Age-related macular degeneration (AMD) is a degenerative retinal disease affecting the macula, leading to progressive central vision loss and is the leading cause of irreversible blindness in people aged 50 and older (Steinmetz et al., 2021). The pathogenesis of AMD is multifactorial, involving environmental and genetic factors. Several early changes are associated with aging, a major risk factor for AMD. Environmental risk factors include sunlight exposure, cigarette smoke and oxidative stress linked to age and diet (Evans, 2005; Jonasson et al., 2014; Lambert et al., 2016; Schick et al., 2016).
Sunlight, specifically the wavelengths associated with ultraviolet (UV) and blue light, impacts the physiology of retinal cells and is, to some extent, comparable to the photo-aging of the skin (Parkinson et al., 2015). An important distinction is that retinal cells may be affected by certain wavelengths of sunlight, such as blue light (400–500 nm), since most of the radiation in the UV range is absorbed by the cornea (100–315 nm) and lens (315–400 nm), thereby helping to protect the retina from UV-induced photo-oxidative damage (Behar-Cohen et al., 2011; Mallet and Rochette, 2011, 2013).
In recent years, there have been growing concerns about the long-term effects of artificial light exposure from light emitting diodes (LEDs) and modern electronic devices which emit a high proportion of their light in the blue wavelength (Behar-Cohen et al., 2011; Wong and Bahmani, 2022). Research in the field indicates that chronic exposure to blue light in the range of 400–490 nm may affect the function of photoreceptor and RPE due to its high energy, and contribute to the pathogenesis of AMD (Roehlecke et al., 2009; Lin et al., 2017; Gea et al., 2018; Baker et al., 2022; Françon et al., 2024). Nevertheless, evidence from clinical studies remains inconclusive and a definitive link between blue light and retinal damage has yet to be established.
Blue light: a risk factor for AMD?Estimating the link between sunlight or blue light exposure and AMD risk is challenging due to inconsistent results from population-based studies and meta-analyses of the epidemiological literature (Cruickshanks et al., 2001; Tomany, 2004; Sui et al., 2013; Schick et al., 2016; Zhou et al., 2018; Achiron et al., 2021). The Beaver Dam Eye Study found a possible association between sunlight exposure and increased risk of retinal pigmented epithelium (RPE) abnormalities and early AMD (Cruickshanks et al., 2001; Tomany, 2004). The Chesapeake Bay study reported that AMD patients with extensive geographic atrophy had significantly higher exposure to blue light over the preceding 20 years, compared to age-matched controls (Taylor et al., 1990). The authors combined personal exposure histories with laboratory investigations and field measurements to determine ocular exposure to sunlight. Published ambient data on intensity and spectral distribution of visible light was used to calculate the yearly ocular exposure to blue light for each individual. In the European Eye Study (EUREYE), blue light exposure was estimated by combining meteorologic and questionnaire data regarding outdoor exposure. No link was found between blue light exposure and neovascular or early AMD. However, significant associations were found between blue light exposure and wet AMD in participants with low levels of antioxidants, specifically dietary zinc and zeaxanthin, vitamins C and E in blood. These nutrients are known to work synergistically to protect the retina from light-induced oxidative damage (Thomson et al., 2002; Wrona et al., 2003, 2004). This study emphasizes the complexity of these associations, and the need for further research in this direction (Fletcher, 2008).
Unlike sunlight, artificial lighting contains a fixed spectral distribution that peaks in the blue portion of the electromagnetic spectrum, and the long-term impact of chronic exposure to artificial light on retinal health is unclear (Contín et al., 2016; Wong and Bahmani, 2022). A recent case–control study conducted using nationwide population-based data in South Korea found that artificial light exposure at night significantly increased the risk of developing exudative AMD (Kim et al., 2024). Excessive blue light exposure consistently damages photoreceptors and RPE in culture and animal models, as detailed in the following two sections. However, this correlation has not yet been fully validated in human studies and remains an area of active research.
Effect of blue light on photoreceptorsPhotoreceptors are specialized sensory cells in the retina and interact with the RPE to convert incoming light into neural signals, a process known as phototransduction (Molday and Moritz, 2015). Photoreceptor health is crucial for vision. Light-induced damage is classified into two types based on total dose, which includes irradiance (mW/cm2) and exposure duration: Class I, or Noell damage, predominantly studied in rats but also observed in primates and other species, occurs with longer exposures (>1.5 h) and lower irradiance (<1 mW/cm2), and primarily affects photoreceptors, although RPE damage can occur with extended exposure (>8 days) (Noell et al., 1966; Noell, 1980; Hunter et al., 2012). Class II, or Ham damage, predominantly studied in primates but also observed in rats and other species, results from shorter exposures (<5 h) at much higher irradiances (>10 mW/cm2), and mainly affects the RPE (Ham et al., 1979; Ham, 1983; Hunter et al., 2012; Cougnard-Gregoire et al., 2023; Youssef et al., 2024; Zhang et al., 2024).
In rats, blue light damages photoreceptors through several cellular mechanisms, including reactive oxygen species (ROS) production, mitochondrial damage, and apoptosis (Busch et al., 1999; Theruveethi et al., 2022). In primates, blue light caused significantly more damage to the RPE and cone outer segments compared to longer wavelengths (Zhang et al., 2024). In rats,12 h/day of blue light for 28 days significantly disrupted the outer retinal structure, notably reducing photoreceptor nuclei, damaging their outer segments, and disrupting the outer plexiform layers (Theruveethi et al., 2022). Photoreceptor transcriptome profiling by RNA-seq after blue light exposure in Drosophila demonstrated an upregulation of a broad range of genes involved in oxidative stress response and neuroprotective pathways, with concomitant downregulation of genes required for light response including voltage-gated calcium, potassium and chloride ion channels. Interestingly, mature flies were more susceptible to these blue light-induced transcriptomic changes compared to very young flies (Hall et al., 2018).
In cultured murine photoreceptor-derived cells, blue light increased production of ROS, altered protein expression, and caused photoreceptor damage (Kuse et al., 2014). Photoreceptor cell death from blue light exposure was mainly caused by inducing mitochondrial dysfunction and apoptosis through Bax and caspase-3 activation (Xu et al., 2023). Similarly, mouse retinal explant cultures showed increased ROS production, morphological changes including the disorganization of the outer segments, cell membrane disruption, and cell death with prolonged blue light exposure (Figure 1) (Roehlecke et al., 2011).
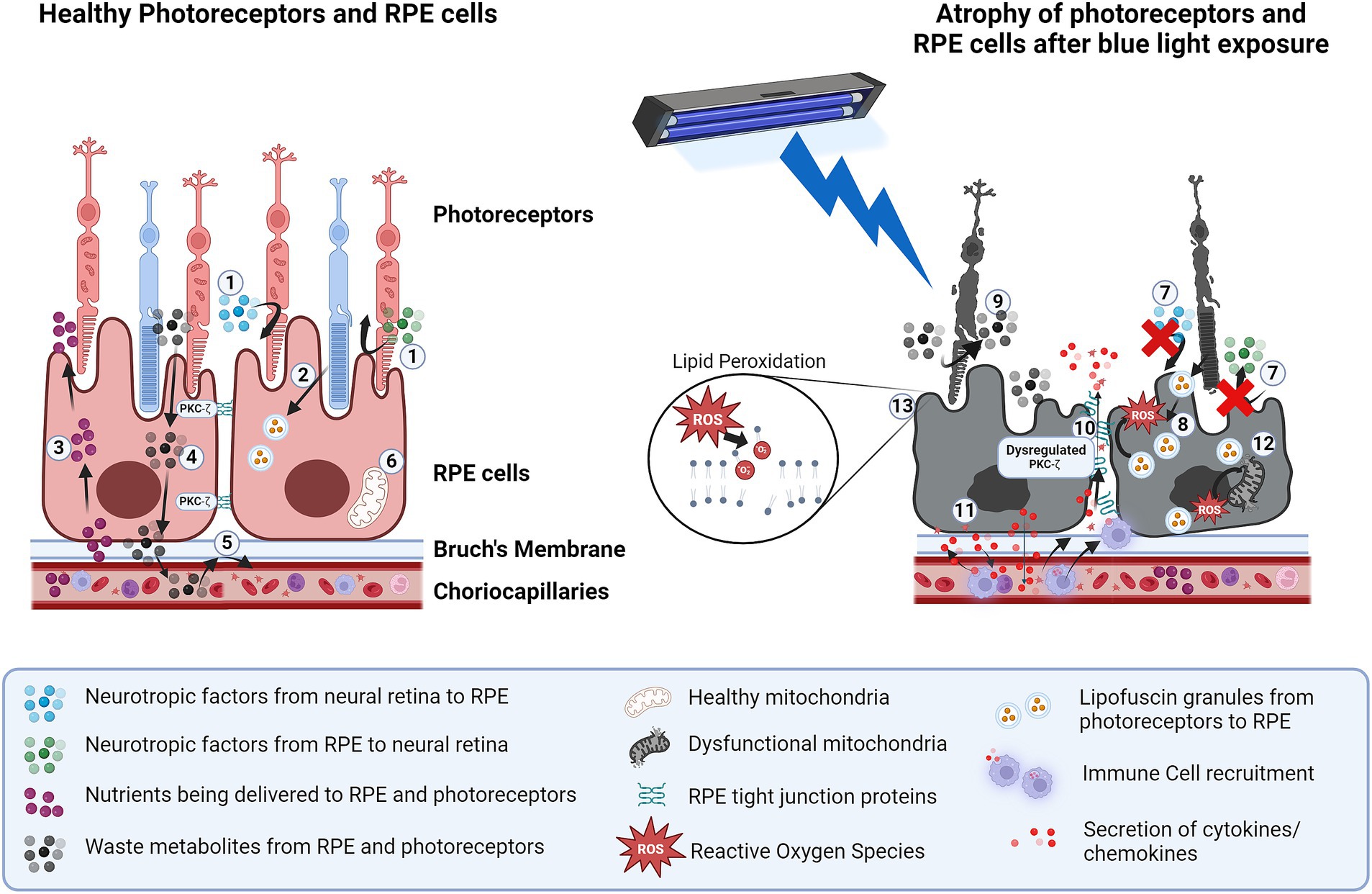
Figure 1. Structural and functional impairments in photoreceptors and RPE cells due to blue light exposure. In healthy photoreceptors and RPE cells: 1. secreted neurotropic factors such as HGF, VEGF and angiogenin help maintain homeostasis of the neural retina and RPE. 2. Phagocytosis of photoreceptor outer segments by RPE into lysosomes helps preserve photoreceptor function. 3. Nutrient uptake is controlled by RPE. 4. Metabolite exchange from retina to choroid is controlled by RPE. 5. Outer blood-retinal barrier is maintained due to RPE tight junctions. 6. Healthy mitochondria help manage oxidative stress in RPE. Blue light exposure of RPE causes: 7. Dysfunctional neurotrophic factors secretion between neural retina and RPE. 8. Dysfunction in phagocytosis of photoreceptor outer segments 9. Metabolite accumulation in the retina due to lack of uptake by RPE. 10. Breakdown of tight junctions due to blue light-induced dysfunction of PKC-ζ. 11. Secretion of cytokines/chemokines causes inflammation and exacerbates the recruitment of immune cells which further damage RPE. 12. Increase in oxidative stress from lipofuscin granules and ROS resulting in mitochondrial damage. 13. Lipid peroxidation from ROS resulting in cellular toxicity and reduced viability. Created in BioRender. BioRender.com/e21v624.
Effect of blue light exposure on retinal pigmented epitheliumThe RPE forms a crucial interface between the neural retina and choroid, the vascular supply of the outer retina. The RPE controls the transportation of nutrients, ions, and water to photoreceptors, absorbs light and safeguards against photooxidation, converts all-trans-retinal to 11-cis-retinal to support the visual cycle, engulfs and discards photoreceptor membranes through phagocytosis, and secretes vital factors contributing to the structural integrity of the retina. The RPE is also important in combating lipid photooxidation and the generation of ROS, both of which are toxic to the retina, serving as the main defense system to counterbalance the high oxidative stress in the retina (Simó et al., 2010).
The outer blood-retinal barrier (oBRB), consisting of RPE monolayer and their tight junctions with Bruch’s membrane, controls nutrient and metabolite exchange vital for photoreceptor survival. Blue light exposure disrupts RPE tight junctions, resulting in loss of barrier function in rodents, rabbits and cultured human RPE cells. The decline in barrier function is associated with alterations in RPE tight junction proteins, specifically zonula occludens-1 (ZO-1) (Putting et al., 1992; Ozkaya et al., 2019; Xie et al., 2021). Blue light (400–520 nm) at 50 J/cm2energy (0.014 W/cm2 for 1 h) causes oBRB dysfunction in rabbits, 30 times more effectively than yellow light (510–740 nm) at the same energy (Putting et al., 1992, 1994). In human RPE cells, this effect is attributed to the dysfunction of Protein Kinase C-ζ (PKC-ζ), a component of a protein scaffold complex that regulates the polarity of RPE tight junctions (Ozkaya et al., 2019).
Morphological changes observed in cultured human RPE cells exposed to blue light include the presence of cracked nuclei, swollen mitochondria, disappearance of the inner limiting membrane of the mitochondria, vacuole formation, and dilation of the rough endoplasmic reticulum (Busch et al., 1999; Zhang, 2005; Roehlecke et al., 2009; Song et al., 2022; Françon et al., 2024). Blue light also impairs the secretion of retinal neurotrophic factors important for the maintenance of retinal cells such as hepatocyte growth factor and angiogenin by human RPE cells and vascular endothelial growth factor-A (VEGF-A) by porcine RPE cells (Chu et al., 2006; Vila et al., 2017; Marie et al., 2019). Transcriptome profiling by RNA-seq following blue light exposure of cultured human RPE cells demonstrated an upregulation of a broad range of genes involved not only in cell survival and cell cycle regulation, but also cell–cell interactions, cell morphology, inflammation and oxidative stress (Cheng et al., 2021).
Blue light can also cause a reversible inhibition of phagocytic activity and reduce receptor proteins, likely arising from oxidative modifications of phagocytic machinery in human RPE (Olchawa et al., 2022). The build-up of lipofuscin (a fluorophore that increases with age in RPE and results in increased oxidative stress) contributes to this reduced ability of cultured rabbit RPE cells to phagocytose the photoreceptor outer segments (Brunk et al., 1995; Sundelin et al., 1998). Loss of this critical function of RPE leads to photoreceptor dysfunction (Olchawa et al., 2017) (Figure 1). A more recent study in Japanese quail demonstrates that blue light exposure-induced photo-oxidative stress contributes to lipofuscin accumulation in RPE cells in the form of melanolipofuscin-like granules, which is suggested to impair RPE function (Serejnikova et al., 2024).
Potential mechanisms of blue light-induced retinal damage Activation of inflammatory pathwaysIt is well established that blue light induces RPE to secrete cytokines and chemokines and growth factors. Specifically, blue light exposure results in an increased release of IL-6, IL-8, IL-17a and basic fibroblast growth factor (bFGF) in human RPE cells compared to other wavelengths (Sato et al., 2021). Additionally, chemokines involved in recruiting immune cells such as monocyte chemotactic protein-1(MCP-1) were also shown to be elevated in RPE-choroid complexes of mice exposed to blue light compared to other wavelengths (Narimatsu et al., 2015). This increase in cytokine secretion from blue light exposure has been shown to induce local proliferation and migration of activated immune cells such as microglia and macrophages in mice, which can contribute to further damage to the RPE (Figure 1) (Nakamura et al., 2018). Activation of these innate immune cells is not seen with white light (400–700 nm) exposure in mice, further highlighting the heightened effects of blue light on immune activation (Ebert et al., 2012).
Oxidative stressA key mechanism of blue light-induced RPE injury is the generation of ROS, resulting in oxidative damage and reduced viability through increased lipid peroxidation in cultured human and bovine RPE cells (Nakanishi-Ueda et al., 2013; Abdouh et al., 2022). The major source of ROS in human RPE is the mitochondria, particularly the endogenous fluorophores in the inner mitochondrial membrane (King et al., 2004). By making RPE more vulnerable to oxidative stress, blue light exposure can thus promote necroptosis, a form of programmed cell death that has features of both necrosis and apoptosis, and is independent of caspase activity in human RPE cells (Song et al., 2022). Antioxidant compounds such as lipoxins reduce RPE oxidative stress injury in cultured human RPE and in mice exposed to blue light (Xie et al., 2021).
RPE photoreactivity increases with age due to lipofuscin accumulation, which arises from incomplete degradation of photoreceptor outer segments, as observed in primary porcine, bovine and human RPE cells (Rozanowska et al., 1996; Marie et al., 2018). Lipofuscin toxicity is driven by bisretinoid fluorophores, which oxidize in response to oxygen and blue light, forming toxic aldehydes and ketones (Feldman et al., 2022). The main fluorophore, N-retinylidene-N-retinylethanolamine (A2E), accumulates with age in humans and acts as a photosensitizer, increasing ROS levels and RPE damage (Sparrow et al., 2000). A2E can also disrupt lysosomal membrane permeability, leading to mitochondrial damage, DNA damage, and apoptotic signaling (Xu et al., 2022) It triggers apoptosis by increasing calcium leakage from mitochondria and lysosomes into the cytosol (Brini et al., 2013), ultimately activating the mitochondrial apoptotic pathway.
Mitochondrial dysfunctionMitochondria play a crucial role in RPE damage from blue light exposure. At lower levels of blue light (1–3 mW/cm2), mitochondrial respiratory chain activity increases in RPE cells in Japanese quails (Serezhnikova et al., 2017). Human in vitro RPE models show a rise in mitochondria numbers and morphological changes, with larger, ring-shaped mitochondria forming, increasing membrane surface area. These adaptations likely enhance cellular resistance to ROS by boosting energy transfer efficiency and metabolic activity (Roehlecke et al., 2009).
At higher blue light intensities (>4 mW/cm2), the balance of mitochondrial fusion and fission is disrupted in mice and in cultured human RPE cells, leading to increased fragmentation (Anitua et al., 2023; Wang et al., 2023). This effect occurs in human RPE even without A2E loading (Alaimo et al., 2019). The fusion-fission balance is crucial for maintaining a healthy mitochondrial network, and its dysregulation marks an early step in apoptosis (McBride and Scorrano, 2013). Mitochondrial pathways, rather than purely ROS signaling, play a significant role in blue light-induced cell death in human RPE (Moon et al., 2017).
Overactivation of autophagyAutophagy is a protective mechanism in RPE that maintains cellular homeostasis by the degradation of harmful material from the ER, mitochondria or lysosomes (Intartaglia et al., 2022). However, autophagy competes with LC3-associated phagocytosis (LAP), a form of non-canonical autophagy that combines components of autophagy with phagocytosis to facilitate clearance of cellular debris. LAP is the primary process utilized by RPE to degrade the shed photoreceptor outer segments (POS), produce 11-cis retinal for the visual cycle and recycle essential components for photoreceptor disc renewal (Kim et al., 2013). Therefore, autophagy must be tightly regulated to ensure LAP occurs efficiently, prevent accelerated lipofuscin buildup, maintain POS clearance, and support the visual cycle (Kim et al., 2013; Ferguson and Green, 2014). Excessive autophagy activation has been observed in human and rodent RPE exposed to blue light, potentially due to the increased ROS and lysosomes and mitochondria permeability, disrupting the balance of autophagy and LAP. This imbalance reduces RPE viability and promotes lipofuscin buildup (Figure 1) (Fujita et al., 2007; Shen et al., 2013; Lee et al., 2015; Galluzzi et al., 2018).
Experimental models of blue light stimulationExperimental models are used to mimic environmental stressors associated with blue light stimulation through a variety of methods which are summarized below:
In vitro modelsThe ARPE-19 cell line is the most commonly used cell model, along with primary RPE cell cultures. Study parameters such as exposure duration and irradiance intensity vary extensively in the literature (Table 1). In vitro experiments range from low intensity (0.04–3.7 mW/cm2) and long duration (3–48 h) (Roehlecke et al., 2009; Moon et al., 2017; Marie et al., 2018; Cheng et al., 2021; Anitua et al., 2023; Françon et al., 2024) to high intensity (4.4–100 mW/cm2) and short duration (1–90 min) (Pautler and BIOPHYSICS, 1990; Rozanowska et al., 1996; Sparrow and Cai, 2001; King et al., 2004; Alaimo et al., 2019; Abdouh et al., 2022; Olchawa et al., 2022). Despite these variations, similar effects on RPE have been observed. For example, Jeong et al exposed A2E-laden ARPE-19 to 6,000 lux blue light for 5 min/day for 120 days, while Burght et al used a single 15 h exposure of 1 mW/cm2 of blue light, yet both found similar changes in apoptosis and inflammation and complement-related gene expression (Van Der Burght et al., 2013; Jin and Jeong, 2022).
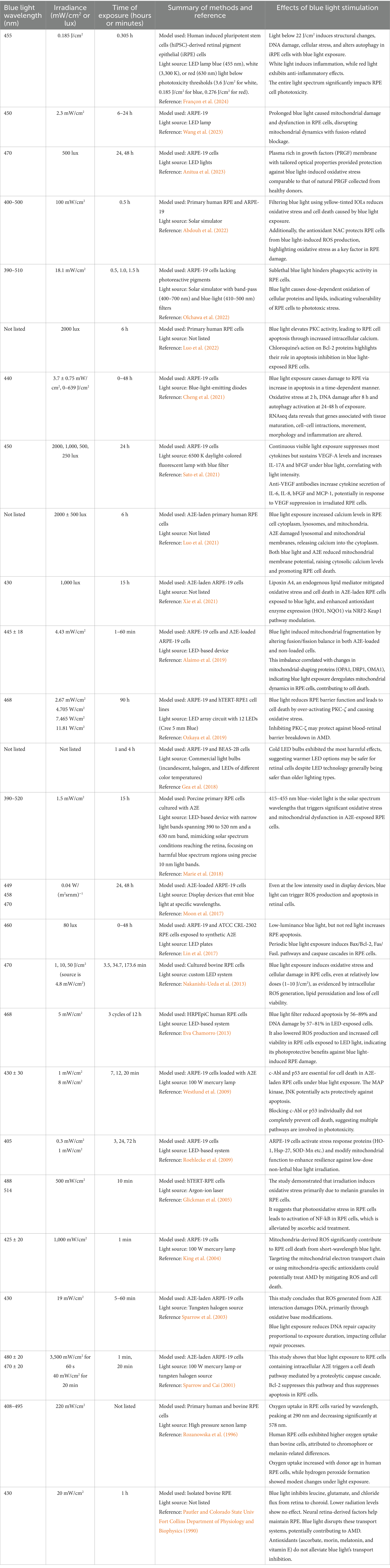
Table 1. Blue light wavelengths, irradiance and times of exposure from in vitro experimental studies reviewed here are compiled to compare and highlight the variability in the methodologies used.
The wavelength of blue light also varies between studies. Blue light typically refers to wavelengths around 445 nm, the “Blue Light Hazard” (BLH) wavelength which causes photochemical damage (Van Norren and Gorgels, 2011). BLH refers to the damage caused by light with a polychromatic profile containing peaks at 445 nm, often leading to visible morphological damage (Françon et al., 2024). Wavelength variations affect results, and using LED light sources with known peaks at 445 nm is recommended to prevent inconsistencies in experimental studies. Some studies use white fluorescent lamps or solar simulators, but their peak wavelengths are often unknown, complicating accurate irradiance calculations. Although less common in in vitro models compared to in vivo, white fluorescent lamps with a blue-light filter or solar light simulators may be used (Gea et al., 2018; Sato et al., 2021; Olchawa et al., 2022). Narrow-band interference filters or blue film filters allow for light exposure in the range of 400-490 nm, broad-band pass filters provide a range of 400-520 nm or 400-700 nm, and UV and infrared (IR) blocking filters remove wavelengths below 400 nm and above 740 nm, respectively (Putting et al., 1994; Olchawa et al., 2022). Neutral-density filters are used to standardize the intensity of light without altering its spectral composition (Ozkaya et al., 2019; Sato et al., 2021). However, the peak wavelength of these light sources is often unknown, leading to difficulties in accurate calculation of their irradiance intensities. Therefore, light sources with more uniform peak wavelengths such as LED devices should be utilized to prevent variance of wavelengths in blue light studies.
A common baseline for irradiance intensity is the phototoxic threshold, which is the threshold of irradiance intensity at which microscopic phototoxic damage occurs. The phototoxic threshold for blue light at 445 nm in humans was initially determined to be 22 J/cm2 (Van Norren and Gorgels, 2011). However, this threshold of the BLH may have been overestimated, as phototoxic damage has been observed in animal models at intensities lower than the estimated threshold by a factor of 20 (Hunter et al., 2012; Jaadane et al., 2020). Sub-threshold exposure in human in vitro RPE models also shows phototoxic changes in RPE morphology and immunological responses, suggesting that lower irradiance levels should be further explored (Françon et al., 2024).
A majority of studies measure blue light intensity in mW/cm2, which measures the intensity per unit area, but not the total amount of light energy received by the cells. This is problematic since exposure duration varies between studies, making it challenging to compare results. Measuring the total energy (J/cm2) would better reflect the cumulative light exposure, enabling easier comparisons across studies (Van Norren and Gorgels, 2011). Furthermore, the use of lux, a measure of illuminance, instead of mW/cm2, adds another layer of complexity, as lux measures light emitted rather than received. Therefore, providing mW/cm2, duration and total energy (J/cm2) in studies will allow for more consistent comparisons across studies.
In vivo and ex vivo modelsWhile cell-based blue light experiments are easier to design and provide more reliable, reproducible results, in vivo models offer significant advantages over cultured RPE cells: (1) Animal studies offer more physiologically relevant insights into the complex structural and functional interactions between different cell types in retinal tissue which are often lacking in cultured systems, since these model systems preserve systemic responses such as neuronal activity, blood circulation and immune activation. (2) Cells in the neural retina such as photoreceptors lose their morphological integrity in vitro. This issue can be addressed by using in vivo or ex vivo models to study blue light-mediated effects on the retina and its interactions with the RPE/choroid in a more complex physiologic environment. (3) In vivo models also allow longitudinal studies for studying the effects of low intensity, long-term blue light exposure and tracking changes over time in the same animal.
Ex vivo models containing neural retina, RPE-Bruch’s membrane-choroid complexes or whole eyes can be used to assess chronic or intermittent blue light effects on ocular tissue under controlled conditions. One study utilized whole eyeball cultures to examine the impact of blue light exposure on mouse photoreceptors (Roehlecke et al., 2011). Eyeballs were punctured with a needle to enable fluid exchange and maintained in serum-containing medium at 37°C and 5%CO2. The eyes were positioned with corneas facing the blue light diodes and were irradiated from 0.5–24 h. In another study, whole porcine eyes were irradiated with blue light for 3 h and incubated for further 6 h in PBS. Isolated neural retina was also exposed to blue light for 1–2 h and maintained on cell culture inserts for 24–48 h (Fietz et al., 2023). Standardization of ex vivo models would greatly benefit the field of blue light study. Additionally, ex vivo models used for studying choroidal microvascular angiogenesis could be adapted for blue light research. Rodent or human RPE/choroid/scleral tissue can be readily isolated and cultured for up to 6 days, allowing for reproducible evaluation of specific pathways involved in blue light-induced responses (Shao et al., 2013; Tomita et al., 2020). Such explants can also be used to compare differences between genetically modified mouse tissue and wild type following blue light stimulation.
One limitation of rodent models for AMD and blue light research is the absence of a macula, an area critical to high-resolution vision in humans. Another challenge with using animal models is the inherent variability in irradiance intensities on the ocular tissue (Table 2) (Narimatsu et al., 2015; Lin et al., 2017; Xie et al., 2021; Wang et al., 2023). Ex vivo models allow a more precise manipulation of illumination conditions, compared to animal models, thereby improving reproducibility and reducing complexity due to systemic influences. Despite some limitations, both in vivo and ex vivo model systems provide significant advantages.
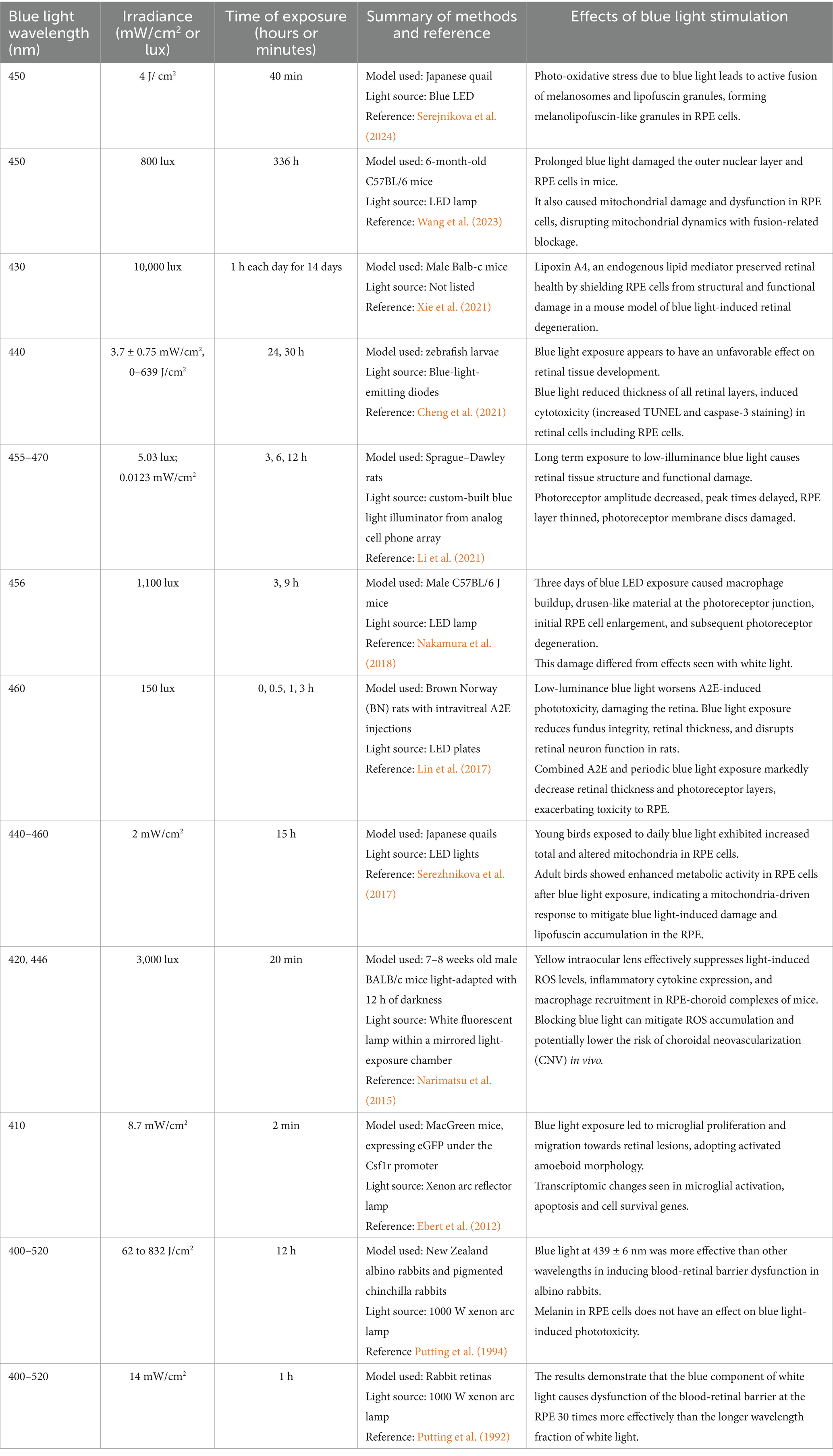
Table 2. Blue light wavelengths, irradiance and times of exposure from in vivo experimental studies reviewed here are compiled to compare and highlight the variability in the methodologies used.
Conclusion and future perspectivesIn conclusion, while some evidence suggests a potential association between prolonged blue light exposure and increased AMD risk, more epidemiological studies are needed to establish a definitive link. Blue light induces oxidative stress, disrupts cell structures, and impairs essential RPE functions, leading to apoptosis and the AMD progression. Further research could also focus on protective strategies, including therapeutic interventions targeting pathways activated by blue light and improving blue-filtering technology to prevent retinal damage. Moreover, refining blue light exposure experiments—through standardized protocols, precise light irradiance measurements, and the development of novel in vivo and ex vivo models—will improve the consistency and relevance of findings across studies and enhance our understanding of blue light’s effects on the retina and ocular tissues.
Author contributionsHC: Conceptualization, Supervision, Visualization, Writing – original draft, Writing – review & editing. VG: Data curation, Visualization, Writing – original draft, Writing – review & editing. CW: Data curation, Methodology, Writing – original draft, Writing – review & editing. AH: Writing – original draft, Writing – review & editing, Data curation. JM: Conceptualization, Funding acquisition, Resources, Supervision, Writing – review & editing, Writing – original draft.
FundingThe author(s) declare financial support was received for the research, authorship, and/or publication of this article. We thank Fighting Blindness Canada, Vancouver Coastal Health Research Institute, Natural Sciences and Engineering Research Council, Canadian Institutes of Health Research and Faculty of Medicine UBC for funding.
Conflict of interestThe authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statementThe author(s) declare that no Gen AI was used in the creation of this manuscript.
Publisher’s noteAll claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
ReferencesAbdouh, M., Lu, M., Chen, Y., Goyeneche, A., Burnier, J. V., and Burnier, M. N. (2022). Filtering blue light mitigates the deleterious effects induced by the oxidative stress in human retinal pigment epithelial cells. Exp. Eye Res. 217:108978. doi: 10.1016/j.exer.2022.108978
PubMed Abstract | Crossref Full Text | Google Scholar
Achiron, A., Elbaz, U., Hecht, I., Spierer, O., Einan-Lifshitz, A., Karesvuo, P., et al. (2021). The effect of blue-light filtering intraocular lenses on the development and progression of Neovascular age-related macular degeneration. Ophthalmology 128, 410–416. doi: 10.1016/j.ophtha.2020.07.039
PubMed Abstract | Crossref Full Text | Google Scholar
Alaimo, A., Liñares, G. G., Bujjamer, J. M., Gorojod, R. M., Alcon, S. P., Martínez, J. H., et al. (2019). Toxicity of blue led light and A2E is associated to mitochondrial dynamics impairment in ARPE-19 cells: implications for age-related macular degeneration. Arch. Toxicol. 93, 1401–1415. doi: 10.1007/s00204-019-02409-6
PubMed Abstract | Crossref Full Text | Google Scholar
Anitua, E., Muruzabal, F., De La Fuente, M., Del Olmo-Aguado, S., Alkhraisat, M. H., and Merayo-Lloves, J. (2023). PRGF membrane with tailored optical properties preserves the Cytoprotective effect of plasma rich in growth factors: in vitro model of retinal pigment epithelial cells. Int. J. Mol. Sci. 24:11195. doi: 10.3390/ijms241311195
PubMed Abstract | Crossref Full Text | Google Scholar
Baker, J., Putnam, N., Kozlowski, R. E., Anderson, M., Bird, Z., Chmielewski, J., et al. (2022). Effects of chronic, daily exposures to low intensity blue light on human retinal pigment epithelial cells: implications for the use of personal electronic devices. J. Photochem. Photobiol. 10:100118. doi: 10.1016/j.jpap.2022.100118
Crossref Full Text | Google Scholar
Behar-Cohen, F., Martinsons, C., Viénot, F., Zissis, G., Barlier-Salsi, A., Cesarini, J. P., et al. (2011). Light-emitting diodes (LED) for domestic lighting: any risks for the eye? Prog. Retin. Eye Res. 30, 239–257. doi: 10.1016/j.preteyeres.2011.04.002
PubMed Abstract | Crossref Full Text | Google Scholar
Brunk, U. T., Wihlmark, U., Wrigstad, A., Roberg, K., and Nilsson, S.-E. (1995). Accumulation of lipofuscin within retinal pigment epithelial cells results in enhanced sensitivity to photo-oxidation. Gerontology 41, 201–212. doi: 10.1159/000213743
PubMed Abstract | Crossref Full Text | Google Scholar
Busch, E. M., Gorgels, T. G. M. F., and Van Norren, D. (1999). Temporal sequence of changes in rat retina after UV-A and blue light exposure. Vis. Res. 39, 1233–1247. doi: 10.1016/S0042-6989(98)00233-8
PubMed Abstract | Crossref Full Text | Google Scholar
Cheng, K.-C., Hsu, Y.-T., Liu, W., Huang, H.-L., Chen, L.-Y., He, C.-X., et al. (2021). The role of oxidative stress and autophagy in blue-light-induced damage to the retinal pigment epithelium in zebrafish in vitro and in vivo. Int. J. Mol. Sci. 22:1338. doi: 10.3390/ijms22031338
PubMed Abstract | Crossref Full Text | Google Scholar
Chu, R., Zheng, X., Chen, D., and Hu, D. (2006). Blue light irradiation inhibits the production of HGF by human retinal pigment epithelium cells in vitro. Photochem. Photobiol. 82, 1247–1250. doi: 10.1562/2006-04-19-RA-880
PubMed Abstract | Crossref Full Text | Google Scholar
Contín, M. A., Benedetto, M. M., Quinteros-Quintana, M. L., and Guido, M. E. (2016). Light pollution: the possible consequences of excessive illumination on retina. Eye 30, 255–263. doi: 10.1038/eye.2015.221
PubMed Abstract | Crossref Full Text | Google Scholar
Cougnard-Gregoire, A., Merle, B. M. J., Aslam, T., Seddon, J. M., Aknin, I., Klaver, C. C. W., et al. (2023). Blue light exposure: ocular hazards and prevention—a narrative review. Ophthalmol. Ther. 12, 755–788. doi: 10.1007/s40123-023-00675-3
PubMed Abstract | Crossref Full Text | Google Scholar
Cruickshanks, K. J., Klein, R., Klein, B. E., and Nondahl, D. M. (2001). Sunlight and the 5-year incidence of early age-related maculopathy: the beaver dam eye study. Arch. Ophthalmol. Chic. Ill 1960, 246–250.
Ebert, S., Walczak, Y., Remé, C., and Langmann, T. (2012). Microglial activation and transcriptomic changes in the blue light-exposed mouse retina, 619–632. doi: 10.1007/978-1-4614-0631-0_79
Crossref Full Text | Google Scholar
Eva Chamorro, S. F. C. (2013). Photoprotective effects of blue light absorbing filter against LED light exposure on human retinal pigment epithelial cells in vitro. J. Carcinog. Mutagen. 1–7. doi: 10.4172/2157-2518.S6-008
Crossref Full Text | Google Scholar
Evans, J. R. (2005). 28 000 cases of age related macular degeneration causing visual loss in people aged 75 years and above in the United Kingdom may be attributable to smoking. Br. J. Ophthalmol. 89, 550–553. doi: 10.1136/bjo.2004.049726
PubMed Abstract | Crossref Full Text | Google Scholar
Feldman, T., Ostrovskiy, D., Yakovleva, M., Dontsov, A., Borzenok, S., and Ostrovsky, M. (2022). Lipofuscin-mediated photic stress induces a dark toxic effect on ARPE-19 cells. Int. J. Mol. Sci. 23:12234. doi: 10.3390/ijms232012234
PubMed Abstract | Crossref Full Text | Google Scholar
Fietz, A., Corsi, F., Hurst, J., and Schnichels, S. (2023). Blue light damage and p53: unravelling the role of p53 in oxidative-stress-induced retinal apoptosis. Antioxidants 12:2072. doi: 10.3390/antiox12122072
PubMed Abstract | Crossref Full Text | Google Scholar
Fletcher, A. E. (2008). Sunlight exposure, antioxidants, and age-related macular degeneration. Arch. Ophthalmol. 126:1396. doi: 10.1001/archopht.126.10.1396
Crossref Full Text | Google Scholar
Françon, A., Delaunay, K., Jaworski, T., Lebon, C., Picard, E., Youale, J., et al. (2024). Phototoxicity of low doses of light and influence of the spectral composition on human RPE cells. Sci. Rep. 14:6839. doi: 10.1038/s41598-024-56980-9
PubMed Abstract | Crossref Full Text | Google Scholar
Fujita, E., Kouroku, Y., Isoai, A., Kumagai, H., Misutani, A., Matsuda, C., et al. (2007). Two endoplasmic reticulum-associated degradation (ERAD) systems for the novel variant of the mutant dysferlin: ubiquitin/proteasome ERAD(I) and autophagy/lysosome ERAD(II). Hum. Mol. Genet. 16, 618–629. doi: 10.1093/hmg/ddm002
PubMed Abstract | Crossref Full Text | Google Scholar
Galluzzi, L., Vitale, I., Aaronson, S. A., Abrams, J. M., Adam, D., Agostinis, P., et al. (2018). Molecular mechanisms of cell death: recommendations of the nomenclature committee on cell death 2018. Cell Death Differ. 25, 486–541. doi: 10.1038/s41418-017-0012-4
PubMed Abstract | Crossref Full Text | Google Scholar
Gea, M., Schilirò, T., Iacomussi, P., Degan, R., Bonetta, S., and Gilli, G. (2018). Cytotoxicity and genotoxicity of light emitted by incandescent, halogen, and LED bulbs on ARPE-19 and BEAS-2B cell lines. J. Toxicol. Environ. Health A 81, 998–1014. doi: 10.1080/15287394.2018.1510350
PubMed Abstract | Crossref Full Text | Google Scholar
Glickman, R. D., Natarajan, M., Rockwell, B. A., Denton, M., Maswadi, S., Kumar, N., et al. (2005) in Intracellular signaling mechanisms responsive to laser-induced photochemical and thermal stress. eds. S. L. Jacques and W. P. Roach.
Hall, H., Ma, J., Shekhar, S., Leon-Salas, W. D., and Weake, V. M. (2018). Blue light induces a neuroprotective gene expression program in Drosophila photoreceptors. BMC Neurosci. 19:43. doi: 10.1186/s12868-018-0443-y
PubMed Abstract | Crossref Full Text | Google Scholar
Ham, W. T., Mueller, H. A., Ruffolo, J. J., and Clarke, A. M. (1979). Sensitivity of the retina to radiation damage as a function of wavelength. Photochem. Photobiol. 29, 735–743. doi: 10.1111/j.1751-1097.1979.tb07759.x
Crossref Full Text | Google Scholar
Hunter, J. J., Morgan, J. I. W., Merigan, W. H., Sliney, D. H., Sparrow, J. R., and Williams, D. R. (2012). The susceptibility of the retina to photochemical damage from visible light. Prog. Retin. Eye Res. 31, 28–42. doi: 10.1016/j.preteyeres.2011.11.001
PubMed Abstract | Crossref Full Text | Google Scholar
Intartaglia, D., Giamundo, G., and Conte, I. (2022). Autophagy in the retinal pigment epithelium: a new vision and future challenges. FEBS J. 289, 7199–7212. doi: 10.1111/febs.16018
Crossref Full Text | Google Scholar
Jaadane, I., Villalpando Rodriguez, G., Boulenguez, P., Carré, S., Dassieni, I., Lebon, C., et al. (2020). Retinal phototoxicity and the evaluation of the blue light hazard of a new solid-state lighting technology. Sci. Rep. 10:6733. doi: 10.1038/s41598-020-63442-5
PubMed Abstract | Crossref Full Text | Google Scholar
Jin, H. L., and Jeong, K. W. (2022). Transcriptome analysis of long-term exposure to blue light in retinal pigment epithelial cells. Biomol. Ther. 30, 291–297. doi: 10.4062/biomolther.2021.155
留言 (0)