Enterococci are Gram-positive, resilient bacteria that are widely distributed in the natural environment and commonly found in the intestinal tracts of animals; they can be easily isolated from a wide range of hosts (Fiore et al., 2019; Kang et al., 2019). Enterococci, particularly Enterococcus faecalis and E. faecium, are opportunistic pathogens that are among the main causes of human nosocomial infections, including urinary tract infections, intra-abdominal infections, surgical infections and even life-threatening conditions, such as endocarditis, septicemia, and bacteremia (Fiore et al., 2019; Sood et al., 2008). Moreover, Enterococcus spp. exhibit intrinsic resistances to several antimicrobials, such as cephalosporins, sulphonamides, clindamycin, and low concentrations of aminoglycosides. In addition, enterococci can acquire mobile genetic elements carrying antimicrobial resistant genes (ARGs), especially those against vancomycin, which limits the treatment options for infections caused by these bacteria (Zou et al., 2020; Xiong et al., 2022; Ben Braiek and Smaoui, 2019).
Linezolid, the first commercially available oxazolidinone, was approved by the Food and Drug Administration (FDA) in 2000. It is considered as a last-resort drug for treating severe infections caused by Gram-positive bacteria, such as vancomycin-resistant enterococci (VRE), methicillin-resistant staphylococci (MRSA) and multidrug-resistant pneumococci (Sadowy, 2018; Hashemian et al., 2018; Bain and Wittbrodt, 2001). Over the past decades, the prevalence of linezolid-resistant E. faecalis and E. faecium has increased in hospitals worldwide, posing a significant challenge to the treatment of VRE (Kang et al., 2019; Vorobieva et al., 2017). Transferable resistance to linezolid is based on the multi-resistance gene cfr (Schwarz et al., 2000), and the ATP-binding cassette (ABC)-F protein encoding genes optrA and poxtA (Wang et al., 2015; Antonelli et al., 2018). The multi-resistance gene cfr could encode a 23S rRNA methyltransferase that mediates resistance to five classes of antimicrobials (phenicols, lincosamides, oxazolidinones, pleuromutilins and streptogramin A) (Long et al., 2006). Moreover, several variants of cfr had been reported, including cfr(B), cfr(C), cfr(D) and cfr(E) (Guerin et al., 2020), of which only cfr(B) and cfr(D) could been detected in enterococci by searching NCBI pubmed. And unlike poxtA, which can cause bacteria to decrease susceptibility to phenicols, oxazolidinones and tetracyclines (Antonelli et al., 2018), optrA only confers resistance or decreased susceptibility to phenicols and oxazolidinones. However, optrA can also cause bacterial resistance to tedizolid, a second-generation oxazolidinone that was approved by the FDA in 2014 (Wang et al., 2015; Locke et al., 2014; Zhanel et al., 2015). Among the three transferable linezolid resistance genes, optrA has been the main driver of the recent increase in the prevalence of linezolid-resistant enterococci (LRE) in humans (Freitas et al., 2020). Since its first identification in enterococci from both humans and animals in 2015, optrA has been detected in various bacteria, including Enterococcus, Staphylococcus, Streptococcus, Clostridium, Campylobacter, Fusobacterium, Listeria, and Salmonella (Schwarz et al., 2021; Brenciani et al., 2022). Compared to the high prevalence of cfr in staphylococci, Enterococcus spp., such as Enterococcus faecalis, are the major host bacteria carrying optrA (Schwarz et al., 2021). According to NCBI Nucleotide databases, the gene optrA is present in bacteria (mainly Enterococcus spp.) from six continents, originating from humans, animals, animal-derived food, vegetables, and environmental sources (Schwarz et al., 2021). Reports have indicated that optrA-carrying LRE have been circulating globally in hospitals since at least 2005 (Deshpande et al., 2018).
While optrA has been distributed worldwide, especially in enterococci of animal origin, and optrA-positive enterococci (OPEs) pose an increasing threat to public health (Brenciani et al., 2022), the molecular characteristics of OPEs from humans have been rarely reported. Cai et al. screened for optrA-positive bacteria isolated from healthy individuals in Hangzhou, China in 2015 and 2022 (Cai et al., 2019; Shen et al., 2022; Shen et al., 2024), and Nüesch-Inderbinen et al. isolated enterococci harboring oxazolidinone resistance genes among healthy humans in a Swiss community in 2021 (Nuesch-Inderbinen et al., 2022). In our previous study, we found that 18.10% (102/565, 95% CI: 14.90–21.20%) of individual fecal samples were positive for optrA in a large community (>20,000 residents) in Shenzhen from 2018 to 2019 (Xiang et al., 2022), and suggested that higher daily consumption of pork (>50 g/day) [Odds ratio (OR) = 1.62, 95% CI: 1.02–2.57, p = 0.042] and hospitalization within 3 months (OR = 11.55, 95% CI: 2.15–61.96, p = 0.004) were associated with a higher risk of optrA carriage. On the basis of the previous study, we compared the antimicrobial resistance phenotypes and genotypes between OPEs and optrA-negative enterococci (ONEs) at present study. We also deciphered the optrA-carrying plasmids, the genetic environment of optrA, and phylogenetic relationships among optrA-positive enterococci using based on whole-genome sequencing (Illumina and GridION platform) and bioinformatics analysis.
2 Materials and methods 2.1 Sample collection and isolation of OPEs and ONEsA total of 719 volunteers were recruited for sample collection from 1st of March, 2018 to 31st of December 2019 in a community in Shenzhen, Guangdong province, China. Ethical approval (R2018021) was granted by the ethics committee of the Shenzhen CDC on 19 January 2018. In total, 565 individual fecal samples accompanied by complete questionnaire information were selected for this study, and the sample selection criteria have been described in our previous studies (Xiang et al., 2022; Lv et al., 2022), in which 102 (102/565, 18.10, 95%CI: 14.90–21.20%) fecal samples were positive for optrA (Xiang et al., 2022) and 102 non-duplicate OPEs were isolated from these samples. The OPEs were identified using the following process: in brief, samples were cultured in LB broth (Luqiao, Beijing, China), then PCR was applied to detect the optrA gene in those isolates using the primers optrA-F: 5’-GCACCAGACCAATACGATACAA-3′, optrA-R: 5’-TCCTTCTTAACCTTCTCCTTCTCA-3′ (annealing temperature 52°C, amplicon size 794 bp) (Li, 2016). The optrA-positive broth was subsequently enriched in enterococcal culture-medium (SP3954, Luqiao, Beijing, China) containing 10 μg/mL florfenicol (ECM + FFC10). The enrichment was further inoculated onto the same plates (ECM + FFC10). Colonies on selective agar were confirmed for optrA and the species of enterococcal colonies positive for the optrA gene were identified by MALDI-ToF MS (MALDI Biotyper, Bruker, Germany). Moreover, ONEs were also isolated from optrA-positive fecal samples, following the same procedure, except that florfenicol was not added to the medium.
2.2 Antimicrobial susceptibility testingAST of enterococci was conducted using the broth microdilution method. Eleven antimicrobial agents were included: linezolid (LNZ, 64–0.25 μg/mL), florfenicol (FFC, 128–0.5 μg/mL), amoxicillin-clavulanate (A/C, 128/64–0.5/0.25 μg/mL), ampicillin (AMP, 128–0.5 μg/mL), ciprofloxacin (CIP, 64–0.25 μg/mL), daptomycin (DAP, 128–0.5 μg/mL), erythromycin (ERY, 128–0.5 μg/mL), tigecycline (TGC, 64–0.25 μg/mL), doxycycline (DOX, 128–0.5 μg/mL), nitrofurantoin (FM, 256-8 μg/mL) and vancomycin (VAN, 128–0.5 μg/mL). The antimicrobial microtiter plates were customized by local merchants (IN. KING, Tianjin, China) according to our requirements for dilution ranges of 11 antimicrobial agents. Antimicrobial resistance was interpreted according to the clinical breakpoints in EUCAST (version v13.1) guidelines and the CLSI document M100 (33rd ed). E. faecalis ATCC 29212 was included as a quality control strain.
2.3 Whole genome sequencing and analysisEnterococcal genomic DNA was extracted using the HiPure Bacterial DNA Kit (Guangzhou Magen Biotechnology Co., Ltd., China). Whole Genome Sequencing (WGS) with PE150 short-reads sequencing strategy (a minimum of 150-fold coverage) for each isolate was performed using the Illumina Novaseq 6,000 sequencing platform. Long-read sequencing of optrA-positive enterococci was conducted on the Oxford Nanopore Technologies (ONT) GridION platform. Long-read libraries of optrA-positive enterococci were prepared using the Ligation Sequencing Kit (SQK-LSK110) with EXP-NBD104 and EXP-NBD114. The constructed library was then subjected to ONT long-read sequencing in R9.4.1 flow cells on the ONT GridION platform according to the manufacturer’s protocol. Short-reads de-novo assembly or short- and long-reads hybrid de-novo assembly were performed using Unicycler v0.4.8 (Wick et al., 2017). The resulting contig sets (chromosomal or plasmid) were annotated with Bakta v1.8.2 (Schwengers et al., 2021) (accessed on 1 July, 2024). Multilocus Sequence Typing (MLST) was identified using mlst v2.19.0 (Seemann, 2020a) based on assembled contigs. ARGs, Virulence factors genes (VFGs) and plasmid types were identified using Abricate v1.0.1 (Seemann, 2020b) (accessed on 1 July, 2024). Single-nucleotide polymorphisms (SNPs) were identified using Snippy v4.6.0 (Seemann, 2020c), and SNP distances between isolates were calculated based on concatenated SNPs using snp-dists v0.8.2 (Seemann and Klötzl, 2021). The population structure of those isolates was delineated using Bayesian model-based population structures (BAPs) analysis via Fastbaps v1.0.8 (Tonkin-Hill et al., 2019). Maximum likelihood based phylogenetic trees were constructed using IQ-TREE v2.2.2.7 (Minh et al., 2020). Phylogenetic trees were then visualized using Interactive Tree of Life (iTOL) v6.7.6 (Letunic and Bork, 2024) with the corresponding features of each isolate. Genetic characteristics of optrA-positive isolates were compared with BLAST Ring Image Generator (BRIG) 0.95 and Easyfig 2.2.5 (Sullivan et al., 2011; Alikhan et al., 2011). All optrA-harboring enterococcal genomes available in the NCBI database were downloaded on 7 April, 2024 for further analysis. Moreover, some optrA-carrying plasmid sequences in NCBI database were downloaded for comparison of plasmid structures (accessed on 10 July, 2024). To ascertain whether there are any novel optrA-carrying plasmids, all of the circular optrA-carrying plasmids identified herein were deposited in NCBI BLASTn and compared with the NCBI nonredundant (nr) database (accessed on 10 July, 2024). When the reference sequence contained optrA, but the coverage was <60%, or the reference sequences did not contain optrA, or the coverage and identity were both >90%, but the plasmid type was different, the respective plasmids have been considered as novel optrA-carrying plasmids.
2.4 Statistical analysesThe statistical analyses were conducted in R v.4.3.1 (R Core Team, 2023). Differences between the numbers of distinct ARGs/VFGs per isolate in different groups were assessed using the Kruskal-Wallis test. Gene prevalence differences between groups were assessed with the χ2 test (n ≥ 40) or Fisher’s exact test (n < 40 or minimum expected frequency < 1).
3 Results 3.1 High antimicrobial resistance rates in OPEs, especially Enterococcus faecalisWe previously identified 102 optrA-positive fecal samples from the community population and 102 non-duplicate optrA-positive enterococci were selected for further analysis (Xiang et al., 2022). Enterococcus faecalis (n = 75/102, 73.5%) was the predominant species among the OPEs, followed by E. faecium (n = 7/102, 6.9%), E. avium (n = 6/102, 5.9%), E. casseliflavus (n = 6/102, 5.9%), E. gallinarum (n = 4/102, 3.9%) and E. hirae (n = 4/102, 3.9%). Concurrently, for species matching, optrA-negative E. faecalis were also isolated from the 75 optrA-positive fecal samples from which we isolated optrA-positive E. faecalis because of its high prevalence rate. A total of 26 optrA-negative E. faecalis (paired samples) were selected from 26 optrA-positive E. faecalis fecal samples to explore the differences between optrA-positive (optrA+_Match, n = 26) and optrA-negative (optrA-_Match, n = 26) isolates.
All of the optrA-positive enterococci were resistant to florfenicol (102/102, 100%) and susceptible to vancomycin, tigecycline, amoxicillin-clavulanate, ampicillin, daptomycin and nitrofurantoin (Supplementary Tables S1, S2). The overall resistance rate for linezolid was 33.3% (34/102). Compared to optrA-_Match isolates, high resistance rates were observed in OPEs, with higher resistance rates against linezolid, florfenicol, doxycycline, erythromycin, and ciprofloxacin, in optrA+_Match (LNZ: 38.5%, FFC: 100%, DOX: 88.5%, ERY: 100%, CIP: 15.4%, p < 0.001, except for CIP) and all of OPEs in this study (optrA+_Total, n = 102) (LNZ: 33.3%, FFC: 100%, DOX: 63.7%, ERY: 97.0%, CIP: 27.5%, p < 0.001 for all but p < 0.01 for CIP; Figure 1A; Supplementary Table S2). We observed that the linezolid resistance rate varied in different enterococcal species, 41.3% (31/75) for E. faecalis, 14.3% (1/7) for E. faecium and 10.0% (2/20) for other enterococcal species (E. avium, E. casseliflavus, E. gallinarum, and E. hirae, Figure 1B; Supplementary Table S3). The resistance rates of different OPEs species to ciprofloxacin were relatively low (25.3–35.0%), but the erythromycin resistance rates were high (90.0–100%). Particularly, the doxycycline resistance rate was significantly higher in E. faecalis (56/75, 74.7%; p < 0.001) than in other enterococcal species.
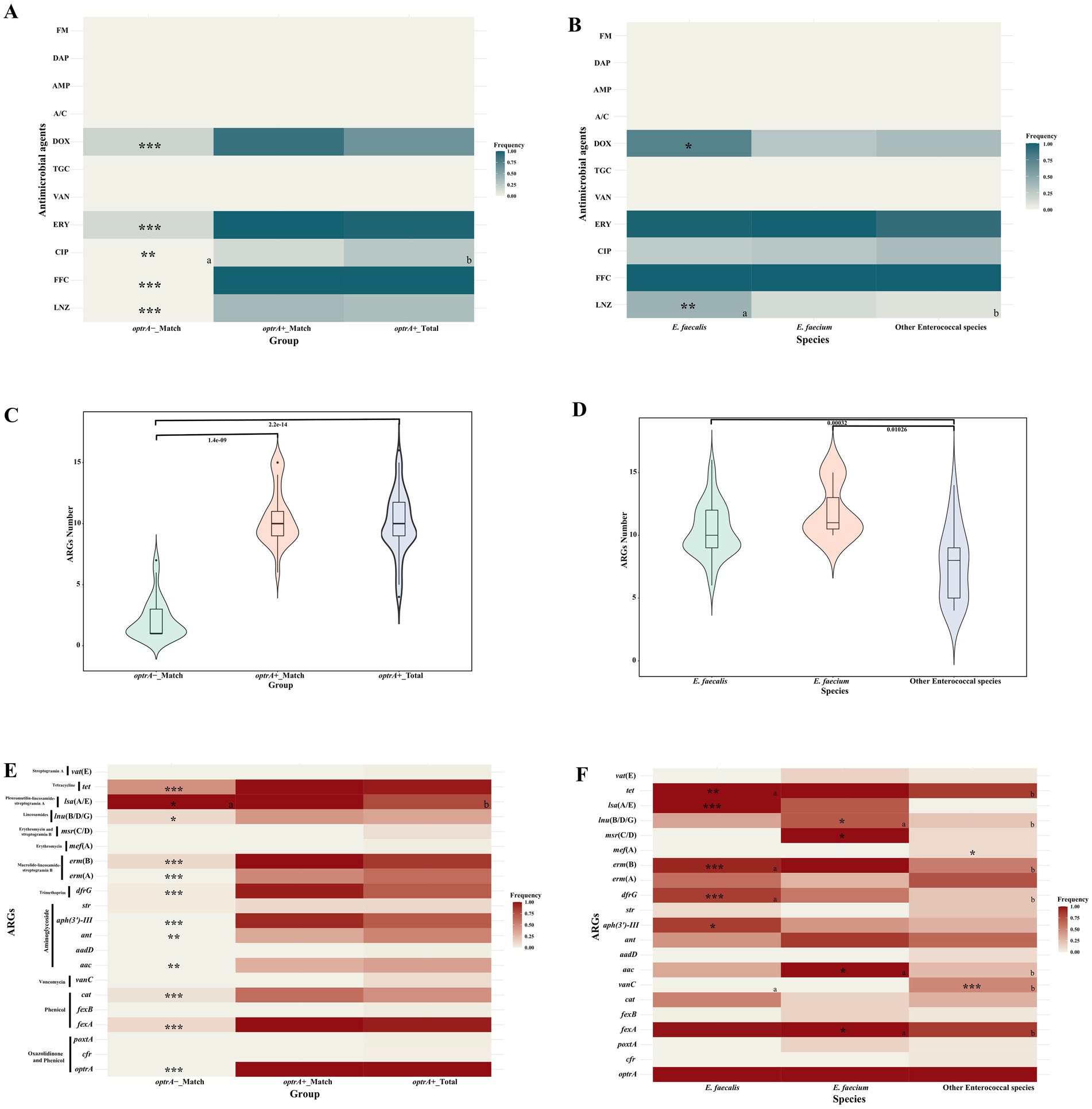
Figure 1. Comparison of antimicrobial susceptibility, ARGs numbers and profiles of OPEs and ONEs (A,C,E), and of different enterococcal species (B,D,F). cat--cat or cat(pC221); vanC--vanC1XY, vanC2XY or vanC4XY; aac--aac(6′)-Ii, aac(6′)-Iid, or aac(6′)-aph(2″); ant--ant(6)-Ia and ant(9)-Ia; msr(C/D)--msr(C) or msr(D); lnu(B/D/G)--lnu(B), lnu(D) or lun(G); lsa(A/E)--lsa(A) or lsa(E); tet--tet(L), tet(M), tet(O/W/32/O) or tet(S). a and b Represent significant differences between the two groups; only asterisks indicated that the group showed significant differences with other groups; “*”: p < 0.05; “**”: p < 0.01; “***”: p < 0.001.
3.2 High presence of ARGs in OPEs, especially Enterococcus faecalis and Enterococcus faeciumA total of 21 ARGs were identified in the different enterococcal species (Figures 1C,F; Supplementary Table S4). The number of ARG in optrA+_Match (median 10, interquartile range (IQR) 9–11) and optrA+_Total (median 10, IQR 9–11.75) isolates were distinctly higher than that in optrA-_Match isolates (median 1, IQR 1–3; p < 0.0001, Figure 1C). Notably, the phenicol resistance gene fexA, the aminoglycoside resistance genes aac, ant and aph(3′)-III, the trimethoprim resistance gene dfrG, the macrolide-lincosamide-streptogramin B (MLSB) resistance genes erm(A) and erm(B), the lincosamides resistance genes lnu(B/D/G), and the tetracycline resistance genes tet were more prevalent in optrA+_Match and optrA+_Total than that in optrA-_Match isolates, especially fexA, cat, ant(9)-Ia, aph(3′)-III, erm(A), erm(B), lsa(A), tet(L) and tet(M) (Figure 1E). Conversely, no significant differences in the 42 VFGs were observed between optrA+_Match or optrA+_Total and optrA-_Match isolates (Supplementary Figure S1A; Supplementary Table S5).
Among the different species, ARGs in E. faecalis (median 10, interquartile range (IQR) 9–12) and E. faecium (median 11, IQR 10.5–13) were more frequently observed than in other enterococcal species (median 8, IQR 5–9) (p < 0.001 and p < 0.05 respectively) (Figure 1D). Both fexA (85–100%) and tet (85–100%) were predominant in all of the optrA-positive species (Figure 1F). In addition, poxtA was found in one E. faecium, and cfr was identified in one E. hirae and one E. avium isolate. However, all three isolates carrying two types of linezolid resistance genes showed susceptibility to linezolid. Moreover, the prevalence of other ARGs in different optrA-positive enterococci varied substantially. The pleuromutilin-lincosamide-streptogramin A resistance gene lsa(A/E) was detected in all E. faecalis isolates, but less frequently in E. faecium (71.4%) and other enterococcal species (0%) (p < 0.001). The MLSB resistance gene erm(B) was present in 93.3% E. faecalis and 100% E. faecium isolates, but at lower frequency (55%) in other enterococcal species. The vancomycin resistance gene clusters vanC were absent in either E. faecalis or E. faecium, but were found in 50% of other enterococcal species because E. casseliflavus and E. gallinarum are intrinsically resistant to vancomycin by carrying the vanC gene cluster on their chromosomes (Garcia-Solache and Rice, 2019) (p < 0.05). The aminoglycoside resistance gene aac and the erythromycin and streptogramin B resistance gene msr(C/D) were identified in all of the E. faecium isolates, but at a lower frequency in E. faecalis (25.0–34.6%) and other enterococcal species (0–10%). In addition, VFGs were more frequently detected in E. faecalis (median 25, IQR 17–26) than in E. faecium (median 1, IQR 0–1) and other enterococcal species (median 0, IQR 0–0) (p < 0.001, Supplementary Figures S1B,D).
3.3 Heterogeneous population structure of OPEs and ONEsMLST revealed that both optrA-positive and optrA-negative E. faecalis isolates exhibited a heterogeneous population structure (Supplementary Table S6). Among the 75 optrA-positive E. faecalis, 52 isolates were assigned to 26 STs, including ST16 (n = 10, 13.3%), ST376 (n = 5, 6.6%), ST93 (n = 4, 5.3%), ST632/ST256 (n = 3, 4.0%), ST59/ST202/ST330/ST618/ST631/ST633 (n = 2, 2.6%), and 15 STs were represented by one isolate each, while the remaining 23 optrA-positive E. faecalis isolates belonged to novel STs. The STs differed across the optrA-positive and optrA-negative E. faecalis in 26 paired samples (Figure 2A). In addition, the optrA-positive and optrA-negative E. faecalis isolates were phylogenetically distant from each other (Figure 2A). Among seven optrA-positive E. faecium, ST66, ST55, ST944 and ST194 were identified in four isolates and novel STs in other three isolates. To explore the phylogenetic relationship between OPEs isolated from the tested community and other sources, we downloaded 945 whole genome sequences using the keywords “optrA” from NCBI and screened E. faecalis isolates from China with their sources clearly documented in the respective metadata. A total of 339 E. faecalis were included in the phylogenetic analysis along with the 75 E. faecalis isolates from this study (Figure 2B). The BAPs analysis divided these 414 optrA-positive E. faecalis isolates into 17 clusters with no prominent BAPs, suggesting a heterogeneous population structure of optrA-positive E. faecalis. In line with the phylogenetic analysis, high diversity was also found in MLST typing, 51.93% (215/414) of the isolates, belonging to nine STs, were obtained from different origins and only ST506 was predominately isolated from pigs (38/39). There was no significant correlation between neither the BAPs clusters nor the sequence types with the source of optrA-positive E. faecalis. However, high similarly between optrA-positive E. faecalis isolated from the study community and other origins (human clinics, pigs, chickens, pets, and environment) was observed in the same BAPs cluster according to low core genome diversity reflected by a limited number of SNPs (19–338 SNPs difference).
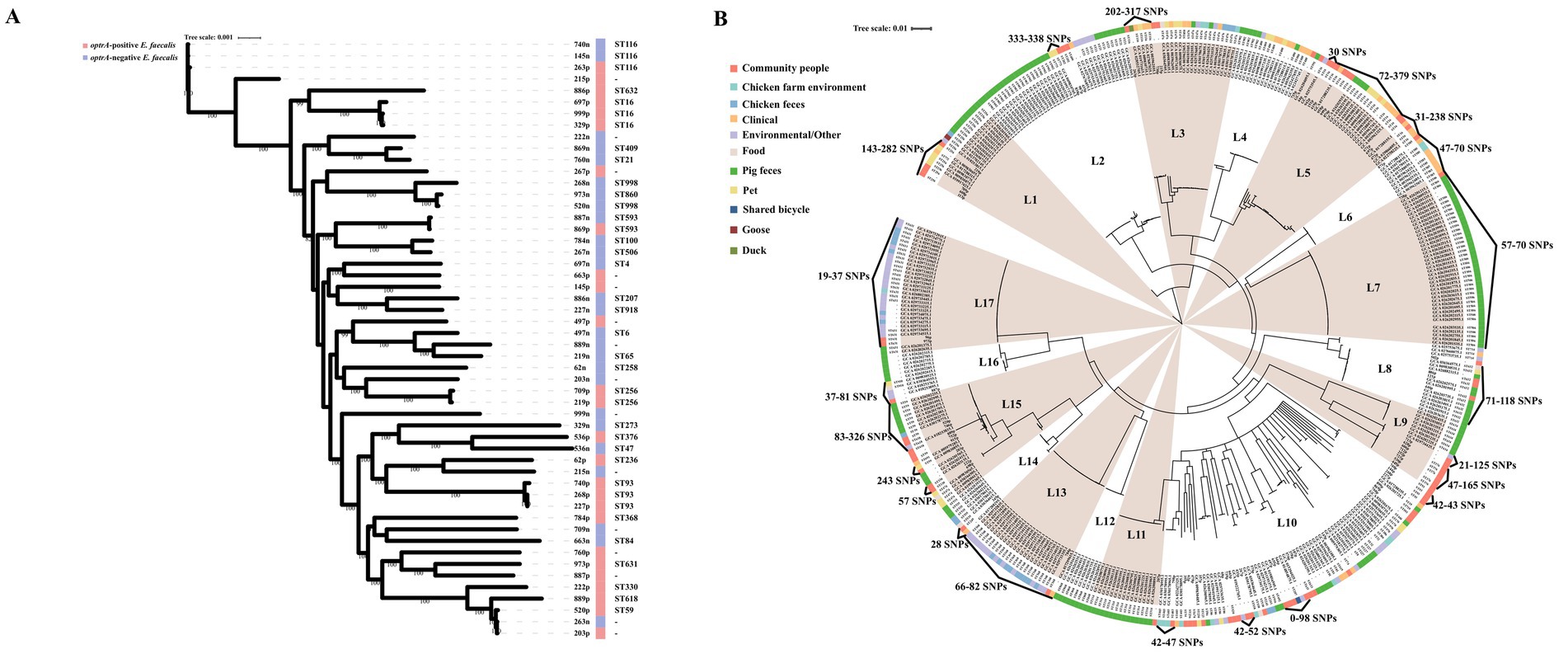
Figure 2. Phylogenetic trees based on maximum likelihood analysis of 26 pairs optrA-positive and -negative E. faecalis isolated from the same fecal samples (A), and 414 optrA-positive E. faecalis including 75 strains isolated from the present study and 339 strains whose genomes were downloaded from NCBI (B).
3.4 Location of optrA gene and diversity of optrA-carrying plasmid typesTo investigate the genetic basis of optrA dissemination among different isolates, all of the 102 OPEs underwent sequencing via a combination of short-read and long-read WGS, followed by hybrid assembly. The majority of hybrid assemblies produced complete optrA-carrying sequences and only 11 optrA-carrying sequences were incomplete or non-circular (Supplementary Table S6). The optrA was detected on on the chromosome (n = 36), on plasmids (n = 62), or both (n = 4, three E. faecalis and one E. gallinarum, Table 1). A total of 15 distinct plasmid types were identified among 66 optrA-carrying plasmids with sizes varying between 25.9 and 92.8 kb. This highlighted the considerable diversity of optrA-carrying plasmids among enterococci isolated from the community population (Table 1; Supplementary Table S6). The types of replicons carried by different enterococci varied considerably. Half of the optrA-harboring plasmids (33/66) carried multiple replicons, predominant with rep9a_1_repA(pAD1) + repUS43_1_CDS12738(DOp1) (24/66, 36.36%), which were identified exclusively in E. faecalis. One plasmid harbored even three replicons, rep33_1_rep(pSMA198) + pAD1 + DOp1. The rep9 (belonging to repA_N) was the mostly detected replication gene (44/66), followed by repUS43 (belonging to rep_trans; 29/66), both of which were only identified in E. faecalis. In addition, repUS1, associated with the broad host-range plasmids Inc18, was identified in seven optrA-carrying plasmids derived from three E. casseliflavus, two E. avium, one E. gallinarum and one E. faecium isolate. The backbones of 19 out of 24 pAD1 + DOp1 optrA-carrying plasmids all showed high homology (coverage 100% and identity >99.90%) with plasmid pNS2A, located in a Chinese water-borne E. faecalis isolate (GenBank no. CP078163.1, Figure 3A). Most of the pAD1 + DOp1 plasmids carried additional ARGs including ant(6)-Ia, aph(3′)-III, cat, fexA, dfrG, erm(B), tet(L), tet(M) and etc. And the bacitracin resistance cluster bcrABDR was also frequently identified on this type optrA-carrying plasmids through annotation of plasmid sequences and NCBI BLASTn. The backbones of other type plasmids with identical replicons were dissimilar, and only rep9b_2_prgW(EF62pC) + DOp1-type and repUS1_3_rep(pVEF1)-type optrA-carrying plasmids showed relatively high homology (Figures 3B,C). Furthermore, putative rep genes were identified on seven circular-formed optrA-carrying segments but no clear replicons were assigned, which would necessitate further investigation.
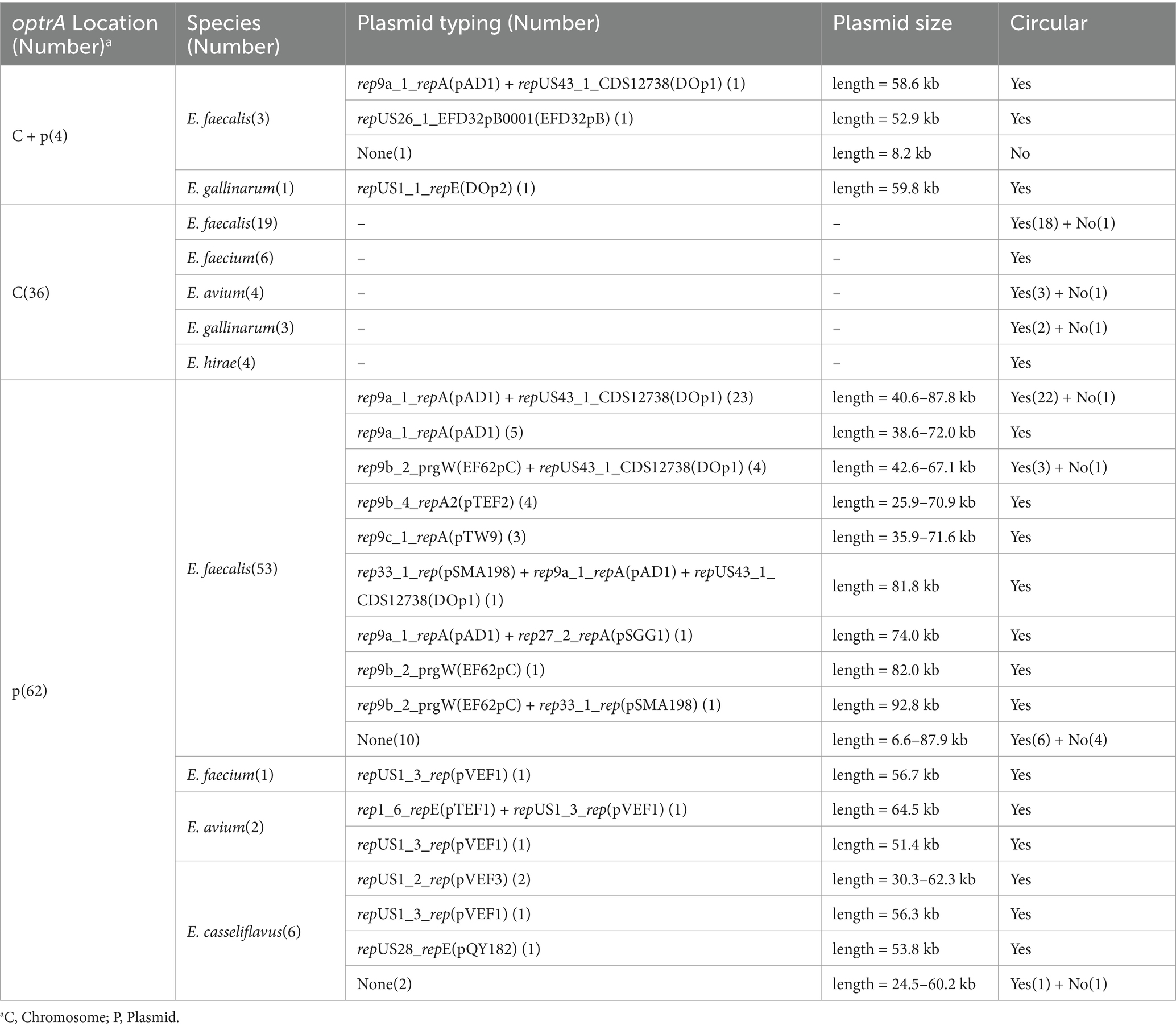
Table 1. Location of the optrA gene as well as typing and sizes of optrA-carrying plasmids.
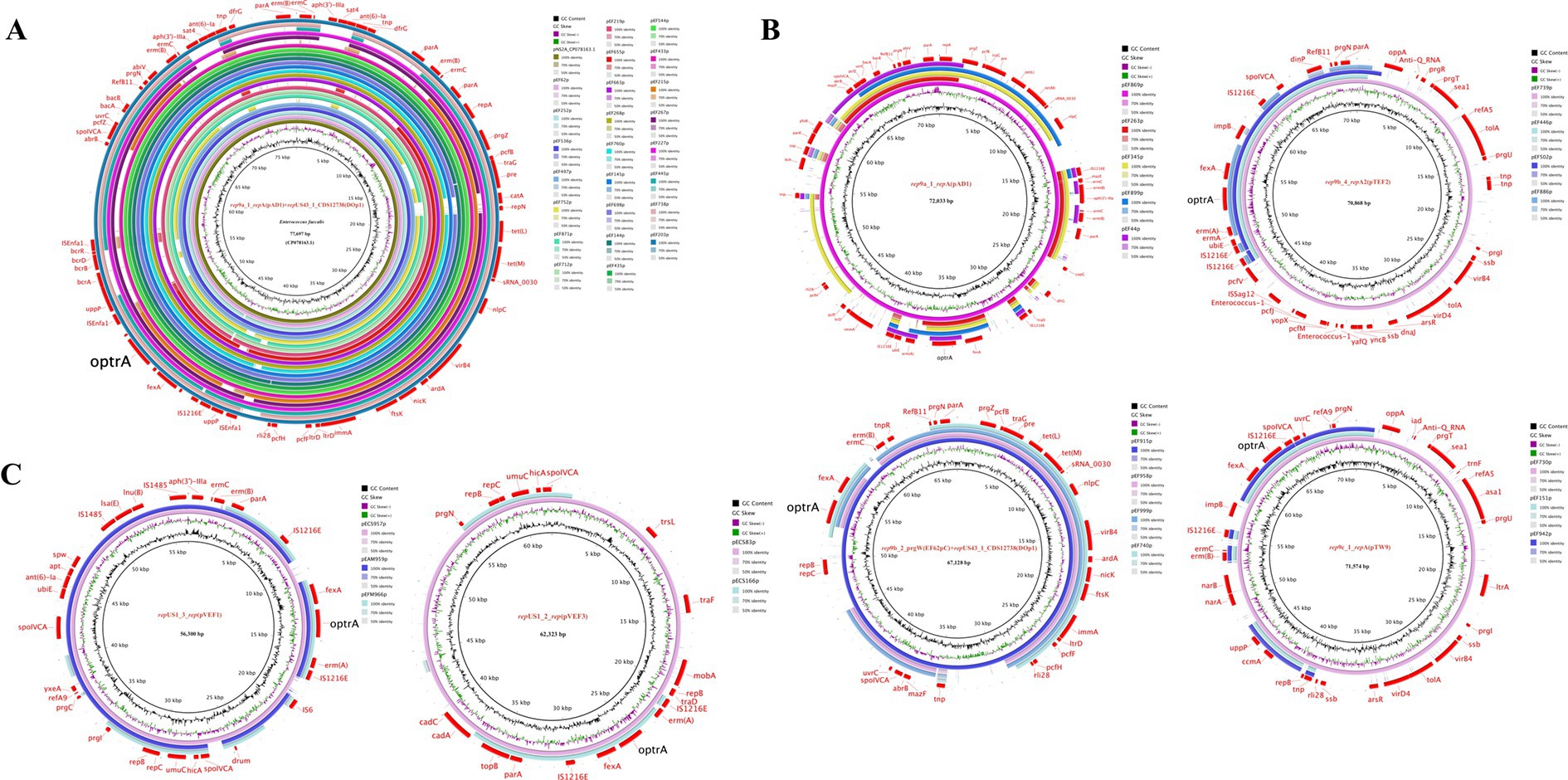
Figure 3. Circular comparison of optrA-carrying plasmids of the same type in this study. (A) pAD1 + DOp1; (B) optrA-carrying plasmids in E. faecalis; (C) optrA-carrying plasmids in other enterococcal species.
3.5 Novel optrA-carrying plasmids were identified and most ARGs were co-located on optrA-carrying plasmidsTo investigate optrA-carrying plasmids, all of the circular optrA-carrying plasmids identified in this study were deposited in NCBI BLASTn and compared with the NCBI nr database (accessed on 10 July, 2024). The pAD1 + DOp1 optrA-carrying plasmids were not only prevalent in the community population but also showed highly homology (coverage >90% and identity >99%) with plasmids from various sources (water, patient urine, cattle, feces, pig, pet food, wastewater and other environments) deposited in NCBI (GenBank accession no. CP078163.1, CP145115.1, CP116554.1, CP116558.1, CP088199.1, CP097007.1, CP116556.1, CP096048.1, MK784777.1, MK465704.1, CP148053.1, CP118757.1, OP046178.1, etc.). Moreover, these plasmids were predominantly found in E. faecalis, with only MK465704.1 identified in E. faecium. Notably, we identified 16 novel optrA-carrying plasmids with their sizes ranging from 30 kb to 92 kb and various replicon types or combinations of replicons. Moreover, circular comparisons of 16 novel optrA-carrying plasmid maps were conducted with the most similar plasmid sequences in the NCBI nr database (Supplementary Figures S2–S4). Among the novel plasmids, the optrA-carrying plasmid pEF575p harboring three replicons (pSMA198 + pAD1 + and DOp1) was detected in E. faecalis. We found that the plasmid pEF575p also showed high similarly with plasmid pNS2A (coverage 89% and identity 99.97%) which same as pAD1 + DOp1-type optrA-carrying plasmids (Supplementary Figure S2A). And in this study, nine circular optrA-carrying plasmids were identified in non-E. faecalis enterococcal species. The result of BLASTn with NCBI nr database revealed that seven were novel, with six carrying the repUS1 replicon (Supplementary Figure S3).
Multiple ARGs were identified on these optrA-carrying plasmids and the heatmap showed that 28.69–83.87% of ARGs were co-located with optrA-carrying plasmids, except for four isolates with optrA located both on the chromosome and plasmid in one isolate (Figure 4). The ARGs co-located with optrA on pAD1 + DOp1, pAD1, EF62pc + DOp1, pTW9, pVEF1 and other types of optrA-carrying plasmids (including EF62pC, EF62pC + pSMA198, pAD1 + rep27_2_repA(pSGG1), repUS28_repE(pQY182), pSMA198 + pAD1 + DOp1, and rep1_6_repE(pTEF1) + pVEF1), accounted for more than 50% of the total ARGs carried by the whole genomes of the isolates. The genes fexA (60/62, 96.77%) was the ARGs most commonly co-located with optrA-carrying plasmids, followed by erm(B) (42/62, 67.74%), bcrABDR (34/62, 54.84%), tet(M) (34/62, 54.84%), tet(L) (34/62, 54.84%), erm(A) (33/62, 53.23%), aph(3′)-III (32/62, 51.61%), dfrG (30/62, 48.39%), cat (24/62, 38.71%) and other ARGs. The results demonstrated that the acquisition of optrA-carrying plasmids facilitates host strains to harbor more ARGs, which poses a significant threat to human health. Notably, up to 83.87% of ARGs were found to be co-located in pAD1 + DOp1-type plasmids, emphasizing the need for increased attention to this type optrA-carrying plasmids. Furthermore, the co-existence of optrA and cfr genes was observed in a novel plasmid pEAM528p from one E. avium isolate and two replicons pTEF1 and pVEF1, both belonging to the broad host Inc18 plasmids, were identified in pEAM528p. This plasmid exhibited a high degree of homology (coverage 75% and identity 99.30%) to the pTEF1-type plasmid pAFL-J5-2, which was carried by porcine E. faecalis isolated from Xinjiang, China (GenBank accession number: CP148054.1, Supplementary Figure S4F). Especially, the flanking region of optrA in pEAM528p was highly similarly to that in pAFL-J5-2, and the only difference was the presence of an aminoglycoside resistance gene aac(6′)-Ie in pEAM528p (Figure 5D).
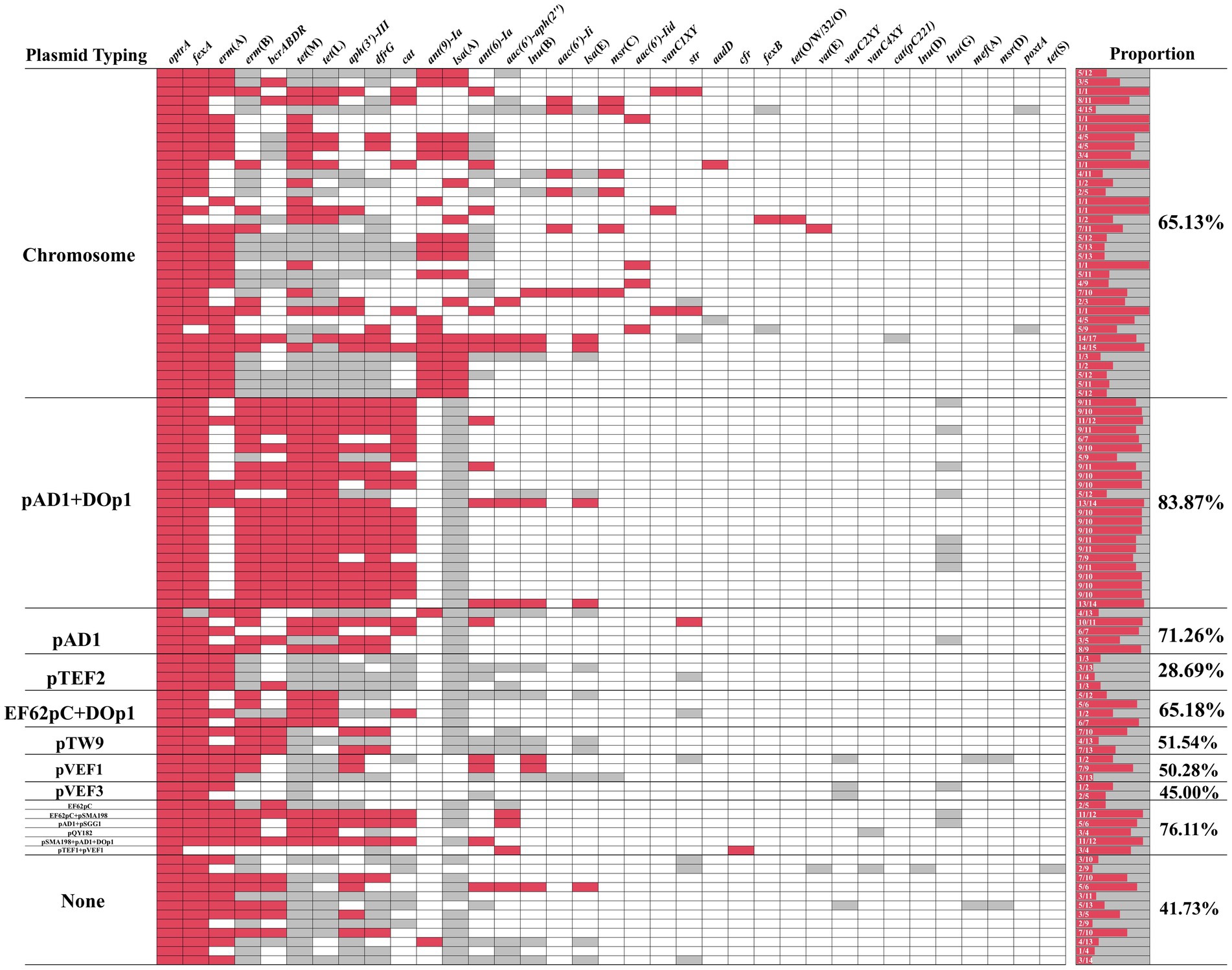
Figure 4. The heatmap of ARGs coexisting with optrA. Red indicates that the ARGs and optrA co-exist on the same contig, gray indicates that the ARGs and optrA do not co-exist on the same contig, and white indicates that the ARGs were not detected in the same isolate.
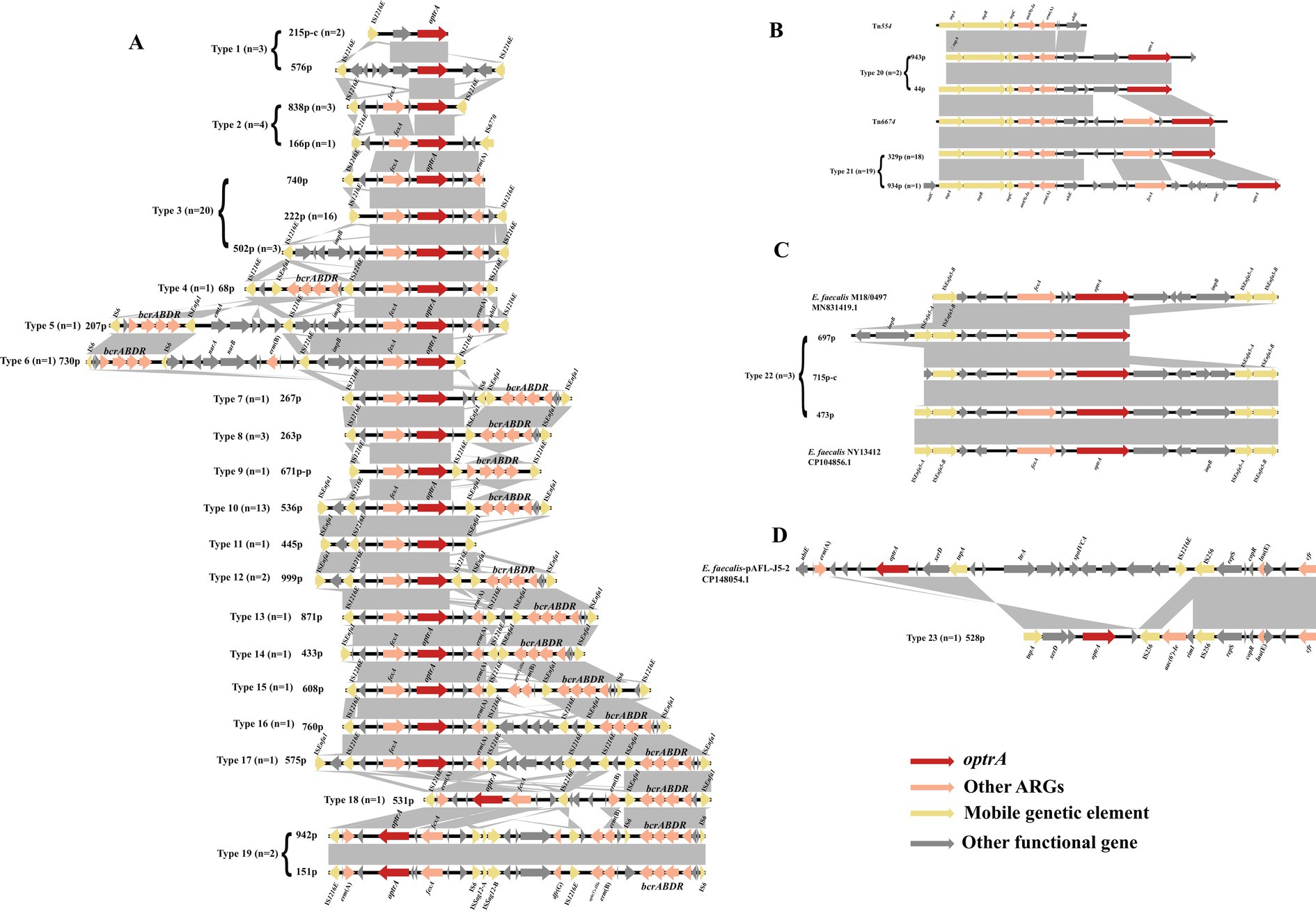
Figure 5. Schematic presentation and comparison of the genetic environment of optrA in the enterococci investigated in this study. The “-c” in the number indicates that the sequence is derived from the chromosome sequences, and other sequences are derived from the plasmid sequences. (A) IS1216E-related; (B) Tn554-related; (C) ISEnfa5-related; (D) optrA and cfr co-existing in the same genetic environment.
3.6 optrA is commonly surrounded by mobile genetic elementsThe genetic contexts of optrA (n = 106) identified in this study can be categorized into four classes and 27 types: IS12126E-related (types 1–19, n = 59, Figure 5A), Tn554-related (types 20–21, n = 21, Figure 5B), ISEnfa5-related (type 22, n = 3, Figure 5C), cfr located in the surrounding of optrA (type 23, n = 1, Figure 5D) and no MGEs (type 24: fexA-optrA, n = 12; type 25: optrA-erm(A), n = 1; type 26: fexA-optrA-erm(A), n = 8; type 27: optrA, n = 1). The IS12126E-related context of optrA was the most prevalent (59/106, 55.66%). IS1216E can be located upstream or downstream of optrA, or both in the same or different orientations. Besides IS1216E, the ISEnfa1 often appears in the surrounding area of optrA as ISEnfa1-bcrABDR-ISEnfa1 (Figure 5A, types 7, 8, 10, 12–14, 16–18; 22/59). Both ISEnfa1 and IS1216E belong to the IS6 family, with ISEnfa1 exhibiting 94% amino acid identity to IS1216E. The bacitracin resistance cluster bcrABDR has been identified in E. faecalis previously and frequently detected in surrounding genetic context of optrA in present study (Matos et al., 2009). It can also appear as IS1216E-bcrABDR-IS1216E structure (type 9). Moreover, IS1216E and ISEnfa1 were interspersed between optrA and bcrABDR in various forms (types 4–19). Besides the IS1216E, Tn554 or the Tn554-related transposon Tn6674 were commonly associated with optrA (Figure 5B, type 21, n = 19). Tn6674 can be active and form circular intermediates to accelerate the transfer of optrA (Li et al., 2019). In addition, ISEnfa5 in the upstream region or both up- and downstream regions of optrA were observed in three optrA-carrying contigs (Figure 5C). Moreover, florfenicol resistance gene fexA and MLSB resistance gene erm(A) were frequently detected in most of genetic contexts surrounding optrA.
A sankey diagram was employed to analysis the relationships among enterococcal species, plasmid types, and genetic environments of optrA (Figure 6). The results showed that optrA was predominantly identified on plasmids (53/72, 73.61%) in E. faecalis and on chromosomes in other enterococci (17/26, 65.38%). Concurrently, most plasmid types (10/15) were identified in E. faecalis, in contrast to only five types were found in other enterococci. Tn554-related genetic contexts of optrA and Tn-ND (MGEs were absent in the surrounding area of optrA) were primarily located on chromosome. IS1216E-related genetic contexts of optrA could be located on the chromosome or plasmids, yet mostly on plasmids. IS1216E + bcrABDR-related genetic contexts of optrA could be located on a variety of plasmids and were mainly detected in E. faecalis (with occasional detection in E. casseliflavus).
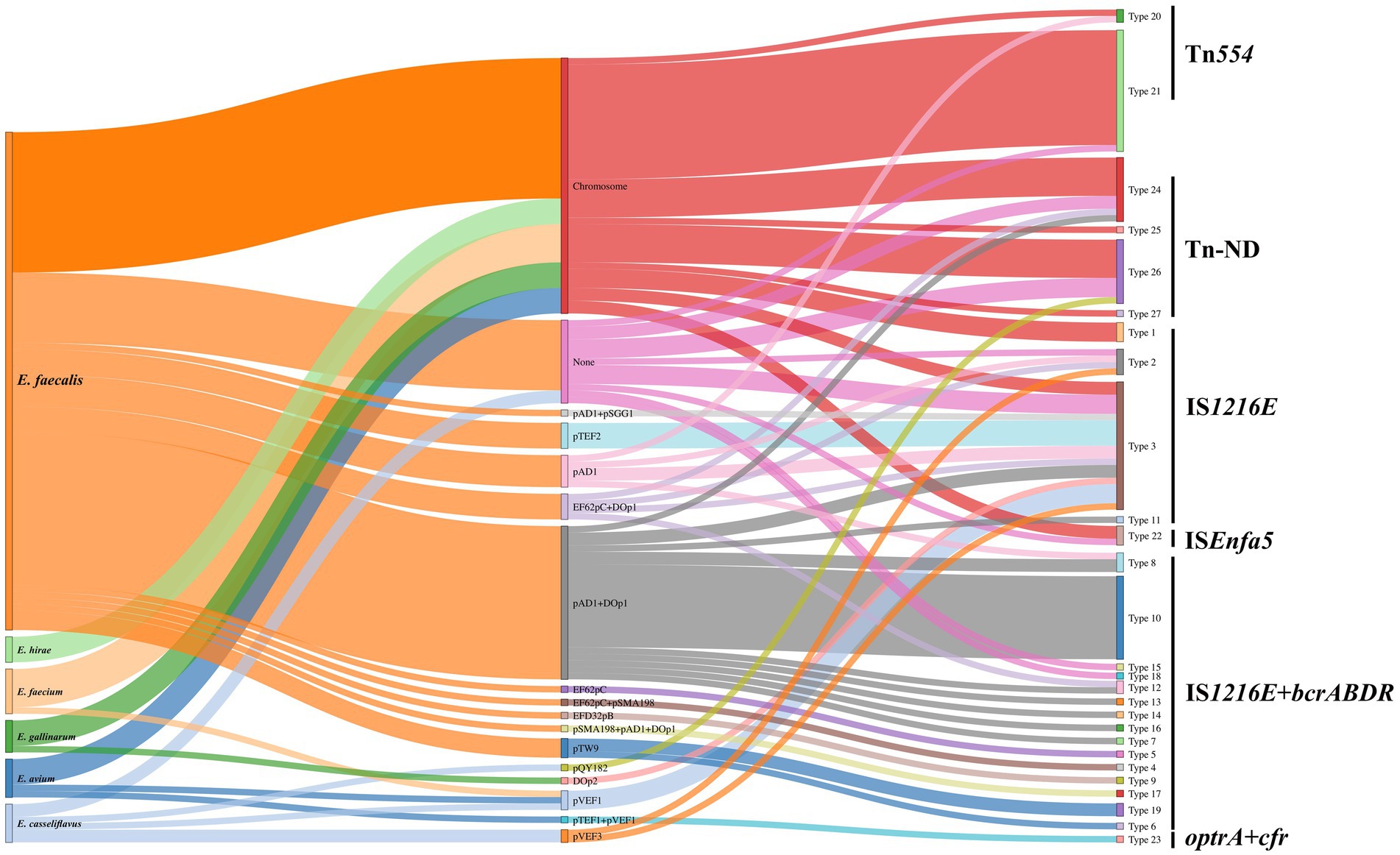
Figure 6. Sankey diagram displaying the flow of enterococcal species, plasmid types, and genetic environment of optrA. None indicated no known replicons were detected. Tn-ND indicated transposons were not identified in the genetic environment of optrA.
4 DiscussionTo date, most studies have focused on OPEs from animals or the environment, with few studies systematically exploring these isolates in healthy humans. The prevalence of optrA in a community population in Shenzhen (18.1%) (Xiang et al., 2022) was higher than that in a healthy population in Switzerland (3.8%) (Nuesch-Inderbinen et al., 2022), but similar to that in Hangzhou, China in 2022 (19.3%) (Shen et al., 2022). According to the China Antimicrobial Surveillance Network (CHINET), the prevalence of clinical LRE was 0.92% (1.9% for E. faecalis and 0.2% for E. faecium) involving 44 Chinese hospitals in 2018 (Fupin et al., 2020), and increased to 1.9% (3.8% for E. faecalis and 0.4% for E. faecium) involving 73 hospitals in 2023 (Fupin et al., 2023). However, the LRE carriage in the community population in this study reached 6.01% (34/565, 2018–2019), indicating that the prevalence of LRE in the Chinese healthy population might be underestimated. OPEs exhibited high resistance rates to linezolid, florfenicol, doxycycline, and erythromycin, with corresponding ARGs like fexA (96.08%), cat (45.10%), tet(M) (95.10%), erm(A) (61.76%), and erm(B) (86.27%) also showing high detection rates. Similar results - as the higher resistance rates in the optrA-positive group - were displayed for multiple types of farm samples in Vietnam (Ha et al., 2023). Our results indicated that not only high resistance rates but also a larger number of ARGs were presented in OPEs, suggesting that OPEs poses a higher risk to public health. Our previous study revealed that higher daily consumption of pork and hospitalization within 3 months were associated with a higher risk of optrA carriage in these healthy population (Xiang et al., 2022). Despite the heterogeneous population structure observed in optrA-positive E. faecalis in the community, high homology was observed in optrA-positive E. faecalis isolates between from the community and other sources (clinics, pigs, chickens, pets and environment), suggesting that multiple sources, including animals and clinics, contribute to the human optrA carriage.
Unlike studies on the presence of optrA in diverse ecological niches (Schwarz et al., 2021), comprehensive studies on optrA-bearing plasmids are still lacking. Diverse types of optrA-carrying plasmids were identified in this study, with the majority found in E. faecalis. Mostly optrA-carrying plasmids in E. faecalis carried the rep9 replicon, which belongs to the repA_N replicon family and is considered specific for E. faecalis (Mikalsen et al., 2015). The repA_N replicon is the most prevalent plasmid family in Gram-positive bacteria and belongs to narrow host range plasmids (Weaver et al., 2009). The rep9 family plasmids contained multiple characterized sex-pheromone response plasmids such as pAD1. The sex-pheromone based conjugation systems were known as very efficient transfer vehicles and could promote the transfer of ARGs (Jensen et al., 2010). All these observations might explain the high detection rate of rep9 replicons in optrA-positive E. faecalis in present study. Moreover, pAD1(rep9 family) + DOp1-type optrA-carrying plasmids were found to be the most prevalent in the present study and were detected exclusively in E. faecalis, which was in agreement with the results of Nuesch-Inderbinen et al., who identified optrA-carrying plasmids in florfenicol-resistant E. faecalis isolated from beef cattle (Nuesch-Inderbinen et al., 2023). Moreover, the pAD1 + DOp1 optrA-carrying plasmids were highly similarly to those from various sources deposited in the NCBI database. Given that the role of pAD1 + DOp1-type plasmids in helping the spread of optrA has not been reported, further investigation into the cross-host transmission of this plasmid type is warranted. The repUS1-containing optrA-carrying plasmid were widely detected in other enterococcal species except E. faecalis, especially pTEF1 + pVEF1(repUS1 family)-type plasmid pEAM528p carrying two linezolid resistance genes cfr and optrA. The repUS1 family plasmids belong to broad host Inc18 plasmid, which has been detected in a wide range of Gram-positive bacteria and was proved to transmit resistance across species, played an important role in ARGs spreading (Jensen et al., 2010; Wardal et al., 2022). In this study, it was found that six repUS1-containing plasmids were identified novel optrA-carrying plasmids, indicating that repUS1-containing optrA-carrying plasmids in other enterococcal species except E. faecalis need more research. Notably, multiple types of optrA-carrying plasmids showed that ARGs co-located with optrA accounted for more than 50% of the total ARGs carried by the isolates (83.87% for pAD1 + DOp1-type plasmids), indicating that with the acquisition of an optrA-carrying plasmid, other ARGs are likely to be co-acquired. It suggested that the acquisition of optrA-carrying plasmids will make humans more vulnerable to antimicrobial resistance, especially MLSB (erm(A) and erm(B)), tetracycline (tet(M) and tet(L)), aminoglycoside (aph(3′)-III), bacitracin (bcrABDR), trimethoprim (dfrG), florfenicol (fexA and cat) and other antimicrobial resistance.
In this study, optrA was surrounded by several MGEs, in which IS1216E appeared most frequently and was consistent with the previously reported structures including IS1216E-fexA-optrA-erm(A)-IS1216E, IS1216E-optrA-erm(A)-IS1216E and IS1216E-fexA-optrA-IS1216E (Schwarz et al., 2021). In addition, ISEnfa5, linked to cfr (Morroni et al., 2018), may be related to optrA as well, as the similar genetic structure was only identified in the study of Weiyi Shen et al. (Shen et al., 2024). In agreement to a previous study (Wu et al., 2019), Tn554-related genetic environments of optrA were predominantly located on chromosomes. Notably, optrA was often co-located with the bacitracin resistance gene cluster bcrABDR as a form of ISEnfa1-bcrABDR-ISEnfa1 or IS1216E-bcrABDR-IS1216E, both of which were located either up or downstream of optrA, and the most commonly observed structure was ISEnfa1-IS1216E-fexA-optrA-ISEnfa1-bcrABDR-ISEnfa1 in present study (Figure 4A, Type 10). This structure was also observed in the porcine enterococcal plasmid pE035 (Hao et al., 2019). The transfer of bcrABDR has previously been identified to be possibly related to ISEnfa1 and IS1216E, especially ISEnfa1 (Matos et al., 2009; Wang et al., 2015; Chen et al., 2016; Huang et al., 2019; Xuan et al., 2023). As ISEnfa1 or IS1216E were interspersed between optrA and bcrABDR in various forms in our study, and considering the high homology of IS1216E and ISEnfa1 (94% aa similar), it is reasonable to speculate that IS1216E and ISEnfa1 can mediate the transfer of optrA, bcrABDR or both in various forms. Bacitracin was widely used as a growth promoter and prophylactic agent in livestock, but generally used to treat local infections caused by Gram-positive bacteria and for occasional oral administration in human clinics (Wang et al., 2015; Tran et al., 2015). The consumption of bacitracin, as a growth promoter, was 1,352 tons and accounted for 4.12% of the total antimicrobial use in animals in China, 2020 (Ministry of Agriculture, 2021). Previous studies have characterized the acquisition of bcrABDR encoding high-level bacitracin resistance in E. faecium and E. faecalis (Wang et al., 2015; Manson et al., 2004). Thus, the extensive use of this antimicrobial agent in animals might have played a role as a vital driver for the co-transfer and prevalence of optrA and bcrABDR in human enterococci. It also suggested that the optrA is likely to spread from livestock to the human gut through the food chain. Moreover, IS1216E+bcrABDR-related genetic contexts of optrA could be located on a variety of plasmids and detected not only in E. faecalis but also in E. casseliflavus, which again highlighted the need to pay attention to the risk of the spread of optrA in the food chain. Moreover, phenicol resistance gene fexA and MLSB resistance gene erm(A) were also frequently identified IS1216E-related genetic contexts of optrA, which was similar to previous study (Ha et al., 2023; He et al., 2016). The widespread use of florfenicol and MLSB in animals could also contribute to the spread of optrA in the food chain.
In summary, the high prevalence of multi-drug resistant OPEs in the human intestinal flora indicated that community populations serve as a significant reservoir of optrA. OPEs showed high resistance rates to linezolid, florfenicol, doxycycline and erythromycin and corresponding ARGs including fexA, cat, tet(M), erm(A) and erm(B) also showed high detection rates. Antimicrobial resistance and presence of ARGs were higher in OPEs than that in ONEs. Specifically, E. faecalis poses a high risk for the spread of optrA in human communities, with larger numbers of ARGs and harboring most types of optrA-carrying plasmids. The rep9-containing optrA-carrying plasmids were often and only detected in E. faecalis. Meanwhile, repUS1-containing optrA-carrying plasmid were widely detected in other enterococcal species. Most ARGs harbored by OPEs were identified on optrA-carrying plasmids, suggesting that the acquisition of optrA-carrying plasmids will make humans more vulnerable to antimicrobial resistance, thereby posing a greater threat to public health. Notably, the pAD1(rep9a_1_repA) + DOp1-type optrA-carrying plasmids should receive more attention for the transfer of optrA given their high prevalence (24/66, 36.36%), high number of co-located ARGs with optrA (83.87% of total ARGs) and presence in multiple sources. The mobility of optrA was mainly associated with multiple MGEs, including IS1216E, ISEnfa1, ISEnfa5 and Tn6674. Tn6674 are primarily located on chromosome but IS1216E or ISEnfa1 are mostly located on the plasmids. The bcrABDR gene cluster, conferring resistance to the animal growth promoter bacitracin, was firstly frequently identified surrounding optrA in present study. It underscores the pressing need to closely monitor and mitigate the risk of optrA dissemination in the food chain. Given that linezolid is a last-resort antimicrobial agent for treating serious infections caused by Gram-positive bacteria, community-acquired optrA-positive enterococci and optrA-carrying plasmids and other origins, especially the optrA-positive E. faecalis and pAD1 + DOp1-type plasmids, should be incorporated into public health monitoring programs.
Data availability statementThe genomic sequences obtained in this study have been deposited in NCBI Genbank database under the BioProject accession number, PRJNA1159101 (https://www.ncbi.nlm.nih.gov/bioproject/PRJNA1159101).
Ethics statementThe studies involving humans were approved by the ethics committee of the Shenzhen CDC (Ethical approval number: R2018021). The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants and participants’ legal guardians/next of kin.
Author contributionsYF: Conceptualization, Data curation, Investigation, Methodology, Visualization, Writing – original draft, Writing – review & editing. ZD: Methodology, Validation, Writing – original draft, Writing – review & editing. YS: Conceptualization, Formal analysis, Methodology, Writing – review & editing. WW: Resources, Writing – review & editing. QX: Investigation, Methodology, Writing – original draft. ZL: Investigation, Writing – review & editing. KH: Resources, Writing – review & editing. SH: Project administration, Supervision, Writing – review & editing. ZL: Investigation, Writing – review & editing. TC: Investigation, Writing – review & editing. CP: Investigation, Writing – review & editing. RZ: Formal analysis, Methodology, Writing – review & editing. XZ: Funding acquisition, Supervision, Writing – review & editing. JS: Supervision, Writing – review & editing. SS: Validation, Writing – review & editing. YW: Conceptualization, Writing – review & editing. DL: Conceptualization, Validation, Writing – review & editing. ZL: Conceptualization, Data curation, Funding acquisition, Project administration, Writing – review & editing. YK: Funding acquisition, Project administration, Resources, Supervision, Writing – review & editing.
FundingThe author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This research was funded by Sanming Project of Medicine in Shenzhen [SZSM202011008], Shenzhen Science and Technology Program [JCYJ20210324124201004], Shenzhen Key Medical Discipline Construction Fund [SZXK066], Shenzhen Science and Technology Program [JSGG20220606141800001].
AcknowledgmentsThe authors thank Sanming Project of Medicine in Shenzhen [SZSM201611068] for providing research samples used in this study.
Conflict of interestThe authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI StatementThe authors declare that no Generative AI was used in the creation of this manuscript.
Publisher’s noteAll claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary materialThe Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmicb.2024.1505107/full#supplementary-material
ReferencesAlikhan, N. F., Petty, N. K., Ben Zakour, N. L., and Beatson, S. A. (2011). BLAST ring image generator (BRIG): simple prokaryote genome comparisons. BMC Genomics 12:402. doi: 10.1186/1471-2164-12-402
PubMed Abstract | Crossref Full Text | Google Scholar
Antonelli, A., D'Andrea, M. M., Brenciani, A., Galeotti, C. L., Morroni, G., Pollini, S., et al. (2018). Characterization of poxtA, a novel phenicol-oxazolidinone-tetracycline resistance gene from an MRSA of clinical origin. J. Antimicrob. Chemother. 73, 1763–1769. doi: 10.1093/jac/dky088
留言 (0)