Plantar fasciitis (PF) is the most common cause of inferior heel pain. The lifetime prevalence of PF has reached 10% in general population, and it is even higher among athletes, military members, obese people, and those with flatfoot or diabetes mellitus (1, 2). PF is characterized by pain exacerbated with the first walking in the morning or after a long period of rest (3). PF can lead to difficulties in standing, walking, sleeping, and in severe cases, to partial loss of walking function (4). The current treatments for PF often involve the use of physical therapy, orthotic devices, nonsteroidal anti-inflammatory drugs, local steroid injection, splinting and walking cast (5). However, PF has long disease course, slow recovery rate and susceptibility to recurrence (6), stirring up great interest among researchers in the study of the treatment for PF.
Myofascial trigger points (MTrPs) are palpable and hyperirritable nodules located in the taut bands of skeletal muscles (7). In the review by Vincenzo and colleagues (8), it was shown that MTrPs had abnormally contracted sarcomeres and formed contractile knots along the muscle fibers. The gaps between the contraction knots reveal the presence of microcracks and clefts in the endomysium. In addition, MTrPs contraction knots are surrounded by high concentrations of glycosaminoglycans, which are very hygroscopicous molecules, resulting in the trapping of toxic chemicals in the extracellular matrix of muscle tissue. Recently, dry needling (DN) based on MTrPs theory has been proved effective in relieving pain caused by muscle strain, including PF. Previously, five systematic reviews (SRs) (9–13) have indicated that DN exerted favorable effects on PF, however, they also addressed the insufficiency of evidence due to the poor design of the randomized controlled trials (RCTs) analyzed. Furthermore, high heterogeneity has been demonstrated across RCTs regarding to their study design, particularly, the controls. Moreover, when the treatment takes effects, a question that many clinicians are frequently asked about, is still uncertain. In addition, the safety issue of DN is a great concern to many. Therefore, the primary purpose of the current SR was to evaluate the effectiveness of DN on pain and functional outcomes in patients with PF, by carrying out meta-analysis on different control methods, and the second purpose was to investigate the effectiveness of DN on PF at different assessment time. Safety issues of DN treating PF were also discussed.
2 MethodsThis systematic review and meta-analysis was conducted in accordance with Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) (14).
2.1.Search strategyPubMed, Embase, the Cochrane Library, EBSCO, web of science, physiotherapy Evidence Database (PEDro) were searched from their inception to December 2022. Subject headings and free terms of plantar fasciitis and dry needling were used in combine in the search. The search was limited to RCTs without language restriction. Additionally, the reference list of the identified articles and relevant review were manually searched for more references.
2.2 Inclusion and exclusion criteriaStudy was included if (1) it was an RCT; (2) the participants were diagnosed with plantar fasciitis. The diagnostic criteria of PF were based on the clinical guidelines linked to the International Classification of Function, Disability and Health from the Orthopedic Section of the American Physical Therapy Association; (3) dry needling was used as the intervention. Study was excluded if full text cannot be obtained, or no available data was presented in the RCT.
2.3 Literature screening and data extractionTwo authors independently screened all titles and abstracts, and any disagreements were then resolved by a third author. Studies that satisfied the inclusion and exclusion criteria were retrieved for full-text assessment. Data extracted included basic information (authors, date of publication), subject information (age, gender, and sample size), intervention regimen (frequency and treatment sites of DN), risk of bias, outcome measures, and the follow-up time. The outcomes were extracted in the form of Mean ± standard deviation. Incomplete data were further researched by contacting the author.
2.4 Quality assessmentMethodologic Quality Criteria List, which was adapted from Cochrane handbook of reviews of interventions and recommended by the updated method guide for systematic reviews in the Cochrane Back and Neck Group, was applied by two reviewers to evaluate the validity of the included studies independently (15, 16). The evaluation outcomes of each research were presented as Yes, No or Unsure (if there were any unsatisfied results or major deficiencies), which represented Low Risk of Bias, High Risk of Bias and Uncertain Risk of Bias, respectively.
2.5 Data synthesis and statistical analysisPain relief measured by VAS or NPRS (Visual Analog Scale, Numerical Pain Rating Scale) and functional improvement measured by FFI (foot functional index) were used to evaluate the effectiveness of DN. Lower scores of VAS/NPRS and FFI indicate less severe pain and higher foot function, respectively. The scores of VAS and NPRS were synthesized, considering the similarity in the mechanism of the two scales (17). For different purposes, meta-analysis was conducted based on different outcomes (pain and foot function), control methods (DN vs. other treatments, DN + routine treatment vs. routine treatment alone), and assessment time (within 1 month, at 1 month and over 1 month).
Data were analyzed using RevMan 5.3 software which was provided by Cochrane Collaboration. Firstly, chi-square test was applied to examine the heterogeneity of the included studies, if p>0.10 and I2<50%, the studies were considered homogenous and a fixed-effect model was used. On the contrary, if p<0.10 and I2>50%, heterogeneity of the studies were considered high and random effect models was used (18). The effect sizes were measured using mean difference (MD) and 95% confidence interval (CI). if p ≤ 0.05, the difference were considered statistically significant; if p>0.05, the difference was considered not statistically significant.
3 Results 3.1 Study selectionA total of 238 studies were retrieved from the mentioned databases. After a strict screening process based on the inclusion and exclusion criteria, 12 studies involving 781 participants were eligible and included in current SR and meta-analysis (Figure 1) (19–30).
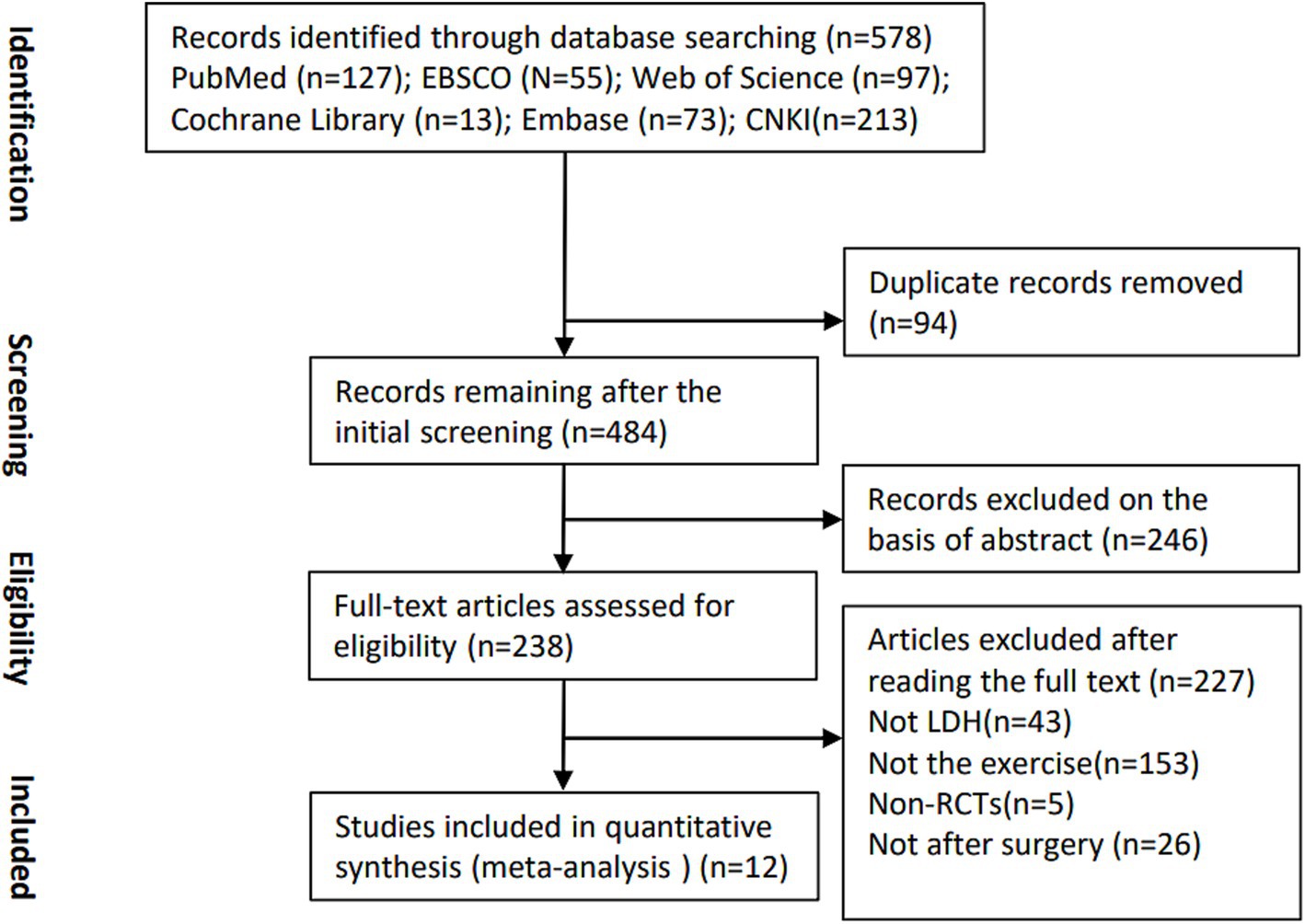
Figure 1. Eligibility of studies for inclusion in meta-analysis.
3.2 Study characteristicsThe study characteristics of the included RCTs were summarized and presented in Table 1. The publication dates of the included studies ranged from 2014 to 2022. The sample size varied from 20 to 111. Both acute and chronic cases were involved. Adverse events of DN were reported in 6 studies, including pain, bleeding, hematomas and bruising, all of which were self-limiting and easy to be managed. As for the selection of treatment sites, 3 studies used both plantar and gastrocnemius trigger points, 5 studies used only plantar trigger points, 2 study used only gastrocnemius trigger points, 1 study used gastrocnemius and soleus trigger points, and 1 study used soleus, gastrocnemius, quadratus plantae, flexor digitorum brevis and abductor hallucis trigger points according to the degree of pain. The frequency of treatment ranged from 1 to 2 times a week, and the patients received at least 3 sessions of treatment in total.
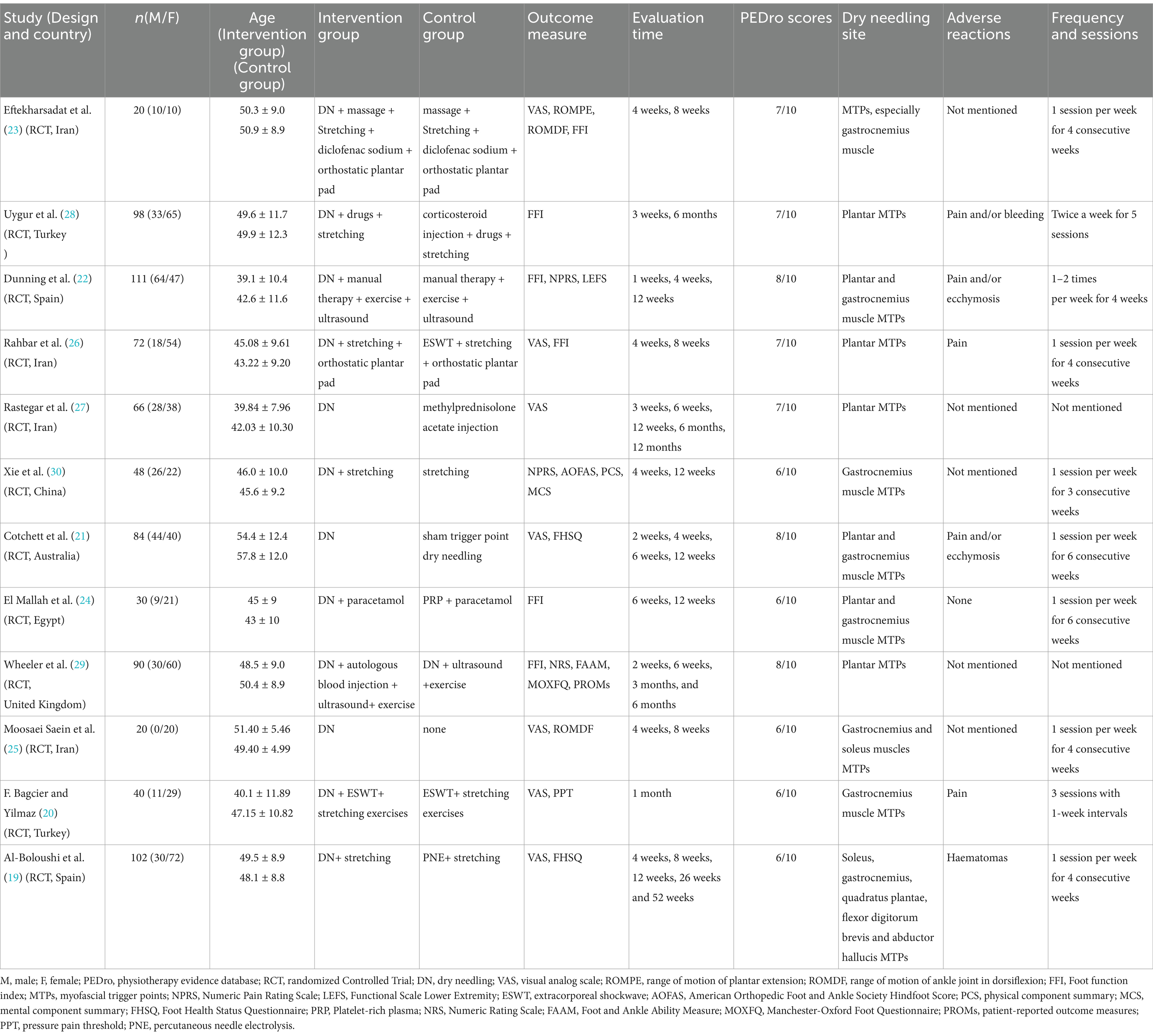
Table 1. Participant characteristics of studies included in this systematic review and meta-analysis.
3.3 Risk of bias within studiesThe risk-of-bias of the 12 RCTs were demonstrated in Figure 2. The risk of bias in random allocation were low in the studies. Nevertheless, the risks of bias in allocation concealment remained unclear in 5 studies. Blinding to patients was applied in 3 studies, and blinding to assessors was applied in 3 studies. Other risks of bias were low in these studies.
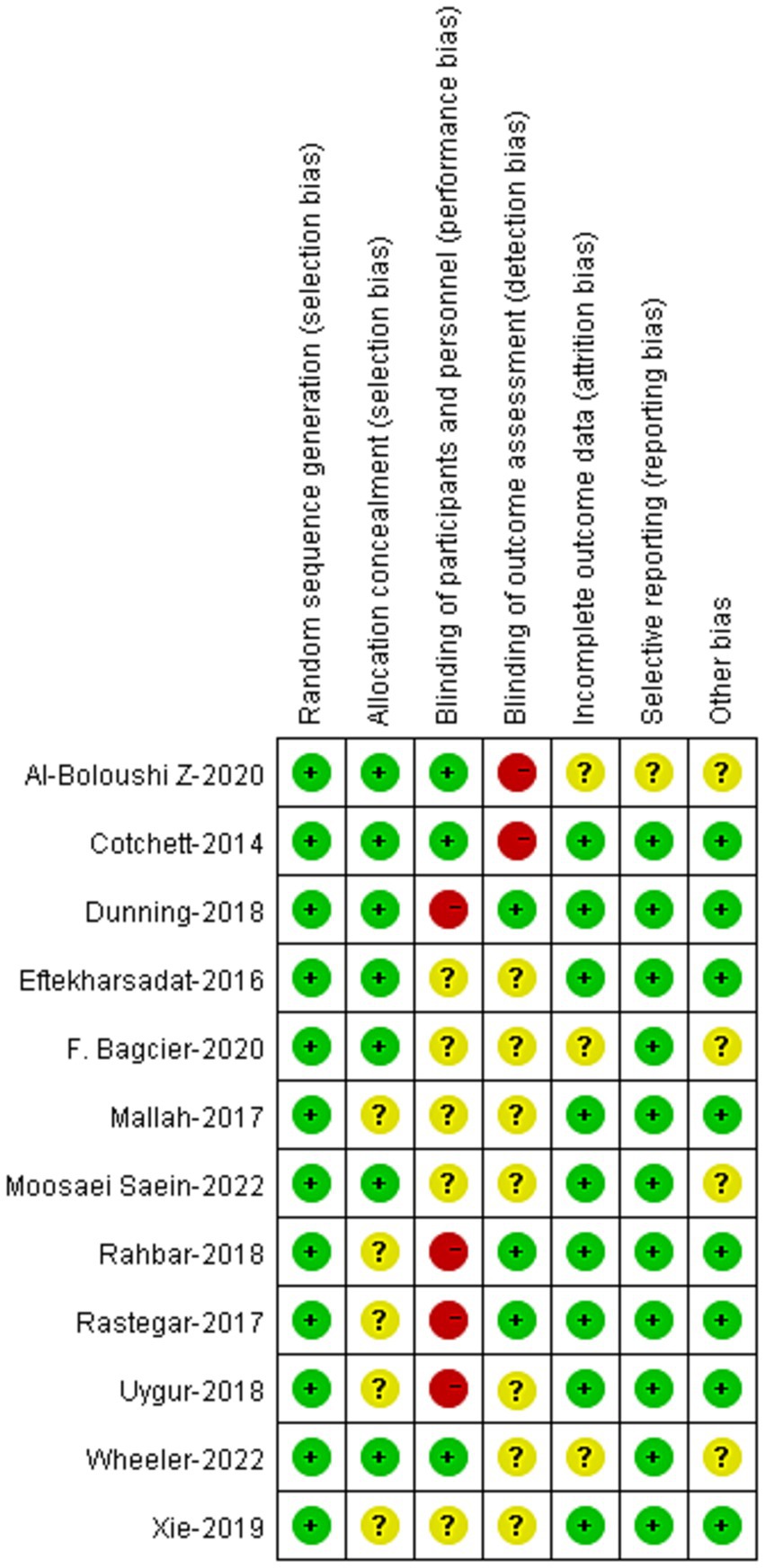
Figure 2. Risk of bias analysis of included studies.
3.4 Meta-analysis based on different control methods 3.4.1 Pain4 RCTs compared the effectiveness of DN + routine treatment vs. routine treatment alone on pain relieving (20, 22, 23, 30). Routine treatments were defined by the RCTs as muscle massage, stretching, exercise, and ultrasound therapy, etc. Meta-analysis demonstrated that patients who received DN + routine treatment had lower VAS/NPRS scores compared to those who received routine treatment alone, and the difference were statistically significant [95%CI (−2.12, −1.76), p < 0.0001] (Figure 3).
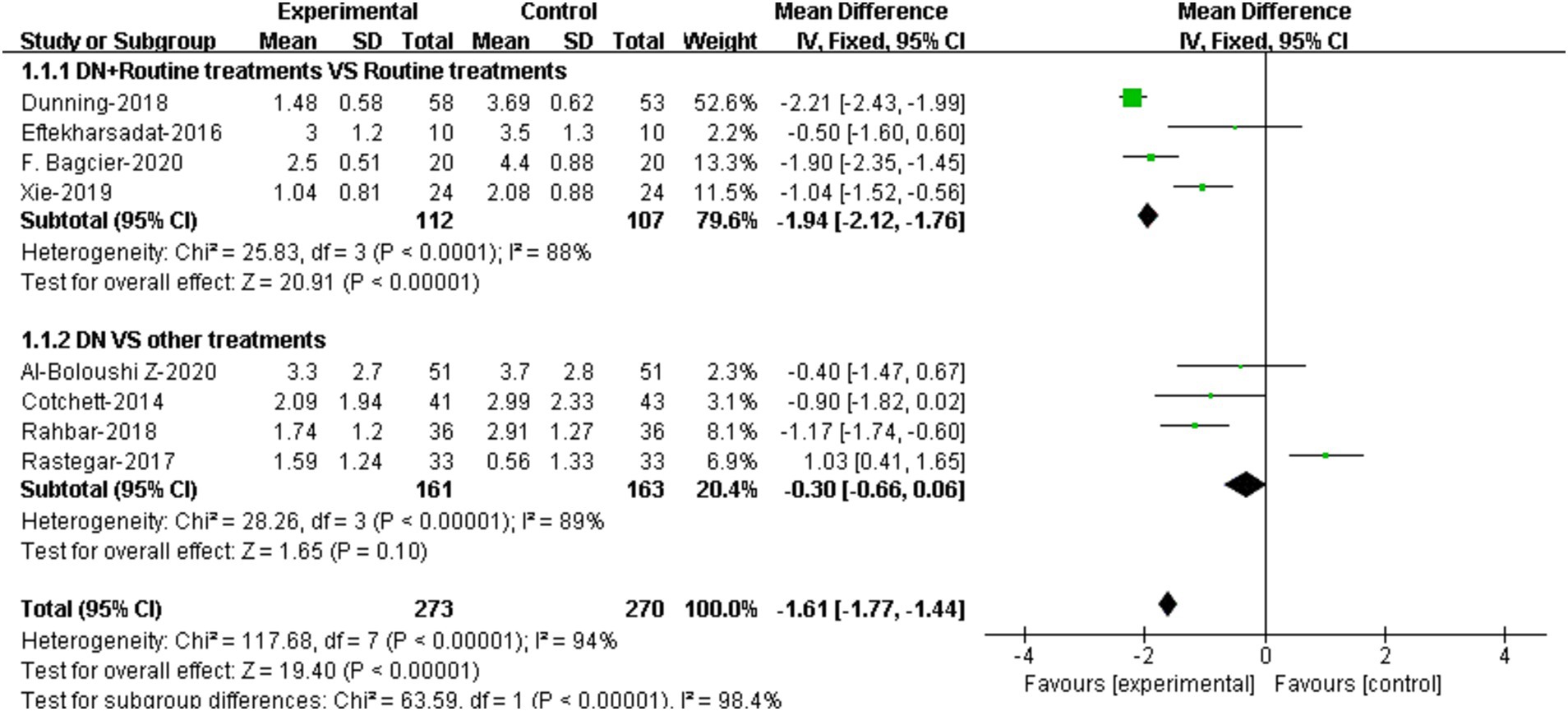
Figure 3. Forest plot illustrating the comparison of effectiveness on pain between DN + routine treatments vs. routine treatments alone, and between DN vs. other treatments.
The comparison of effectiveness of DN vs. other treatments on pain was reported in 4 RCTs (19, 21, 26, 27). Results showed that there was no significant difference in VAS/NPRS scores between DN group and other treatment group [95%CI (−0.66, 0.06), p = 0.10] (Figure 3).
3.4.2 Functional improvementThe comparisons of the effectiveness of DN + routine treatment vs. routine treatment alone on functional improvement were reported in 2 RCTs (22, 23). The results demonstrated that there was significant difference in FFI score between the two groups [95%CI (−12.57, −3.58), p = 0.004] (Figure 4).
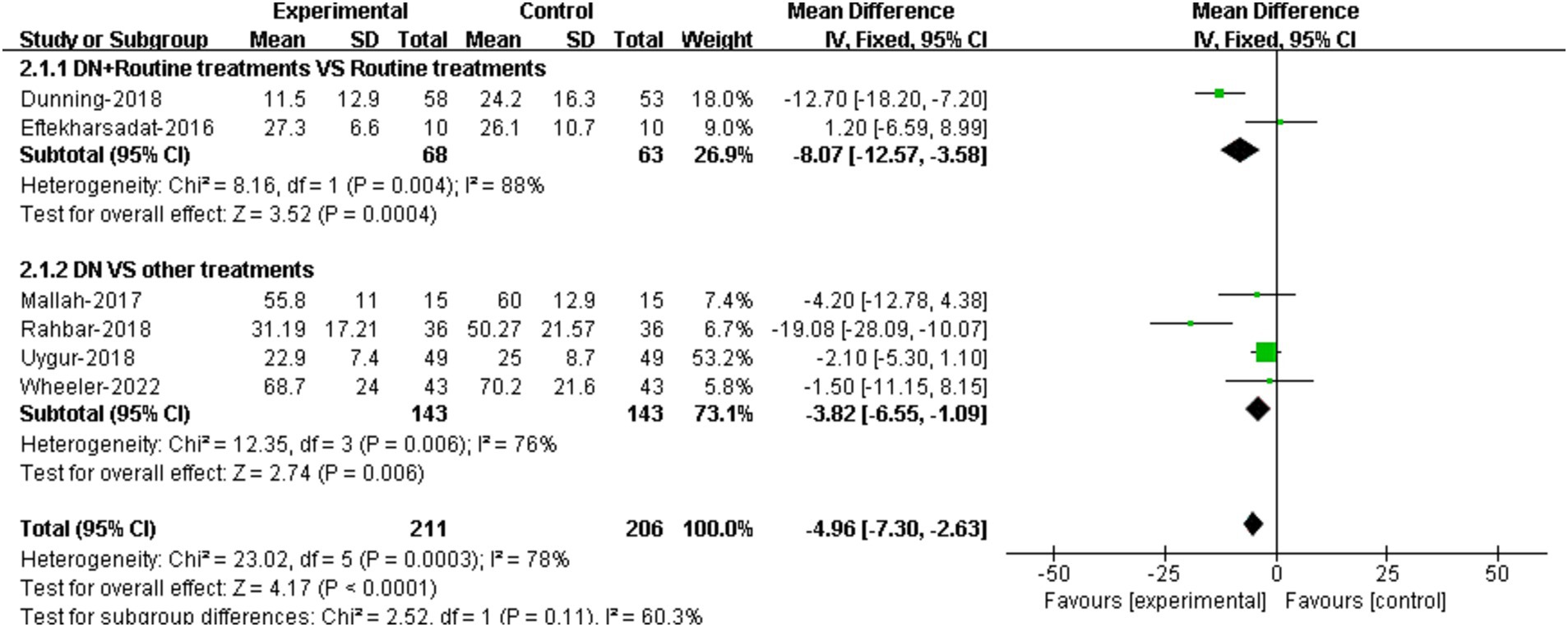
Figure 4. Forest plot illustrating the comparison of effectiveness on functional improvement between DN + routine treatments vs. routine treatments alone, and between DN vs. other treatments.
The comparisons of the effectiveness of DN vs. other treatments on functional improvement were reported in 4 RCTs (24, 26, 28, 29). The results suggested that DN improved patients’ foot function compared to other treatments. The difference in FFI scores between two groups was statistically significant [95%CI (−6.55, −1.09), p = 0.006] (Figure 4).
3.5 Meta-analysis based on different assessment time 3.5.1 Pain3 RCTs provided assessment data of pain within 1 month. (21, 22, 27). Meta-analysis showed that there was significant difference in VAS/NPRS scores within 1 month between the two groups [95%CI (−0.69, −0.32), p < 0.00001] (Figure 5).
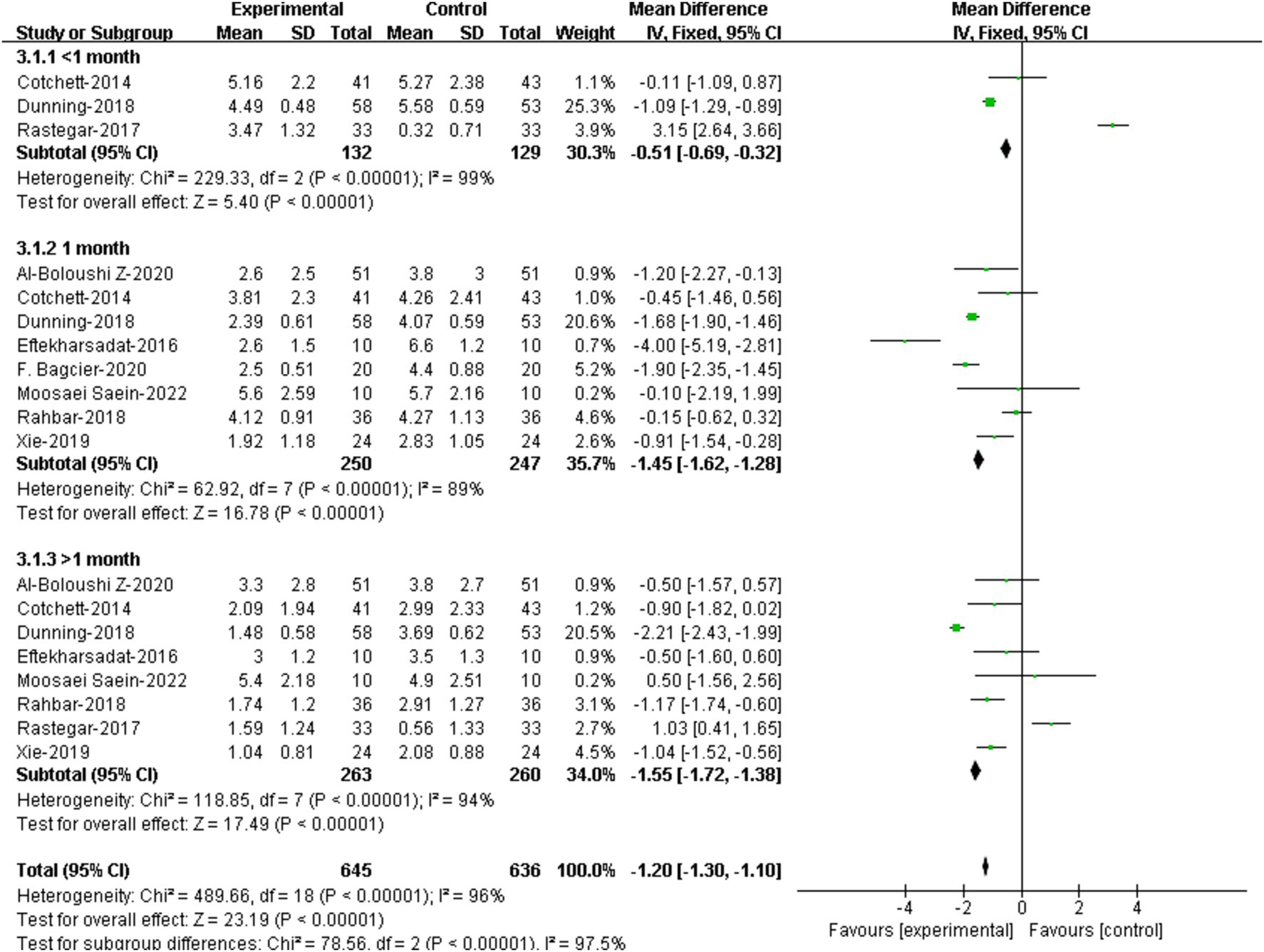
Figure 5. Forest plot illustrating the effectiveness of DN on pain at different assessment time.
8 RCTs assessed pain at 1 month (19–23, 25, 26, 30) Results showed that patients receiving DN had lower VAS/NPRS scores at 1 month compared to that of the controls, and the difference were statistically significant [95%CI (−1.62, −1.28), p < 0.00001] (Figure 5).
8 RCTs assessed pain at over 1 month (19, 21–23, 25–27, 30). Results showed that patients receiving DN had lower VAS/NPRS scores at over 1 month compared to that of the controls, and the difference were statistically significant [95%CI (−1.72, −1.38), p<0.0001] (Figure 5).
3.5.2 Functional improvement3 RCTs reported foot function assessment data within 1 month (22, 28, 29). Results showed that no significant difference was demonstrated between DN group and the control group [95%CI (−4.83, 0.59), p = 0.13] (Figure 6).
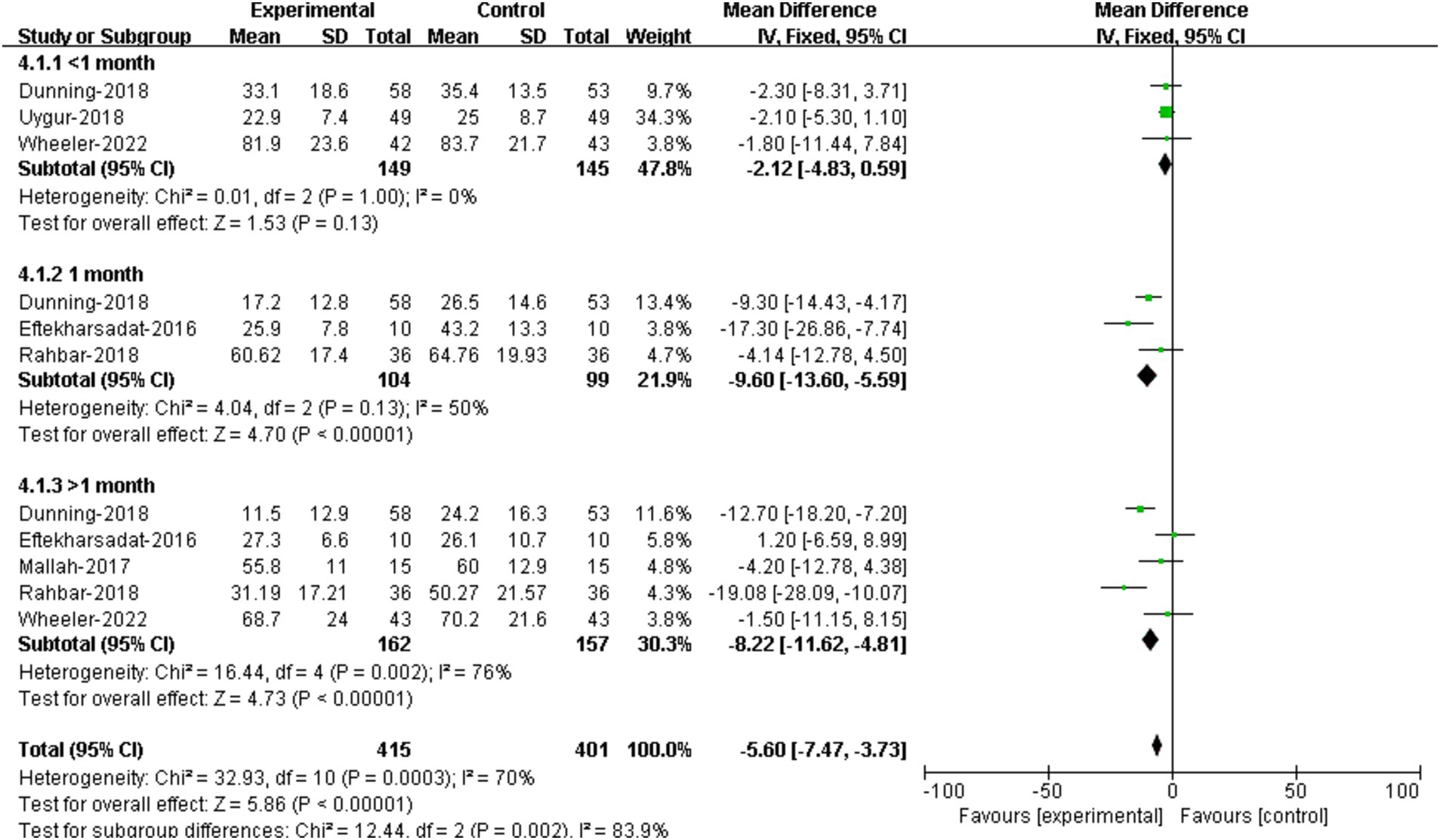
Figure 6. Forest plot illustrating the effectiveness of DN on functional improvement at different assessment time.
The comparison of treatment effectiveness on foot function at 1 month between DN and controls were conducted in 3 RCTs (22, 23, 26). The results suggested that patients receiving DN had lower FFI scores compared to that of the controls, and the difference was statistically significant [95%CI (−13.60, −5.59), p < 0.00001] (Figure 6).
5 RCTs provided assessment data of function improvement at over on month (22–24, 26, 29). The results showed that there was no significant difference in FFI scores at over 1 month between groups [95%CI (−11.62, −4.81), p < 0.00001] (Figure 6).
4 DiscussionIt is recognized that DN is capable of reducing inflammation of the plantar fascia, enhancing local blood circulation, and relieving tissue internal pressure, resulting in extensive use of DN to treat PF clinically (20). The current study evaluated the effectiveness of DN on pain relieving and functional improvement in patients with PF. To gain a more precise and comprehensive understanding of its effectiveness, we conducted meta-analysis based on different control methods and different assessment time.
12 RCTs involving 781 participants with plantar fasciitis were analyzed in our study. In the analysis based on different control methods, the comparison of effectiveness on foot function between DN + routine treatments vs. routine treatments alone did not demonstrate significant difference, but the result favored the use of DN + routine treatments. In all the other comparisons (DN + routine treatments vs. routine treatments on pain), results suggested that patients who received DN had significant improvement in pain and foot function. These results were in line with the previous SRs. However, there was no significant difference between the DN group and the other treatment group.
In the analysis based on different assessment time, current evidence indicated that DN exhibited no obvious advantage over other treatments within 1 month. But at 1 month and at over 1 month, patients receiving DN had lower VAS/NPRS and FFI scores compared to that of the controls. These results suggested that DN relieved heel pain and improved foot function in a time-dependent manner. Possible reasons for the absence of effectiveness of DN within 1 month may be: (1) needling itself can cause pain in the heel, which may make confusion with the pain caused by the disease and thus interfering with the pain assessment (31). (2) DN has a cumulative effect in treating PF, the mechanical effects of the needle induce a remodeling of the collagen fibers of the plantar fascia that requires several weeks. (3) The repeated movements of the back and forward and rotations of the needle release the myofascial trigger points of the intrinsic muscles of the foot modulating their perfusion. (4) The dry needling of the posterior compartment of the leg modulates the tension of the fascial elements (especially the deep fascia located in between the muscles and the subcutaneous fat tissue) which are in the histological continuum with the plantar fascia (32). In other words, it takes time for DN to exert significant benefits on PF patients (33).
Even though our study supports the use of DN in treating PF, optimal management of PF requires other composite measures, including weight control (34), gait correction (35), and wearing appropriate shoes (36), to maintain the effects of DN and prevent disease recurrence. Adverse events, mainly including pain and bruise, were reported in 6 RCTs (50%). DN is generally safe given the fact that these adverse events were considered to be mild and easy to recover without the need of special care. However, in order to ensure patient safety, ultrasound-guided dry needling therapy may be considered in future studies to reduce local bleeding caused by accidental vascular rupture and avoid hematoma.
Our study has several strengths. Firstly, we have updated the literature by including 8 latest RCTs, all of which were published in the past 5 years, in our study. Secondly, we performed meta-analysis based on different control methods and assessment time, which may provide more precise information as to the effectiveness of DN. Nevertheless, there are limitations in this study. Most included RCTs have high risks of selective bias due to the lack of allocation concealment and blinding to patients. On the other hand, the included RCT articles were highly heterogeneous. Firstly, the control group intervention measures adopted in different studies are very different, with significant heterogeneity. The differences were great in the treatment sites, frequency, and operation methods of DN in the 12 RCTs. Secondly, using only clinical features to select the patients with “plantar fasciitis” some atypical pathologies such as the heel fat pad syndrome (37) and the disruption of the plantar fascia (38) may be included in the sample of fasciitis. Moreover, most RCTs have small sample size. All these limitations may influence the power of our conclusion.
Despite its limitations, this systematic review and meta-analysis provided moderate evidence supporting the use of dry needling to relieve pain and restore function in patients with plantar fasciitis. Furthermore, dry needling may take at least 1 month to take effects in patients with plantar fasciitis. More multi-center RCTs with high-quality, large sample size are needed to further conform our conclusion.
Data availability statementThe original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author/s.
Author contributionsAY: Data curation, Writing – original draft, Writing – review & editing. RL: Data curation, Methodology, Software, Writing – review & editing. WX: Data curation, Writing – review & editing. HS: Data curation, Writing – review & editing. YH: Supervision, Writing – review & editing.
FundingThe author(s) declare that financial support was received for the research, authorship, and/or publication of this article.
Conflict of interestThe authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statementThe authors declare that no Generative AI was used in the creation of this manuscript.
Publisher’s noteAll claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
AbbreviationsDN, Dry Needling; PF, Plantar Fasciitis; RCT, Randomized Control Trials; VAS, Visual Analog Scale; NPRS, Numerical Pain Rating Scale; FFI, Foot Function Index; MTrPs, Myofascial Trigger Points; SR, Systematic Review; PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses; PEDro, Physiotherapy Evidence Database; MD, Mean Difference; CI, Confidence Interval
References1. Scher, DL, Belmont, PJ, Bear, R, Mountcastle, SB, Orr, JD, and Owens, BD. The incidence of plantar fasciitis in the United States military. J Bone Joint Surg Am. (2009) 91:2867–72. doi: 10.2106/jbjs.I.00257
PubMed Abstract | Crossref Full Text | Google Scholar
2. Shashua, A, Flechter, S, Avidan, L, Ofir, D, Melayev, A, and Kalichman, L. The effect of additional ankle and midfoot mobilizations on plantar fasciitis: a randomized controlled trial. J Orthop Sports Phys Ther. (2015) 45:265–72. doi: 10.2519/jospt.2015.5155
PubMed Abstract | Crossref Full Text | Google Scholar
4. Greve, C, Schuitema, D, Otten, B, van Kouwenhove, L, and Hijmans, JM. Biomechanical effects of rocker shoes on plantar aponeurosis strain in patients with plantar fasciitis and healthy controls. PLoS One. (2019) 14:e0222388. doi: 10.1371/journal.pone.0222388
PubMed Abstract | Crossref Full Text | Google Scholar
5. Luffy, L, Grosel, J, Thomas, R, and So, E. Plantar fasciitis: a review of treatments. JAAPA. (2017) 31:20–4. doi: 10.1097/01.JAA.0000527695.76041.99
Crossref Full Text | Google Scholar
6. Filippou, DK, Kalliakmanis, A, Triga, A, Rizos, S, and Shipkov, CD. Sport related plantar fasciitis. Current diagnostic and therapeutic advances. Folia Med. (2004) 46:56–60.
PubMed Abstract | Google Scholar
7. Sikdar, S, Shah, JP, Gilliams, E, Gebreab, T, and Gerber, LH. Assessment of myofascial trigger points (MTrPs): a new application of ultrasound imaging and vibration sonoelastography. Conf Proc IEEE Eng Med Biol Soc. (2008) 2008:5585–8. doi: 10.1109/iembs.2008.4650480
PubMed Abstract | Crossref Full Text | Google Scholar
8. Ricci, V, Ricci, C, Gervasoni, F, Cocco, G, Andreoli, A, and Özçakar, L. From Histoanatomy to sonography in myofascial pain syndrome: a EURO-MUSCULUS/USPRM approach. Am J Phys Med Rehabil. (2023) 102:92–7. doi: 10.1097/phm.0000000000001975
PubMed Abstract | Crossref Full Text | Google Scholar
9. Cotchett, MP, Landorf, KB, and Munteanu, SE. Effectiveness of dry needling and injections of myofascial trigger points associated with plantar heel pain: a systematic review. J Foot Ankle Res. (2010) 3:18. doi: 10.1186/1757-1146-3-18
PubMed Abstract | Crossref Full Text | Google Scholar
10. He, C, and Hua, M. Effectiveness of trigger point dry needling for plantar heel pain: a meta-analysis of seven randomized controlled trials. J Pain Res. (2017) 10:1933–42. doi: 10.2147/JPR.S141607
PubMed Abstract | Crossref Full Text | Google Scholar
11. Salvioli, S, Guidi, M, and Marcotulli, G. The effectiveness of conservative, non-pharmacological treatment for plantar heel pain: a systematic review with meta-analysis. Foot. (2017) 33:57–67. doi: 10.1016/j.foot.2017.05.004
Crossref Full Text | Google Scholar
12. Salehi, S, Shadmehr, A, Olyaee, G, Tajali, SB, and Mir, SM. Effectiveness of dry needling for the Management of Plantar Fasciitis: a review study. J Mod Rehabil. (2019) 2019:1–10. doi: 10.32598/JMR.13.1.1
Crossref Full Text | Google Scholar
13. al-Boloushi, Z, López-Royo, MP, Arian, M, Gómez-Trullén, EM, and Herrero, P. Minimally invasive non-surgical management of plantar fasciitis: A systematic review. J Bodyw Mov Ther. (2018) 23:122–37. doi: 10.1016/j.jbmt.2018.05.002
PubMed Abstract | Crossref Full Text | Google Scholar
14. Moher, D, Liberati, A, Tetzlaff, J, and Altman, DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. (2009) 6:e1000097. doi: 10.1371/journal.pmed.1000097
PubMed Abstract | Crossref Full Text | Google Scholar
15. Furlan, AD, Malmivaara, A, Chou, R, Maher, CG, Deyo, RA, Schoene, M, et al. 2015 updated method guideline for systematic reviews in the Cochrane Back and neck group. Spine. (2015) 40:1660–73. doi: 10.1097/BRS.0000000000001061
PubMed Abstract | Crossref Full Text | Google Scholar
16. Tarsilla, M. Cochrane handbook for systematic reviews of interventions. J Multidiscip Eval. (2008) 6:142–8. doi: 10.56645/jmde.v6i14.284
Crossref Full Text | Google Scholar
17. Bijur, PE. Validation of a verbally administered numerical rating scale of acute pain for use in the emergency department. Acad Emerg Med. (2003) 10:390–2. doi: 10.1197/aemj.10.4.390
PubMed Abstract | Crossref Full Text | Google Scholar
18. Deeks, JJ, and Altman, DG. Analysing data and undertaking meta-analyses In: JP Higgins and S Green, editors. Cochrane Handbook for Systematic Reviews of Interventions Cochrane Book. London: Cochrane Collaboration (2011)
19. Al-Boloushi, Z, Gómez-Trullén, EM, Arian, M, Fernández, D, Herrero, P, and Bellosta-López, P. Comparing two dry needling interventions for plantar heel pain: a randomised controlled trial. BMJ Open. (2020) 10:e038033. doi: 10.1136/bmjopen-2020-038033
PubMed Abstract | Crossref Full Text | Google Scholar
20. Bagcier, F, and Yilmaz, N. The impact of extracorporeal shock wave therapy and dry needling combination on pain and functionality in the patients diagnosed with plantar fasciitis. J Foot Ankle Surg. (2020) 59:689–93. doi: 10.1053/j.jfas.2019.09.038
Crossref Full Text | Google Scholar
21. Cotchett, MP, Munteanu, SE, and Landorf, KB. Effectiveness of trigger point dry needling for plantar heel pain: a randomized controlled trial. J Foot Ankle Res. (2014) 94:1083–94. doi: 10.2522/ptj.20130255
Crossref Full Text | Google Scholar
22. Dunning, J, Butts, R, Henry, N, Mourad, F, Brannon, A, Rodriguez, H, et al. Electrical dry needling as an adjunct to exercise, manual therapy and ultrasound for plantar fasciitis: a multi-center randomized clinical trial. PLoS One. (2018) 13:e0205405. doi: 10.1371/journal.pone.0205405
PubMed Abstract | Crossref Full Text | Google Scholar
23. Eftekharsadat, B, Babaei-Ghazani, A, and Zeinolabedinzadeh, V. Dry needling in patients with chronic heel pain due to plantar fasciitis: a single-blinded randomized clinical trial. Med J Islam Repub Iran. (2016) 30:401.
PubMed Abstract | Google Scholar
24. El Mallah, EEM, Elattar, E, and Zidan, H. Platelet-rich plasma versus dry needling of myofascial meridian trigger points in the treatment of plantar fasciitis. Egypt Rheumatol Rehabil. (2017) 44:58–68.
25. Moosaei Saein, A, Safavi-Farokhi, Z, Aminianfar, A, and Mortezanejad, M. The effect of dry needling on pain, range of motion of ankle joint, and Ultrasonographic changes of plantar fascia in patients with plantar fasciitis. J Sport Rehabil. (2022) 31:299–304. doi: 10.1123/jsr.2021-0156
PubMed Abstract | Crossref Full Text | Google Scholar
26. Rahbar, M, Eslamian, F, Toopchizadeh, V, Aminabad, F, Kargar, A, and Dolatkhah, N. A comparison of the efficacy of dry-needling and extracorporeal shockwave therapy for plantar fasciitis: a randomized clinical trial. Iran Red Crescent Med J Press. (2018) 2018:908. doi: 10.5812/ircmj.68908
Crossref Full Text | Google Scholar
27. Rastegar, S, Mahdavi, SB, Hoseinzadeh, B, and Badiei, S. Comparison of dry needling and steroid injection in the treatment of plantar fasciitis: a single-blind randomized clinical trial. Int Orthop. (2017) 42:109–16. doi: 10.1007/s00264-017-3681-1
Crossref Full Text | Google Scholar
28. Uygur, E, Aktaş, B, Eceviz, E, Yilmazoğlu, EG, and Poyanli, O. Preliminary report on the role of dry needling versus corticosteroid injection, an effective treatment method for plantar fasciitis: a randomized controlled trial. J Foot Ankle Surg. (2019) 58:301–5. doi: 10.1053/j.jfas.2018.08.058
PubMed Abstract | Crossref Full Text | Google Scholar
29. Wheeler, PC, Dudson, C, Gregory, KM, Singh, H, and Boyd, KT. Autologous blood injection with dry-needling vs dry-needling alone treatment for chronic plantar fasciitis: a randomized controlled trial. Foot Ankle Int. (2022) 43:646–57. doi: 10.1177/10711007211061365
PubMed Abstract | Crossref Full Text | Google Scholar
30. Xie, N, Shen, N, Cong, X, and Zheng, Y. Ultrasound-guided dry needling for myofascial trigger points in treatment of plantar fasciitis. Chin J Med Imag Technol. (2019) 38:1128–32.
31. Martín-Pintado-Zugasti, A, Del Moral, OM, Gerwin, RD, and Fernández-Carnero, J. Postneedling soreness after myofascial trigger point dry needling: current status and future research. J Bodyw Mov Ther. (2018) 22:941–6. doi: 10.1016/j.jbmt.2018.01.003
Crossref Full Text | Google Scholar
33. Cagnie, B, Dewitte, V, Barbe, T, Timmermans, F, Delrue, N, and Meeus, M. Physiologic effects of dry needling. Curr Pain Headache Rep. (2013) 17:348. doi: 10.1007/s11916-013-0348-5
PubMed Abstract | Crossref Full Text | Google Scholar
35. Glazer, LJ. An approach to the diagnosis and treatment of plantar fasciitis. Phys Sportsmed. (2009) 37:74–9. doi: 10.3810/psm.2009.06.1712
Crossref Full Text | Google Scholar
36. van Leeuwen, KD, Rogers, J, Winzenberg, T, and van Middelkoop, M. Higher body mass index is associated with plantar fasciopathy/'plantar fasciitis': systematic review and meta-analysis of various clinical and imaging risk factors. Br J Sports Med. (2016) 50:972–81. doi: 10.1136/bjsports-2015-094695
PubMed Abstract | Crossref Full Text | Google Scholar
37. Ricci, V, Abdulsalam, AJ, and Özçakar, L. From plantar fasciitis to heel fat pad syndrome: sonographic kaleidoscope for heel pain. Am J Phys Med Rehabil. (2024) 103:e172–3. doi: 10.1097/phm.0000000000002518
PubMed Abstract | Crossref Full Text | Google Scholar
38. Cocco, G, Ricci, V, Boccatonda, A, Abate, M, Guagnano, MT, and Schiavone, C. Ultrasound follow-up of spontaneous tears of the plantar fascia treated with conservative therapies: two case reports. Medicine (Baltimore). (2019) 98:e18428. doi: 10.1097/md.0000000000018428
留言 (0)