Nickel is a silver-white, ferromagnetic, corrosion-resistant metal element that is widely distributed in the lungs, liver, kidneys, and other tissues and organs. It is also one of the essential trace elements in the human body. A deficiency of nickel can lead to a decrease in enzyme activity and can impact human growth and development (1). However, excessive exposure to nickel can pose health hazards such as neurasthenic reactions, inflammatory responses, and oxidative stress injuries (2, 3). Studies have indicated that occupational populations exposed to high levels of nickel for prolonged periods are at an increased risk of developing lung cancer, nasopharyngeal cancer, and occupational asthma (4–10).
In 1990, the International Agency for Research on Cancer (IARC) classified nickel compounds as Group I carcinogens and metal nickel as a Group II B carcinogen. Na et al. (11) conducted a retrospective cohort study on four nickel-receiving factories and mines, revealing an increased risk of lung cancer death among workers in nickel refining and smelting. Pesch et al. (12) carried out a case-control study within a German occupational population involved in nickel welding, finding a dose-response relationship between nickel exposure and the risk of lung cancer. The mechanism by which nickel exposure induces lung cancer has also been verified. Several studies have demonstrated that molecular mechanisms such as apoptosis and autophagy are induced by nickel exposure (13–16). Other studies have found that epigenetic changes, activation of hypoxia signaling pathways, and production of reactive oxygen species are also implicated in how nickel and its compounds induce cancer at a molecular level (17).
Disability-adjusted life years (DALY) serves as an indicator of the disease burden, calculating all healthy years of life lost from the onset to death. This includes years of life lost due to premature death (YLL) and years of healthy life lost due to disability from illness (YLD). The global burden of disease (GBD) database has been in operation since 1988, its purpose is to analyze the global burden of disease, encompassing health burdens related to disease, risk factors, disabilities, and mortality (18).
China is a leading global producer of nickel compounds, according to the 2022 report on the prevalence of malignant tumors in China, lung cancer has the highest incidence among all malignant tumors (19). However, there is a lack of analysis on the burden of lung cancer related to nickel exposure. Therefore, this paper aims to extract mortality and disease burden data related to nickel-induced lung cancer from the GBD database. The study will analyze the disease burden of nickel-related lung cancer from 1990 to 2019 and its changing trend. Additionally, relevant software will be used to predict the disease burden of nickel-related lung cancer over the next 15 years. The findings aim to provide suggestions for developing occupational disease prevention measures and improving the quality of life for current patients.
2 Materials and methods 2.1 Study dataData from this study is obtained from the GBD2019 database and the global health data exchange (GHDx) website, which provides access to public data. The Institute for Health Metrics and Evaluation (IHME) systematically estimates the incidence, prevalence, mortality, DALY, YLL, and YLD for 369 diseases and injuries in 204 countries and territories (20, 21). The cancer codes used in the GBD correspond to the International Statistical Classification of Diseases and Related Health Problems (Tenth Revision) (ICD-10) (22).
2.2 Statistical analysisIn this study, the number of deaths and standardized mortality were chosen to reflect the risk of death in a specific population during a certain period. YLL represents the years of life lost, while YLD reflects years lived with disability. DALY was utilized as an indicator of disease burden for nickel-associated lung cancer, comprehensively reflecting the impact of life loss caused by both YLL and YLD.
ASR stands for the age-standardized rate, while ASMR refers to the age-standardized mortality rate and ASDR represents the age-standardized DALY rate. The 95% uncertainty interval (UIs) was employed to estimate the number of deaths and other indicators, while 100% annual change (APC) and average annual change percentage (AAPC) were used to analyze trends in statistical indicators such as ASDR (abbreviations are utilized following the text). Joinpoint software was utilized for calculating and statistically analyzing AAPC, with R software being used for data cleaning, visual display, and testing at a significance level α = 0.05.
3 Results 3.1 Mortality and DALY in nickel-associated lung cancer in China and globallyWe conducted an analysis of the trends in nickel-associated lung cancer deaths and DALY in China and globally from 1990 to 2019. During this period, the total number of nickel-associated lung cancer deaths in China was 83,720, accounting for 40.2% of the global nickel-associated lung cancer deaths. The death rate of nickel-associated lung cancer in China increased by 145.8%, rising from 1,668 to 4,100. Globally, nickel-associated lung cancer deaths increased from 5,248 to 9,330, representing a rise of 77.8% (Table 1 and Figures 1A, B). Furthermore, the DALY caused by nickel-associated lung cancer in China increased from 52,043 person-years to 114,606 person-years, marking an increase of 120.2%. The global DALY due to nickel-associated lung cancer increased from 158,519 person-years to 260,986 person-years, representing a significant increase of 64.6% (Table 1 and Figures 1C, D). As depicted in Figure 2, the ASMR of nickel-associated lung cancer in the Chinese population showed an initial increase followed by a decrease during the period of 1990–2019 (AAPC = 0.22%, P < 0.05, Figure 2A). Conversely, there was an overall downward trend globally (AAPC = −0.43%, P < 0.05, Figure 2B). The ASDR of nickel-associated lung cancer in China exhibited an initial increase followed by a decrease with no statistical significance (AAPC = −0.08%, P > 0.05, Figure 2C), while there was an overall downward trend globally (AAPC = −0.65%, P < 0.05, Figure 2D).
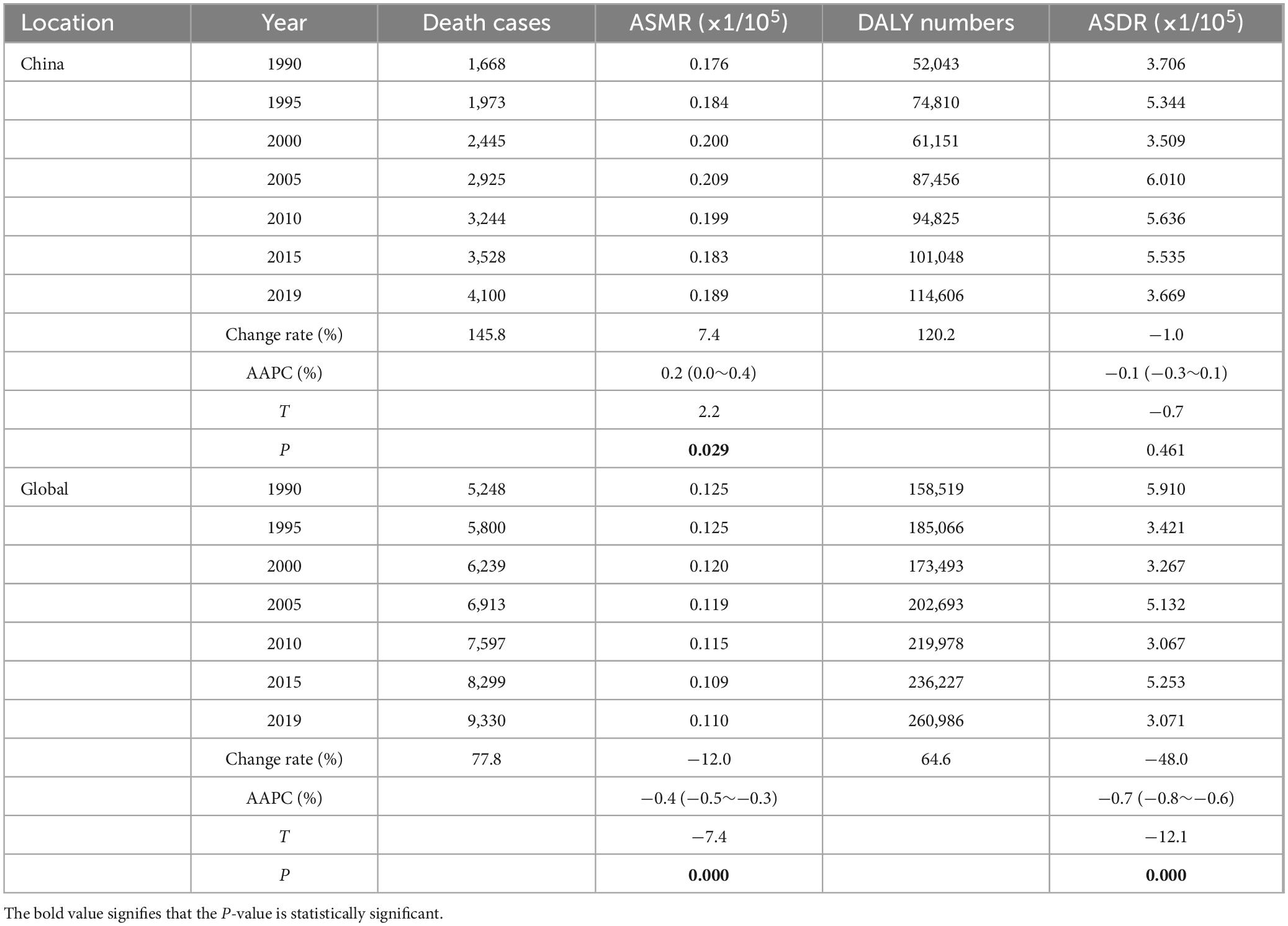
Table 1. Mortality and DALY in nickel-associated lung cancer in China and globally from 1990 to 2019.
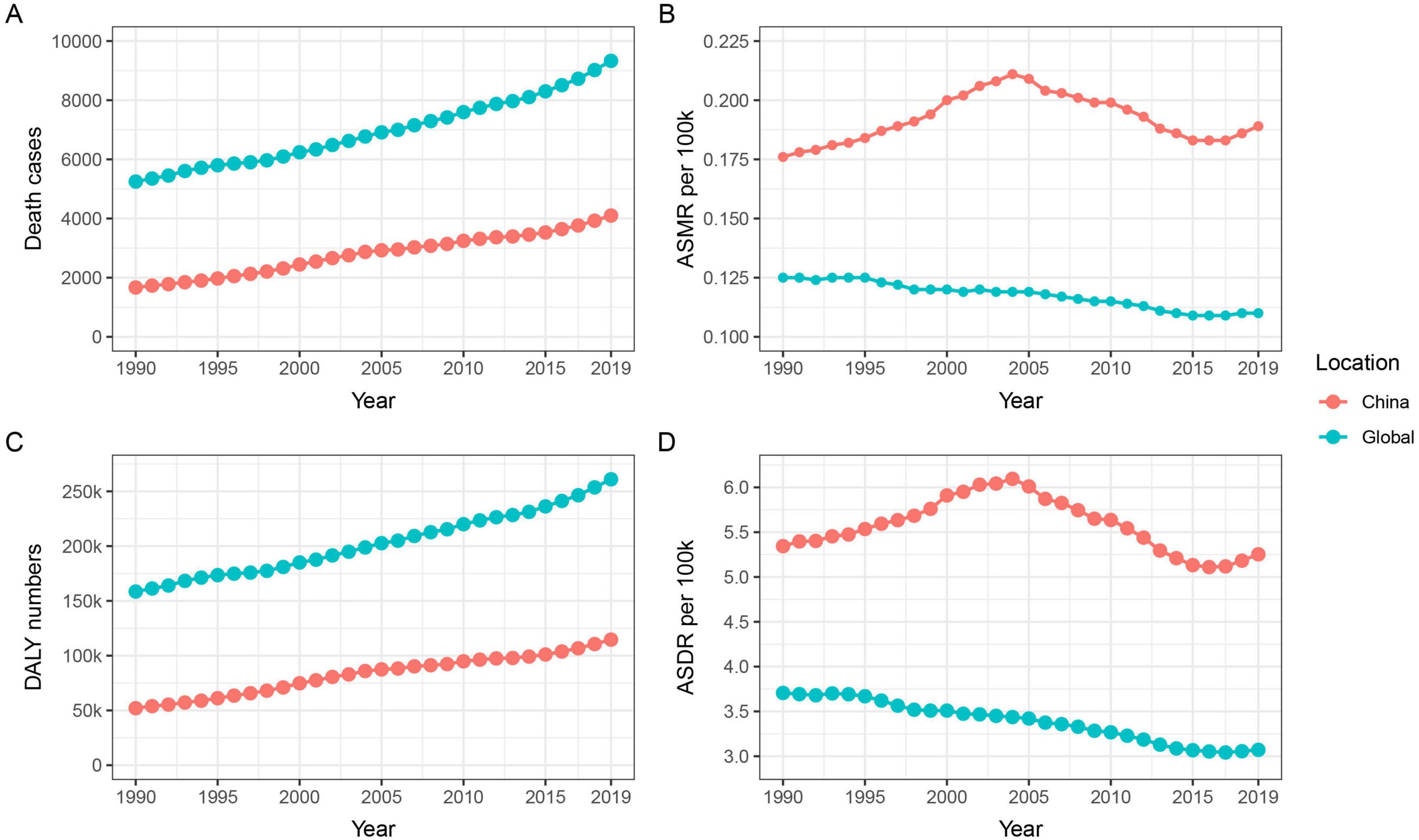
Figure 1. Trends in mortality and DALY for nickel-associated lung cancer in China and globally from 1990 to 2019. (A) Temporal trends of the cases of deaths; (B) Temporal trends of ASMR; (C) Temporal trends of DALY; (D) Temporal trends of ASDR.
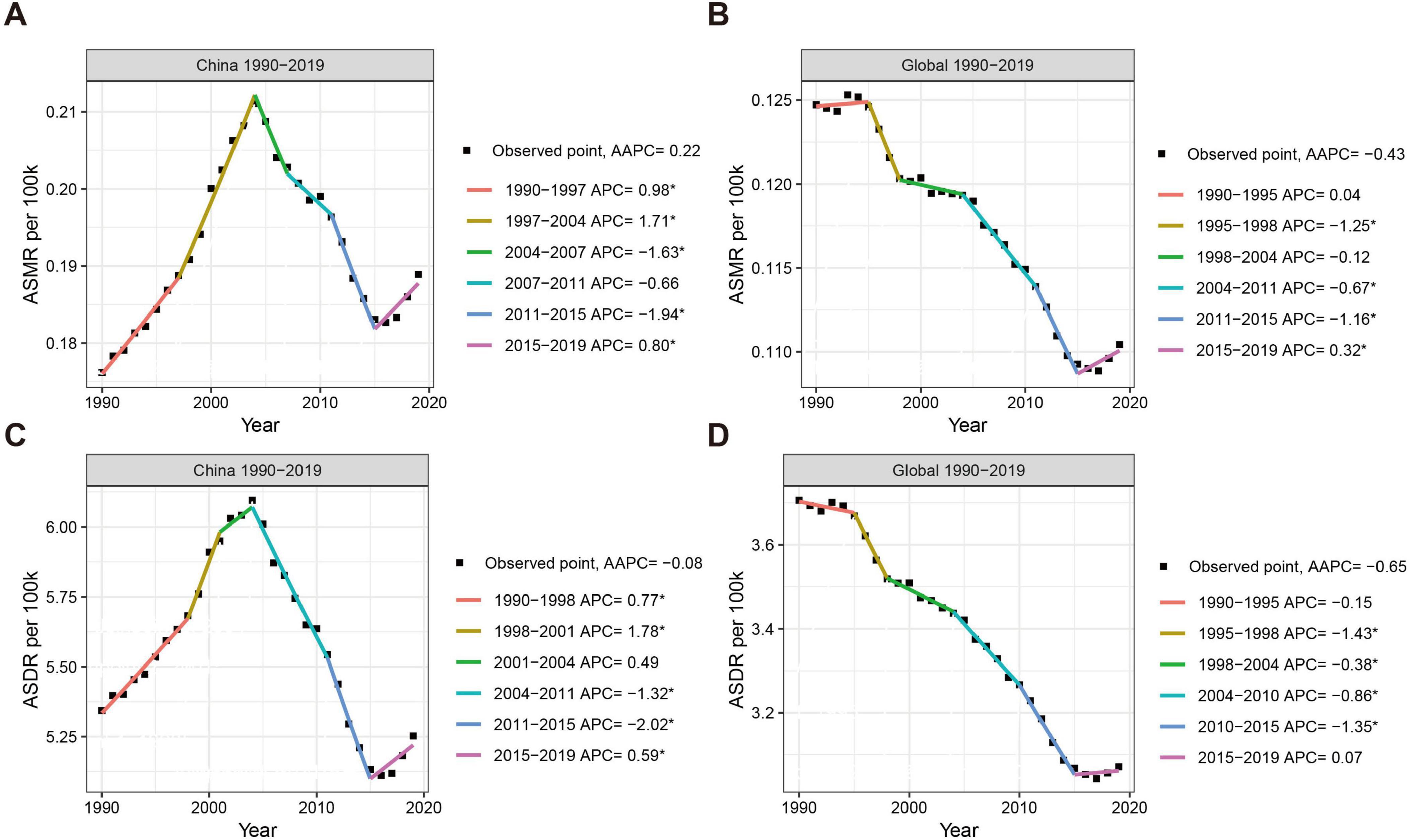
Figure 2. Trends in ASMR and ASDR for nickel-associated lung cancer in China and globally from 1990 to 2019. (A) Temporal trends of ASMR and average annual change percentage in China; (B) Temporal trends of ASMR and average annual change percentage in global; (C) Temporal trends of ASDR and average annual change percentage in China; (D) Temporal trends of ASDR and average annual change percentage in global.
3.2 Analysis of the disease burden caused by lung cancer attributable to nickel exposureFrom an alternative perspective, the YLL and YLD caused by nickel-associated lung cancer in the Chinese population increased from 51,625 person-years and 418 person-years to 113,365 person-years and 1,241 person-years, respectively, between 1990 and 2019 (Table 2 and Figure 3A, C). The global population saw an increase from 157,118 and 1,402 to 258,195 and 2,790 respectively. It is worth noting that the growth rate of China was higher than that of the world. The age-standardized YLD rate (ASYLDR) increased from 0.044/100,000 to 0.057/100,000 with AAPC = 0.9% (P < 0.001), while the global AAPC decreased from 0.033/100,000 to 0.033/100,000 with AAPC = −0.0% (P = 0.737) (Table 2 and Figure 3B). The age-standardized YLL rate (ASYLLR) decreased from 5.300/100,000 in 1990 to 5.196/100,000 in 2019, with an AAPC of −0.1% (P = 0.408). The global AAPC also showed a decrease from 3.673/100,000 to 3.037/100,000, with an AAPC of −0.7% (P < 0.001) (Table 2 and Figure 3D). The increase in YLD/YLL for nickel-associated lung cancer in the Chinese population (35.1%) was higher than that in the global population (21.2%), and by 2018 the YLD/YLL in the Chinese population had surpassed that of the global population for the first time (Table 2 and Figure 3E).
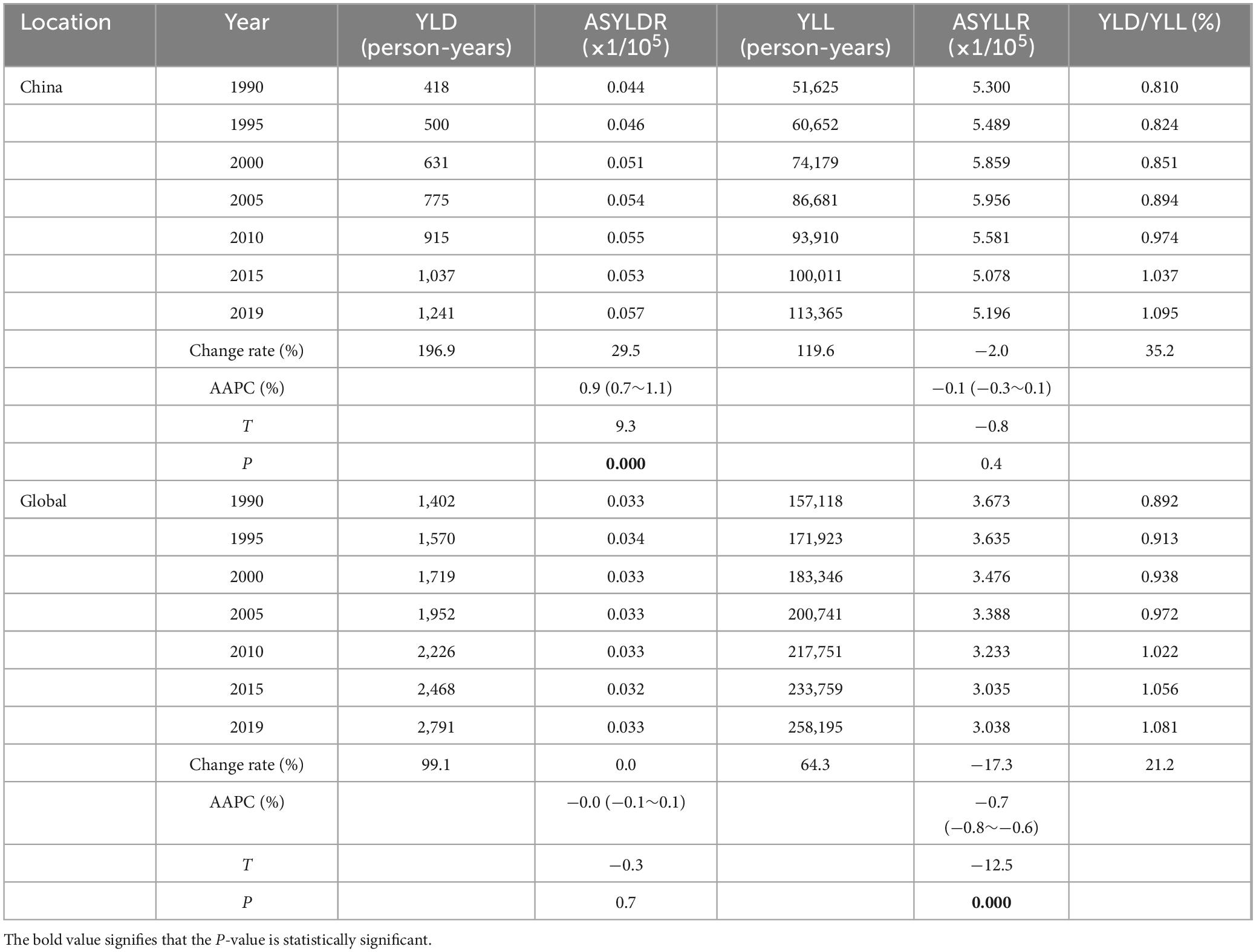
Table 2. YLD and YLL of lung cancer attributable to nickel from 1990 to 2019.
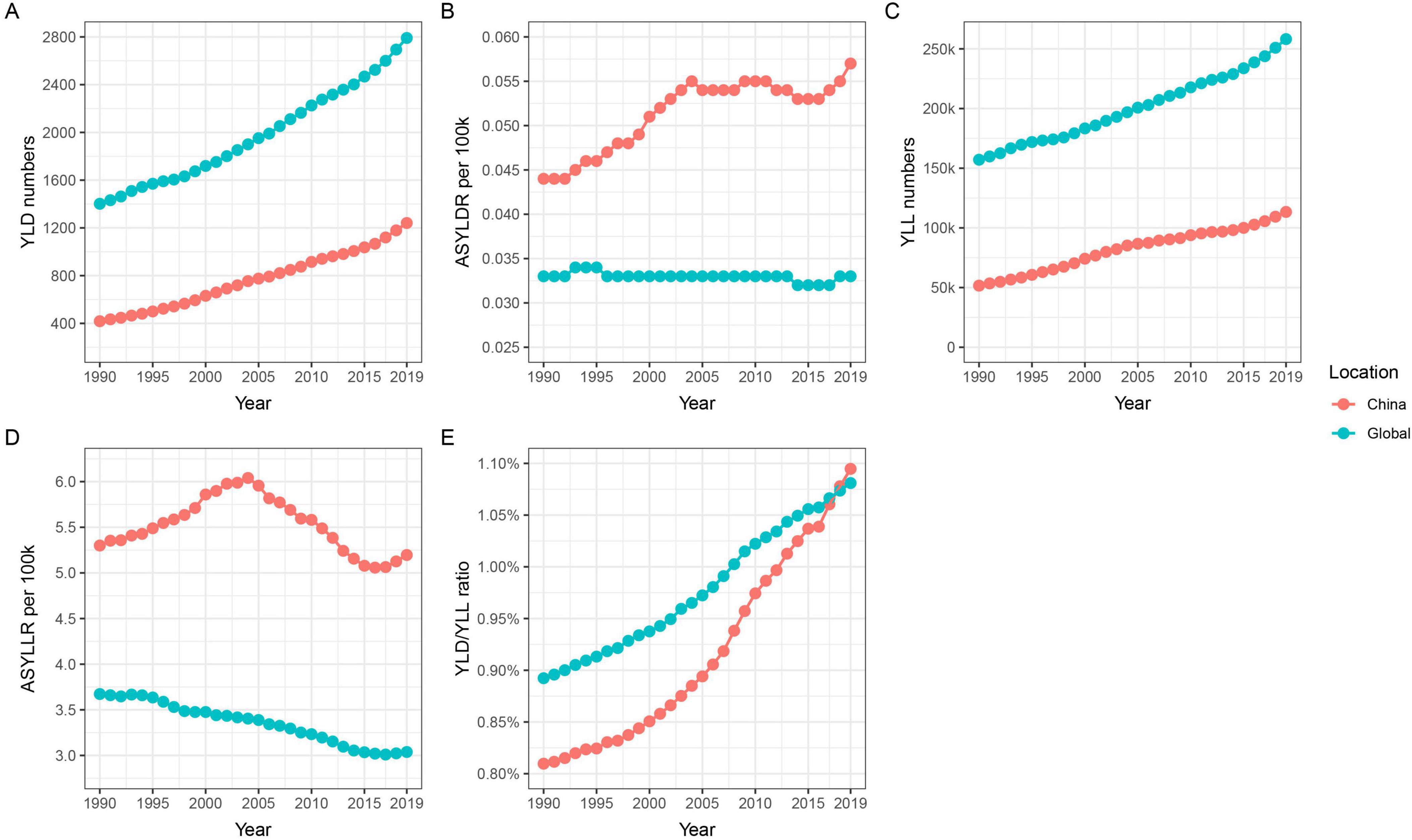
Figure 3. Trends in YLD, YLL, ASYLDR, ASYLLR, and YLD/YLL for nickel-associated lung cancer in China and globally from 1990 to 2019. (A) Temporal trends of YLD; (B) Temporal trends of ASYLDR; (C) Temporal trends of YLL; (D) Temporal trends of ASYLLR; (E) Temporal trends of YLD/YLL.
3.3 Trends in the global burden of disease by regionFrom a global perspective, there is significant variation in the changes in the burden of nickel-associated lung cancer disease from 1990 to 2019 across different countries. The proportion of lung cancer deaths attributable to nickel has seen substantial increases in Egypt, Pakistan, and Ethiopia, ranging from 0.10 to 0.76%. Conversely, countries such as Russia, Sweden, and Saudi Arabia have experienced notable declines in the nickel attributable proportion of lung cancer deaths, ranging from 0.19 to 0.66% (Figure 4A). When considering the nickel attribution ratio in DALY caused by lung cancer, it is evident that Egypt, Pakistan, Vietnam and other countries have also witnessed significant increases ranging from 0.09 to 0.80%. On the other hand, Russia, Sweden and Saudi Arabia stand out for experiencing the highest declines in this ratio ranging from 0.17 to 0.65% (Figure 4B).
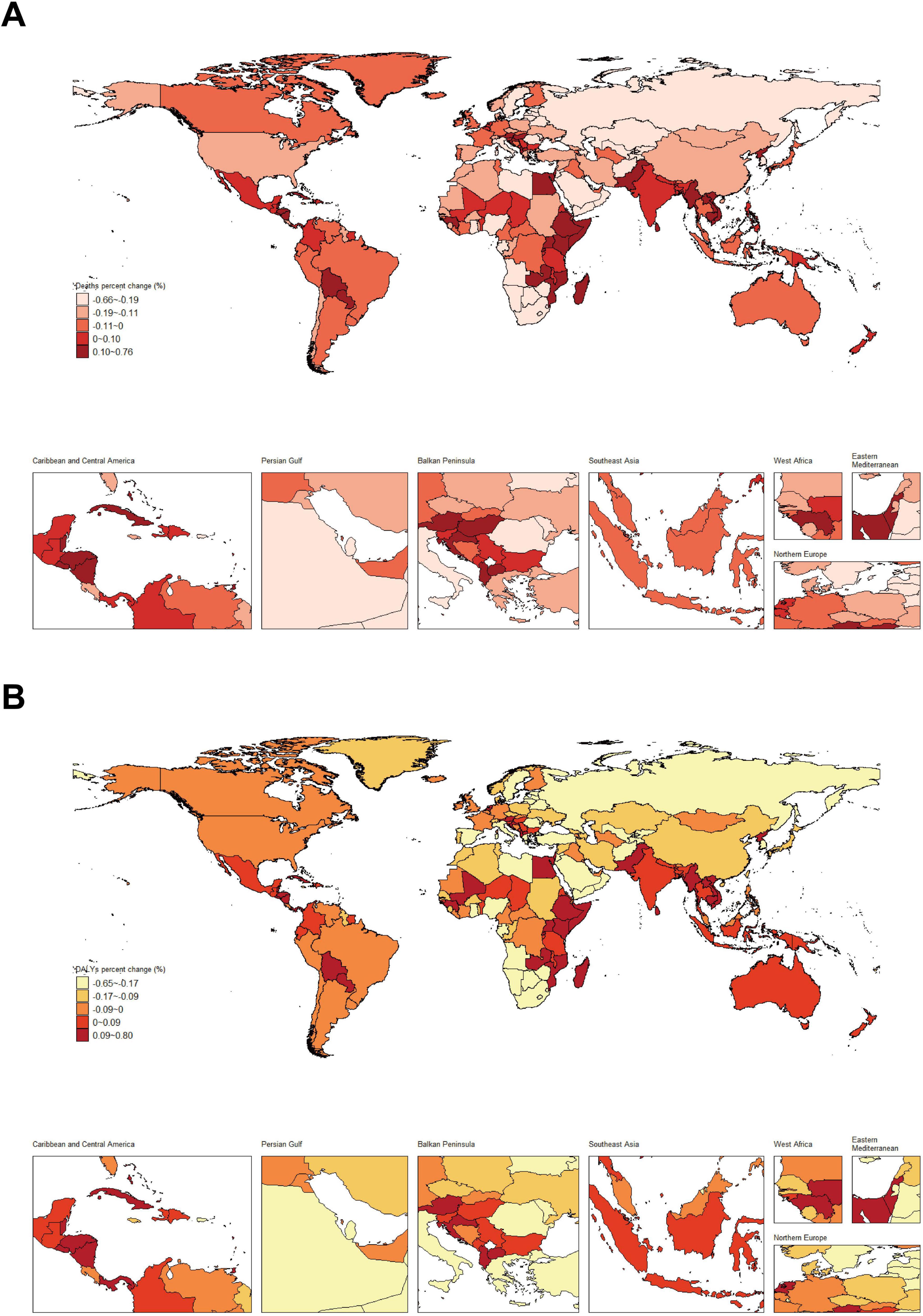
Figure 4. Changes in the proportion of nickel attributable to lung cancer deaths and DALY from 1990 to 2019. (A) Proportional changes in nickel-associated lung cancer mortality; (B) Trends in nickel attribution for DALY associated with lung cancer.
From the perspective of ASMR for nickel-associated lung cancer, countries such as India, Pakistan, and Egypt have shown the largest annual average increase, ranging from 0.26 to 1.10%. Conversely, the United States, Russia, and South Africa experienced the largest annual average declines in this regard, with percentages ranging from 0.32 to 0.70% (Figure 5A). In terms of ASDR for nickel-associated lung cancer, Indonesia, Egypt, Serbia and other countries demonstrated the largest annual average increase at a range of 0.12–0.98%. On the other hand, countries such as the United States, Canada and Russia experienced significant annual average declines in ASDR of nickel-associated lung cancer at a range of 0.34 to 0.72% (Figure 5B). Overall trends indicate an upward trend in African and Southeast Asian countries while European and East Asian countries show a downward trend for nickel-associated lung cancer rates.
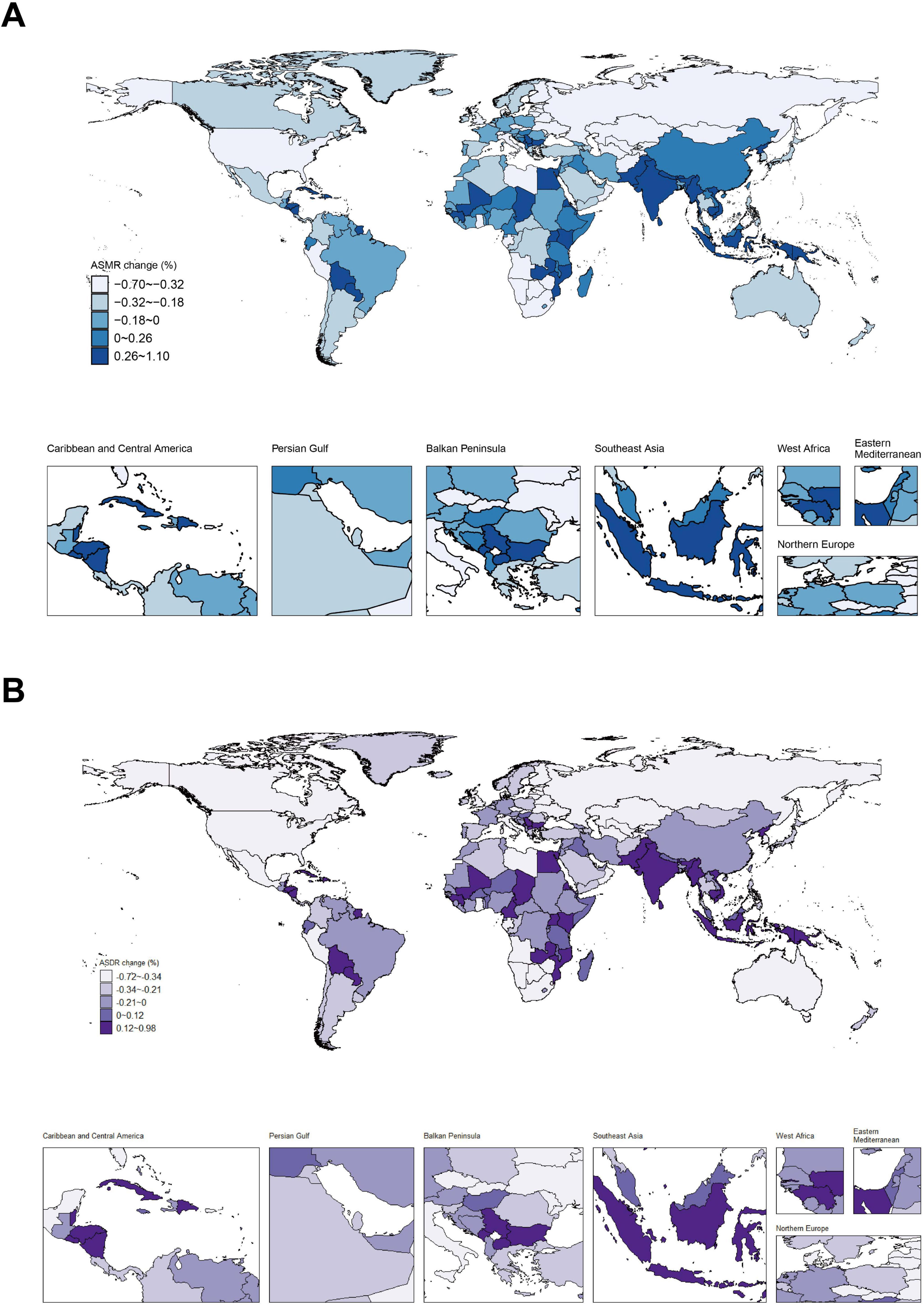
Figure 5. Distribution of ASMR and ASDR in nickel-associated lung cancer from 1990 to 2019. (A) Annual trend of ASMR in nickel-associated lung cancer; (B) Annual trend of ASDR in nickel-associated lung cancer.
3.4 Age and gender distribution of the burden of lung cancer attributable to nickel exposureIn the analysis of the burden of nickel-associated lung cancer by sex, it was observed that the ASMR and ASDR of nickel-associated lung cancer in China and the global population exhibited a pattern of initially increasing and then decreasing with advancing age, with higher rates in men than in women. The ASMR of nickel-associated lung cancer in Chinese men and women peaked in the 60–64 age group in 1990 and in the 70–74 age group in 2019 (Tables 3, 4 and Figure 6). In the global population, the ASMR of nickel-associated lung cancer reached its zenith in 1990 among women aged 65–69 and men aged 60–64. However, by 2019, both men and women showed a peak ASMR in the 70–74 age group (Tables 3, 4 and Figure 6). Similarly, the ASDR of nickel-associated lung cancer among Chinese men and women also peaked between the ages of sixty to sixty-four during both time periods (Tables 3, 4 and Figure 7); while globally, the ASDR reached its pinnacle at ages between sixty to sixty-four years old during both time periods (Tables 3, 4 and Figure 7).
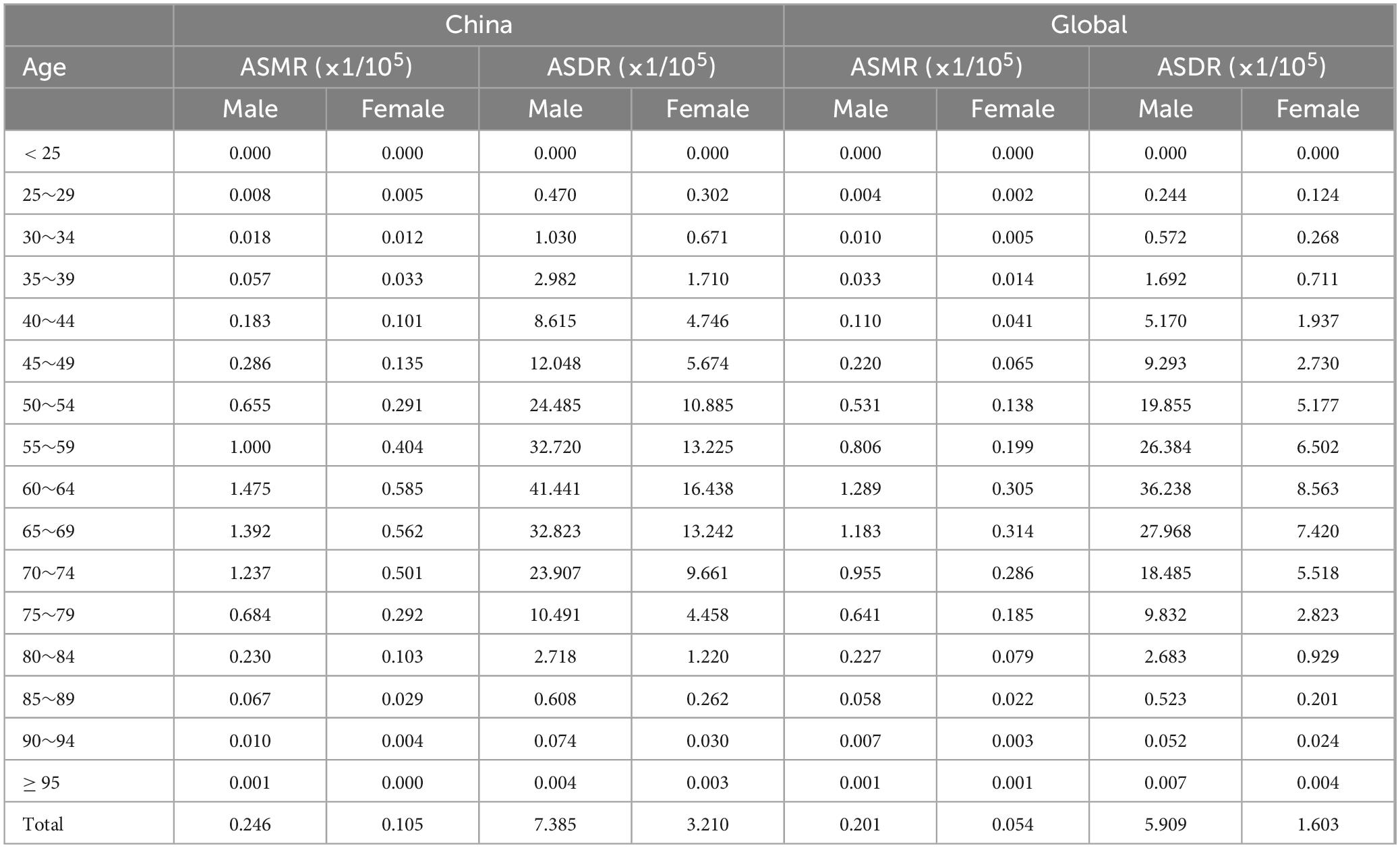
Table 3. The age distribution on ASRs of lung cancer attributable to nickel in China, 1990.
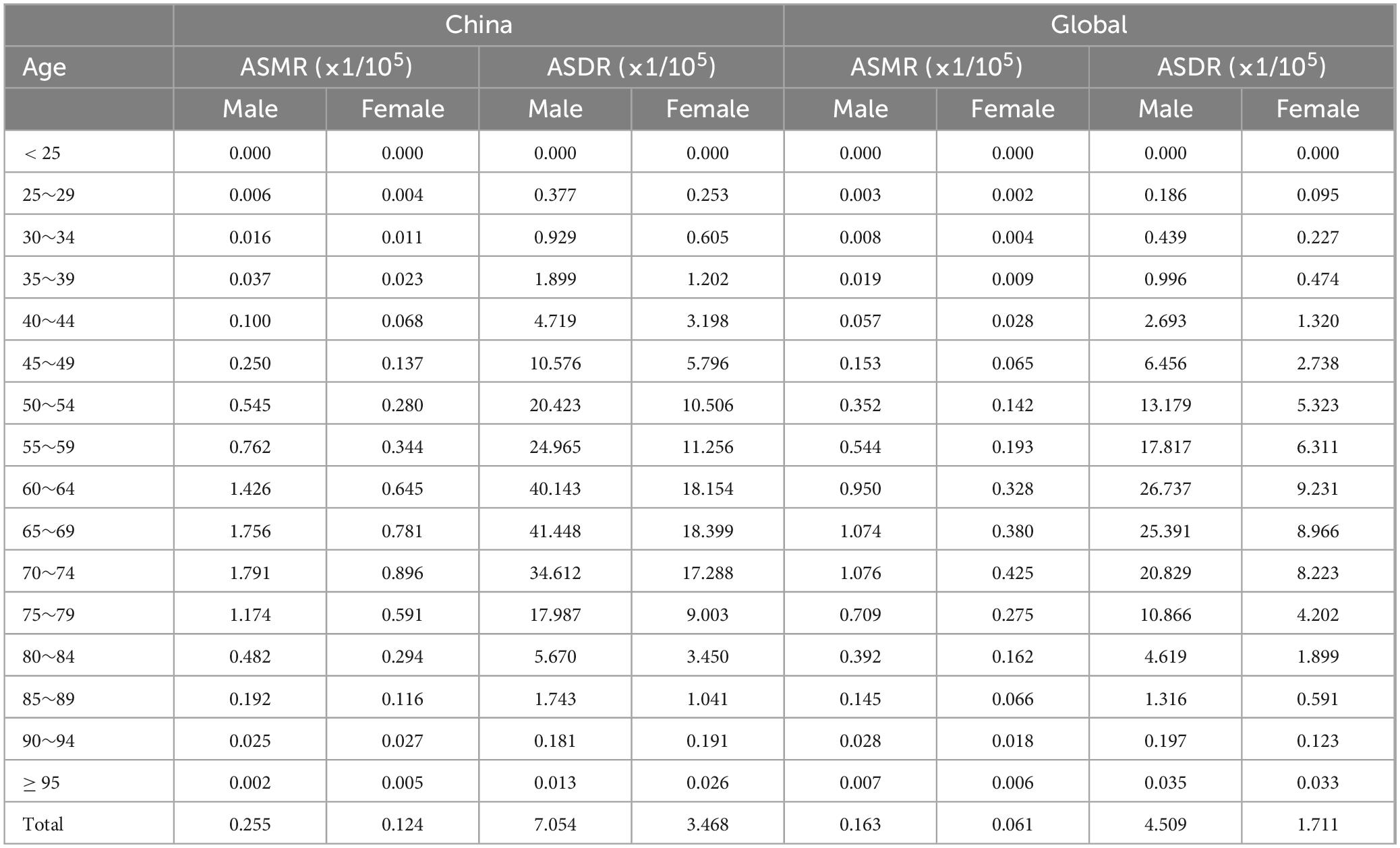
Table 4. The age distribution on ASRs of lung cancer attributable to nickel in China, 2019.
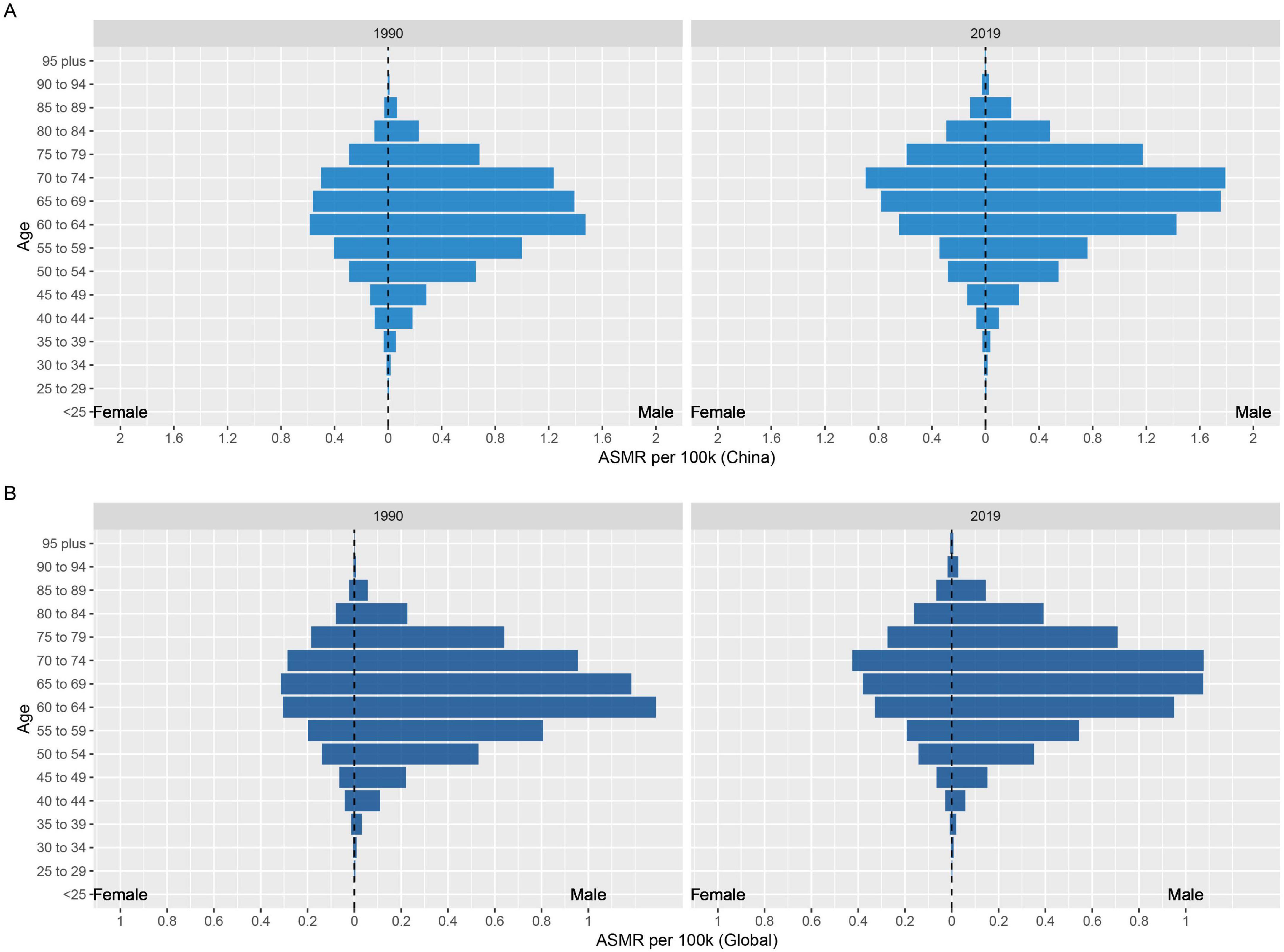
Figure 6. Age and sex-specific distribution of ASMR for nickel-associated lung cancer in 1990 and 2019. (A) In China; (B) In worldwide.

Figure 7. Age and sex-specific distribution of ASDR for nickel-associated lung cancer in 1990 and 2019. (A) In China; (B) In worldwide.
3.5 Prediction of the burden of lung cancer attributable to nickel exposure in the next 15 yearsThis study is based on the Nordpred model to predict the ASMR and ASDR of nickel-associated lung cancer in China and globally from 2020 to 2035. The results indicate that deaths related to nickel-associated lung cancer in China and globally are expected to reach their peak in 2027, with 4,087 and 9,874 cases, respectively (Table 5 and Figure 8A). The DALY of nickel-associated lung cancer in both China and worldwide shows a pattern of initial increase followed by decrease, reaching its peak in 2027 at 114,942 person-years and 268,731 person-years, respectively (Table 5 and Figure 8B). Table 5 and Figure 9A demonstrate that the ASMR of nickel-associated lung cancer in China exhibits an initial increase followed by decrease. Globally, the ASMR for nickel-associated lung cancer shows an initial increase followed by stabilization. Table 5 and Figure 9B show that the ASDR of nickel-associated lung cancer in China demonstrates an increasing trend; however, globally it shows a decreasing trend.
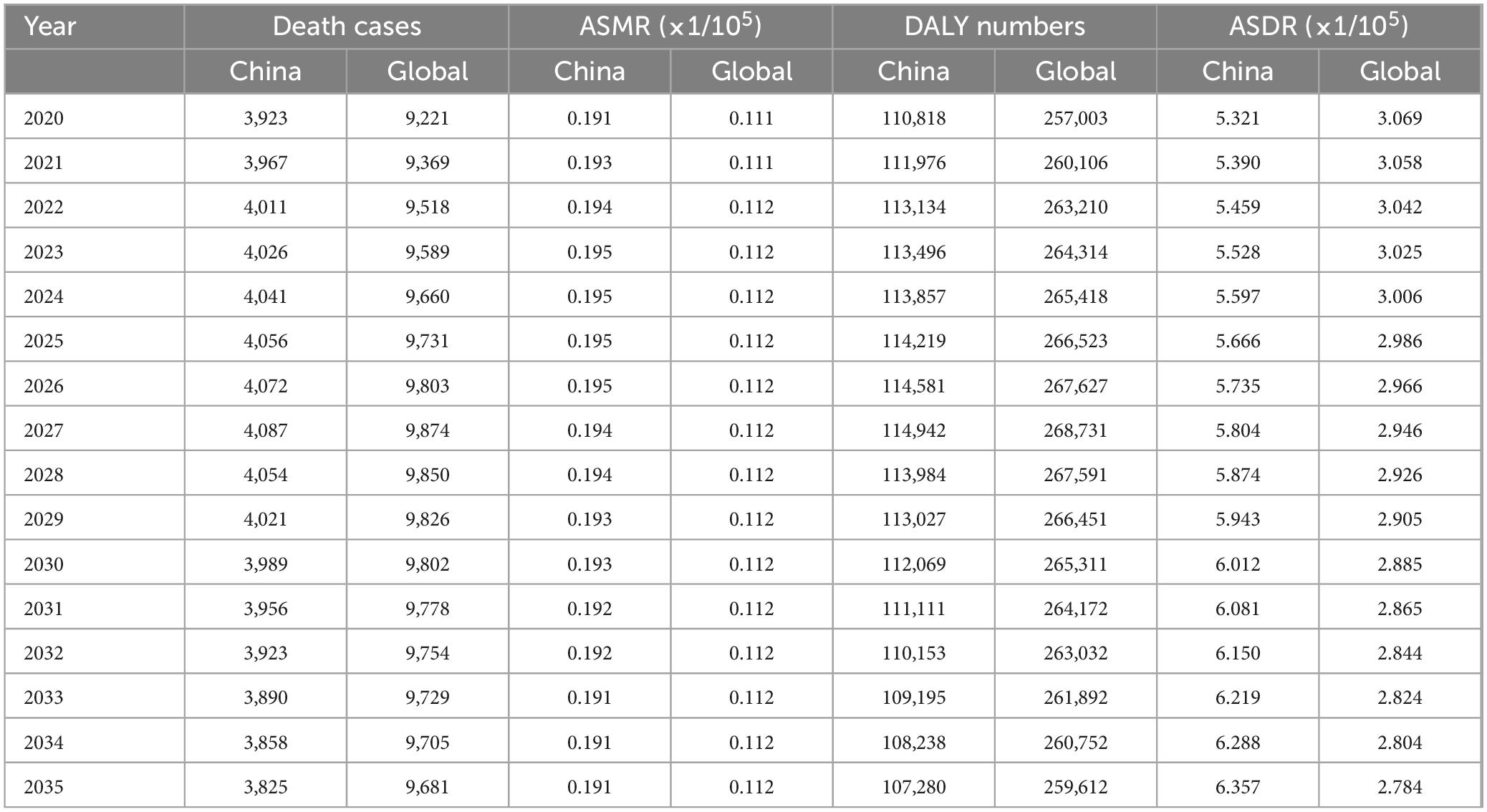
Table 5. Death and DALY of lung cancer attributable to nickel predicted by Norpred model from 2020 to 2035.
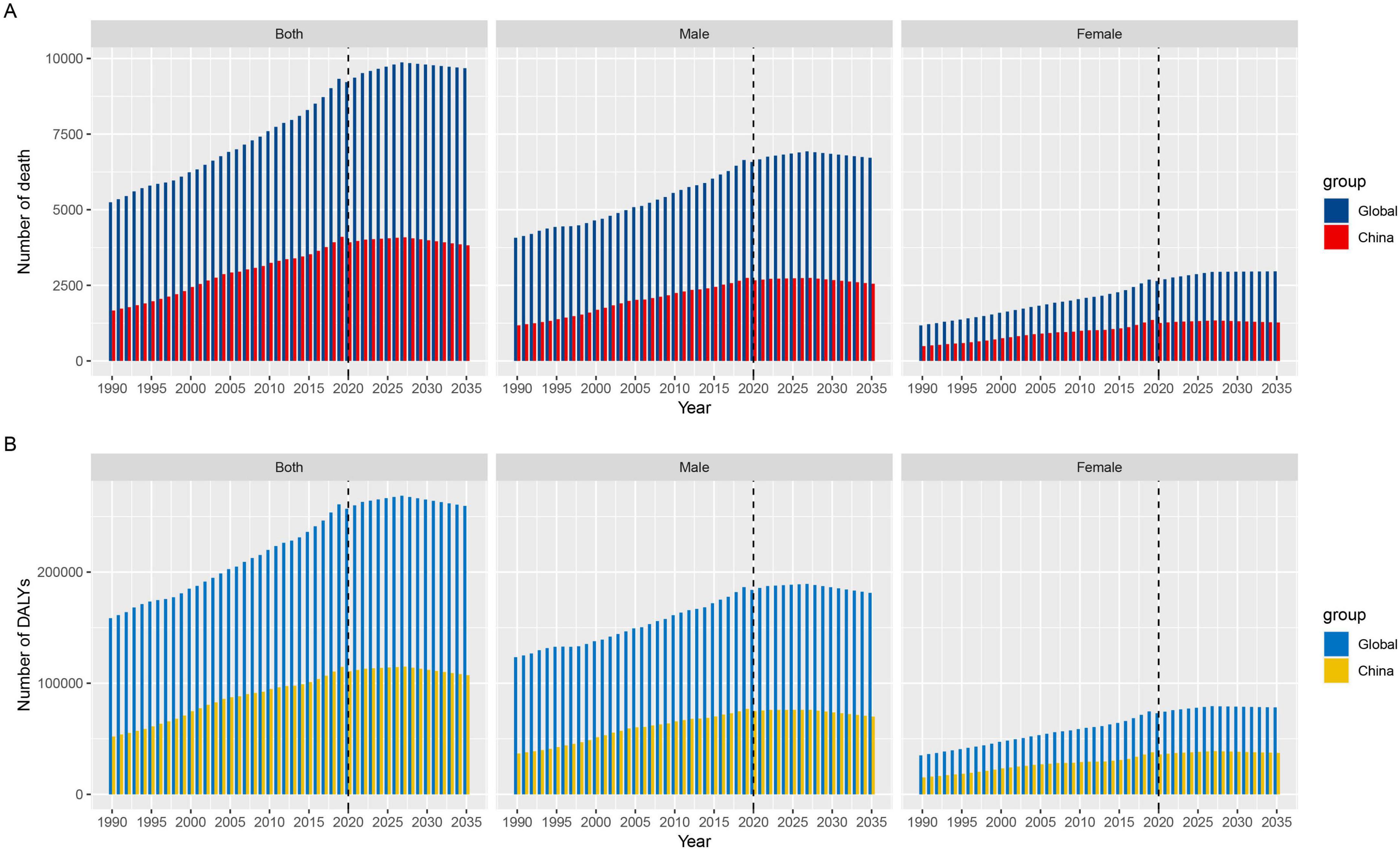
Figure 8. Projected trends in nickel-associated lung cancer mortality and DALY by sex in China and globally from 1990 to 2035. (A) Number of death; (B) Number of DALYs.
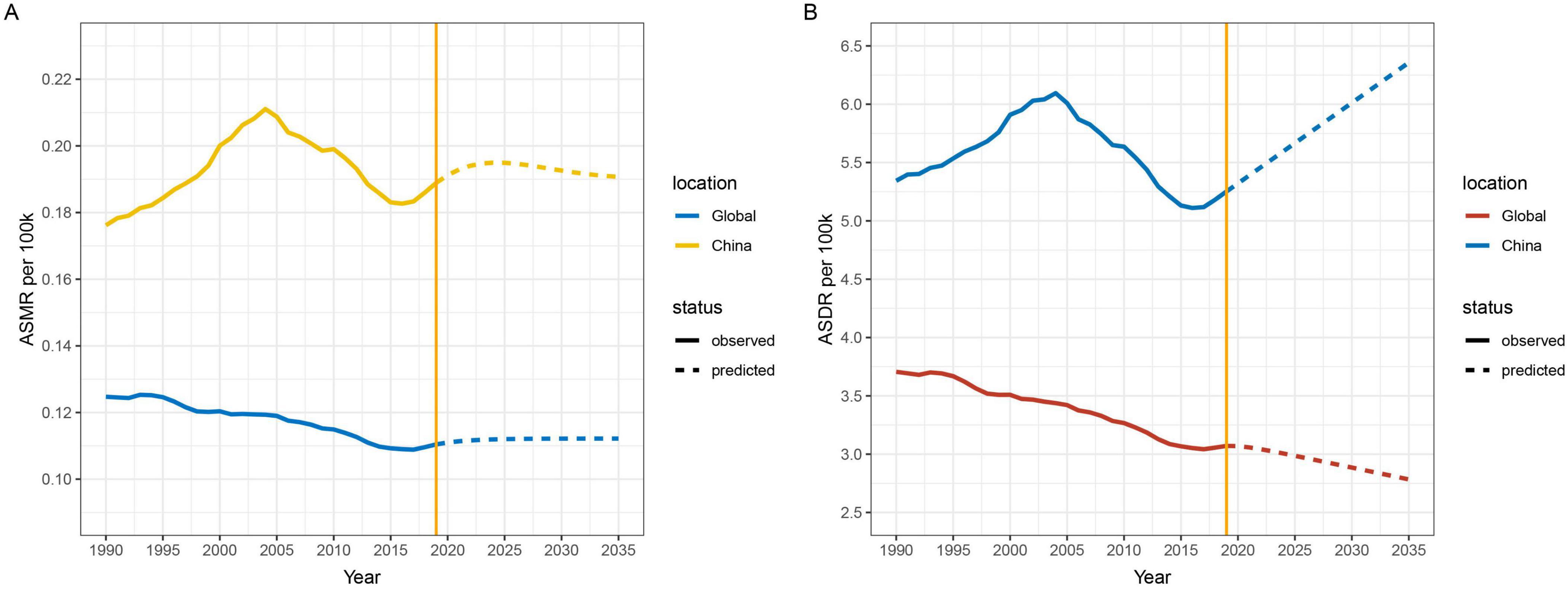
Figure 9. Projected trends in nickel-associated ASMR and ASDR by sex in China and globally from 1990 to 2035. (A) ASMR; (B) ASDR.
4 DiscussionBased on the death toll, ASMR, DALY and other disease burden indicators in China and the world in GBD2019 database, this study analyzed and predicted the disease burden data and its changing trend of nickel-associated lung cancer. The normalized DALY, YLL and YLD rates of nickel-associated lung cancer in the Chinese population were calculated using the average age structure of the world population as the standard population, so as to form a comparable result with the global population. Compared with previous studies that mainly focused on the disease burden of lung cancer caused by risk factors such as tobacco and air pollution, this study focused on the disease burden of occupational nickel-exposed populations, paying more attention to occupational populations. It supplemented current neglected studies on the disease burden of nickel-associated lung cancer.
In 2019, the DALY of nickel-associated lung cancer in China accounted for 0.03% of the total disease burden, represented 43.91% of the global nickel-associated lung cancer disease burden (23). The disease burden of lung cancer caused by occupational nickel exposure is relatively small compared to other widely distributed risk factors for lung cancer (24, 25). However, it is important to note that our study found an increasing number of nickel-associated lung cancer deaths and ASDR in China and globally, with China experiencing a greater increase, the potential risk of developing lung cancer due to exposure to nickel cannot be overlooked, particularly within the context of China. On the contrary, there were no significant changes in the ASMR and ASDR of nickel-associated lung cancer, indicating there was no significant change in the impact of nickel on lung cancer within the population. The increasing burden of nickel-associated lung cancer is attributed to the aging age structure of patients.
The findings indicate that from 1990 to 2019, there was a consistent decrease in ASYLLR and an increase in YLD/YLL for both the Chinese and global populations affected by nickel-associated lung cancer. This suggests a reduction in the premature mortality impact of nickel-associated lung cancer, but an increase in the burden of disability. Furthermore, analysis of ASMR and ASDR for nickel-associated lung cancer in the Chinese population revealed a shift toward older age groups between 1990 and 2019. The reasons for this trend are twofold. Firstly, there is the acceleration of China’s population aging process (26, 27). Secondly, there has been an improvement in occupational health protection and medical care levels, as well as the implementation of effective three-level prevention measures (28–30). These factors have led to a reduction in disease mortality rates and an extension of patient survival times. However, a new challenge we face is how to improve the quality of life for patients.
Then, we projected the future trend of ASMR and ASDR in nickel-associated lung cancer in the Chinese population. It is anticipated that ASMR will initially increase and then decrease over the next 15 years, while ASDR is expected to show a continuous upward trend. At present, China’s new energy industry is developing rapidly, the scale of nickel industry is expanding, the occupational exposure of nickel and the general population exposure are increasing (31, 32), and China is in the aging process, the ASDR of nickel-associated lung cancer may highlight the rising trend. Compared with a general lung cancer risk analysis, this study focuses more on the harm caused by nickel as an occupational exposure factor to the population, which is also the key population exposed to nickel. With the robust development of the new energy industry in recent years, nickel has found extensive applications across various industrial sectors, including electroplating, battery manufacturing, and aerospace. This widespread utilization can be attributed to its exceptional corrosion resistance, physical strength, and distinctive ferromagnetic properties. Therefore, we contend that to effectively manage the disease burden of nickel-associated lung cancer, it is essential to prioritize the protection of occupational nickel-exposed populations. Additionally, it is crucial to address the impact of the aging age structure of patients on nickel-induced lung cancer. Finally, efforts should be made to mitigate the disease burden resulting from disability.
First and foremost, it is essential to implement effective primary prevention strategies aimed at reducing the incidence and mortality associated with nickel-associated lung cancer. Enhance the working environment and monitor nickel concentration levels in the workplace to ensure compliance with national safety standards. Implement advanced ventilation systems and utilize personal protective equipment in the workplace to effectively minimize workers’ direct exposure to nickel. In addition, it is essential to provide regular occupational health training for workers to enhance their awareness of the risks associated with nickel exposure, and user-friendly health education models specifically tailored for older workers should be developed. Second, enhance the routine occupational health assessments for workers exposed to nickel, and ensure effective implementation of secondary prevention measures. Enhance the monitoring and assessment of occupational health, and establish as well as improve personal health records for workers exposed to nickel. In particular, it is essential to increase the frequency of occupational health examinations for older workers. For individuals exhibiting mild symptoms or identified potential risks, early intervention measures such as job reassignment or a reduction in work intensity should be enacted. Third, it is essential to not only focus on the disease itself but also to consider the quality of life of patients with nickel-associated lung cancer, do a good job of tertiary prevention, and reduce the burden of disease. Timely identification of patients’ disabilities and mental health conditions, along with the provision of professional rehabilitation guidance and services for workers affected by disability or illness, is essential to assist them in regaining their physical function to the greatest extent possible. Enhance mental health support and offer psychological counseling to affected workers and their family members in order to alleviate the mental stress induced by the disease.
There are certain limitations to this study. First, all the data used in our study came from GBD 2019, which originated from various sources (33). Consequently, there may be inconsistencies and incompatibilities between these data, potentially leading to bias. Second, as a measure of disease burden, DALY is restricted to the patient group level and does not evaluate the burden caused by out-patient groups such as family, society, and the state. Finally, these data rely on the calculation of monitoring data, and there may be lags in estimating the current BOD.
5 ConclusionIn summary, although the DALY of nickel-associated lung cancer in the Chinese population is relatively low compared with other causes, it accounts for a high proportion globally. Additionally, the age structure of nickel-associated lung cancer patients shows an aging trend, and the ASDR in the Chinese population indicates a potential upward trend when projecting the disease burden of nickel-associated lung cancer over the next 15 years. Therefore, in the future, China should focus on occupational health prevention, strengthen protection for those in occupational contact with nickel, increase basic scientific research on nickel-associated lung cancer, pay attention to improving patients’ quality of life and mental health protection, and reduce the disease burden of nickel-related lung cancer within our population.
Data availability statementThe original contributions presented in this study are included in this article/supplementary material, further inquiries can be directed to the corresponding authors.
Ethics statementEthical review and approval was not required for the study on human participants in accordance with the local legislation and institutional requirements. Written informed consent from the patients/participants or the patients’/participants’ legal guardian/next of kin was not required to participate in this study in accordance with the national legislation and the institutional requirements.
Author contributionsHL: Conceptualization, Data curation,. Formal analysis, Visualization, Writing – original draft. LH: Project administration, Supervision, Validation, Writing – review and editing, Conceptualization. PW: Data curation, Formal analysis, Visualization, Writing – review and editing. XL: Conceptualization, Funding acquisition, Methodology, Visualization, Writing – review and editing. QJ: Funding acquisition, Project administration, Resources, Writing – review and editing.
FundingThe authors declare that financial support was received for the research, authorship, and/or publication of this article. This study was supported by the Jiangsu Provincial Social Development Program of Key R&D Project (BE2022803), Jiangsu Provincial Key Medical Discipline (ZDXK202249), and Scientific Research Project of Jiangsu Health Commission (M2022036).
AcknowledgmentsWe express our gratitude to all the staff who participated in this study.
Conflict of interestThe authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s noteAll claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Footnotes References1. Rana SV. Perspectives in endocrine toxicity of heavy metals-a review. Biol Trace Elem Res. (2014) 160:1–14.
2. Alarifi S, Ali D, Alakhtani S, Al Suhaibani ES, Al-Qahtani AA. Reactive oxygen species-mediated DNA damage and apoptosis in human skin epidermal cells after exposure to nickel nanoparticles. Biol Trace Elem Res. (2014) 157:84–93.
3. Rehman K, Fatima F, Waheed I, Akash MSH. Prevalence of exposure of heavy metals and their impact on health consequences. J Cell Biochem. (2018) 119:157–84.
4. Denkhaus E, Salnikow K. Nickel essentiality, toxicity, and carcinogenicity. Crit Rev Oncol Hematol (2002) 42:35–56.
5. Coogan TP, Latta DM, Snow ET, Costa M. Toxicity and carcinogenicity of nickel compounds. Crit Rev Toxicol. (1989) 19:341–84.
6. Xu Z, Ren T, Xiao C, Li H, Wu T. Nickel promotes the invasive potential of human lung cancer cells via TLR4/MyD88 signaling. Toxicology. (2011) 285:25–30.
7. Bright P, Burge PS, O’Hickey SP, Gannon PF, Robertson AS, Boran A. Occupational asthma due to chrome and nickel electroplating. Thorax. (1997) 52:28–32.
8. So R, Chen J, Stafoggia M, de Hoogh K, Katsouyanni K, Vienneau D, et al. Long-term exposure to elemental components of fine particulate matter and all-natural and cause-specific mortality in a Danish nationwide administrative cohort study. Environ Res. (2023) 224:115552.
9. Boysen M, Solberg LA, Andersen I, Høgetveit AC, Torjussen W. Nasal histology and nickel concentration in plasma and urine after improvements in the work environment at a nickel refinery in Norway. Scand J Work Environ Health. (1982) 8:283–9.
10. Iskandar IYK, Gawkrodger DJ, Byrne L, Gittins M, Carder M, Fishwick D, et al. Trends in work-related respiratory diseases attributed to nickel, chromium and cobalt in the UK: Descriptive findings from The Health and Occupation Research (THOR) network 1996-2019. Occupat Environ Med. (2024) 81:220–4.
11. Na CY, Chen L, Wang XL, Xing SD, Gang BQ. The epidemiological study on lung cancer of nickel smeltery workers. Chin J Industr Hygiene Occup Dis. (1993) 11:261–4.
12. Pesch B, Kendzia B, Pohlabeln H, Ahrens W, Wichmann HE, Siemiatycki J, et al. Exposure to welding fumes, hexavalent chromium, or nickel and risk of lung cancer. Am J Epidemiol. (2019) 188:1984–93.
13. Pan YL, Xin R, Wang SY, Wang Y, Zhang L, Yu CP, et al. Nickel-smelting fumes induce mitochondrial damage and apoptosis, accompanied by decreases in viability, in NIH/3T3 cells. Arch Biochem Biophys. (2018) 660:20–8.
14. Cao X, Tian S, Fu M, Li Y, Sun Y, Liu J, et al. Resveratrol protects human bronchial epithelial cells against nickel-induced toxicity via suppressing p38 MAPK, NF-κB signaling, and NLRP3 inflammasome activation. Environ Toxicol. (2020) 35:609–18.
15. Zheng GH, Liu CM, Sun JM, Feng ZJ, Cheng C. Nickel-induced oxidative stress and apoptosis in Carassius auratus liver by JNK pathway. Aquat Toxicol. (2014) 147:105–11.
16. Guo H, Cui H, Peng X, Fang J, Zuo Z, Deng J, et al. Modulation of the PI3K/Akt pathway and Bcl-2 family proteins involved in Chicken’s tubular apoptosis induced by Nickel Chloride (NiCl2). Int J Mol Sci. (2015) 16: 22989–3011.
17. Son YO. Molecular mechanisms of nickel-induced carcinogenesis. Endocr Metab Immune Disord Drug Targets. (2020) 20:1015–23.
18. Murray CJL. The Global Burden of Disease Study at 30 years. Nat Med. (2022) 28:2019–26.
19. Zheng RS, Chen R, Han BF, Wang SM, Li L, Sun KX, et al. Cancer incidence and mortality in China, 2022. Chin J Oncol. (2024) 46:221–31.
20. GBD 2019 Universal Health Coverage Collaborators. Measuring universal health coverage based on an index of effective coverage of health services in 204 countries and territories, 1990-2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet. (2020) 396:1250–84.
21. GBD 2017 SDG Collaborators. Measuring progress from 1990 to 2017 and projecting attainment to 2030 of the health-related Sustainable Development Goals for 195 countries and territories: A systematic analysis for the Global Burden of Disease Study 2017. Lancet. (2018) 392:2091–138.
22. GBD 2019 Respiratory Tract Cancers Collaborators. Global, regional, and national burden of respiratory tract cancers and associated risk factors from 1990 to 2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet Respir Med. (2021) 9:1030–49.
23. Xin WY, Guo XL, He XS, Wang B, Xu LN. Epidemiological trends and projections of lung cancer deaths levels and disease burden in Chinese residents, 1990-2019. Chin J Cancer Prev Treatm. (2024) 31:65–73.
24. Ding LL, Zhu J, Zhang YH, Xu YY, Chen YS, Wang J. Disease burden of lung cancer attributable to smoking in China from 1990 to 2019. J Mod Oncol. (2024) 32:141–6.
留言 (0)