The heart rhythm of a normal fetus is regular, with a rate of 110–160 beats per minute. Fetal tachyarrhythmias (FTs) is defined as a fetal heart rate persistently exceeding 180 beats per minute in the absence of uterine contractions. The most common types are supraventricular tachycardia (SVT) and atrial flutter (AFL), while ventricular tachycardia (VT) is rare (1). FTs are important causes of intrauterine fetal nonimmune edema, preterm birth, and increased perinatal morbidity and mortality. Compared to fetuses with intermittent SVT or AFL, those with persistent SVT or AFL (tachycardia >50% of the time or >12 h/day) or fetal edema have poorer outcomes, with increased mortality and risk during the perinatal period (2). A 10-year retrospective cohort study showed that compared to nonedematous fetuses, edematous fetuses have a higher rate of in utero treatment but significantly lower survival rates, earlier delivery, lower cardiovascular scores at diagnosis and birth, lower birth weight, and higher termination of pregnancy rates (3). The American Heart Association recommends that (4), in addition to near-term fetuses, in utero treatment is recommended for all fetuses with persistent tachyarrhythmias, with or without edema, or with intermittent tachyarrhythmias associated with heart failure or edema. A study from Japan showed that (5), compared to untreated fetuses, in utero treatment significantly reduces the rates of cesarean section, preterm birth, and neonatal arrhythmias. In ∼80% of cases, transplacental drug therapy is effective, allowing for a smooth postponement of delivery into the third trimester (6).
Currently, there is no consensus on the optimal treatment strategy for FTs. Prenatal treatment primarily consists of drug therapy, and digoxin, flecainide, sotalol, amiodarone, and other drugs are supported by clinical data as first-line treatments (7–10) (Table 1). However, the treatment of FTs still faces many unknown issues, such as drug selection, dosage, effective routes of administration, and monitoring of drug toxicity and side effects.
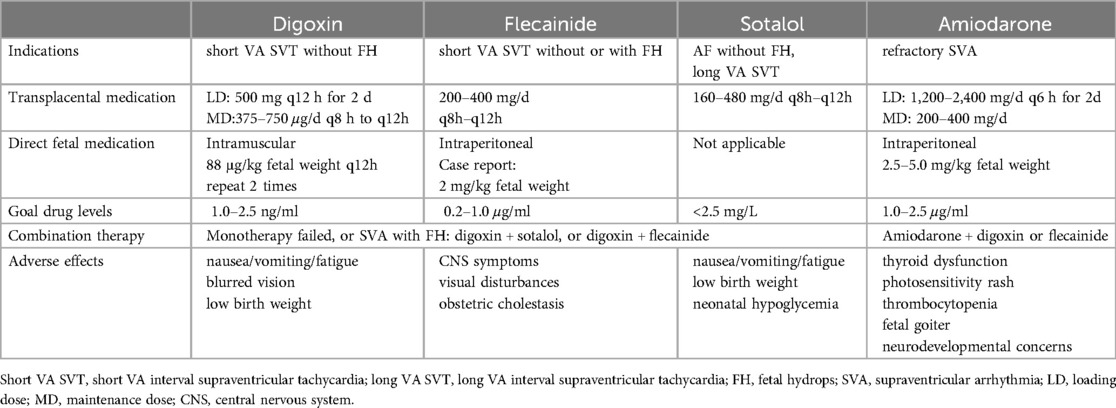
Table 1. Antiarrhythmic drugs.
2 Types and etiology of FTs 2.1 Sinus tachycardiaSinus tachycardia (ST) refers to a fetal heart rate of 180–200 beats per minute with normal atrioventricular conduction. ST is usually associated with intrauterine distress and underlying maternal diseases, including hyperthyroidism, anemia, fever, use of β-receptor agonists or stimulants, fetal hypoxia, chorioamnionitis, and intrauterine cytomegalovirus infection (1). The prenatal treatment strategies for ST focus on correcting the underlying causes, with good prognosis in most cases.
2.2 SVTSVT is the most common FTs, accounting for 60%–90% of cases (11). The incidence of fetal edema in SVT is as high as 30%–40% (6, 9), making it a significant cause of fetal morbidity and mortality. SVT often occurs between weeks 24 and 32 of gestation, with a fetal atrial rate of 220–300 beats per minute and 1:1 atrioventricular conduction. The electrophysiological mechanism is mainly accessory pathway conduction forming a re-entrant circuit. Based on the relationship between ventriculoatrial (VA) interval and atrioventricular(AV) interval, SVT can be further classified into short and long VA SVT. Some studies found a significant increase in the incidence of fetal SVT during the COVID-19 pandemic (12). Abdallah et al. reported two cases of fetal SVT after maternal COVID-19 vaccination (13), possibly related to severe fetal hypoxia caused by SARS CoV-2 infection of the placenta (14) and immune metastasis and immune response of the placenta.
2.3 AFLApproximately 30% of FTs are AFL (2), which often occurs in late gestation with a fetal atrial rate >300 beats per minute and an atrioventricular conduction ratio of 2:1, 3:1, or even 4:1. AFL is associated with myocarditis, anti-Ro (SSA), anti-La (SSB) autoantibodies, and congenital heart disease, with ∼15% of fetuses with AFL developing fetal edema (1, 4).
2.4 VTVT is rare in the fetal period, accounting for <2% of FTs (15). The ventricular rate is between 180 and 300 beats per minute, with a ventriculoatrial ratio <1. Its electrophysiological mechanism is ectopic ventricular foci caused by inflammation or abnormal myocardial oxygen supply. VT can occur in fetuses with atrioventricular block, cardiac tumors, myocarditis, ventricular aneurysm, and cardiomyopathy (2). When VT coexists with sinus bradycardia or second-degree block, the possibility of long QT syndrome (LQTS) needs to be considered (1).
3 Treatment of FTsIn recent years, with the rapid development of fetal medicine, the intrauterine treatment methods for fetal diseases have gradually increased. However, prenatal treatment for fetal arrhythmias still mainly relies on drug therapy, mainly including: transplacental drug therapy; administration via umbilical vein injection; administration via the fetal abdominal cavity; administration via the amniotic cavity; and administration via fetal intramuscular injection. Transplacental drug therapy is the preferred approach, while other methods are limited in clinical application due to their invasiveness, and are only considered when the placental transfer rate of severe fetal arrhythmias is low.
3.1 Transplacental drug transport 3.1.1 Treatment for SVT and AFL 3.1.1.1 DigoxinDigoxin is the longest-used medication for treating FTs. It has a high protein-binding rate, but its ability to cross the edematous placenta is poor, requiring larger and more frequent doses to achieve therapeutic levels in the fetus (16). A multicenter prospective study from Japan showed that (7), for treating short VA SVT without fetal edema, the single-drug relief rate of digoxin was 46.7%, while the total relief rate of digoxin combined with sotalol or flecainide was 86.7%; for AFL without fetal edema, the single-drug relief rate of digoxin was 59.3%, and the total relief rate of digoxin combined with sotalol or flecainide was 92.6%. The efficacy of digoxin treatment for long VA SVT patients is poor (7, 17). In cases of FTs with accompanying edema, the relief rate of digoxin also decreases significantly to <20%, and the occurrence of intrauterine or neonatal death in edematous fetuses treated with digoxin alone reaches 43% (9, 18).
3.1.1.2 FlecainideFlecainide is a lipophilic compound with no significant protein binding, and it has good placental transfer (16). Several studies have suggested that flecainide may be more effective than sotalol or digoxin, and fetal intrauterine death is rare (8, 9, 19). It has been reported that the relief rate of flecainide monotherapy for fetal SVT can be as high as 88.2% (20). Two meta-analyses have shown that (8, 19) flecainide and sotalol are superior to digoxin in cases of FTs with or without fetal edema. Especially, when fetal edema is present, the effectiveness of flecainide compared to digoxin is more apparent. Additionally, in cases of long VA SVT, flecainide is also superior to digoxin (9), and placental administration of digoxin combined with flecainide is an effective treatment (21). Therefore, flecainide monotherapy and combination of digoxin and flecainide should be considered the optimal strategies for FTs (22).
3.1.1.3 SotalolSotalol is rapidly absorbed and crosses the placenta almost completely (16). Studies have shown that compared with digoxin or flecainide, the use of sotalol alone or in combination with digoxin is associated with a higher rate of prenatal AFL termination. Sotalol can be recommended as the first-choice for treating fetal AFL (23). Jaeggi et al. (24) conducted a multicenter nonrandomized study on fetuses with AFL and found that sotalol was a better choice for treating fetal AFL. For long VA SVT, sotalol has a higher rate of successful conversion compared to digoxin or flecainide (7).
3.1.1.4 AmiodaroneAmiodarone, either alone or in combination with other drugs, is a safe and effective approach for refractory FTs, even in the presence of fetal edema or cardiac dysfunction (25). It is worth noting that amiodarone is associated with maternal and neonatal thyroid dysfunction, and caution is needed regarding the potential risk of fetal neurodevelopmental issues (16).
3.1.2 Treatment for VTFetal VT is rare. It should be noted that, while treating fetal VT, it is necessary to exclude the possibility of LQTS through fetal magnetocardiography (fMGG) (26). If LQTS can be ruled out, sotalol, amiodarone, or flecainide may be considered (16). Studies have shown that intravenous administration of magnesium sulfate to the mother can be used as a first-line option for fetal VT (2, 15, 27), but its use should be limited to within 48 h. Vaksmann et al. (28) suggested that beta-blockers should be used as first-line therapy for isolated fetal VT (i.e., without tumors or cardiomyopathy), as LQTS may be a potential cause of arrhythmia.
3.2 Direct fetal medicationFor FTs that fail to respond to placental drug transfer therapy, direct fetal medication can be administered through fetal intramuscular injection, umbilical vein injection, intra-amniotic injection, or fetal abdominal cavity injection. Lin et al. reported successful cases of repeated fetal abdominal and intra-amniotic injection of amiodarone for treating AFL (29). Munoz et al. reported a case of fetal intramuscular injection of digoxin for treating refractory SVT with fetal edema (30). However, considering the potential trauma to the fetus associated with direct medication, these approaches have not yet gained widespread acceptance and application in clinical practice.
3.3 Fetal transesophageal pacingStirnemann et al. reported a case of successful in utero transesophageal pacing for treatment of severe refractory tachyarrhythmias (31). The case was a fetus at 27 + 5 weeks’ gestation with AFL and fetal edema. After treatment with digoxin combined with flecainide and amiodarone, fetal edema worsened, accompanied by subcutaneous edema, persistent AFL, and tricuspid and mitral valve regurgitation. At 29 + 4 weeks’ gestation, fetal cardiac pacing via the esophagus was performed under fetoscopic guidance and ultrasound monitoring. Sinus rhythm was restored postoperatively, and there was no recurrence. In experienced cardiac centers, fetal transesophageal pacing can be considered as a salvage therapy for refractory tachyarrhythmias when medical treatment fails, but caution must be exercised regarding iatrogenic premature rupture of membranes and related complications, as well as the risk of ventricular fibrillation caused by transesophageal pacing.
4 ConclusionPresently, the prenatal treatment of FTs is still mainly based on drug therapy. Transplacental transport drug therapy commonly employed as a first-line treatment. While drug therapy via placental transfer exposes the mother to the side effects and risks of antiarrhythmic medications, its efficacy may be limited, particularly in cases of fetal edema. The treatment of FTs depends on a comprehensive analysis and assessment of gestational age, evidence of fetal edema, and potential risks to the mother. Currently, there is no randomized clinical study demonstrating the superiority of one antiarrhythmic drug over another, but flecainide and sotalol (rather than digoxin) are increasingly becoming the first-line treatment for fetal SVT and AFL.
Another significant consideration is the current ethical challenges. As all prenatal treatment approaches involve passing through the maternal body due to the fetus being located within the uterus, they inevitably affect the mother to varying degrees, thus raising numerous ethical concerns. In 2017, the International Fetal Medicine and Surgery Society (IFMSS) together with the North American Fetal Therapy Network (NAFTNet) issued fundamental principles for fetal in utero therapy in response to emerging needs (32). These principles emphasize that the goal of fetal therapy is no longer solely focused on improving fetal survival rates but increasingly on the short- and long-term effects on maternal health. Therefore, before deciding on prenatal intervention for fetal arrhythmias, a comprehensive assessment should be conducted to weigh the benefits of restoring rhythm against the potential adverse effects of medications on both the mother and fetus, aiming to achieve the most favorable outcome, ensuring the safety of both mother and baby, and minimizing complications to the greatest extent possible.
Understanding the indications and clinical outcomes for prenatal treatment of FTs helps facilitate timely intervention and prevent adverse outcomes. However, current research on prenatal treatment of FTs mainly consists of retrospective clinical case studies, with only a few prospective, single-center studies, limited case numbers, and variations in medication methods, dosages, and efficacy among different research centers. Furthermore, systematic analysis of medication dosages and side effects are lacking. The FAST RCT trial, initiated in 2016, is a prospective randomized clinical trial investigating the treatment of fetal AFL and SVT. Its aim is to determine the effectiveness and safety of commonly used drug regimens administered via placental transfer in treating AFL and SVT in fetuses with or without edema (33). This trial aims to bridge the knowledge gap regarding drug efficacy and adverse effects on both pregnant patients and fetuses, ultimately establishing systematic treatment protocols for FTs. It is believed that once the results are published, they will provide valuable insights into individualized prenatal drug therapy for FTs.
Author contributionsJT: Conceptualization, Data curation, Investigation, Methodology, Software, Writing – original draft, Writing – review & editing, Formal Analysis. PH: Conceptualization, Data curation, Investigation, Methodology, Software, Writing – original draft, Writing – review & editing, Formal Analysis. XD: Data curation, Investigation, Writing – review & editing. LZ: Data curation, Investigation, Writing – review & editing. YZ: Data curation, Investigation, Writing – review & editing. TW: Funding acquisition, Project administration, Resources, Supervision, Validation, Visualization, Writing – review & editing, Conceptualization.
FundingThe author(s) declare financial support was received for the research, authorship, and/or publication of this article. The study was supported by National Natural Science Foundation of China with Grant Numbers 81701888, Science-technology Support Plan Projects of Sichuan province with Grant Numbers 2019YFS0239 and 2023YFS0206.
Conflict of interestThe authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statementThe author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher's noteAll claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References2. Joglar JA, Kapa S, Saarel EV, Dubin AM, Gorenek B, Hameed AB, et al. 2023 HRS expert consensus statement on the management of arrhythmias during pregnancy. Heart Rhythm. (2023) 20(10):e175–264. doi: 10.1016/j.hrthm.2023.05.017
PubMed Abstract | Crossref Full Text | Google Scholar
3. Hu Q, Liao H, Xu T, Liu H, Wang X, Yu H. Perinatal outcomes of intrauterine fetal arrhythmias: a 10-year retrospective cohort study. Medicine (Baltimore). (2023) 102(10):e33244. doi: 10.1097/MD.0000000000033244
PubMed Abstract | Crossref Full Text | Google Scholar
4. Donofrio MT, Moon-Grady AJ, Hornberger LK, Copel JA, Sklansky MS, Abuhamad A, et al. American Heart Association Adults with congenital heart disease joint committee of the council on cardiovascular disease in the young and council on clinical cardiology, council on cardiovascular surgery and anesthesia, and council on cardiovascular and stroke nursing. Diagnosis and treatment of fetal cardiac disease: a scientific statement from the American Heart Association. Circulation. (2014) 129(21):2183–242. doi: 10.1161/01.cir.0000437597.44550.5d Erratum in: Circulation. 2014 May 27;129(21):e512.24763516
PubMed Abstract | Crossref Full Text | Google Scholar
5. Ueda K, Maeno Y, Miyoshi T, Inamura N, Kawataki M, Taketazu M, et al. on behalf of Japan fetal arrhythmia group. The impact of intrauterine treatment on fetal tachycardia: a nationwide survey in Japan. J Matern Fetal Neonatal Med. (2018) 31(19):2605–10. doi: 10.1080/14767058.2017.1350159
PubMed Abstract | Crossref Full Text | Google Scholar
6. Ekici H, Ökmen F, İmamoğlu M, İmamoğlu AG, Ergenoğlu AM. Fetal arrhythmias: ten years’ experience and review of the literature. Turk J Obstet Gynecol. (2022) 19(4):302–7. doi: 10.4274/tjod.galenos.2022.61818
PubMed Abstract | Crossref Full Text | Google Scholar
7. Miyoshi T, Maeno Y, Hamasaki T, Inamura N, Yasukochi S, Kawataki M, et al. Japan Fetal arrhythmia group. Antenatal therapy for fetal supraventricular tachyarrhythmias: multicenter trial. J Am Coll Cardiol. (2019) 74(7):874–85. doi: 10.1016/j.jacc.2019.06.024
PubMed Abstract | Crossref Full Text | Google Scholar
8. Alsaied T, Baskar S, Fares M, Alahdab F, Czosek RJ, Murad MH, et al. First-Line antiarrhythmic transplacental treatment for fetal tachyarrhythmia: a systematic review and meta-analysis. J Am Heart Assoc. (2017) 6(12):e007164. doi: 10.1161/JAHA.117.007164
PubMed Abstract | Crossref Full Text | Google Scholar
9. Sridharan S, Sullivan I, Tomek V, Wolfenden J, Škovránek J, Yates R, et al. Flecainide versus digoxin for fetal supraventricular tachycardia: comparison of two drug treatment protocols. Heart Rhythm. (2016) 13(9):1913–9. doi: 10.1016/j.hrthm.2016.03.023
PubMed Abstract | Crossref Full Text | Google Scholar
10. Refaat M, El Dick J, Sabra M, Bitar F, Tayeh C, Abutaqa M, et al. Sotalol as an effective adjunct therapy in the management of supraventricular tachycardia induced fetal hydrops fetalis. J Neonatal Perinatal Med. (2020) 13(2):267–73. doi: 10.3233/NPM-190268
PubMed Abstract | Crossref Full Text | Google Scholar
12. Samples S, Patel S, Lee S, Gotteiner N, Patel A. Incidence of fetal arrhythmia before and during the COVID-19 pandemic: a single-center experience. Pediatr Cardiol. (2024). doi: 10.1007/s00246-024-03439-3
PubMed Abstract | Crossref Full Text | Google Scholar
13. Abdallah W, Rechdan JB, Lakkis R, Nassar M, Daou L, Kassis NE, et al. Fetal supraventricular tachycardia and maternal COVID-19 vaccination: is there any relationship? Future Sci OA. (2022) 8(7):FSO812. doi: 10.2144/fsoa-2022-0007
PubMed Abstract | Crossref Full Text | Google Scholar
14. Mullins J, Bewley DJ, Oviedo A. COVID-19 and placental infection: are fetal survivors at risk of long-term cardiovascular complications? Cureus. (2023) 15(4):e38077. doi: 10.7759/cureus.38077
PubMed Abstract | Crossref Full Text | Google Scholar
15. Simpson JM, Maxwell D, Rosenthal E, Gill H. Fetal ventricular tachycardia secondary to long QT syndrome treated with maternal intravenous magnesium: case report and review of the literature. Ultrasound Obstet Gynecol. (2009) 34(4):475–80. doi: 10.1002/uog.6433
PubMed Abstract | Crossref Full Text | Google Scholar
16. Batra AS, Silka MJ, Borquez A, Cuneo B, Dechert B, Jaeggi E, et al. American Heart Association Clinical pharmacology committee of the council on clinical cardiology, council on basic cardiovascular sciences, council on cardiovascular and stroke nursing, council on genomic and precision medicine, and council on lifelong congenital heart disease and heart health in the young. Pharmacological management of cardiac arrhythmias in the fetal and neonatal periods: a scientific statement from the American Heart Association: endorsed by the pediatric & congenital electrophysiology society(PACES). Circulation. (2024) 149(10):e937–52. doi: 10.1161/CIR.0000000000001206
PubMed Abstract | Crossref Full Text | Google Scholar
18. Krapp M, Kohl T, Simpson JM, Sharland GK, Katalinic A, Gembruch U. Review of diagnosis, treatment, and outcome of fetal atrial flutter compared with supraventricular tachycardia. Heart. (2003) 89(8):913–7. doi: 10.1136/heart.89.8.913
PubMed Abstract | Crossref Full Text | Google Scholar
19. Hill GD, Kovach JR, Saudek DE, Singh AK, Wehrheim K, Frommelt MA. Transplacental treatment of fetal tachycardia: a systematic review and meta-analysis. Prenat Diagn. (2017) 37(11):1076–83. doi: 10.1002/pd.5144
PubMed Abstract | Crossref Full Text | Google Scholar
20. Ekiz A, Kaya B, Bornaun H, Acar DK, Avci ME, Bestel A, et al. Flecainide as first line treatment for fetal supraventricular tachycardia. J Matern Fetal Neonatal Med. (2018) 31(4):407–12. doi: 10.1080/14767058.2017.1286317
PubMed Abstract | Crossref Full Text | Google Scholar
21. Karmegeraj B, Namdeo S, Sudhakar A, Krishnan V, Kunjukutty R, Vaidyanathan B. Clinical presentation, management, and postnatal outcomes of fetal tachyarrhythmias: a 10-year single center experience. Ann Pediatr Cardiol. (2018) 11(1):34–9. doi: 10.4103/apc.APC_102_17
PubMed Abstract | Crossref Full Text | Google Scholar
22. Qin J, Deng Z, Tang C, Zhang Y, Hu R, Li J, et al. Efficacy and safety of various first-line therapeutic strategies for fetal tachycardias: a network meta-analysis and systematic review. Front Pharmacol. (2022) 13:935455. doi: 10.3389/fphar.2022.935455
PubMed Abstract | Crossref Full Text | Google Scholar
23. Gozar L, Gabor-Miklosi D, Toganel R, Fagarasan A, Gozar H, Toma D, et al. Fetal tachyarrhythmia management from digoxin to amiodarone-A review. J Clin Med. (2022) 11(3):804. doi: 10.3390/jcm11030804
PubMed Abstract | Crossref Full Text | Google Scholar
24. Jaeggi ET, Carvalho JS, De Groot E, Api O, Clur SA, Rammeloo L, et al. Comparison of transplacental treatment of fetal supraventricular tachyarrhythmias with digoxin, flecainide, and sotalol: results of a nonrandomized multicenter study. Circulation. (2011) 124(16):1747–54. doi: 10.1161/CIRCULATIONAHA.111.026120
PubMed Abstract | Crossref Full Text | Google Scholar
25. Strasburger JF, Cuneo BF, Michon MM, Gotteiner NL, Deal BJ, McGregor SN, et al. Amiodarone therapy for drug-refractory fetal tachycardia. Circulation. (2004) 109(3):375–9. doi: 10.1161/01.CIR.0000109494.05317.58
PubMed Abstract | Crossref Full Text | Google Scholar
26. Strasburger JF, Eckstein G, Butler M, Noffke P, Wacker-Gussmann A. Fetal arrhythmia diagnosis and pharmacologic management. J Clin Pharmacol. (2022) 62 Suppl 1(Suppl 1):S53–66. doi: 10.1002/jcph.2129
PubMed Abstract | Crossref Full Text | Google Scholar
27. Miyoshi T, Sakaguchi H, Shiraishi I, Yoshimatsu J, Ikeda T. Potential utility of pulsed-wave Doppler for prenatal diagnosis of fetal ventricular tachycardia secondary to long QT syndrome. Ultrasound Obstet Gynecol. (2018) 51(5):697–9. doi: 10.1002/uog.18819
PubMed Abstract | Crossref Full Text | Google Scholar
28. Vaksmann G, Lucidarme S, Henriet E. Fetal ventricular tachycardia: betablockers should be the first line treatment. J Gynecol Obstet Hum Reprod. (2021) 50(6):101946. doi: 10.1016/j.jogoh.2020.101946
PubMed Abstract | Crossref Full Text | Google Scholar
29. Lin PH, Wu HH, Tsai HD, Hsieh CT. Successful treatment of atrial flutter by repeated intraperitoneal and intra-amniotic injections of amiodarone in a fetus with hydrops. Taiwan J Obstet Gynecol. (2016) 55(3):434–6. doi: 10.1016/j.tjog.2016.04.022
PubMed Abstract | Crossref Full Text | Google Scholar
30. Munoz JL, Lewis AL, Song J, Ramsey PS. Fetal intervention for refractory supraventricular tachycardia complicated by hydrops Fetalis. Case Rep Obstet Gynecol. (2022) 2022:5148250. doi: 10.1155/2022/5148250
PubMed Abstract | Crossref Full Text | Google Scholar
31. Stirnemann J, Maltret A, Haydar A, Stos B, Bonnet D, Ville Y. Successful in utero transesophageal pacing for severe drug-resistant tachyarrhythmia. Am J Obstet Gynecol. (2018) 219(4):320–5. doi: 10.1016/j.ajog.2018.07.018
PubMed Abstract | Crossref Full Text | Google Scholar
32. Moon-Grady AJ, Baschat A, Cass D, Choolani M, Copel JA, Crombleholme TM, et al. Fetal treatment 2017: the evolution of fetal therapy centers- A joint opinion from the international fetal medicine and surgical society (IFMSS) and the north American fetal therapy network (NAFTNet). Fetal Diagn Ther. (2017) 42(4):241–8. doi: 10.1159/000475929
留言 (0)