The paraspinal muscles (PM) are composed of muscle groups adjacent to the vertebrae and are responsible for movement and stabilization of the spine. In the lumbar spine, these muscles include the multifidus, erector spinae, interspinales, intertransversarii, psoas major and quadratus lumborum (1). The multifidus muscles are important stabilizers of the lumbar spine (2).
The lumbar multifidus originates from the mammillary processes of the lumbar vertebrae and the posterior surface of the sacrum. It courses medially and cranially to insert on the spinous processes, two to five levels above their origin. Among the lumbar paraspinal muscles, the multifidus is the most crucial muscle as it contributes to almost two-thirds of spinal stability and is predominantly atrophied in patients with chronic low back pain (3).
Reduced range of motion in the spine, particularly in lumbar flexion, is associated with a high degree of fatty infiltration of the PM (4).
There are two hypotheses for the development of PM atrophy. The first one considers the atrophy to be caused by disuse, and the second one—by denervation due to spinal nerve root compression by a herniated intervertebral disc. In the latter case, the atrophic process is local (5). In patients with unilateral chronic radicular pain, the PM cross-sectional area is smaller on the symptomatic side (6). Moreover, histology reveals changes in the multifidus muscles at the level of the affected segment of the spine, which are caused by compression of the spinal nerve root. Histopathological data suggest a relatively low regenerative capacity of the multifidus muscles in patients with long-lasting radicular pain (7).
Electromyography (EMG) remains an important neurophysiological method for diagnosing radiculopathy and can be used to assess the functional state of compressed spinal nerve roots. EMG of the PM stands out as a way to identify electrographic signs of denervation and reinnervation processes in the deep muscles of the back (8). Denervation manifests as fibrillation potentials and positive sharp waves, whereas reinnervation can be observed as an increase in the amplitude, duration, and phases of MUAPs. Neuroanatomical and pathophysiological studies indicate that the innervation of the m. multifidus is very specific, whereas m. longissimus and m. iliocostalis may have multi-spinal nerve innervation.
The m. multifidus is innervated by the medial branch nerve of the posterior ramus of the spinal nerve at each level, which exits the spinal canal superolateral to the facet joint (1). Spinal nerve root compression leads to localized denervation changes in this particular muscle.
The purpose of this study was to determine the relationship between PM neuropathic changes measured by EMG and the degree of fatty infiltration on the affected and unaffected sides.
Materials and methodsA retrospective observational study was performed in the Clinical Neurophysiology Laboratory of the Emergency Neurosurgery Department, N.V. Sklifosovsky Research Institute for Emergency Medicine.
Inclusion criteriaClinical manifestations of unilateral radiculopathy L5 (radicular pain syndrome (leg pain), motor deficit in the form of weakness of the extensor muscles of the foot and/or big toe, sensory loss in the L5 dermatome, decrease/loss of the medial hamstring tendon reflex) caused by L4-L5 intervertebral disc herniation, confirmed by MRI. Disease duration was from one to 12 months after onset.
Exclusion criteriaPatients with severe pain (VAS score of 9 to 10), disease duration of less than 4 weeks, history of spinal surgery at any segment, anatomical variants of the spine (S1 lumbarization, L5 sacralization), and neuromuscular disease were not included in the study.
The study designThe study design covered standard neurological examination, radicular pain localization and intensity evaluation (VAS score), estimation of motor deficit according to the Medical Research Council Weakness Scale (MRC), and sensory loss assessment.
The participants with clinical manifistation of L5 radiculopathy were consecutively selected in order of hospitalization in neurosurgery department (consecutive sampling). All patients were to undergo spinal surgery. The sampling process came to an end when the time limit (1 year) was reached.
Сlinical assessmentThe clinical assessment in the study involved the following key points:
Patient Characteristics: The study included patients with unilateral L5 radiculopathy, characterized by radicular pain, motor deficits, and sensory loss as confirmed by clinical evaluations and MRI.
Neurological Examination: Each patient underwent a standard neurological examination, which included evaluation of radicular pain localization and intensity using a Visual Analog Scale (VAS), as well as assessment of motor deficits according to the Medical Research Council Weakness Scale (MRC).
Grouping by Disease Duration: Patients were stratified based on the duration of symptoms prior to EMG testing. The groups are as follows:
1) Less than 3 months: Patients in this group had a disease duration of fewer than 3 months before EMG testing.
2) Four to 6 months: This group included patients whose symptoms had persisted for between 4 to 6 months.
3) More than 6 months: Patients in this group had been experiencing symptoms for more than 6 months prior to the EMG.
This division was made to analyze the correlation between the duration of the disease and the electrodiagnostic and MRI findings related to the paraspinal muscles.
EMG assessmentEMG was performed via Skybox equipment (Neurosoft, Russian Federation). А coaxial bipolar electrodes (l = 75 mm, d = 0.6 mm, Neurosoft, Russian Federation) were used. The protocol included the examination of the multifidus muscles at the L5 level on the affected side and L4 level on the opposite side to evaluate the neurophysiological differences. Specifically:
Affected Side (L5): The focus was on the L5 level on the affected side to assess the impact of spinal nerve root compression. This allowed for evaluation of motor unit action potentials (MUAPs) to identify neuropathic changes associated with the nerve injury.
Opposite Side (L4): The L4 level on the opposite side served as a control to observe how the muscle’s electrical activity was affected by the unilateral radiculopathy. This comparison offered insight into how the neuromuscular function might differ in regions not directly affected by nerve compression, thus providing a baseline for understanding the changes that occur on the symptomatic side.
A sample of 20 MUAPs were extracted. MUAP duration was defined as the time between the start and end points of the MUAP when observed at a sensitivity of 100 μV/cm and a sweep screen of 10 ms/cm. We did not use the software-based assessment of MUAPs and made changes such as excluding inappropriate software-selected MUAPs. The mean and maximum amplitude and duration of MUAPs, the number of phases and interference pattern analysis (quantitative and qualitative) were investigated (Figure 1). An increase in mean MUAPs duration combined with a reduced interference pattern were classified as neurogenic findings. At least three different sites within each muscle were sampled and analyzed for MUAP morphology and recruitment.
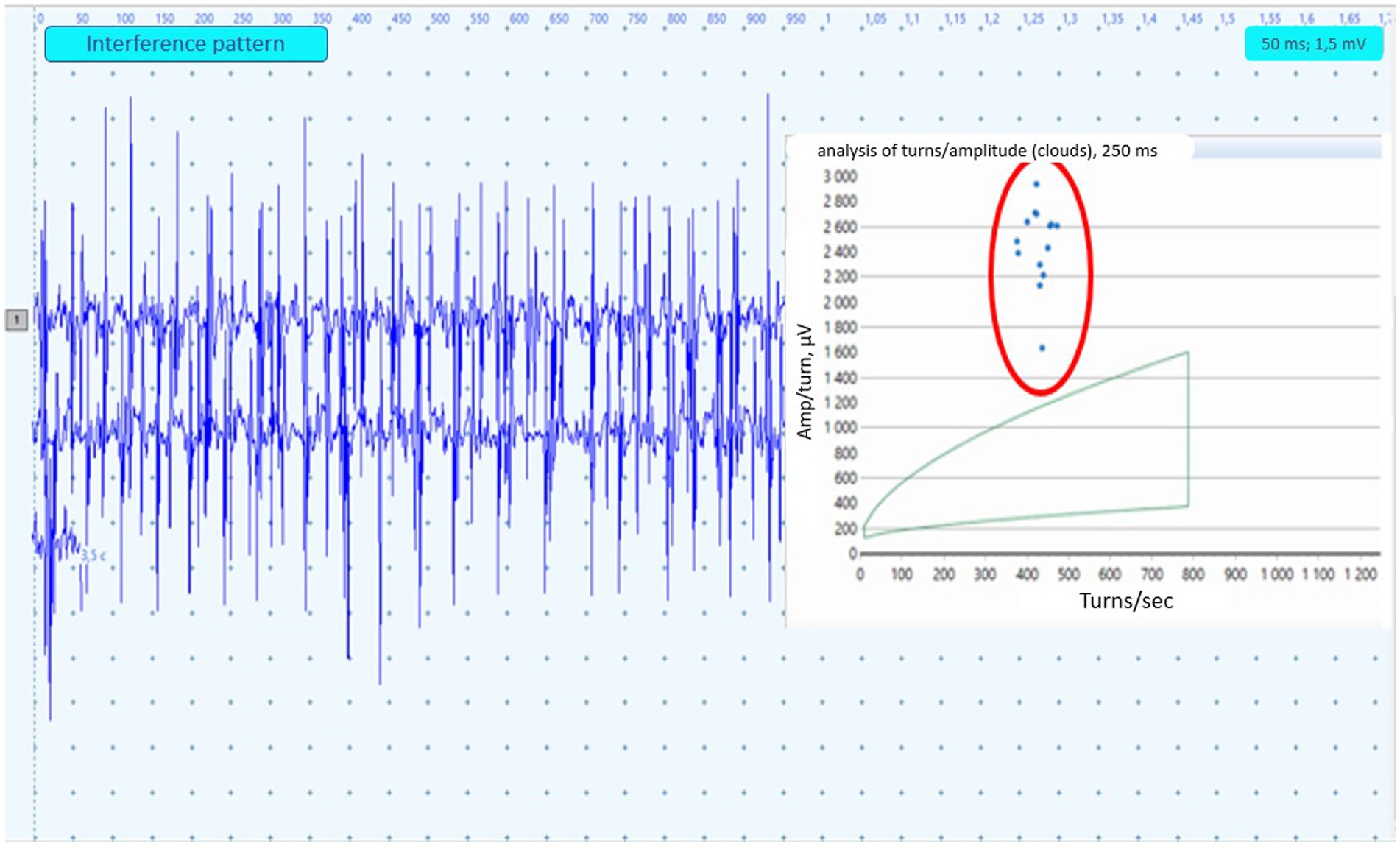
Figure 1. Example of the EMG neuropathic interference pattern in paraspinal muscles. The turns-amplitude analysis cloud corresponds to 95% confidence age-matched limits. Dots circled in red represent the result of PM analysis from 250 msec. Epochs.
The patient was in the prone position throughout the study. Muscle activation was achieved by lifting the homologous leg. The skin penetration point was located 2.5 cm lateral to the spinous process of the L4 and L5 vertebrae, correspondingly. The coaxial bipolar electrode was inserted at a 45-degree angle to the skin surface.
The reference values used for MUAPs analysis (amplitude and duration) are presented in Table 1.

Table 1. Mean MUAPs amplitude and duration in m. multifidus at L5 level in healthy people according to Marco Tomasella (10).
MRI assessmentVisual assessment of the PM structure was based on axial T2-weighted MRI sections at the level of the herniated L4-L5 disc. The severity of PM fatty infiltration was used to divide the patients into 3 groups: “Grade 0” - 0-10%, “Grade 1” - 10-50%, and “Grade 2” - more than 50%. In general, the degree of fatty infiltration of PM was previously reported to be correlated with that in isolated m. multifidus (Figure 2) (9).
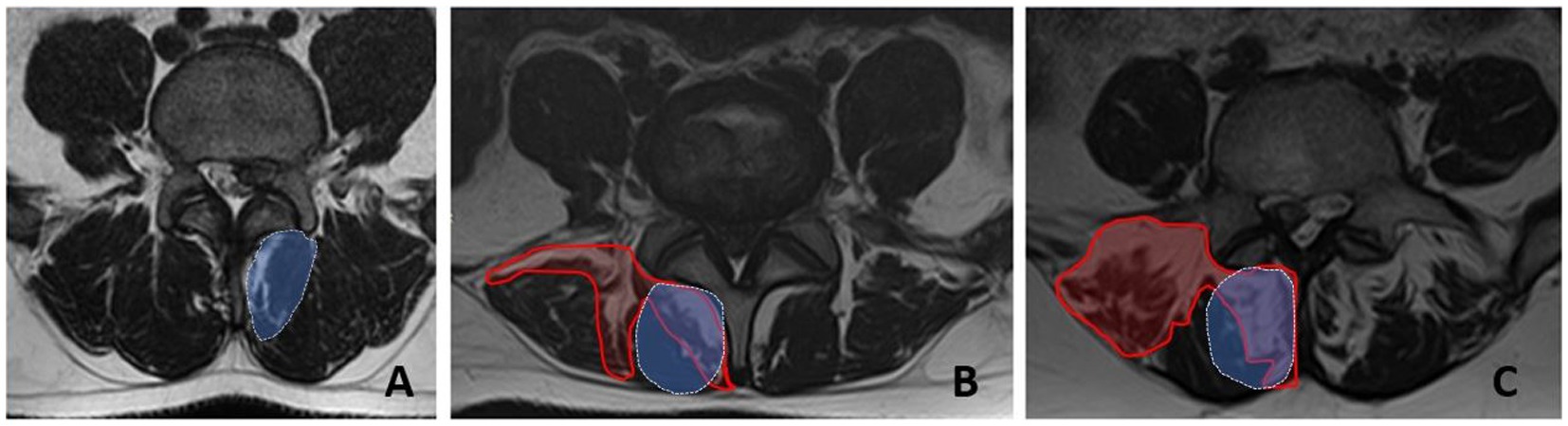
Figure 2. Fatty infiltration grades for the paraspinal muscles at the L5 level measured by MRI (axial sections). A - grade 0, B - grade 1, C - grade 2. The area of fatty infiltration is circled in red. The area of m.multifidus is circled in blue.
Statistical data processingStatistical data processing was performed using the SPSS 26 application package. Median, 25th and 75th percentiles were calculated for participants’ characteristics. The nonparametric Wilcoxon test (for pairwise comparisons) and the Friedman test (for multiple comparisons) were used to compare electromyography parameters. Chi square test was used to evaluate the differences between the groups. The regression equations linking the EMG parameters of PM (mean amplitude and duration of MUAPs at the L5 level on the side of spinal nerve root compression) with the degree of fatty infiltration of PM were estimated.
Differences were considered statistically significant at p < 0.05.
ResultsThe study cohort comprised 58 patients aged between 26 and 73 years, with a median age of 44 years (interquartile range: 41–54). Among these participants, 32 (55.2%) were female (Table 2). Radicular pain of varying severity was observed in all patients. Furthermore, motor deficits were present in 51.7% of the patients. Numbness in the L5 dermatome was reported in 26 patients (44.8%). The duration of disease before spinal surgery ranged from four to 48 weeks (Me 12, Q1-Q3:4–16).
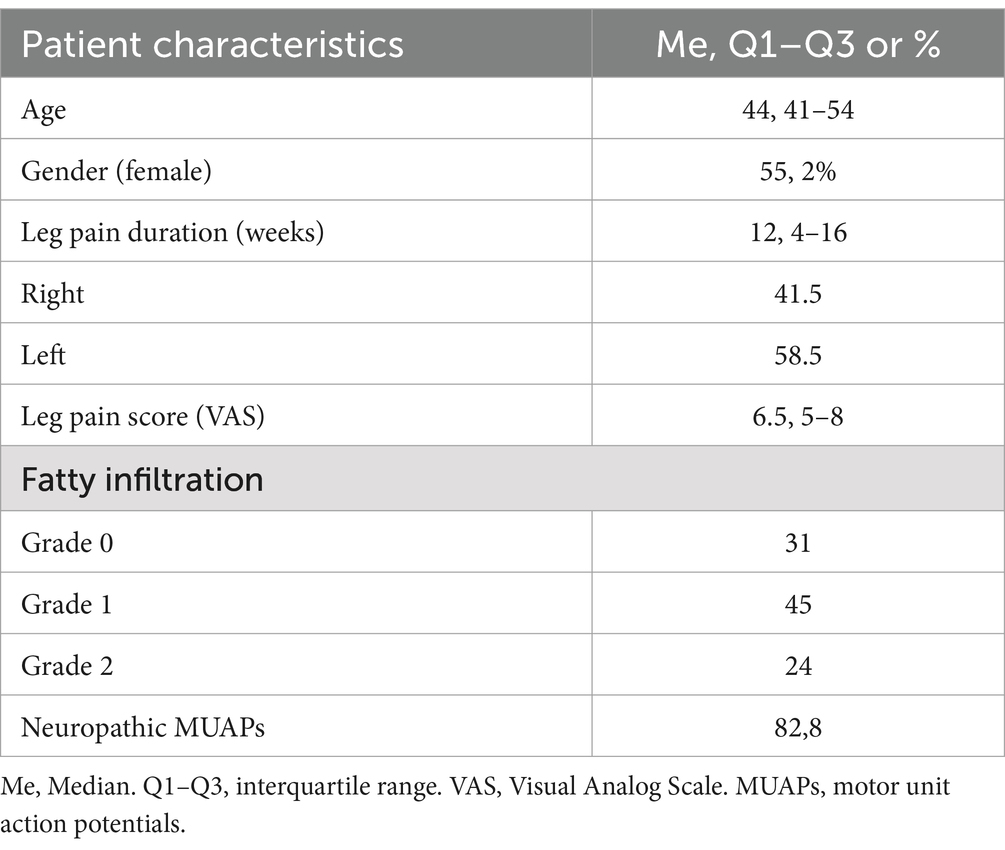
Table 2. Patients’ characteristics (n = 58).
The mean and maximal MUAPs amplitude and duration at the L5 level on the affected side and L4 level on the opposite side are presented in Table 3.
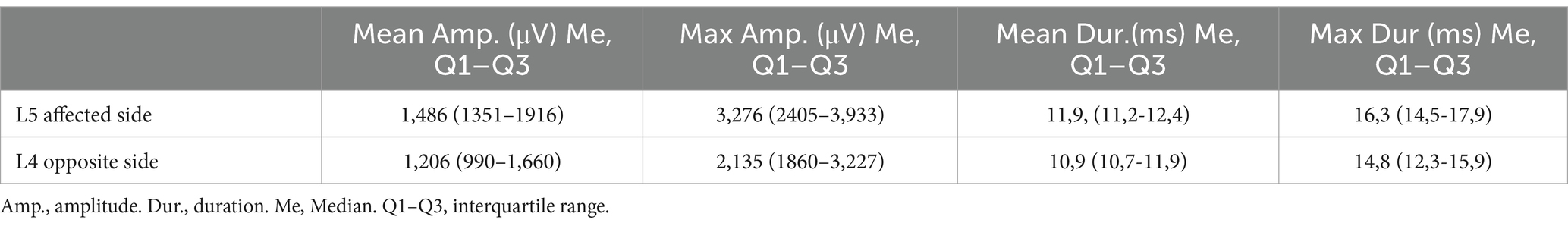
Table 3. Comparison of the mean and maximal MUAPs amplitude and duration of the paraspinal muscle at L5 level on the affected side and L4 level on the opposite side.
No statistically significant differences in the mean and maximum MUAPs amplitude at the L4 and L5 levels on the symptomatic and contralateral sides were found (p > 0.05). The mean and maximum duration of MUAPs at the L5 level on the affected side were statistically significantly different from of MUAPs at the L4 level on the opposite side (p < 0.001). The mean duration of MUAPs on the contralateral side corresponded to the normative values calculated by Marco Tomasella (10). On the symptomatic side they were abnormal and a total of 48 (82.8%) patients demonstrated a neuropathic pattern in the PM (Figure 3A).

Figure 3. (A) Distribution of patients with neurogenic remodeling of MUAPs in the paraspinal muscles. Pie chart representing the percentage of normal (blue) and neuroparhic (red) EMG pattern is shown. (B) Distribution of patients depending on the degree of PM fatty infiltration (FI). Pie chart representing the percentage of patients with Grade 0 (blue), Grade 1 (red) or Grade 2 (green) FI is shown. (C) Different degrees of PM fatty degeneration in male and female patients. Bar plots representing 26 of male (left) and 32 female (right) patients with different FI grades are shown. (D) Distribution of patients with different degrees of PM fatty infiltration and disease duration. Bar plots representing patients with different FI grades depending on the disease duration are shown. (E) Distribution of patients with different degrees of PM fatty infiltration and age. Bar plots representing patients with different FI grades depending on patient’s age are shown.
The degree of fatty infiltration was almost identical on the symptomatic and non-symptomatic sides. The PM structure did not change in 18 (31%) patients. The severity of fatty infiltration corresponded to Grade 1 in 26 (45%) patients and to Grade 2 in 14 (24%) patients (Figure 3B). Fatty infiltration (FI) in the PM was significantly more often observed in females (p < 0.05; Figure 3C). The presence of FI in the PM did not depend on the disease duration (p > 0.05; Figure 3D). In patients over 45 years old, fatty infiltration of the PM was statistically more frequent (p < 0.05; Figure 3E).
The rate of neurogenic remodeling of MUAPs was equal in patients with different degrees of FI (p > 0.05; Figure 4).
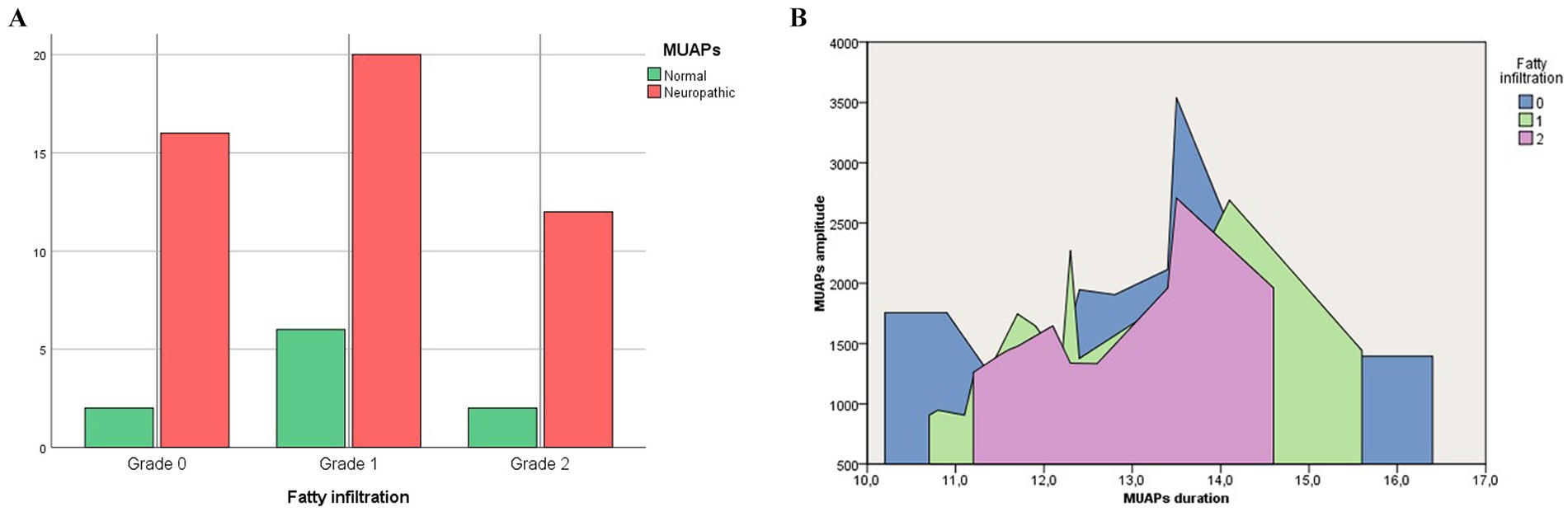
Figure 4. Rate of neurogenic remodeling of MUAPs (A) and the average amplitude (mV) and duration (ms) of MUAPs (B) in patients with different degrees of fatty infiltration.
Correlation analysis did not reveal any relationship between the severity of fatty infiltration and the presence of neurogenic remodeling of MUAPs in the PM (Table 4).
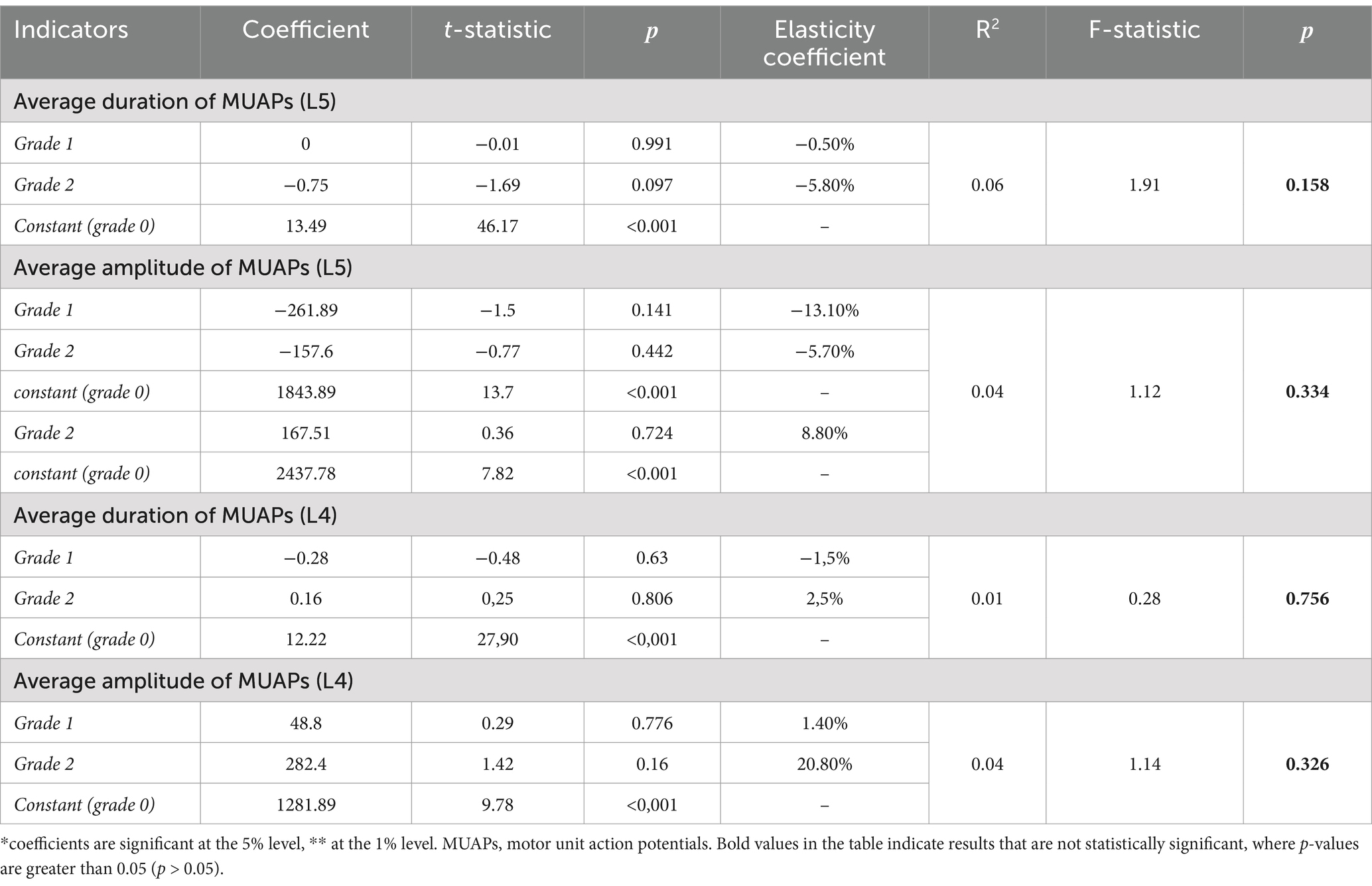
Table 4. The results of assessing regression equations relating PM EMG parameters (the mean amplitude and duration of MUAPs at the L5 level on the affected side and at the L4 level on the unaffected side) to the degree of PM fatty infiltration.
DiscussionRecent publications have described fatty infiltration of the PM as a natural ageing process (11). The development of muscle disuse atrophy is also promoted by a sedentary lifestyle. Muscles undergo adaptive remodeling in response to physical inactivity and aging (12). Several studies have shown that PM fatty infiltration can be detected both in patients with acute or chronic low back pain and healthy individuals and, therefore it is not a pain-specific feature.
Current data on the factors influencing the development of PM fatty infiltration is contradictory. For example, M. Hildebrandt et al. reported that PM fatty infiltration is not dependent on the duration of the back pain, patient’s age, sex, and body mass index (BMI) (4). On the other hand, a study by X. Peng et al. showed that PM fatty infiltration increases with age and BMI (13). According to another study by V. G. Felipe et al., the area and severity of PM fatty infiltration does not correlate with age and is more often observed in women (14).
There are two ways for adipocyte accumulation in skeletal muscles (15). First, lipids can be accumulated within myofibrils (intramyocellular lipids) and such accumulation is associated with insulin resistance, inflammation, and lack of muscle functional activity (16).
The second mechanism of myosteatosis is associated with intermuscular fat accumulation. There are several populations of stem cells in skeletal muscles, the most well-defined of which are muscle satellite cells, which lie beneath the basal lamina of muscle fibers and contribute to myogenesis during muscle regeneration. Another cell population, which was recently described, is called fibroadipogenic progenitors or mesenchymal interstitial cells, which readily differentiate into adipocytes under various conditions such as muscle injury or glucocorticoid treatment (17). Endogenous glucocorticoid levels increase with age, which may contribute to intermuscular fat deposition (18). Moreover, anxiety and depressive disorders are associated with the hypersecretion of corticotropin-releasing hormone, which leads to high levels of circulating endogenous glucocorticoids (19).
Estrogen deficiency increases skeletal muscle lipid content and adipogenic gene expression and decreases the relative share of satellite cells in ovariectomized rodents (20). In contrast, androgen deprivation therapy also increases fatty skeletal muscle infiltration in men with prostate cancer, although computed tomography does not distinguish between intramyocellular and intermuscular lipid accumulation; therefore, the actual location of lipid deposition is unclear in this case (21). Taken together, these data indicate that many conditions that induce bone marrow adipogenesis and bone loss in men and women, such as disuse atrophy, sex hormone deficiency, and changes in endogenous glucocorticoid levels, also stimulate the accumulation of adipocytes and intramyocellular lipids in skeletal muscles.
The available data may serve as a basis for further research into the correlation between hormonal status and the severity of fatty infiltration, as well as for clarifying the mechanism of myosteatosis to identify potential therapeutic strategies and prevention.
We hypothesized that the cause of fatty infiltration of the PM could be neurogenic changes due to spinal nerve root compression. After denervation, muscle passes through immediate loss of voluntary function and rapid loss of mass, increasing atrophy and muscle fiber degeneration and replacement of muscle by fibrous connective tissue and fat. In the PM, specifically in the multifidus muscles, we have identified neurogenic changes of MUAPs on the side of L5 radiculopathy. In contrast, the MUAPs parameters on the unaffected side were within normal limits, regardless of the degree of fatty infiltration. These findings allowed us to conclude that the processes of neurogenic remodeling of MUAPs in multifidus muscles and their fatty infiltration are independent.
Our study indicated that the frequency of fatty infiltration in PM was higher in females, potentially due to hormonal differences, such as estrogen deficiency, which can influence muscle lipid content.
The findings lend support to the hypothesis that more research is needed to explore the mechanisms behind fatty infiltration, to better understand their implications for treatment and prevention strategies in clinical settings.
Limitation of the studyThere may be some possible limitations in this study.
First, patients experiencing severe pain (VAS score of 9 to 10), were excluded (due to EMG intolerance), which could lead to a selection bias and limit the applicability of the results to individuals with more moderate pain levels. Additionally, participants with a disease duration of less than 4 weeks were also excluded, potentially omitting data on early-stage patients. Furthermore, the inclusion of participants who underwent spinal surgery may limit the generalizability of the findings, as the surgical context may not reflect the experiences of patients with similar conditions who have not undergone such interventions. These limitations highlight the necessity for further research to validate the findings across a more diverse population and to incorporate longitudinal assessments that capture long-term outcomes.
ConclusionThere is an absence of correlation between the degree of fatty infiltration and the presence of neuropathic EMG findings. Thus, we should probably consider fatty infiltration and neurogenic changes in the muscles as two independent processes both requiring further in-depth research.
Data availability statementThe raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statementThe study was approved by the Ethics Committee of Sklifosovsky Research Institute for Emergency Medicine No. 3–22 dated March 29, 2022. All patients signed an informed consent form to participate in the study.
Author contributionsES: Conceptualization, Investigation, Methodology, Software, Writing – original draft. MS: Methodology, Project administration, Supervision, Writing – review & editing. AG: Funding acquisition, Project administration, Resources, Supervision, Writing – review & editing.
FundingThe author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This study was sponsored by the Moscow Center for Innovative Technologies in Healthcare.
Conflict of interestThe authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s noteAll claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References1. Moore, KL, Agur, AMR, and Dalley, AF. Clinically oriented anatomy. 7th ed. Baltimore: Lippincott Williams & Wilkins. (2014). 1134.
3. Hung, CY, Wang, B, Chang, HC, Wu, WT, Liu, PT, Chang, KV, et al. Pictorial essay on ultrasound and magnetic resonance imaging of Paraspinal muscles for myofascial pain syndrome. Life (Basel). (2024) 14:499. doi: 10.3390/life14040499
PubMed Abstract | Crossref Full Text | Google Scholar
4. Hildebrandt, M, Fankhauser, G, Meichtry, A, and Luomajoki, H. Correlation between lumbar dysfunction and fat infiltration in lumbar multifidus muscles in patients with low back pain. BMC Musculoskelet Disord. (2017) 18:12. doi: 10.1186/s12891-016-1376-1
PubMed Abstract | Crossref Full Text | Google Scholar
5. Hyun, JK, Lee, JY, Lee, SJ, and Jeon, JY. Asymmetric atrophy of multifidus muscle in patients with unilateral lumbosacral radiculopathy. Spine (Phila Pa 1976). (2007) 32:E598–602. doi: 10.1097/BRS.0b013e318155837b
PubMed Abstract | Crossref Full Text | Google Scholar
6. Hides, J, Stanton, W, Mendis, MD, and Sexton, M. The relationship of transversus abdominis and lumbar multifidus clinical muscle tests in patients with chronic low back pain. Man Ther. (2011) 16:573–7. doi: 10.1016/j.math.2011.05.007
Crossref Full Text | Google Scholar
7. Zhi-Jun, H, Wen-Bin, X, Shuai, C, Zhi-Jie, Z, Feng-Dong, Z, Xiao-Jing, Y, et al. Accuracy of magnetic resonance imaging signal intensity ratio measurements in the evaluation of multifidus muscle injury and atrophy relative to that of histological examinations. Spine. (2014) 39:E623–9. doi: 10.1097/BRS.0000000000000286
PubMed Abstract | Crossref Full Text | Google Scholar
8. Seliverstova, EG, Sinkin, MV, Kordonsky, AY, Aleinikova, IB, Tikhomirov, IV, and Grin, AA. Electrodiagnostic evaluation in differential diagnosis and neurosurgical treatment of radiculopathies caused by spine disorders. Diagnostic value and methodology Zhurnal Voprosy Neirokhirurgii Imeni NN Burdenko. (2022) 86:109–18. doi: 10.17116/neiro202286021109
PubMed Abstract | Crossref Full Text | Google Scholar
9. Joseph, А, McGuinness, К, Welch, N, Coyle, J, and Franklyn-Miller, A. “Fat quantification in MRI-defined lumbar muscles.” In 4th international conference on image processing theory, tools and applications (IPTA). Paris, France; (2014).
10. Tomasella, M, Crielaard, J-M, and Wang, F-C. Dorsal and lumbar paraspinal electromyographic study. Multi-MUP analysis and drawing up normal values in a reference population. Neurophysiol Clin. (2002) 32:109–17. doi: 10.1016/s0987-7053(02)00295-2
PubMed Abstract | Crossref Full Text | Google Scholar
11. Dallaway, A, Hattersley, J, Diokno, M, Tallis, J, Renshaw, D, Wilson, A, et al. Age-related degeneration of lumbar muscle morphology in healthy younger versus older men. Aging Male. (2020) 23:1583–97. doi: 10.1080/13685538.2021.1878130
PubMed Abstract | Crossref Full Text | Google Scholar
12. Gibbons, D, McDonnell, JM, Ahern, DP, Cunniffe, G, Kenny, RA, Romero-Ortuño, R, et al. The relationship between radiological paraspinal lumbar measures and clinical measures of sarcopenia in older patients with chronic lower back pain. J Frailty Sarcopenia Falls. (2022) 7:52–9. doi: 10.22540/JFSF-07-052
PubMed Abstract | Crossref Full Text | Google Scholar
13. Peng, X, Li, X, Xu, Z, Wang, L, Cai, W, Yang, S, et al. Age-related fatty infiltration of lumbar paraspinal muscles: a normative reference database study in 516 Chinese females. Quant Imaging Med Surg. (2020) 10:1590–601. doi: 10.21037/qims-19-835
PubMed Abstract | Crossref Full Text | Google Scholar
14. Felippe, VG, Amaral, CAB, and Labronici, PJ. Correlation between low back pain due to fatty degeneration and sex and age: study by MRI. Coluna/Columna. (2021) 20:272–7. doi: 10.1590/s1808-185120212004250500
Crossref Full Text | Google Scholar
15. Hamrick, MW, McGee-Lawrence, ME, and Frechette, DM. Fatty infiltration of skeletal muscle: mechanisms and comparisons with bone marrow adiposity. Front Endocrinol (Lausanne). (2016) 7:69. doi: 10.3389/fendo.2016.00069
PubMed Abstract | Crossref Full Text | Google Scholar
16. Rivas, DA, McDonald, DJ, Rice, NP, Haran, PH, Dolnikowski, GG, and Fielding, RA. Diminished anabolic signaling response to insulin induced by intramuscular lipid accumulation is associated with inflammation in aging but not obesity. Am J Phys Regul Integr Comp Phys. (2016) 310:R561–9. doi: 10.1152/ajpregu.00198.2015
PubMed Abstract | Crossref Full Text | Google Scholar
17. Dong, Y, Silva, KA, Dong, Y, and Zhang, L. Glucocorticoids increase adipocytes in muscle by affecting IL-4 regulated FAP activity. FASEB J. (2014) 28:4123–32. doi: 10.1096/fj.14-254011
PubMed Abstract | Crossref Full Text | Google Scholar
18. Zhou, H, Cooper, MS, and Seibel, MJ. Endogenous glucocorticoids and bone. Bone Res. (2013) 1:107–19. doi: 10.4248/BR201302001
Crossref Full Text | Google Scholar
19. Schulkin, J. Evolutionary conservation of glucocorticoids and corticotropin releasing hormone: behavioral and physiological adaptations. Brain Res. (2011) 1392:27–46. doi: 10.1016/j.brainres.2011.03.055
PubMed Abstract | Crossref Full Text | Google Scholar
20. Frechette, DM, Krishnamoorthy, D, Adler, BJ, Chan, ME, and Rubin, CT. Diminished satellite cells and elevated adipogenic gene expression in muscle as caused by ovariectomy are averted by low-magnitude mechanical signals. J Appl Physiol. (2015) 119:27–36. doi: 10.1152/japplphysiol.01020.2014
Crossref Full Text | Google Scholar
21. Chang, D, Joseph, DJ, Ebert, MA, Galvão, DA, Taaffe, DR, Denham, JW, et al. Effect of androgen deprivation therapy on muscle attenuation in men with prostate cancer. J Med Imaging Radiat Oncol. (2014) 58:223–8. doi: 10.1111/1754-9485.12124
留言 (0)