In 2023, foremost societies, including the American Heart Association (AHA), the American College of Cardiology (ACC), and the European Society of Cardiology (ESC) endorsed its formal definition (1). Diabetic cardiomyopathy (DMCM) is defined as left ventricle (LV) dysfunction in the presence of diabetes mellitus (DM), whether it is type 1 (DM1) or type 2 (DM2), and in the absence of hypertension (HTN), obstructive epicardial coronary artery disease (CAD), and valvular heart disease (VHD) (2, 3).
Worldwide, the prevalence of DM increased from 151 million in 2000 to 537 million in 2021, and it is projected to increase to 643 million by 2030 (4). Among diabetic patients, the prevalence of DMCM ranges from 16.9% (5) to about 67% (6), depending on the criteria used for definition.
In this review, we will briefly discuss the pathophysiology, staging, and therapeutics, with a dedicated focus on the role of multi-modality imaging.
Pathophysiology & stagingThe pathophysiology of DMCM involves a complex interplay of insulin resistance mediating hyperglycemia and lipotoxicity (Figure 1). In that milieu, oxidative stress ensues, with accompanying inflammation, resulting in cardiomyocyte calcium dyshomeostasis (7, 8), cardiomyocyte death (9, 10) and, later, hypertrophy (11), along with endothelial damage (12, 13) and interstitial fibrosis (14–16).
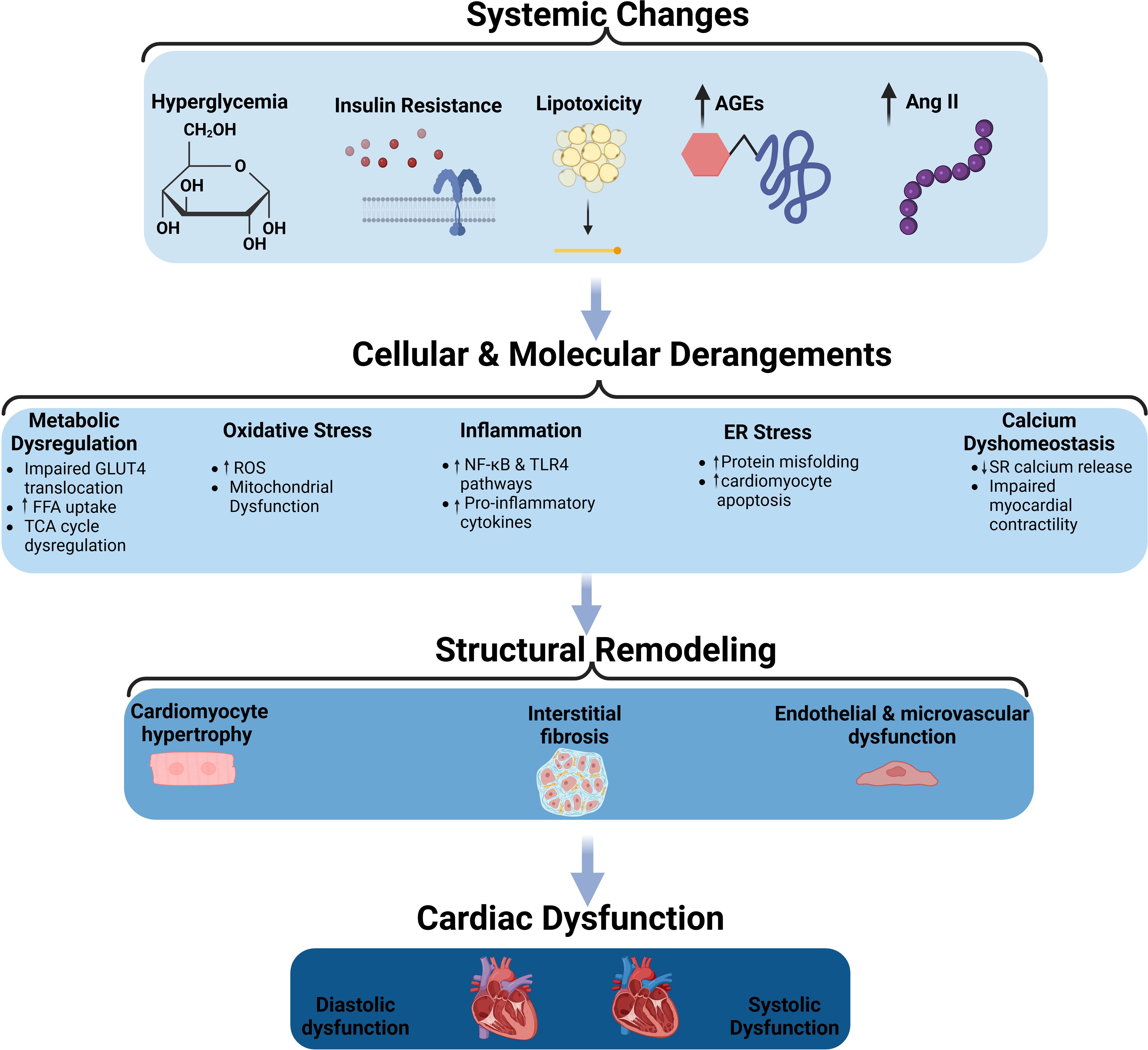
Figure 1. The pathophysiological mechanisms of diabetic cardiomyopathy. Schematic representation of the systemic, cellular, and molecular changes leading to structural remodeling and cardiac dysfunction in diabetic cardiomyopathy. Systemic changes such as hyperglycemia, insulin resistance, lipotoxicity, advanced glycation end-products (AGEs), and increased angiotensin II (Ang II) contribute to cellular and molecular derangements, including metabolic dysregulation, oxidative stress, inflammation, endoplasmic reticulum (ER) stress, and calcium dysregulation. These changes promote structural remodeling characterized by cardiomyocyte hypertrophy, interstitial fibrosis, and endothelial/microvascular dysfunction, ultimately leading to diastolic and systolic cardiac dysfunction. AGEs, Advanced Glycation End-products; Ang II, Angiotensin II; FFA, Free Fatty Acids; GLUT4, Glucose Transporter Type 4; TCA cycle, Tricarboxylic Acid Cycle; ROS, Reactive Oxygen Species; NF-κB, Nuclear Factor Kappa-Light-Chain-Enhancer of Activated B Cells; TLR4, Toll-like Receptor 4; ER, Endoplasmic Reticulum; SR, Sarcoplasmic Reticulum.
Early, patients usually experience impaired myocardial relaxation, which manifests as mild diastolic dysfunction (10, 17). As the disease progresses, patients develop left ventricular hypertrophy (LVH) in the setting of cardiomyocyte hypertrophy, interstitial fibrosis, and maladaptive inflammatory response. Clinically, this manifests as more advanced diastolic dysfunction and possibly early systolic dysfunction (10). In the late stages of the disease, severe neurohormonal disturbances, such as activation of both the Angiotensin-II and sympathetic nervous systems (17), lead to significant increases in LV thickness, mass, and size, with an accompanying impairment in both systolic and diastolic function (10, 17, 18).
Role of multimodality imagingFrom a clinical perspective, the diagnosis of DMCM requires the utilization of at least one imaging modality to confirm LV structural and functional impairments. Imaging also aids in monitoring the course of the disease and assess the impact of treatment. Furthermore, different imaging modalities allow the assessment of different mechanisms contributing to the development and progression of DMCM (3, 19, 20).
EchocardiographyEchocardiography is the gold standard in diagnosing DMCM, owing to its high temporal and spatial resolutions, accessibility, affordability, and harmlessness (19, 21). One of the earliest features of DMCM is the impairment of diastolic function (19). In a case-control study among patients with DM2 with a median duration of > 5 years, systolic function was preserved among all patients. However, 54% of the diabetic patients had diastolic dysfunction, compared to 11% among non-diabetic controls, with more incident diastolic dysfunction correlating with duration of diabetes (22).
Further, Somaratane et al. inspected the prevalence of LVH in a cohort of DM2, and found that 56% of the diabetic population exhibited this structural abnormality. Interestingly, electrocardiograms (EKG) only detected 5% of LVH cases, and while NT-proBNP was superior to EKG, it remained inadequate; this study underscores the significant utility of echocardiography in detecting LVH (23).
Additionally, in patients with DM1 and average HbA1c of 8%, the lateral mitral annular early diastolic velocity was lower compared to non-diabetic controls, which is a marker of diastolic dysfunction (24). Moreover, in DM2 patients with a mean diabetes duration of 6.3 years, 45% demonstrated abnormal global longitudinal strain, which conferred a higher risk for the development of all-cause mortality or hospitalization (25).
Impaired diastolic parameters among diabetic patients are evidently associated with worse clinical outcomes. Rorth et al. showed that, among DM1 patients, higher filling pressures, as measured by early mitral inflow velocity and mitral annular early diastolic velocity ratio (e/e’), was associated with a higher risk of non-fatal myocardial infarction, cerebrovascular accidents, and death (26). Similarly, From et al. demonstrated that, among DM2 patients, the cumulative probability of both the incidence of heart failure and death more than doubled among diabetics with higher filling pressures as measured by e/e’ ratios (27).
In summary, echocardiography remains indispensable in the early detection and monitoring of DMCM, providing critical prognostic information that guides clinical decision-making and management. Its ability to identify subtle changes in cardiac structure and function underscores its role as the gold standard imaging modality in this patient population.
However, this technique is not without its challenges; the diagnostic accuracy can be operator-dependent, and image quality may be compromised in patients with poor acoustic windows, such as those with obesity or chronic lung disease (28). Additionally, while echocardiography is excellent for structural assessment, it provides limited information on myocardial tissue characterization and metabolism, which are crucial for understanding the pathophysiology of DMCM (3).
Cardiac magnetic resonance imagingCardiac magnetic resonance imaging (CMR) possesses higher spatial and temporal resolution than echocardiography, in addition to allowing the assessment of myocardial fibrosis and altered metabolism, which are hallmarks of DMCM (3, 19, 21).
Among DM1 patients, average HbA1c levels were inversely associated with stroke volume and positively correlated with the presence of myocardial scar tissue (29). Additionally, myocardial perfusion reserve and diastolic strain rate were abnormal in diabetic patients when compared to controls; further, increased myocardial triglyceride content, but not impaired myocardial flow reserve, was associated with abnormal diastolic function, suggesting that steatosis, but not necessarily small vessel disease, may be responsible for early diastolic dysfunction in DMCM (30). In a cohort derived from the general population, CMR revealed that pre-DM and DM were associated with LV remodeling compared to non-diabetic controls (31). Fibrotic tissue, as determined by late gadolinium enhancement, can be easily assessed using CMR and it has been associated with worse major adverse cardiovascular events (MACE) in diabetic patients (32).
However, CMR remains underutilized, largely owing to its perceived expense burden, patient comfort issues, and longer examination times (33). Additionally, traditional, older generation Gadolinium-based contrast media (CGBM) were associated with a low but serious risk of nephrogenic systemic fibrosis. Thankfully, with group II CGMB, this risk is significantly diminished (34). With further advancements in imaging and technology, it may be feasible in the future to incorporate CMR as a cornerstone in DMCM management.
Nuclear imagingNuclear imaging allows the detection of low-density processes, making it possible to measure myocardial metabolism and to assess molecular imaging (20). Gated SPECT possesses the capability to assess myocardial perfusion and LV function; however, limited data exist on its widespread clinical utility in DMCM (3).
Positron emission tomography (PET) allows flexibility in radiotracer design radiolabeled with a variety of radionuclides, and radiotracers administered at low doses do not alter metabolic processes (20). In order to circumvent the low spatial resolution observed in PET, it is usually combined with computer tomography or CMR to allow for accurate radiotracer localization (3, 20).
PET imaging has been utilized to study a multitude of metabolic parameters in DMCM. Using PET-CMR in DM2 patients with average HbA1c of 7.1% and mean diabetes duration of 4 years, Rijzewijk et al. demonstrated impaired diastolic parameters among diabetics; more importantly, they revealed increased myocardial fatty acid uptake and oxidation compared to controls, indicating myocardial metabolic remodeling (35). Similarly, in DM1 patients, PET imaging revealed increased free fatty acid utilization and decreased myocardial glucose uptake (36). Further, phase analysis of gated SPECT MPI revealed that asymptomatic DM2 patients with normal perfusion scans exhibited significant left ventricular mechanical dyssynchrony, particularly in those with a diabetes duration of more than 15 years (37). In a study investigating the association between diabetes mellitus and myocardial glucose uptake using 18F-FDG PET/CT, it was demonstrated that DM is significantly associated with decreased myocardial glucose metabolism, with up to 84% of diabetic patients showing poor FDG uptake. Furthermore, multivariate logistic regression analysis revealed that gender (male), Homeostatic Model Assessment of Insulin Resistance, and metabolic dysfunction-associated steatotic liver disease were independent risk factors for poor myocardial FDG uptake in diabetic patients (38).
In summary, nuclear imaging techniques like PET and gated SPECT are valuable in assessing myocardial metabolism and function in DMCM, revealing significant alterations such as impaired diastolic parameters, increased myocardial fatty acid uptake and oxidation, decreased myocardial glucose uptake, and left ventricular mechanical dyssynchrony, particularly in patients with prolonged diabetes duration.
The main challenges associated with nuclear imaging include its high cost and limited availability (20). Additionally, the relatively low spatial resolution of PET compared to CMR can limit its ability to detect small areas of myocardial scar or fibrosis (19). The use of ionizing radiation in both PET and SPECT raises concerns about radiation exposure, particularly in younger patients and those requiring repeated imaging studies (20). Furthermore, the interpretation of nuclear imaging studies requires specialized expertise, which may not be readily available in all clinical settings (3).
In conclusion, while each imaging modality offers unique benefits in the assessment of DMCM, they also come with specific challenges (Table 1). A multimodality approach, leveraging the strengths of each technique, can provide a comprehensive evaluation of diabetic cardiomyopathy, improving diagnostic accuracy and informing therapeutic strategies. Future advancements in imaging technology and reductions in cost may further enhance the integration of these modalities into routine clinical practice.
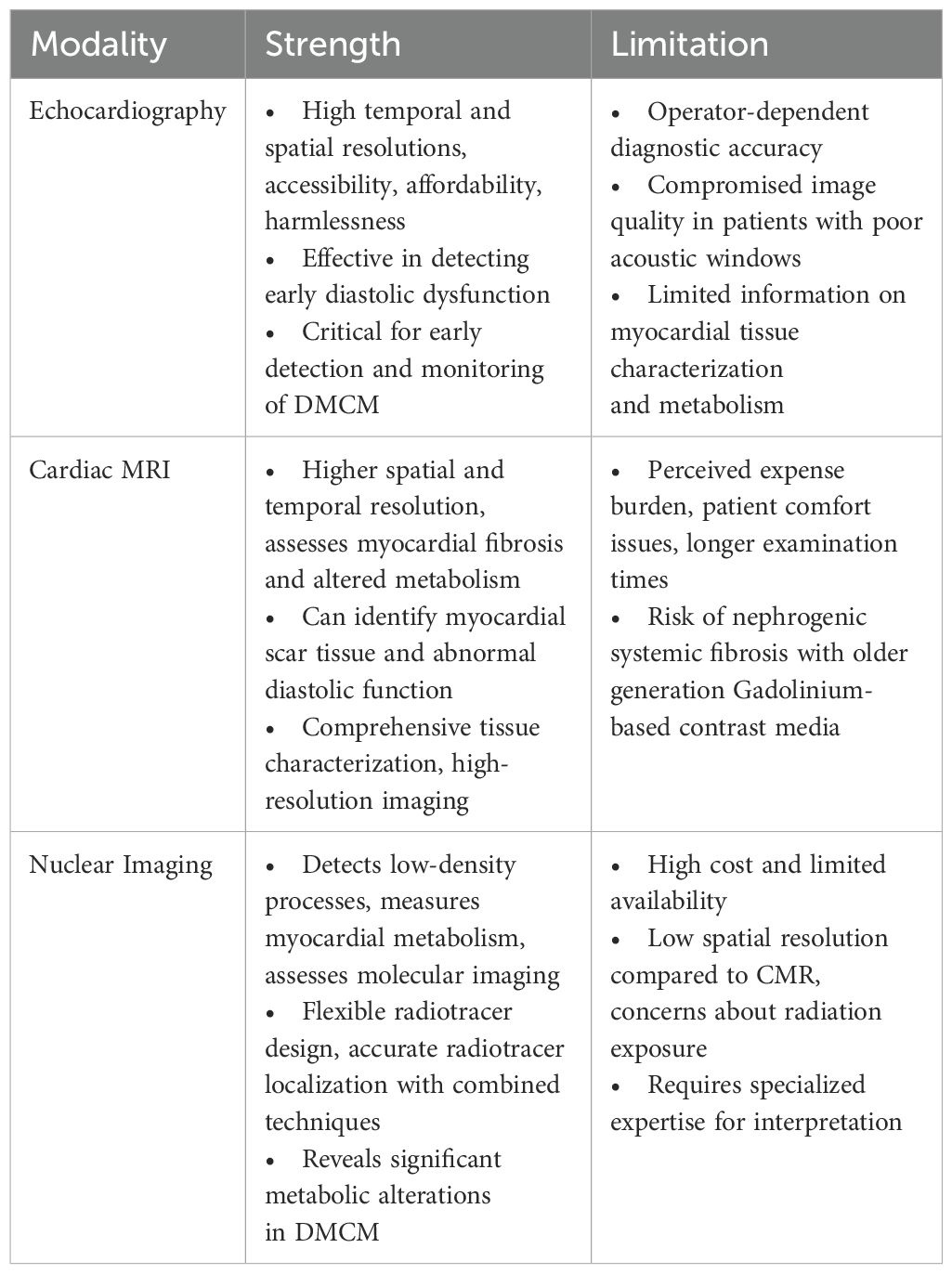
Table 1. Strengths and limitations of multimodality imaging techniques in diabetic cardiomyopathy.
TherapeuticsIn the UK Prospective Diabetes Study (UKPDS), a 1% decrease in HbA1c was associated with a 16% decrease in risk of myocardial infarction (39). Yet, it has become apparent that there is a U-curve relationship between HbA1c and mortality in diabetic patients with heart failure (40). Indeed, intensive glycemic control did not reduce cardiovascular events, and it was associated with a 47% increase in incident heart failure (41). Exogenous insulin therapy, which is utilized by all DM1 patients and 1/3rd of DM2 with heart failure patients (42), has been shown to be a risk factor for incident heart failure (43). Similarly, exogenous insulin use is associated with a higher risk of all-cause mortality, and HF rehospitalization (44). In a preclinical rodent model of experimental DM, insulin use was associated with increased interstitial fibrosis and cardiomyocyte apoptosis compared to both non-diabetic and untreated diabetic controls, further supporting the hypothesis that long-term exogenous insulin may adversely affect the myocardium (45).
Metformin use has yielded inconsistent results. In a metanalysis including 13,110 DM patients, metformin use did not bestow any HF benefit (46). On the other hand, in the UKPDS, metformin use was associated with a 39% reduction in the risk of myocardial infarction (47).
Sulfonylureas and thiazolidinediones have been associated with an increase in all-cause mortality and/or HF hospitalization among DM2 patients (48, 49). Dipeptidyl Peptidase 4 Inhibitors (DPP-4i) have not been associated with any cardiovascular benefit. In fact, saxagliptin has been associated with an increased risk of heart failure hospitalization (50). In the American Diabetes Association (ADA) Consensus Report in 2022, DPP-4i should be avoided in DM patients with ACC/AHA stage B and C (51). Glucagon-like Peptide-1 Receptor Agonists (GLP-1 RAs) use has been associated with a decreased risk of MACE in DM2 patients with established cardiovascular disease (52). While randomized clinical trials for GLP-1 RAs have shown no benefit when it comes to heart failure (53–56), the HARMONY Outcomes trial suggested a 29% reduction in heart failure hospitalization as a secondary outcome (57); further, in one meta-analysis, there was a 9% reduction in heart failure hospitalization (58). In STEP-HFpEF, heart failure hospitalization, as an exploratory end-point, seemed to be lower in the semaglutide group compared to placebo (59).
Sodium–Glucose Cotransporter 2 Inhibitors (SGLT2i) have been shown to decrease the risk of MACE and heart failure hospitalization among diabetics (60, 61). In fact, the ADA recommends SGLT2i use among DM patients with heart disease (62).
ConclusionIn conclusion, DMCM is a major and increasing health concern, fueled by the global rise in DM incidence (4–6). The complex pathophysiology of DMCM, characterized by insulin resistance, hyperglycemia, and lipotoxicity (18), leads to oxidative stress, inflammation, cardiomyocyte death, and fibrosis. These processes result in LVH and dysfunction (7–14). Multimodality imaging, encompassing echocardiography, CMR, and nuclear imaging, is crucial for the diagnosis, staging, and management of DMCM. Each imaging modality provides distinct insights into cardiac structure and function, metabolic changes, and tissue characterization, thus enhancing our comprehension and management of this intricate condition.
Author contributionsFA: Writing – original draft, Writing – review & editing. HC: Writing – review & editing.
FundingThe author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
AcknowledgmentsWe acknowledge the use of ChatGPT-4o for assistance with grammar and language refinement during the preparation of this manuscript. The authors also acknowledge that Figure 1 was created in BioRender. Adel, F. (2024) https://BioRender.com/f43t492.
Conflict of interestHC reports being a co-Founder of Zumbro Discovery, receiving grants from Scios Inc, and receiving royalties from UpToDate, and that his institution has patents for designer natriuretic peptides.
The remaining author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s noteAll claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References1. Authors/Task Force Members, Rydén L, Grant PJ, Anker SD, Berne C, Cosentino F, Danchin N, et al. ESC Guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD: the Task Force on diabetes, pre-diabetes, and cardiovascular diseases of the European Society of Cardiology (ESC) and developed in collaboration with the European Association for the Study of Diabetes (EASD). Eur Heart J. (2013) 34:3035–87. doi: 10.1093/eurheartj/eht108
PubMed Abstract | Crossref Full Text | Google Scholar
2. Jia G, Hill MA, Sowers JR. Diabetic cardiomyopathy: an update of mechanisms contributing to this clinical entity. Circ Res. (2018) 122:624–38. doi: 10.1161/CIRCRESAHA.117.311586
PubMed Abstract | Crossref Full Text | Google Scholar
3. Lorenzo-Almorós A, Tuñón J, Orejas M, Cortés M, Egido J, Lorenzo Ó. Diagnostic approaches for diabetic cardiomyopathy. Cardiovasc Diabetol. (2017) 16:28. doi: 10.1186/s12933-017-0506-x
PubMed Abstract | Crossref Full Text | Google Scholar
5. Dandamudi S, Slusser J, Mahoney DW, Redfield MM, Rodeheffer RJ, Chen HH. The prevalence of diabetic cardiomyopathy: A population-based study in Olmsted County, Minnesota. J Cardiac Failure. (2014) 20:304–9. doi: 10.1016/j.cardfail.2014.02.007
PubMed Abstract | Crossref Full Text | Google Scholar
6. Segar MW, Khan MS, Patel KV, Butler J, Tang Wilson WH, Vaduganathan M, et al. Prevalence and prognostic implications of diabetes with cardiomyopathy in community-dwelling adults. J Am Coll Cardiol. (2021) 78:1587–98. doi: 10.1016/j.jacc.2021.08.020
PubMed Abstract | Crossref Full Text | Google Scholar
7. Lebeche D, Davidoff AJ, Hajjar RJ. Interplay between impaired calcium regulation and insulin signaling abnormalities in diabetic cardiomyopathy. Nat Clin Pract Cardiovasc Med. (2008) 5:715–24. doi: 10.1038/ncpcardio1347
PubMed Abstract | Crossref Full Text | Google Scholar
8. Al Kury L, Smail M, Qureshi MA, Sydorenko V, Shmygol A, Oz M, et al. Calcium signaling in the ventricular myocardium of the Goto-Kakizaki type 2 diabetic rat. J Diabetes Res. (2018) 2018:2974304. doi: 10.1155/2018/2974304
PubMed Abstract | Crossref Full Text | Google Scholar
9. Cai L, Wang Y, Zhou G, Chen T, Song Y, Li X. Attenuation by metallothionein of early cardiac cell death via suppression of mitochondrial oxidative stress results in a prevention of diabetic cardiomyopathy. J Am Coll Cardiol. (2006) 48:1688–97. doi: 10.1016/j.jacc.2006.07.022
PubMed Abstract | Crossref Full Text | Google Scholar
13. Vulesevic B, McNeill B, Giacco F, Maeda K, Blackburn NJR, Brownlee M, et al. Methylglyoxal-induced endothelial cell loss and inflammation contribute to the development of diabetic cardiomyopathy. Diabetes. (2016) 65:1699–713. doi: 10.2337/db15-0568
PubMed Abstract | Crossref Full Text | Google Scholar
14. Van Linthout S, Seeland U, Riad A, Eckhardt O, Hohl M, Dhayat N, et al. Reduced MMP-2 activity contributes to cardiac fibrosis in experimental diabetic cardiomyopathy. Basic Res Cardiol. (2008) 103:319–27. doi: 10.1007/s00395-008-0715-2
PubMed Abstract | Crossref Full Text | Google Scholar
15. Biernacka A, Cavalera M, Wang J, Russo I, Shinde A, Kong P, et al. Smad3 signaling promotes fibrosis while preserving cardiac and aortic geometry in obese diabetic mice. Circ Heart Fail. (2015) 8:788–98. doi: 10.1161/CIRCHEARTFAILURE.114.001963
PubMed Abstract | Crossref Full Text | Google Scholar
16. Zhang Y, Wang JH, Zhang YY, Wang YZ, Wang J, Zhao Y, et al. Deletion of interleukin-6 alleviated interstitial fibrosis in streptozotocin-induced diabetic cardiomyopathy of mice through affecting TGFβ1 and miR-29 pathways. Sci Rep. (2016) 6:23010. doi: 10.1038/srep23010
PubMed Abstract | Crossref Full Text | Google Scholar
18. Tan Y, Zhang Z, Zheng C, Wintergerst KA, Keller BB, Cai L. Mechanisms of diabetic cardiomyopathy and potential therapeutic strategies: preclinical and clinical evidence. Nat Rev Cardiol. (2020) 17:585–607. doi: 10.1038/s41569-020-0339-2
PubMed Abstract | Crossref Full Text | Google Scholar
19. Wamil M, Goncalves M, Rutherford A, Borlotti A, Pellikka PA. Multi-modality cardiac imaging in the management of diabetic heart disease. Front Cardiovasc Med. (2022) 9:1043711. doi: 10.3389/fcvm.2022.1043711
PubMed Abstract | Crossref Full Text | Google Scholar
21. Cosentino F, Grant PJ, Aboyans V, Bailey CJ, Ceriello A, Delgado V, et al. 2019 ESC Guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD. Eur Heart J. (2020) 41:255–323. doi: 10.1093/eurheartj/ehz486
PubMed Abstract | Crossref Full Text | Google Scholar
22. Patil VC, Shah KB, Vasani JD, Shetty P, Patil HV. Diastolic dysfunction in asymptomatic type 2 diabetes mellitus with normal systolic function. J Cardiovasc Dis Res. (2011) 2:213–22. doi: 10.4103/0975-3583.89805
PubMed Abstract | Crossref Full Text | Google Scholar
23. Somaratne JB, Whalley GA, Poppe KK, ter Bals MM, Wadams G, Pearl A, et al. Screening for left ventricular hypertrophy in patients with type 2 diabetes mellitus in the community. Cardiovasc Diabetol. (2011) 10:29. doi: 10.1186/1475-2840-10-29
PubMed Abstract | Crossref Full Text | Google Scholar
24. Weber TR, Silva RLD, Cossul S, Lofrano Alves MS, Lee SVDS, Brum Marques JL. Echocardiographic evaluation in type 1 diabetes mellitus. Rev Portuguesa Cardiologia (English Edition). (2021) 40:757–65. doi: 10.1016/j.repce.2021.08.003
PubMed Abstract | Crossref Full Text | Google Scholar
25. Holland DJ, Marwick TH, Haluska BA, Leano R, Hordern MD, Hare JL, et al. Subclinical LV dysfunction and 10-year outcomes in type 2 diabetes mellitus. Heart. (2015) 101:1061–6. doi: 10.1136/heartjnl-2014-307391
PubMed Abstract | Crossref Full Text | Google Scholar
26. Rørth R, Jørgensen PG, Andersen HU, Christoffersen C, Gøtze JP, Køber L, et al. Cardiovascular prognostic value of echocardiography and N terminal pro B-type natriuretic peptide in type 1 diabetes: the Thousand & 1 Study. Eur J Endocrinol. (2020) 182:481–8. doi: 10.1530/EJE-19-1015
PubMed Abstract | Crossref Full Text | Google Scholar
27. From AM, Scott CG, Chen HH. The development of heart failure in patients with diabetes mellitus and pre-clinical diastolic dysfunction. J Am Coll Cardiol. (2010) 55:300–5. doi: 10.1016/j.jacc.2009.12.003
PubMed Abstract | Crossref Full Text | Google Scholar
28. Powell-Wiley TM, Poirier P, Burke LE, Després JP, Gordon-Larsen P, Lavie CJ, et al. Obesity and cardiovascular disease: A scientific statement from the American heart association. Circulation. (2021) 143:e990–991. doi: 10.1161/CIR.0000000000000973
PubMed Abstract | Crossref Full Text | Google Scholar
29. Turkbey EB, Backlund JYC, Genuth S, Jain AJ, Miao CM, Cleary PA, et al. Myocardial structure, function, and scar in patients with type 1 diabetes mellitus. Circulation. (2011) 124:1737–46. doi: 10.1161/CIRCULATIONAHA.111.022327
PubMed Abstract | Crossref Full Text | Google Scholar
30. Korosoglou G, Humpert PM, Ahrens J, Oikonomou D, Osman NF, Gitsioudis G, et al. Left ventricular diastolic function in type 2 diabetes mellitus is associated with myocardial triglyceride content but not with impaired myocardial perfusion reserve. J Magn Reson Imaging. (2012) 35:804–11. doi: 10.1002/jmri.22879
PubMed Abstract | Crossref Full Text | Google Scholar
31. Storz C, Hetterich H, Lorbeer R, Heber SD, Schafnitzel A, Patscheider H, et al. Myocardial tissue characterization by contrast-enhanced cardiac magnetic resonance imaging in subjects with prediabetes, diabetes, and normal controls with preserved ejection fraction from the general population. Eur Heart J Cardiovasc Imaging. (2018) 19:701–8. doi: 10.1093/ehjci/jex190
PubMed Abstract | Crossref Full Text | Google Scholar
32. Kwong RY, Sattar H, Wu H, Vorobiof G, Gandla V, Steel K, et al. Incidence and prognostic implication of unrecognized myocardial scar characterized by cardiac magnetic resonance in diabetic patients without clinical evidence of myocardial infarction. Circulation. (2008) 118:1011–20. doi: 10.1161/CIRCULATIONAHA.107.727826
PubMed Abstract | Crossref Full Text | Google Scholar
33. Siddiqui TA, Chamarti KS, Tou LC, Demirjian GA, Noorani S, Zink S, et al. The merits, limitations, and future directions of cost-effectiveness analysis in cardiac MRI with a focus on coronary artery disease: A literature review. J Cardiovasc Dev Dis. (2022) 9:357. doi: 10.3390/jcdd9100357
PubMed Abstract | Crossref Full Text | Google Scholar
34. Weinreb JC, Rodby RA, Yee J, Wang CL, Fine D, McDonald RJ, et al. Use of intravenous gadolinium-based contrast media in patients with kidney disease: consensus statements from the American college of radiology and the national kidney foundation. Radiology. (2021) 298:28–35. doi: 10.1148/radiol.2020202903
PubMed Abstract | Crossref Full Text | Google Scholar
35. Rijzewijk LJ, van der Meer RW, Lamb HJ, Jong HWAM, Lubberink M, Romijn JA, et al. Altered myocardial substrate metabolism and decreased diastolic function in nonischemic human diabetic cardiomyopathy: studies with cardiac positron emission tomography and magnetic resonance imaging. J Am Coll Cardiol. (2009) 54:1524–32. doi: 10.1016/j.jacc.2009.04.074
PubMed Abstract | Crossref Full Text | Google Scholar
36. Herrero P, Peterson LR, McGill JB, Matthew S, Lesniak D, Dence C, et al. Increased myocardial fatty acid metabolism in patients with type 1 diabetes mellitus. J Am Coll Cardiol. (2006) 47:598–604. doi: 10.1016/j.jacc.2005.09.030
PubMed Abstract | Crossref Full Text | Google Scholar
37. Hosseinzadeh E, Ghodsirad MA, Alirezaei T, Arfenia M, Pirayesh, Amoiee M, et al. Comparing left ventricular mechanical dyssynchrony between diabetic and non-diabetic patients with normal gated SPECT MPI. Int J Cardiovasc Imaging. (2022) 38:249–56. doi: 10.1007/s10554-021-02358-1
PubMed Abstract | Crossref Full Text | Google Scholar
38. Hu L, Qiu C, Wang X, Xu M, Shao X, Wang Y. The association between diabetes mellitus and reduction in myocardial glucose uptake: a population-based 18F-FDG PET/CT study. BMC Cardiovasc Disord. (2018) 18:203. doi: 10.1186/s12872-018-0943-9
PubMed Abstract | Crossref Full Text | Google Scholar
39. Klonoff DC. United Kingdom prospective diabetes study follow-up studies establish a legacy effect of therapy for hyperglycemia but not hypertension. J Diabetes Sci Technol. (2008) 2:922–4. doi: 10.1177/193229680800200601
PubMed Abstract | Crossref Full Text | Google Scholar
40. Aguilar D, Bozkurt B, Ramasubbu K, Deswal A. Relationship of hemoglobin A1C and mortality in heart failure patients with diabetes. J Am Coll Cardiol. (2009) 54:422–8. doi: 10.1016/j.jacc.2009.04.049
PubMed Abstract | Crossref Full Text | Google Scholar
41. Boussageon R, Bejan-Angoulvant T, Saadatian-Elahi M, Lafont S, Bergeonneau C, Kassaï B, et al. Effect of intensive glucose lowering treatment on all cause mortality, cardiovascular death, and microvascular events in type 2 diabetes: meta-analysis of randomised controlled trials. BMJ. (2011) 343:d4169. doi: 10.1136/bmj.d4169
PubMed Abstract | Crossref Full Text | Google Scholar
42. American Diabetes Association. 2. Classification and diagnosis of diabetes: standards of medical care in diabetes-2020. Diabetes Care. (2020) 43:S14–31. doi: 10.2337/dc20-S002
PubMed Abstract | Crossref Full Text | Google Scholar
43. Wang Y, Negishi T, Negishi K, Marwick TH. Prediction of heart failure in patients with type 2 diabetes mellitus- a systematic review and meta-analysis. Diabetes Res Clin Pract. (2015) 108:55–66. doi: 10.1016/j.diabres.2015.01.011
PubMed Abstract | Crossref Full Text | Google Scholar
44. Cosmi F, Shen L, Magnoli M, Abraham WT, Anand IS, Cleland JG, et al. Treatment with insulin is associated with worse outcome in patients with chronic heart failure and diabetes. Eur J Heart Fail. (2018) 20:888–95. doi: 10.1002/ejhf.1146
PubMed Abstract | Crossref Full Text | Google Scholar
45. Adel FW, Zheng Y, Wan SH, Christie G, Shuchong P, Syed A, et al. Insulin therapy is associated with increased myocardial interstitial fibrosis and cardiomyocyte apoptosis in a rodent model of experimental diabetes. Front Physiol. (2022) 13:890907. doi: 10.3389/fphys.2022.890907
PubMed Abstract | Crossref Full Text | Google Scholar
46. Boussageon R, Supper I, Bejan-Angoulvant T, Kellou N, Cucherat M, Boissel JP, et al. Reappraisal of metformin efficacy in the treatment of type 2 diabetes: a meta-analysis of randomised controlled trials. PloS Med. (2012) 9:e1001204. doi: 10.1371/journal.pmed.1001204
PubMed Abstract | Crossref Full Text | Google Scholar
47. Turner RC, Holman RR, Stratton IM, Cull CA, Matthews DR, Manley SE, et al. Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). UK Prospective Diabetes Study (UKPDS) Group. Lancet. (1998) 352:854–65. doi: 10.1016/S0140-6736(98)07037-8
PubMed Abstract | Crossref Full Text | Google Scholar
48. Tzoulaki I, Molokhia M, Curcin V, Little MP, Millett CJ, Ng A, et al. Risk of cardiovascu
留言 (0)