mmHg (adjusted OR = 1.81, 95% CI: 1.18–2.78, p = 0.007), DBP > 69.44 mmHg (adjusted OR = 1.60, 95% CI: 1.03–2.47, p = 0.036), 2hPG > 7.10 mmol/L (adjusted OR =1.85, 95% CI:1.12–3.04, p = 0.016), HbA1c > 5.20% (adjusted OR = 1.65, 95% CI: 1.03–2.64, p = 0.036), and TG > 2.53 mmol/L (adjusted OR = 1.59, 95% CI: 1.03–2.46, p = 0.038) after adjusting age and pre-BMI (Table 3).
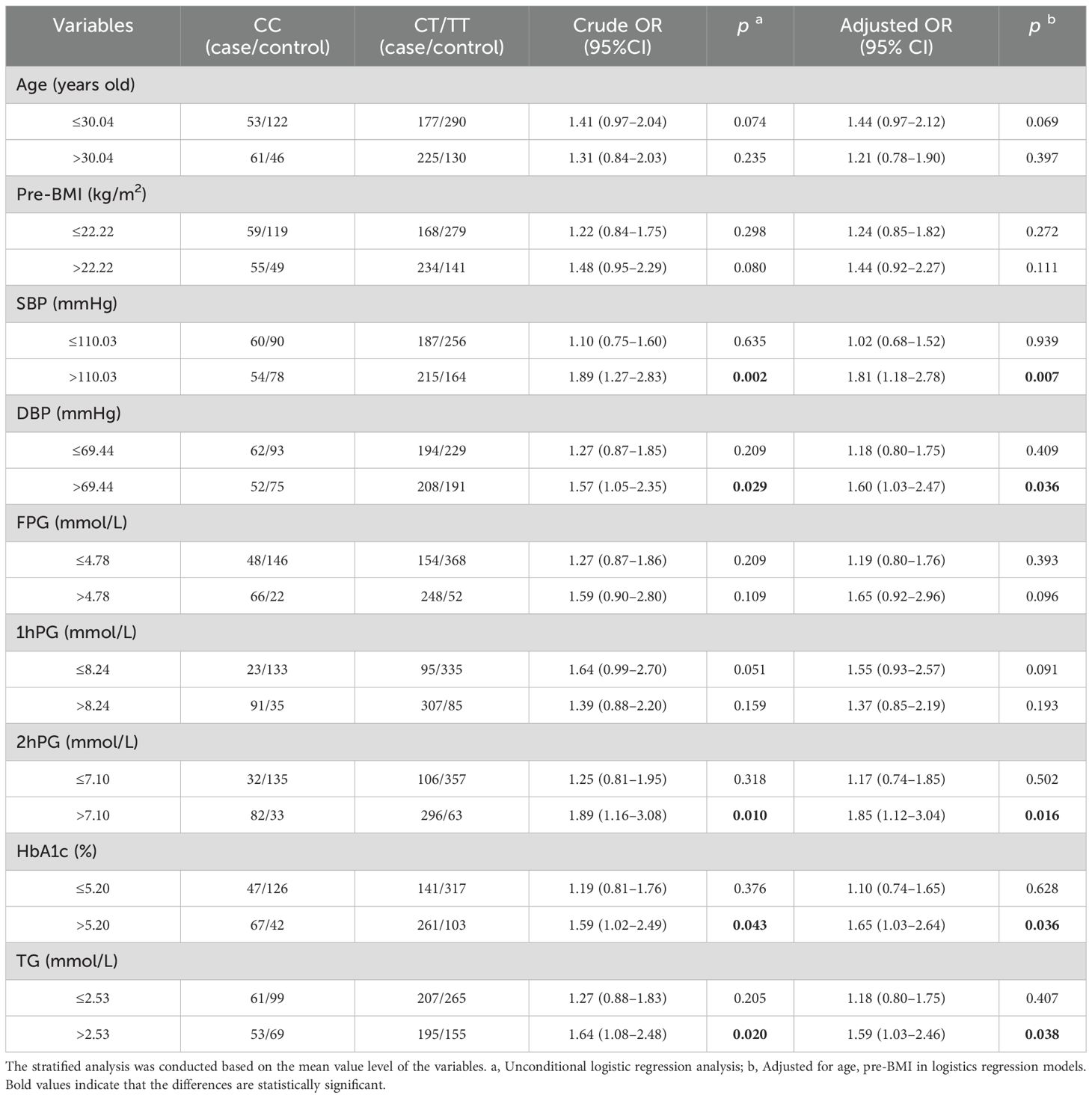
Table 3. Stratified analysis for associations between XAB2 gene rs4134819 and GDM risk.
However, there was no significant association observed between other variants (rs720918, rs2034410, rs11109509, and rs12524768) and the risk of GDM, as shown in Table 2.
3.3 FPRP analysisThe FPRP test was adopted to evaluate the robustness of positive associations with a prior probability setting at 0.1 and an FPRP threshold value setting at 0.2. As demonstrated in Table 4, the association between rs4134819 and GDM risk in the dominant model (CT/TT vs. CC) seems to be reliable correlation (p = 0.147), while other statistically significant results may be detected by chance and should be taken with caution.
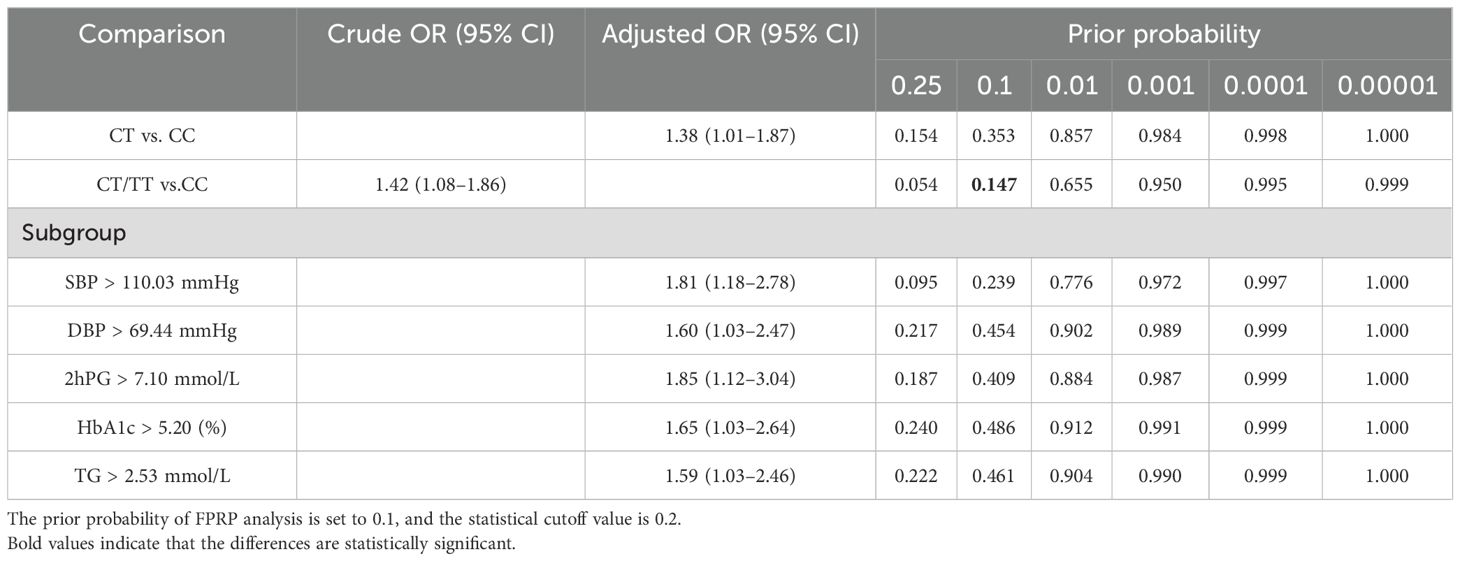
Table 4. FPRP analysis for the positive associations between rs4134819 and GDM risk.
3.4 Biological functional analysisGiven the fact that rs4134819 is located at the TFBS region of XAB2 gene, bioinformatic tools were used to predict the potential functional impact caused by the genetic variant. It can be seen that the rs4134819 C allele binds with the transcription factor “CPE bind” in 91–100 bp. However, when the wild-type allele changes to T allele, the transcription factor attached to it also changes to “GATA-1”, which suggested that rs4134819 may have an impact on the regulation of gene transcription (Figures 3A, B). Moreover, eQTL analysis based on the Genotype-Tissue Expression Project (GTEx V8) indicated that rs4134819 can be an eQTL and affect the expression level of functional genes such as PET100, PCP2, and CTD-3214H19.6 in different tissues (Table 5).
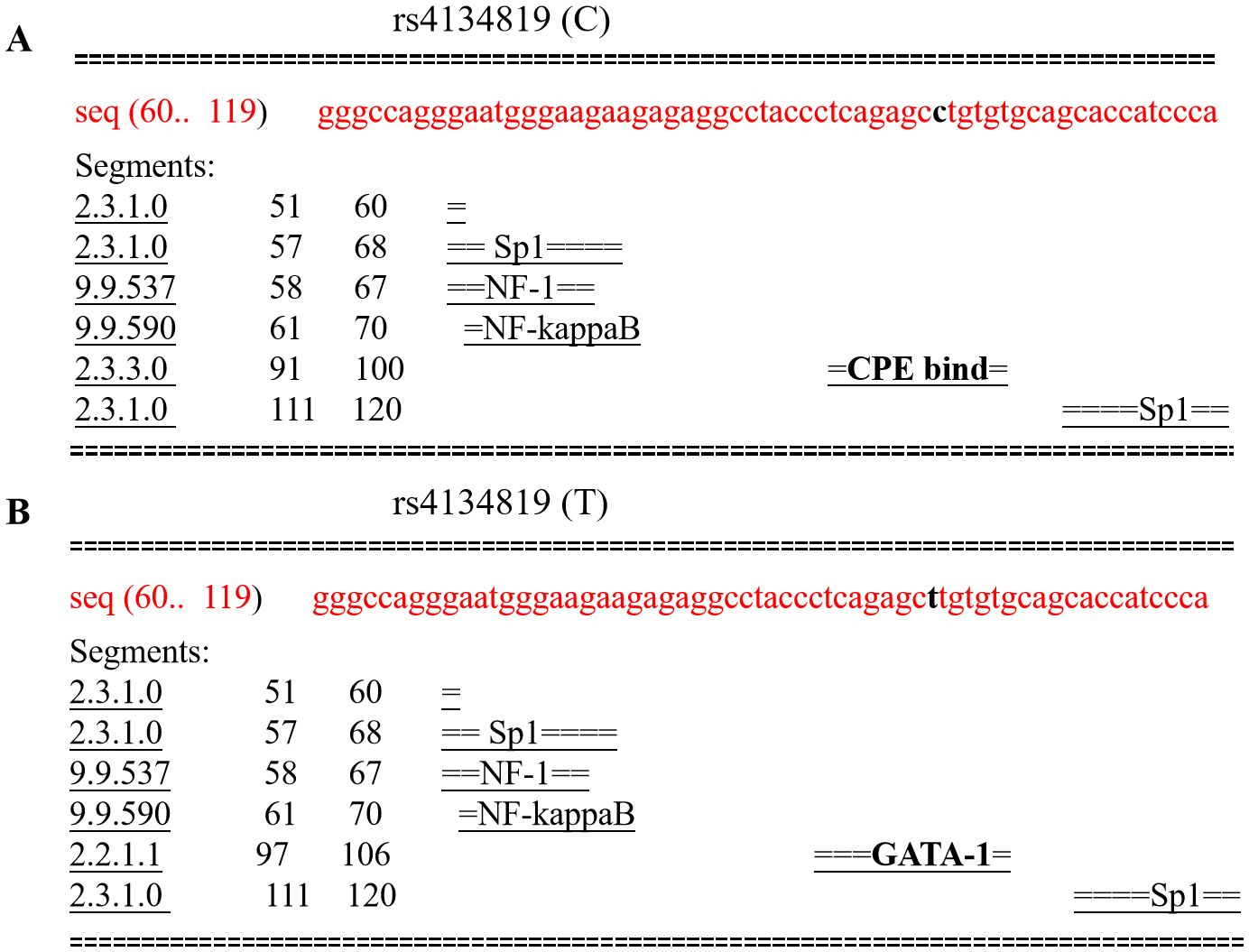
Figure 3. Comparison of transcription factors bound to rs4134819 C>T. (A) The transcription factors bound to the wild allele of rs4134819 in the 60–119 sequence. (B) As the wild allele (C) altered to rs4134819 T, the transcription factor changed from “CPE bind” to “GATA-1” in the 97–106 sequence.

Table 5. The eQTL effect analysis of rs4134819 regulating functional gene expression using online tools.
3.5 Factor selection and the nomogram establishmentClinical variable selection was based on univariate and multivariate logistic regression analysis. The latter was performed by a backward stepwise selection with the Akaike information criterion (AIC). The final results showed that age, FPG, HbA1c, 1hPG, 2hPG, and TG were independent risk factors of GDM with a model AIC of 536.78. Furthermore, considering that rs4134819 was significantly associated with GDM, we attempted to take the variant into the model. Surprisingly, the AIC of the multivariable logistic regression model was decreased to 533.94 (Table 6).
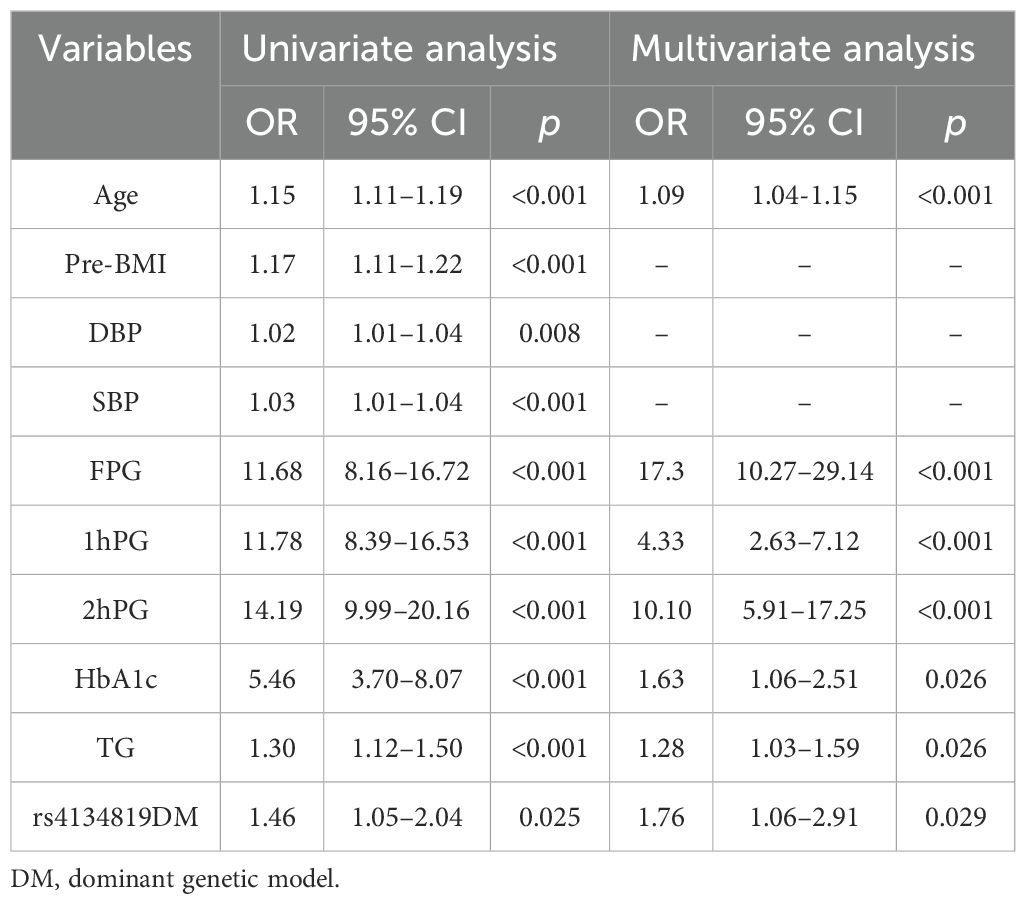
Table 6. GDM risk factors screened using univariate and multivariate logistic regression analysis.
The seven GDM risk factors were ultimately used to construct the predictive nomogram in the training cohort. In the traditionally static nomogram, each value level of the risk factors was given the corresponding score, and the total score obtained by adding up the score of all risk factors can be employed to predict the probability of GDM occurrence (Figure 4). Meanwhile, to make the nomogram more applicable and convenient for clinicians, we developed an online dynamic nomogram (https://qiulianl.shinyapps.io/GDM_risk_prediction/), which was able to visualize the GDM predictive results (Figures 5A, B). For instance, a 31-year-old pregnant woman had the following test results: FPG > 4.78 mmol/L, 1hPG > 8.24 mmol/L, 2hPG ≤ 7.10 mmol/L, HbA1c = 6%, and TG = 3 mmol/L, and carried CT/TT genotypes, whose probability of GDM occurrence was predicted as 66.3%. Interestingly, when the exposure level of clinical indicators remained unchanged and only the genotype was altered to CC, the predictive probability of GDM was 52.9%, which suggested that genetic component played a certain role in GDM occurrence (Figure 5C).
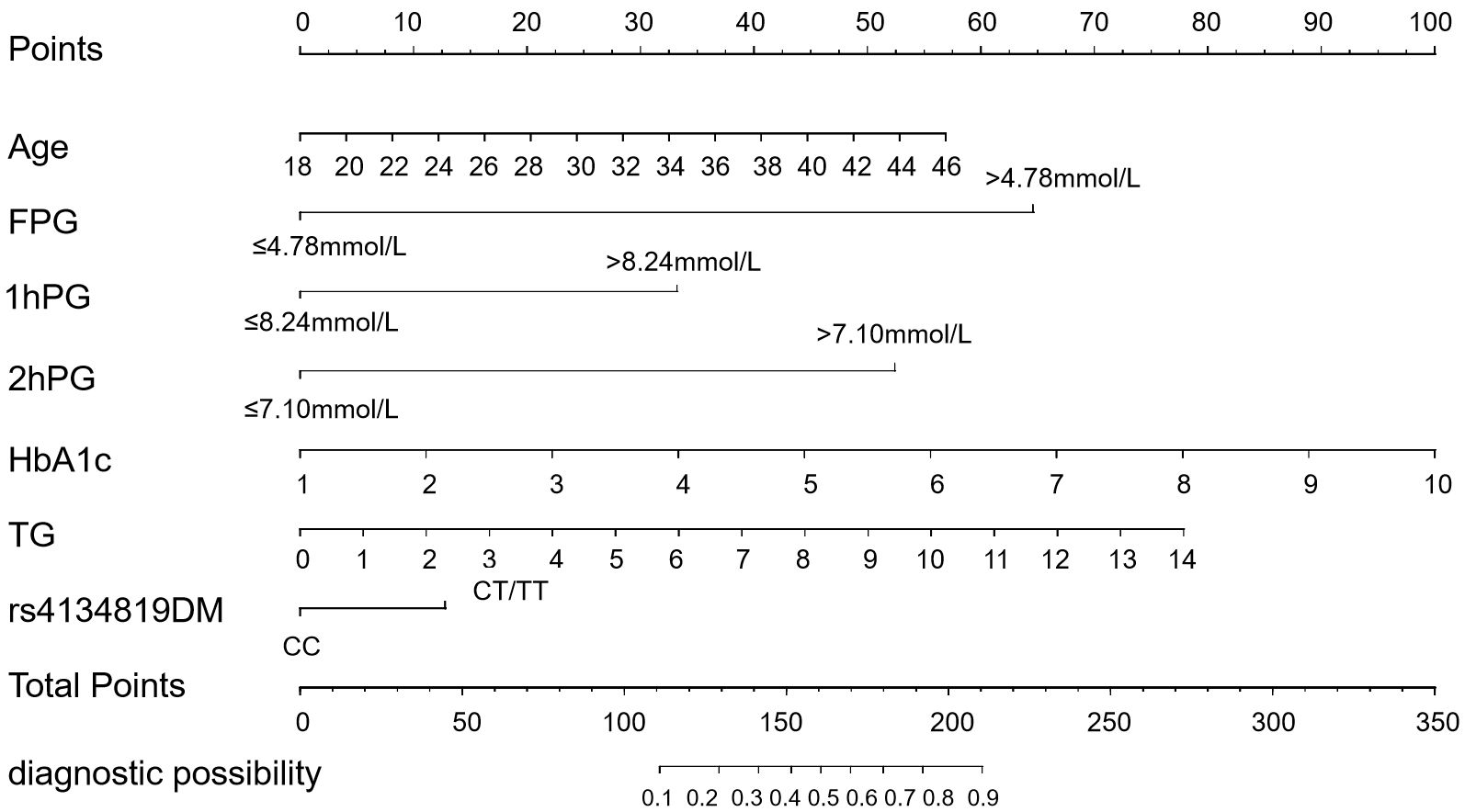
Figure 4. Conventional static nomogram model constructed with age, FPG, 1hPG, 2hPG, HbA1c, TG, and rs4134819DM (dominant model). A standard of scoring based on the regression coefficient (β) of indicators is formulated. Each level of the indicators will be given a specific score, and the scores of each factor are added up to get the total point. The value corresponding to the vertical line of the total points is the probability of GDM occurrence.
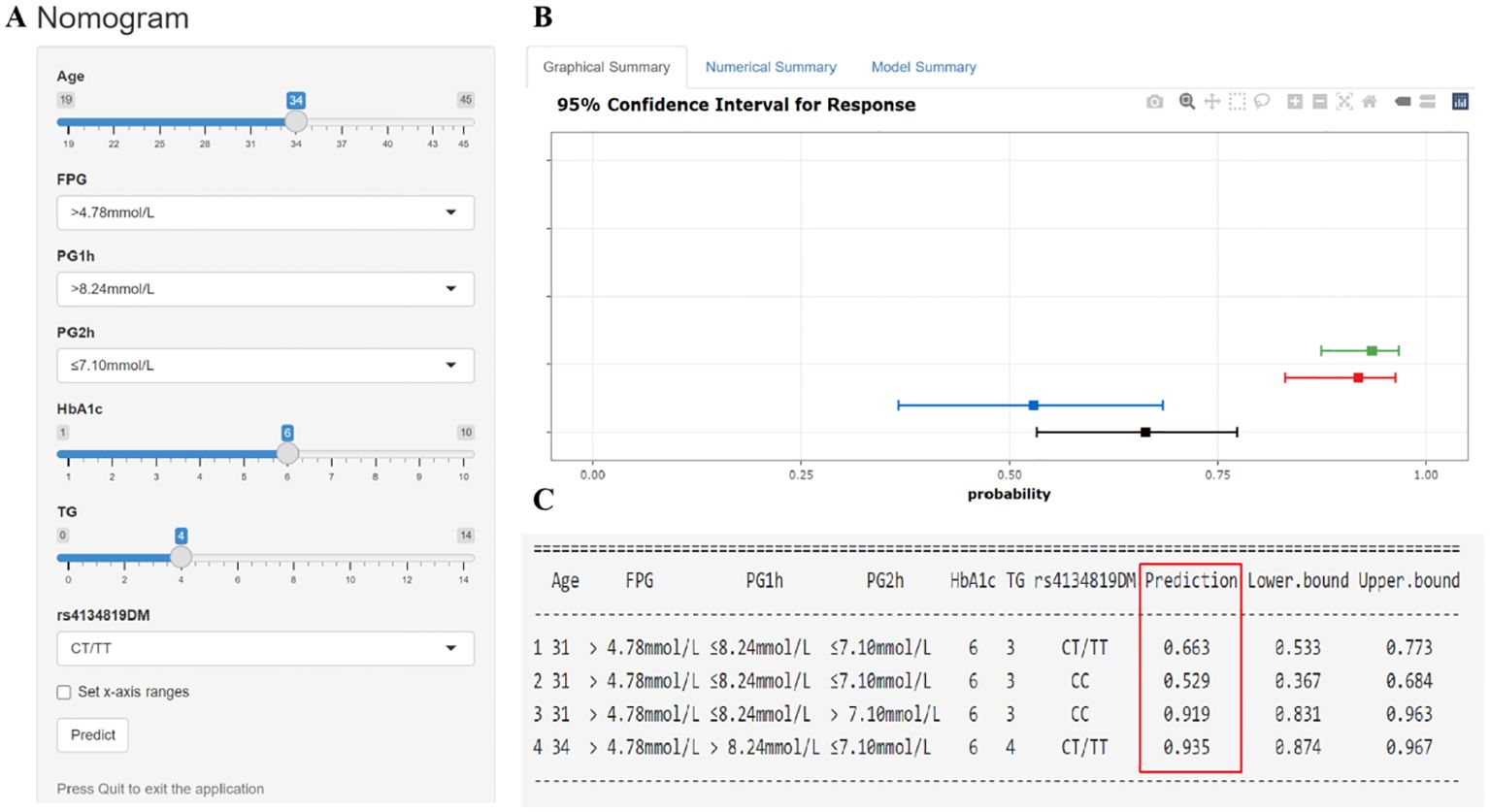
Figure 5. Interactive dynamic nomogram based on a web application “shinyapps” (https://qiulianl.shinyapps.io/GDM_risk_prediction/). (A) The value input plate of pregnant women’s predictive indicators. (B) The results of model prediction are presented in a visual form. A horizontal line represents the predictive results of one subject, the bold square dot is the probability of GDM, and the two ends of the line are 95% confidence intervals. (C) Presentation of specific input values and corresponding predictive result values (GDM incidence and 95% confidence interval).
3.6 Validation of the nomogramThe AUC of the nomogram was 0.931 (95% CI: 0.914–0.948) in the training cohort and 0.902 (95% CI: 0.870–0.935) in the internal validation cohort, suggesting a good predictive power of the model (Figures 6A, B). The calibration curves of the nomogram were close to the ideal line whether in the training cohort or the validation cohort, and the Hosmer–Lemeshow analysis also produced acceptable results (p > 0.05), indicating that there was a good calibration for the risk estimation in the predictive model (Figures 6C, D). The nomogram DCA curves were higher than the “treat all” and “treat none” lines for most of the predicted threshold probabilities, which showed good clinical validity and net benefit (Figures 6E, F).
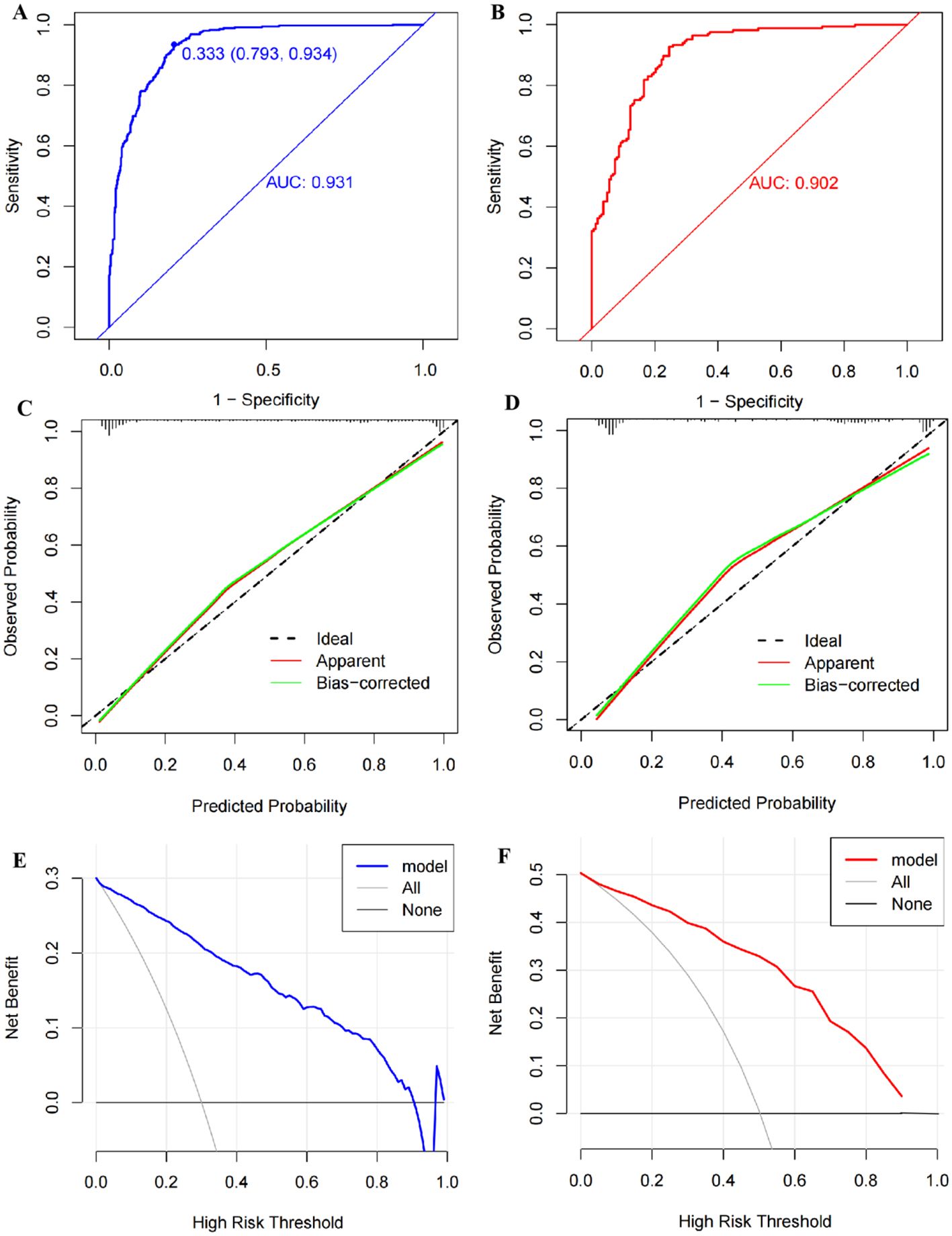
Figure 6. Validation of the nomogram model. (A) The receiver operator characteristic (ROC) curve with an area under the curve (AUC) of 0.931, a cutoff value of 0.333, a sensitivity of 0.934, and a specificity of 0.793 in the training set. (B) The ROC curve with an AUC of 0.902 in the testing set. (C) The calibration curve conducted via a bootstrap method with 1,000 resamples in the training set, and its mean absolute error was 0.027. (D) The calibration curve conducted by a bootstrap method with 1,000 resamples in the testing set, and the mean absolute error was 0.035. (E) Decision curve analysis (DCA) shows the clinical validity of the nomogram model in the training set. (F) DCA curve in the testing set.
4 DiscussionAs is known, chronic insulin resistance and pancreatic β-cell dysfunction are the main physiopathological mechanisms of GDM. As pregnancy progresses, the upregulation of human placental lactogen (hPL), estrogen, progesterone, cortisol, and prolactin can cause progressive insulin resistance, and when pancreatic β-cells fail to compensate for the situation, it will lead to hyperglycemia and even gestational diabetes (32, 33). GDM is affected by the interplay between multiple etiologies and has an obvious genetic tendency (34). Individual genetic susceptibility often interacts with environment factors to participate in the onset of diseases. On the basis of genetic predisposition, complex human diseases can be induced via the acquired environment factors such as personal age, diet, and physical activity (35).
In this case–control study, we confirmed that the TFBS polymorphism rs4134819 in XAB2 gene was significantly associated with an increased GDM risk in all subjects and most subgroups (SBP > 110.03 mmHg, DBP > 69.44 mmHg, 2hPG > 7.10 mmol/L, HbA1c > 5.20%. and TG > 2.53 mmol/L) in the Guilin population. Furthermore, we have detected that age, glucose, and lipid metabolic indicators, including FPG, HbA1c, 1hPG, 2hPG, and TG, are also risk factors for GDM. A nomogram model constructed with the XAB2 rs4134819 and the above clinical indicators suggested a good predictive performance with a diagnostic AUC of 0.931. These findings support the important role of rs4134819 in the pathogenesis of GDM.
Xeroderma Pigmentosum group A-binding protein 2 (XAB2) is a multifunctional protein playing a vital role in cellular processes such as transcription, splicing, DNA repair, and messenger RNA export (36). It was reported that XAB2 may exert as a regulator in hyperglycemia with chronic insulin (37, 38). We observed that XAB2 rs4134819 was correlated with an elevated GDM risk in this study, and the FPRP analysis was performed to confirm the positive association. As predicted by bioinformatic tools, rs4134819 is located in the TFBS region of the XAB2 gene. We further analyzed the potential biological functions of rs4134819 and found that rs4134819 C>T can alter the transcription factors binding to the promoter and act as an eQTL regulating gene transcription. Based on the above findings, it is speculated that this may be one of the biological mechanisms in which XAB2 gene rs4134819 alters individual susceptibility to GDM.
Early pregnancy is a critical period before the onset of GDM and also a critical stage for fetal growth and development. It provides a unique opportunity for the prevention and treatment of uncertain maternal and child diseases in the future. Research supports the circulating biomarkers of the first trimester, such as blood sugar, fasting insulin, adiponectin, HDL-c, triglycerides, and C-reactive protein (39), which may predict an enhanced possibility of GDM risk. The deeper link of indicators of glycolipid metabolism to GDM may be that the glucose homeostasis is affected by disturbed lipid metabolism (40), while high triglycerides and elevated free fatty acids (FFAs) can generate oxidative stress and activate protein kinase C, coupled with the complex combined actions of a series of inflammatory factors, leading to insulin resistance and, ultimately, the development of hyperglycemia (41–43).
The nomogram model based on various risk variables has been regarded as a useful tool for GDM risk prediction in recent years. Wu et al. performed a nomogram of GDM based on maternal age, pre-pregnancy BMI, and OGTT, and obtained a diagnostic AUC of 0.872 (44). There were also several studies constructing GDM predictive models according to general conditions and laboratory indicators, such as TG, HDL-c, being overweight or obese before pregnancy, a family history of diabetes, a history of GDM, and a sedentary lifestyle (45–47). Although these models′ predictive powers were acceptable, lack of precise genetic indicators may decrease the predictive effect of the model. Our study established the dynamic nomogram by combining the genetic variant rs4134819 and significant clinical indicators (age, FPG, HbA1c, 1hPG, 2hPG, and TG), which demonstrated a good performance with an AUC of 0.931, a sensitivity of 0.934, and a specificity of 0.793. This suggests that in the construction of risk prediction models for complex diseases, including GDM, it is necessary to consider key genetic factors.
Our study has some limitations. First, the small sample analyzed in our previous chip screening stage may reduce statistical efficiency and cause the deviation of finding clues. Second, the subjects of this case–control study were selected from the hospital; thus, the selection bias was inevitable. Third, the biological function of the genetic variant was only predicted by bioinformatic tools and was not validated by molecular experiments. Fourth, the risk factors used to construct the nomogram were limited and we did not adopt the multi-center validation for the model’s predictive power. It is hoped that future studies will comprehensively consider multiple factors in establishing nomogram model and validate its predictive effect in different regions and population.
In conclusion, this study supports the significant association between XAB2 rs4134819 C>T and the pathogenesis of GDM. Regulating the binding efficiency of transcription factors (CPE bind and GATA-1) and promoters and affecting the transcription of functional genes may be a potential mechanism. The dynamic nomogram constructed by genetic and clinical risk factors can effectively identify pregnant women with high GDM risk in early pregnancy.
Data availability statementThe data presented in the study are deposited in Dryad repository, accession number https://doi.org/10.5061/dryad.rv15dv4j7.
Ethics statementThe studies involving humans were approved by The Ethics Committee of Guilin Medical University. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributionsQL: Formal analysis, Methodology, Software, Writing – original draft. YS: Formal analysis, Writing – original draft, Funding acquisition, Visualization. ML: Data curation, Formal analysis, Supervision, Writing – original draft. RL: Investigation, Writing – original draft, Data curation, Validation. LN: Data curation, Writing – original draft, Software, Validation. LL: Funding acquisition, Writing – review & editing, Conceptualization. XY: Conceptualization, Funding acquisition, Project administration, Writing – review & editing, Supervision.
FundingThe author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was supported by the Guangxi Natural Science Foundation(2024JJH140351), the Guangxi Science and Technology Base and Talent Special Project (AD24010027), Guilin Science Research and Technology Development Plan Project (20230135-2-1), self-funded research project of the Health Committee of Guangxi (Z-C20241580) and the Fujian Provincial Health Technology Project (2020CXA017).
Conflict of interestThe authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s noteAll claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary materialThe Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2024.1476222/full#supplementary-material.
References1. Diagnostic criteria and classification of hyperglycaemia first detected in pregnancy: a World Health Organization Guideline. Diabetes Res Clin Pract. (2014) 103:341–63. doi: 10.1016/j.diabres.2013.10.012
PubMed Abstract | Crossref Full Text | Google Scholar
2. Wang H, Li N, Chivese T, Werfalli M, Sun H, Yuen L, et al. IDF diabetes atlas: estimation of global and regional gestational diabetes mellitus prevalence for 2021 by international association of diabetes in pregnancy study group’s criteria. Diabetes Res Clin Pract. (2022) 183:109050. doi: 10.1016/j.diabres.2021.109050
PubMed Abstract | Crossref Full Text | Google Scholar
3. Gao C, Sun X, Lu L, Liu F, Yuan J. Prevalence of gestational diabetes mellitus in mainland China: A systematic review and meta-analysis. J Diabetes Investig. (2019) 10:154–62. doi: 10.1111/jdi.12854
PubMed Abstract | Crossref Full Text | Google Scholar
4. Tsakiridis I, Giouleka S, Mamopoulos A, Kourtis A, Athanasiadis A, Filopoulou D, et al. Diagnosis and management of gestational diabetes mellitus: an overview of national and international guidelines. Obstet Gynecol Surv. (2021) 76:367–81. doi: 10.1097/OGX.0000000000000899
PubMed Abstract | Crossref Full Text | Google Scholar
5. Lowe WL Jr., Scholtens DM, Kuang A, Linder B, Lawrence JM, Lebenthal Y, et al. Hyperglycemia and adverse pregnancy outcome follow-up study (HAPO FUS): maternal gestational diabetes mellitus and childhood glucose metabolism. Diabetes Care. (2019) 42:372–80. doi: 10.2337/dc18-1646
PubMed Abstract | Crossref Full Text | Google Scholar
6. Farahvar S, Walfisch A, Sheiner E. Gestational diabetes risk factors and long-term consequences for both mother and offspring: a literature review. Expert Rev Endocrinol Metab. (2019) 14:63–74. doi: 10.1080/17446651.2018.1476135
PubMed Abstract | Crossref Full Text | Google Scholar
7. Juan J, Yang H. Prevalence, prevention, and lifestyle intervention of gestational diabetes mellitus in China. Int J Environ Res Public Health. (2020) 17:9517. doi: 10.3390/ijerph17249517
PubMed Abstract | Crossref Full Text | Google Scholar
8. Wang Y, Luo B. Risk factors analysis of gestational diabetes mellitus based on International Association of Diabetes Pregnancy Study Groups criteria. Nan Fang Yi Ke Da Xue Xue Bao. (2019) 39:572–8. doi: 10.12122/j.issn.1673-4254.2019.05.12
PubMed Abstract | Crossref Full Text | Google Scholar
9. Wan CS, Abell S, Aroni R, Nankervis A, Boyle J, Teede H. Ethnic differences in prevalence, risk factors, and perinatal outcomes of gestational diabetes mellitus: A comparison between immigrant ethnic Chinese women and Australian-born Caucasian women in Australia. J Diabetes. (2019) 11:809–17. doi: 10.1111/1753-0407.12909
PubMed Abstract | Crossref Full Text | Google Scholar
10. Savitz DA, Janevic TM, Engel SM, Kaufman JS, Herring AH. Ethnicity and gestational diabetes in New York City, 1995-2003. BJOG. (2008) 115:969–78. doi: 10.1111/j.1471-0528.2008.01763.x
PubMed Abstract | Crossref Full Text | Google Scholar
11. Claussnitzer M, Cho JH, Collins R, Cox NJ, Dermitzakis ET, Hurles ME, et al. A brief history of human disease genetics. Nature. (2020) 577:179–89. doi: 10.1038/s41586-019-1879-7
PubMed Abstract | Crossref Full Text | Google Scholar
12. Ramirez-Bello J, Jimenez-Morales M. Functional implications of single nucleotide polymorphisms (SNPs) in protein-coding and non-coding RNA genes in multifactorial diseases. Gac Med Mex. (2017) 153:238–50.
PubMed Abstract | Google Scholar
14. Zhen J, Gu Y, Wang P, Wang W, Bian S, Huang S, et al. Genome-wide association and Mendelian randomisation analysis among 30,699 Chinese pregnant women identifies novel genetic and molecular risk factors for gestational diabetes and glycaemic traits. Diabetologia. (2024) 67:703–13. doi: 10.1007/s00125-023-06065-5
PubMed Abstract | Crossref Full Text | Google Scholar
15. Elliott A, Walters RK, Pirinen M, Kurki M, Junna N, Goldstein JI, et al. Distinct and shared genetic architectures of gestational diabetes mellitus and type 2 diabetes. Nat Genet. (2024) 56:377–82. doi: 10.1038/s41588-023-01607-4
PubMed Abstract | Crossref Full Text | Google Scholar
16. Liang Q, Li M, Huang G, Li R, Qin L, Zhong P, et al. Genetic susceptibility, mendelian randomization and nomogram model construction of gestational diabetes mellitus. J Clin Endocrinol Metab. (2024) 109:2802–14. doi: 10.1210/clinem/dgae200
PubMed Abstract | Crossref Full Text | Google Scholar
17. Li R, Wang Y, Yang L, Zhong P, Huang G, Liang Q, et al. Genetic variants of ERBB4 gene and risk of gestational diabetes mellitus: a susceptibility and diagnostic nomogram study. Front Endocrinol (Lausanne). (2023) 14:1283539. doi: 10.3389/fendo.2023.1283539
留言 (0)