Psychiatric disorders, including schizophrenia (SCZ), bipolar disorder (BD), and depression (D), lack quantifiable biomarkers for diagnosis (1–3). These disorders affect approximately 10.7% of the global population, yet diagnostic processes remain subjective (4–7). Treatment is further complicated by severe adverse reactions and resistance to antipsychotic medications (8). Identifying objective biomarkers is crucial to improving diagnosis and understanding these conditions.
Recent research has explored underlying mechanisms of psychiatric disorders to better understand the pathophysiology and identify biomarkers, especially in early disease (9). Studying the neurological foundations of psychiatric disorders can facilitate the development of innovative treatments and improve diagnostic accuracy (10). Chronic low-level inflammation, characterized by a diminished yet ongoing reaction, is commonly observed in emotional, psychiatric, personality, and neurotic conditions (11). Elevated levels of pro-inflammatory cytokines, oxidative stress markers, chemokines, and soluble adhesion molecules have been observed with higher concentrations in the blood and cerebrospinal fluid of individuals with SCZ and BD (12). Similar associations have been reported with cytokines and neuropeptides in depression (13). Biomarker identification for diagnosing and monitoring treatment responses in psychiatric disorders is an essential research focus (9). Serum inflammatory biomarkers offer promising avenues for molecular diagnostics.
The pathogenesis of these illnesses has drawn increased attention to the role of inflammation in recent studies, suggesting a potential link between immune dysregulation and psychiatric symptoms. Specifically, serum inflammatory biomarkers are being explored to understand the causes of the early onset of psychiatric disorder and identify promising diagnostic candidates. Proposed hematological parameters include neutrophil-to-lymphocyte ratio (NLR), systemic immune-inflammation index (SII), systemic inflammation response index (SIRI), aggregate index of systemic inflammation (AISI), lymphocyte/high-density lipoprotein (HDL-C) ratio (LHR), monocytes/HDL-C ratio (MHR), neutrophils/HDL-C ratio (NHR), and platelet/HDL-C ratio (PHR) (14). These cost-effective parameters derived from routine complete blood counts and biochemical assays reflect inflammation and immune balance (15). Although inflammation’s role in mental health is better understood, the diagnostic potential of these serological markers in first-episode psychiatric disorders requires further exploration.
Several studies have examined single or combined indicators in psychiatric disorders (16–18). This study aims to evaluate whether these biomarkers can serve as predictive or early diagnostic tools for psychiatric disorders and enhance understanding of their pathophysiology. We hypothesize that biomarker levels will significantly differ between patients with first-episode psychiatric disorders and healthy controls, with some biomarkers acting as reliable predictors.
2 Materials and methods2.1 Study design and participantsThis retrospective cross-sectional study included patients with SCZ, BD, and D treated at the Mental Health Center of Kunming Medical University from January 2021 to December 2023.
2.1.1 SettingThe study was conducted at the Mental Health Center of Kunming Medical University, which provides comprehensive mental health services.
2.1.2 ParticipantsPatients diagnosed with SCZ, BD, and D based on ICD-10 criteria by psychiatry specialists were included. Inclusion criteria were (1): diagnosis of SCZ, BD, and D based on ICD-10 standards during the first visit and in the acute stage; (2) the presence of complete blood count parameters along with biochemical information; and (3) individuals aged 18 to 65 years at the moment of blood testing. Exclusion criteria were: (1) inflammatory or immunodeficiency disorders, systemic or localized inflammatory diseases, and (2) severe physical illness. Healthy controls (C) were aged between 18 and 65 during the blood analysis and had available biochemical and complete blood count data. Control participants were matched to experimental groups based on sample size.
2.1.3 VariablesThe primary variables were sociodemographic data and hematological parameters. Blood testing: Hematology specimens were tested using standard laboratory equipment (LABOSPECT 008AS, Sysmex XN 1000) following the laboratory’s standard operating procedures to ensure consistency and accuracy. Serum inflammatory biomarkers were calculated using the following formulas: NLR = Neutrophil Count (109/L)/Lymphocyte Count (109/L), SII = Platelet Count (109/L) × Neutrophil Count (109/L)/Lymphocyte Count (109/L), SIRI = Neutrophil Count (109/L) × Monocyte Count (109/L)/Lymphocyte Count (109/L), AISI = Platelet Count (109/L) × Neutrophil Count (109/L) × Monocyte Count (109/L)/Lymphocyte Count (109/L), LHR = Lymphocyte Count (109/L)/HDL-C (mmol/L), MHR = Monocyte Count (109/L)/HDL-C (mmol/L), NHR = Neutrophil Count (109/L)/HDL-C (mmol/L), PHR = Platelet Count (109/L)/HDL-C (mmol/L). Confounding factors, such as age and gender, were also considered.
2.1.4 Data sources/measurementData were extracted from electronic medical records and the Laboratory Information System. Blood samples were collected between 6:00 and 7:30 AM under standardized protocols. Diagnostic data were cross-verified with ICD-10 criteria to minimize selection and information bias, and inclusion/exclusion criteria were strictly applied. Consistent sampling times and standardized diagnostic procedures ensured reliability.
2.2 Ethics statementThe low-risk retrospective observational study was approved by the Ethics Committee of the Mental Health Center of Kunming Medical University (approval number: YNJS-20230615-001). Informed consent was not required, and all data were anonymized to ensure confidentiality.
2.3 Statistical methodsStatistical analyses were conducted using SPSS 23.0. The Kolmogorov-Smirnov test was utilized to assess data normality. For non-normally distributed data and unequal variances, the Kruskal-Wallis test was applied, while one-way analysis of variance (ANOVA) was used for normally distributed data. Post hoc multiple comparisons were conducted using Bonferroni calibration. Descriptive statistics were analyzed with the chi-squared test, and continuous data are presented as mean ± standard deviation. Univariate analysis identified disease risk factors, with variables showing statistical significance (p < 0.05) included in multivariate logistic regression. Binary logistic regression was used to adjust for confounding variables such as gender and age. Receiver operating characteristic (ROC) curve analysis evaluated diagnostic factors, with the Youden index optimized to determine the ideal cutoff value. To minimize the influence of highly variable MHR values, MHR was scaled by multiplying by 10 (19). Combined measurement models constructed via binary logistic regression were assessed for their diagnostic application. A p-value of < 0.05 was considered statistically significant.
3 Results3.1 Participant characteristicsBased on electronic medical records, the preliminary counts of eligible patients were 212 in the D group, 358 in the SCZ group, and 80 in the BD group. After excluding participants with incomplete data, the final analysis included 335 patients with SCZ, 68 with BD, 202 with D, and 282 as C. The SCZ group had an average age of 36.030 ± 13.43 years, consisting of 150 males and 185 females. The BD group had an average age of 34.882 ± 14.547 years, comprising 31 males and 37 females. The D group had an average age of 34.921 ± 13.772 years, comprising 70 male cases and 132 female cases. The C group, averaging 35.755 ± 7.172 years, comprised 108 males and 174 females. Statistical analysis showed no significant differences in age and sex between the patient and C groups (p > 0.05). There were no statistically significant age differences among the patient groups (p > 0.05). However, sex differences were statistically significant between the SCZ and D groups (p < 0.05), with a higher proportion of females in the D group (65%) compared to the SCZ group (55%). No statistically significant sex differences were observed among the other patient groups (p > 0.05).
3.2 Analysis of inflammatory biomarkersIn contrast to the C group, the SCZ group showed higher levels of NLR, NHR, LHR, MHR, PHR, AISI, SII, SIRI, white blood cell (WBC), neutrophils, and monocytes, while cholesterol (CHO), triglycerides (TG), HDL-C, and LDL-C were reduced (p < 0.05). Similarly, the BD group also exhibited increased levels of SII, SIRI, NHR, NLR, AISI, LHR, MHR, PHR, WBC, neutrophils, and monocytes, along with lower CHO, HDL-C, and LDL-C compared with the C-group (p < 0.05). The D group showed decreased CHO, TG, HDL-C, and LDL-C levels and elevated SIRI, NHR, LHR, MHR, PHR, AISI, WBC, and monocytes compared with the C group (p < 0.05).
Comparing the indicators between the SCZ group and those in the BD group, no statistically significant difference was found (p > 0.05). SII, SIRI, NHR, NLR, AISI, WBC, and neutrophils were all higher in the SCZ group than in the D group, while LHR levels were lower in the D group (p < 0.05). The BD group had greater levels of SIRI, NLR, AISI, WBC, neutrophils, and monocytes than the D group, whereas the D group had lower PHR levels. Detailed results for all variables are summarized in Table 1 and Figure 1.
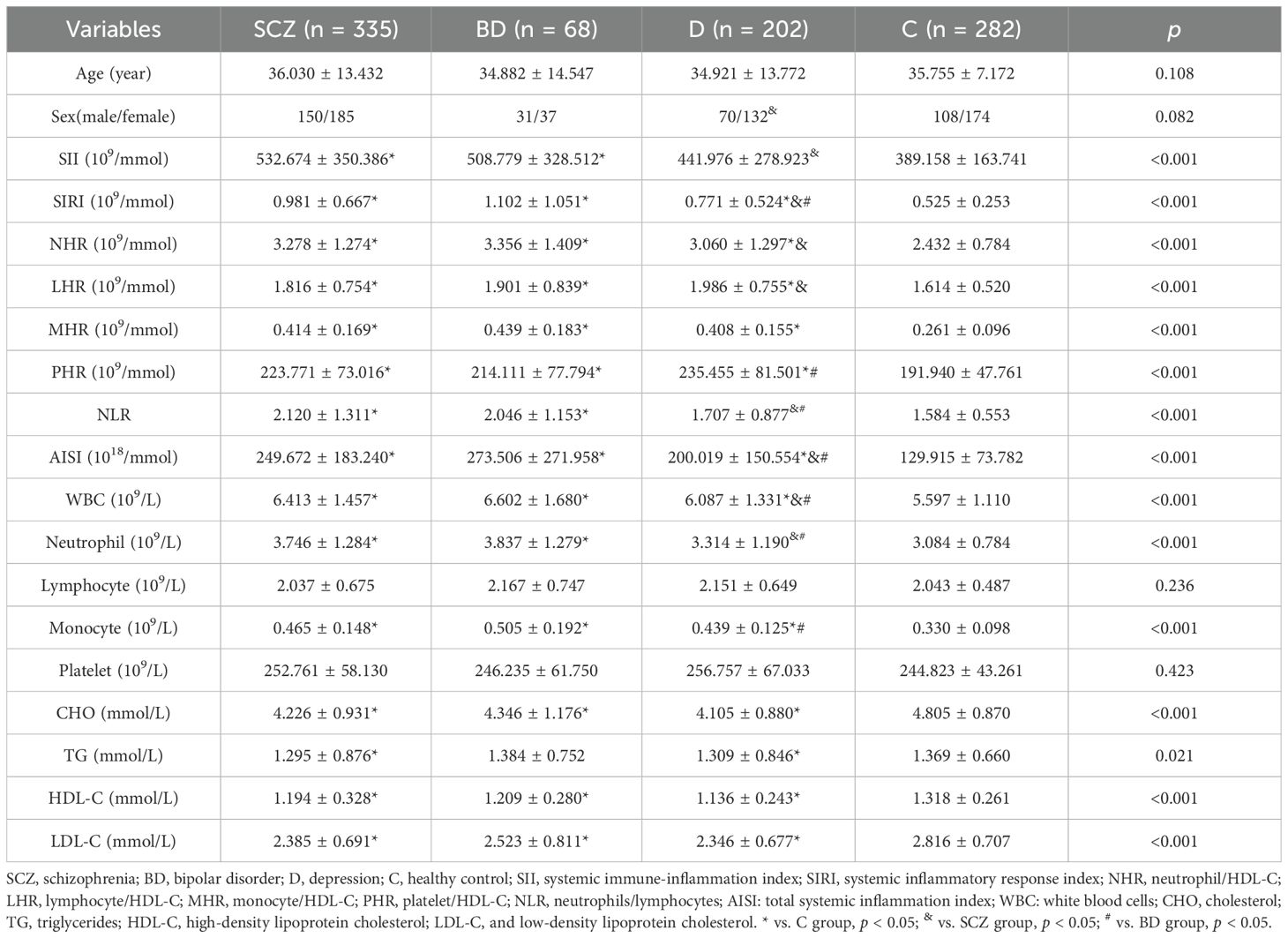
Table 1. shows how the laboratory and demographic traits of healthy controls (C), bipolar disorder (BD), depression (D), and schizophrenia (SCZ) differ from one other.
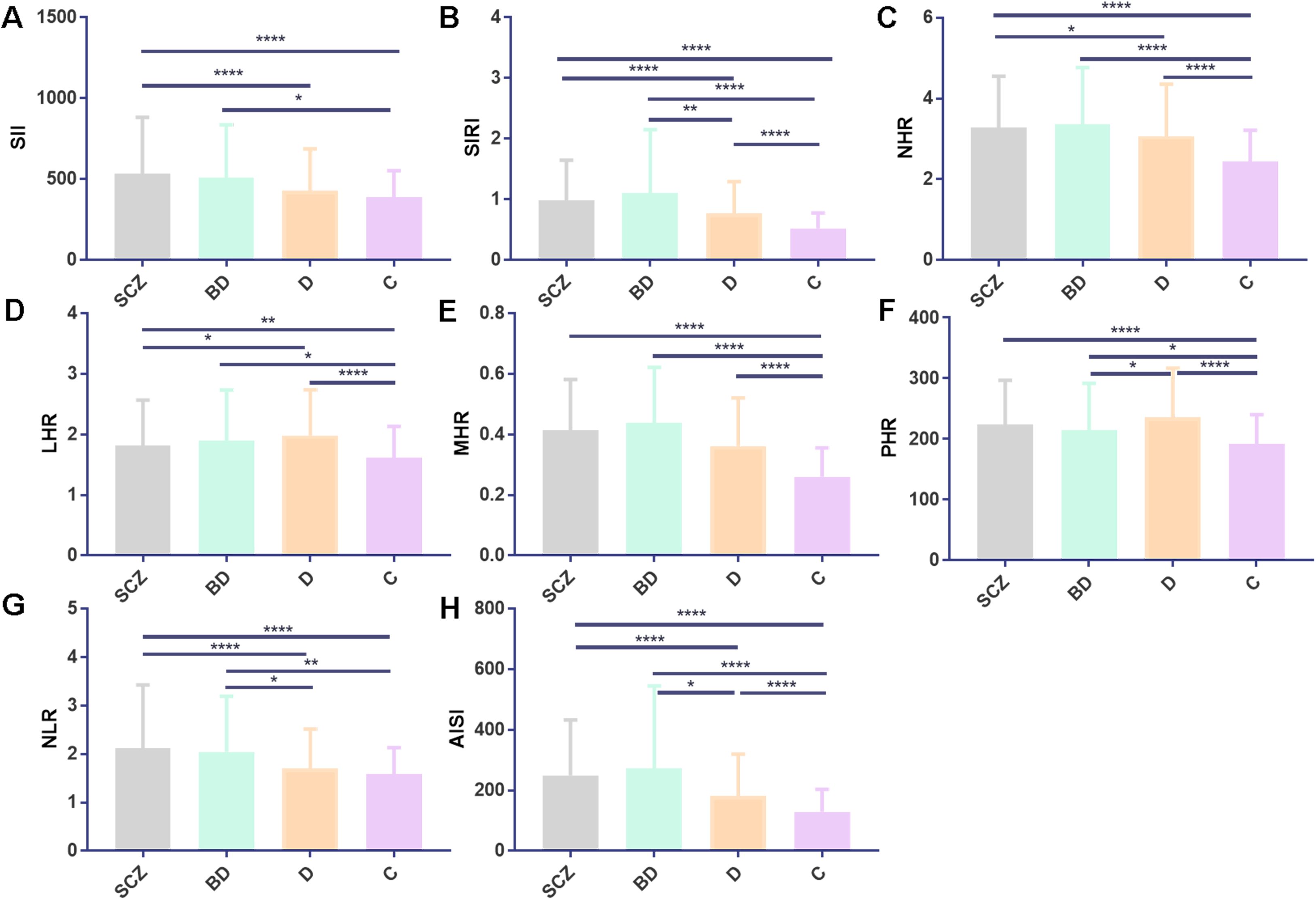
Figure 1. SCZ, BD, D, and C inflammatory ratios comparison. (A–H) depict comparisons for SII, SIRI, NHR, LHR, MHR, PHR, NLR, and AISI. Statistical significance is denoted as follows: *p < 0.05, **p < 0.01, and ****p < 0.0001. All values are presented as mean ± standard deviation. Abbreviations used include SCZ, schizophrenia; BD, bipolar disorder; D, depression; C, healthy control; SII, systemic immune-inflammation index; SIRI, systemic inflammation response index; NHR, neutrophil/HDL-C; LHR, lymphocyte/HDL-C; MHR, monocyte/HDL-C; PHR, platelet/HDL-C; NLR, neutrophils/lymphocytes; and AISI, aggregate index of systemic inflammation.
3.3 Comparison of inflammatory biomarkers between bipolar manic and bipolar depressive episodesThe BD group included 36 individuals with bipolar depressive episodes (BD-D) and 22 individuals with bipolar manic episodes (BD-M). No significant age or gender differences were observed between patient groups and controls, nor within patient subgroups. When comparing the BD-D group with the D group, the BD-D group exhibited higher triglyceride (TG) levels and lower PHR levels (p < 0.05). Detailed findings are presented in Table 2.
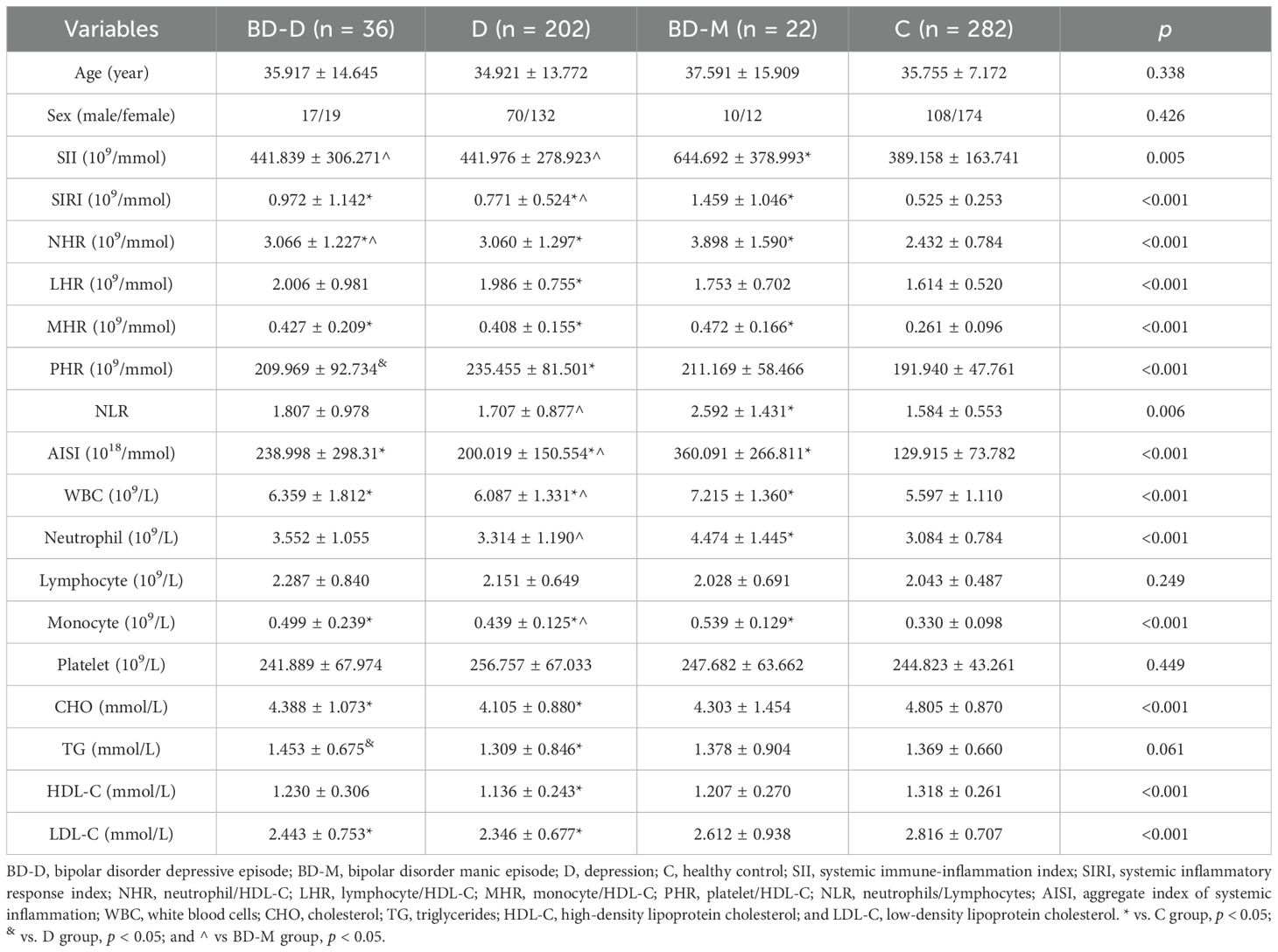
Table 2. Comparison of demographic and laboratory characteristics between bipolar disorder depressive episodes (BD-D), bipolar disorder manic episodes (BD-M), depression (D), and healthy control group (C).
3.4 The contributing factors of SCZ, BD, and D incidence were analyzed using both single-factor and multivariate logistic regressionSignificant variables from univariate logistic regression were included in the multivariate model to mitigate confounding effects to derive adjusted OR. The multivariate analysis identified independent associations between NHR, LHR, MHR, and NLR. Specifically, LHR, MHR, and NLR positively associated with SCZ, while NHR showed a negative association. Moreover, MHR exhibited an independent positive association with BD. Regarding D, NHR, MHR, and sex were identified as independent risk factors, with MHR showing a positive association and NHR showing a negative association. Detailed results are presented in Table 3.

Table 3. Results of SCZ, B, and D binary logistic regression analysis.
3.5 ROC curve analysisIn SCZ, NHR [area under the curve (AUC) 0.703 (0.662–0.743), p < 0.001, critical value 2.831, sensitivity 74.5%, specificity 57.6%] and MHR [AUC 0.790 (0.755–0.826), p < 0.001, critical value 3.15, sensitivity 75.5%, specificity 69.6%] demonstrated AUC values > 0.6, as indicated by the ROC curve. NLR showed AUC 0.625 (0.581–0.669), p < 0.001, critical value 2.123, sensitivity 89.7%, and specificity 35.8%. However, LHR had AUC values < 0.6. The diagnostic efficiency was enhanced by the indicator combination model (NHR + MHR + NLR), showing an AUC value of 0.846 (0.816–0.876), with statistical significance (p < 0.001), a critical value of 0.550, a sensitivity of 82.6%, and a specificity of 72.5%. The data are presented in Figure 2A.
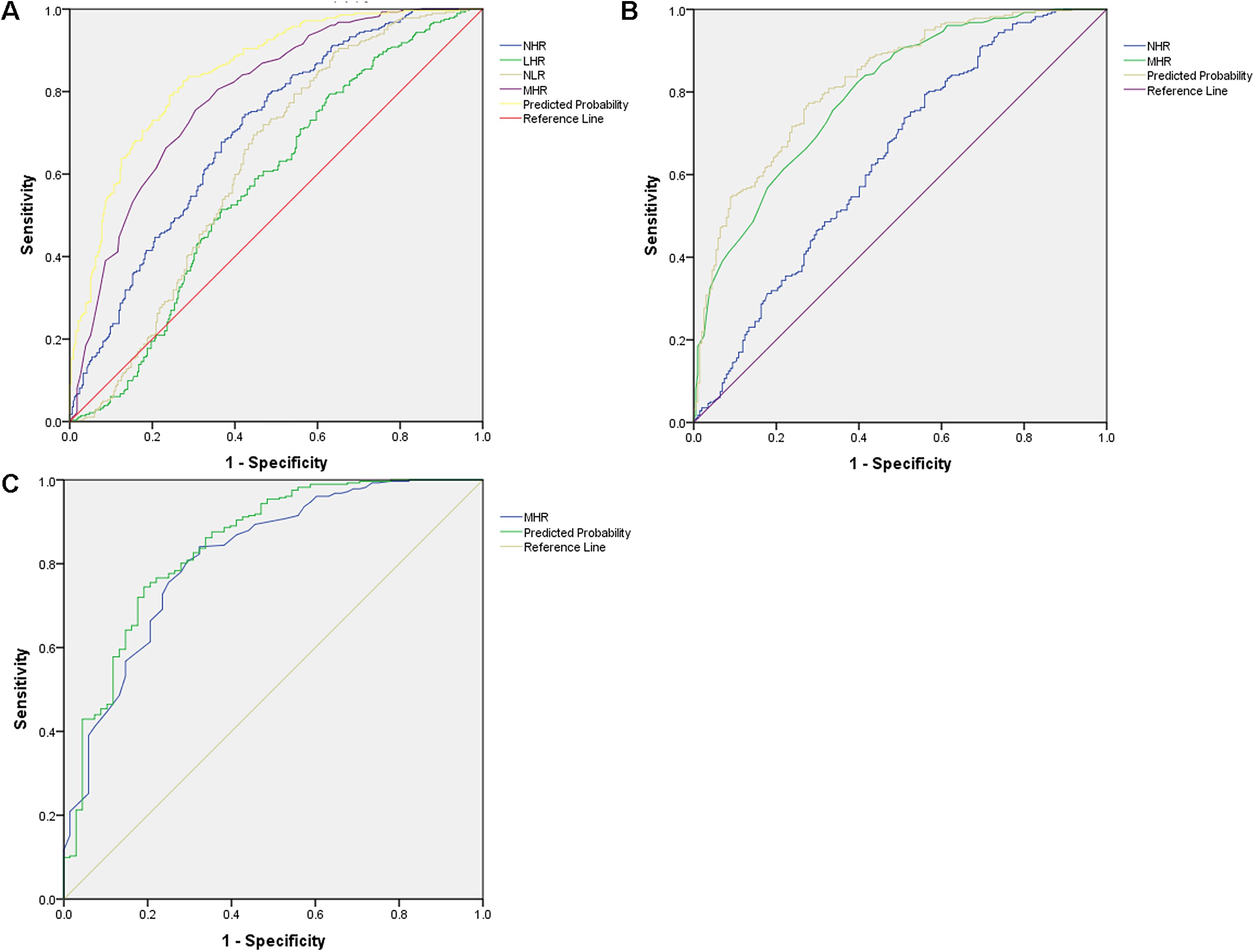
Figure 2. ROC curve analysis of inflammatory markers in predicting psychiatric disorder. (A) ROC curve for SCZ, NHR [AUC 0.703 (0.662–0.743), p < 0.001, critical value 2.831]; MHR [AUC 0.790 (0.755–0.826), p < 0.001, critical value 3.15]; NLR [AUC 0.625 (0.581–0.669), p < 0.001, critical value 2.123]; the AUCs of LHR are lower than 0.6. Indicator combination model (NHR + MHR + NLR) [AUC 0.846 (0.816–0.876), p < 0.001, critical value 0.550]. (B) ROC curve for D, NHR [AUC 0.638 (0.586–0.689), p < 0.001, critical value 2.984]; MHR [AUC 0.795 (0.755–0.834), p < 0.001, critical value 3.450]. Indicator combination model (NHR + MHR) [AUC 0.824 (0.787–0.861), p < 0.001, critical value 0.398]. (C) ROC curve for BD, MHR [AUC 0.816 (0.758–0.875), p < 0.001, critical value 3.550].
For D, NHR showed an AUC of 0.638 (0.586–0.689), with statistical significance (p < 0.001), critical value 2.984, sensitivity 79.4%, and specificity 44.1%. MHR showed a higher AUC of 0.795 (0.755–0.834), with statistical significance (p < 0.001), a critical value of 3.450, a sensitivity of 82.3%, and a specificity of 60.4%. The combined model (NHR + MHR) enhanced diagnostic parameters, yielding an AUC of 0.824 (0.787–0.861), with statistical significance (p < 0.001), a critical value of 0.398, a sensitivity of 77.3%, and a specificity of 72.3%. These findings are illustrated in Figure 2B.
For BD, MHR achieved an AUC of 0.816 (0.758–0.875), with statistical significance (p < 0.001), a critical value of 3.550, a sensitivity of 84%, and a specificity of 67.6%. The data are presented in Figure 2C.
4 DiscussionRecent studies have identified SII, SIRI, AISI, NLR, LHR, MHR, NHR, and PHR as promising inflammation biomarkers. These markers are accessible, cost-effective, and clinically valuable (20, 21). However, their relationship with major first-episode psychiatric disorders remains unexplored. To the best of our knowledge, few studies have examined the association between these biomarkers and first-episode psychiatric disorders. Our retrospective cross-sectional study highlights significant alterations in these biomarkers among patients such patients, suggesting their potential diagnostic utility.
When compared with the C group, there were significant differences in multiple indicators in the SCZ group, BD group, and D group. The SCZ group had higher SII, SIRI, NHR, LHR, MHR, PHR, NLR, and AISI levels than the C group. The BD group also showed higher levels of these biomarkers than the C group. The D group also demonstrated higher SIRI, NHR, LHR, MHR, PHR, and AISI levels than the C group. These findings suggest a generalized inflammatory response across patient populations with first-episode psychiatric disorders, which may contribute to disease onset and progression.
Multivariate logistic regression analysis revealed that NHR, LHR, MHR, and NLR were independently associated with SCZ. MHR was found to be independently associated with BD, while NHR, MHR, and sex were identified as independent risk factors for D. Specifically, MHR, NHR, and NLR effectively distinguished SCZ from C, with MHR alone differentiating BD from C. For D, NHR and MHR served as reliable predictors. Notably, combined indicator models outperformed single-indicator models, with higher AUC values, underscoring the superior predictive power of combined biomarker assessments.
These findings have important clinical implications. Elevated inflammatory biomarkers may guide the development of targeted anti-inflammatory therapies and provide a foundation for personalized treatment strategies. Monitoring these biomarkers could also aid in evaluating therapeutic efficacy and tracking disease progression, offering a more dynamic approach to managing first-episode psychiatric disorders.
When directly compared, no significant statistical differences were noted between the BD and SCZ cohorts. However, the SCZ group displayed elevated levels of several inflammatory biomarkers, including SII, SIRI, NHR, NLR, AISI, WBC, and neutrophils, compared to the D group. In contrast, the SCZ group had lower LHR levels. Similarly, the BD group exhibited higher levels of SIRI, NLR, AISI, white blood cells, neutrophils, and monocytes but lower levels of PHR than the D group. Subgroup analysis within the BD cohort revealed that patients in the BD-D group had higher TG levels and lower PHR levels than the D group. These findings underscore differences in biomarkers and lipid metabolism among patient groups, providing insights into the heterogeneity of biological characteristics and disease manifestations.
NHR and MHR represent key indicators of the interplay between systemic inflammation and lipid metabolism. NHR reflects the dynamic relationship between neutrophils and HDL-C. Neutrophils, when activated, produce oxidants through enzymatic activity, promoting the oxidation of HDL-C and impairing its CHO efflux capability (22). Conversely, HDL-C suppresses neutrophil activation, adhesion, proliferation, and migration (23). Mendelian randomization studies have established a positive association between SCZ and leukocyte counts, including neutrophils (OR 1.013, p = 0.004) and monocytes (OR 1.018, p = 4.60 × 10-4) (24). Additionally, post-hoc analyses have reported elevated immature neutrophil levels in SCZ and BD, positively correlating with TG and inversely correlating with HDL-C (25). MHR signifies the interaction between monocytes and HDL-C levels, reflecting immune and metabolic interactions. Notably, significant differences in immune-related gene expression within monocytes have been documented in SCZ patients, implicating cell heterogeneity and immune dysregulation in the disease pathogenesis (26). Consistent with findings by Zhu et al. (27), in a Chinese cohort, our study observed significantly elevated NLR levels in patients with SCZ, reinforcing the inflammation hypothesis of SCZ. Similarly, Wei et al. (21, 28) demonstrated that MHR was significantly elevated in patients with BD compared to controls. In contrast, MHR was elevated in patients with D. These findings suggest that NHR, MHR, and NLR hold diagnostic promise in distinguishing and understanding the pathophysiological mechanisms of first-episode psychiatric disorders.
We identified dyslipidemia as a significant factor associated with psychiatric disorders. Specifically, we observed that patients with psychiatric disorders such as SCZ, BD, and D had abnormal levels of blood lipid indicators and lower blood lipid levels. SCZ and D patients had lower CHO, TG, HDL-C, and LDL-C levels, while patients with BD showed lower levels of CHO, HDL-C, and LDL-C. These alterations may result from common features of psychiatric disorders, such as malnutrition, anorexia, and metabolic dysregulation. Dyslipidemia may also affect nervous system function through neurotransmitter synthesis and release pathways, nerve cell membrane stability, and neuroinflammatory responses. Lipids are integral to cellular processes, including neurotransmission, neuroplasticity, neural projection myelination, energy metabolism, and neuroinflammation (29). Serum lipid profiles have also been associated with cognitive functioning in patients with SCZ, with TC and TG levels negatively correlated with cognitive scores. Serum lipid profiles may, therefore, serve as indicators for evaluating SCZ symptoms (30, 31). Beyond blood lipid abnormalities, disruptions in other lipid functions have been implicated in SCZ pathophysiology (32). In major depressive disorder, the MANF/EWSR1/ANXA6 pathway has been suggested as a potential link between hypolipidemia and major depression (33). Dyslipidemia in psychiatric disorders is further complicated by the effects of pharmacologic treatments, such as antipsychotics and antidepressants, which can alter lipid metabolism (34–36). These findings emphasize the need for mechanistic studies exploring lipid dysregulation’s role in psychiatric disorders and evaluating whether lipid-level adjustments could aid in preventing and treating these conditions.
However, this study has limitations. As a single-center retrospective analysis, the sample size may not be sufficient to establish definitive causal associations between identified biomarkers and psychiatric pathophysiology. This limitation introduces potential selection bias, restricting the generalizability of the findings. Although our study revealed strong associations between biomarkers and first-episode psychiatric disorders, further research is needed to validate these markers as distinct risk factors. Additionally, the study lacks long-term follow-up data and dynamic monitoring of inflammatory biomarkers. Consequently, it remains uncertain whether changes in these markers correlate with disease severity and progression. Future prospective studies with larger, more diverse sample sizes and longitudinal designs are critical to addressing these limitations. Such investigations could further elucidate the clinical utility of these biomarkers in diagnosing and managing first-episode psychiatric disorders and assess the therapeutic potential of targeting lipid and inflammatory dysregulation.
In conclusion, immune-inflammatory reactions and dyslipidemia are significant factors in the development of psychiatric disorders such as SCZ, BD, and D. These findings enhance our understanding of these conditions and offer insights for future prevention and treatment strategies. Further investigation is required to validate these findings, explore potential molecular pathways, and thoroughly evaluate their therapeutic application.
Data availability statementThe raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statementThe studies involving humans were approved by Mental Health Center of Kunming Medical University. The studies were conducted in accordance with the local legislation and institutional requirements. The ethics committee/institutional review board waived the requirement of written informed consent for participation from the participants or the participants’ legal guardians/next of kin because low-risk retrospective observational study.
Author contributionsMQ: Conceptualization, Data curation, Methodology, Writing – original draft. CZ: Software, Visualization, Writing – original draft. HZ: Formal Analysis, Writing – original draft, Data curation. HC: Formal Analysis, Writing – original draft. YL: Writing – original draft, Investigation. PL: Conceptualization, Data curation, Writing – original draft. SZ: Conceptualization, Funding acquisition, Supervision, Writing – review & editing.
FundingThe author(s) declare that financial support was received for the research, authorship, and/or publication of this article. Yunnan Provincial Department of Science and Technology Kunming Medical University Applied Basic Research Joint Special Fund Project (202401AY070001-313).
AcknowledgmentsWe thank all of the participants for their commitment to this study.
Conflict of interestThe authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s noteAll claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References1. Du Z, Guo S, Sun Y, Zhou Q, Jiang Y, Shen Y, et al. Causal relationships between dietary habits and five major mental disorders: A two-sample mendelian randomization study. J Affect Disord. (2023) 340:607–15. doi: 10.1016/j.jad.2023.08.098
PubMed Abstract | Crossref Full Text | Google Scholar
2. Sullivan PF, Geschwind DH. Defining the genetic, genomic, cellular, and diagnostic architectures of psychiatric disorders. Cell. (2019) 177:162–83. doi: 10.1016/j.cell.2019.01.015
PubMed Abstract | Crossref Full Text | Google Scholar
3. Huang Y, Wang Y, Wang H, Liu Z, Yu X, Yan J, et al. Prevalence of mental disorders in China: A cross-sectional epidemiological study. Lancet Psychiatry. (2019) 6:211–24. doi: 10.1016/s2215-0366(18)30511-x
PubMed Abstract | Crossref Full Text | Google Scholar
5. Global, regional, and national burden of neurological disorders, 1990-2016: A systematic analysis for the global burden of disease study 2016. Lancet Neurol. (2019) 18:459–80. doi: 10.1016/s1474-4422(18)30499-x
PubMed Abstract | Crossref Full Text | Google Scholar
6. Baingana F, al'Absi M, Becker AE, Pringle B. Global research challenges and opportunities for mental health and substance-use disorders. Nature. (2015) 527:S172–7. doi: 10.1038/nature16032
PubMed Abstract | Crossref Full Text | Google Scholar
7. Kessler RC, Demler O, Frank RG, Olfson M, Pincus HA, Walters EE, et al. Prevalence and treatment of mental disorders, 1990 to 2003. New Engl J Med. (2005) 352:2515–23. doi: 10.1056/NEJMsa043266
PubMed Abstract | Crossref Full Text | Google Scholar
8. Chen Y, Guan W, Wang ML, Lin XY. Pi3k-akt/mtor signaling in psychiatric disorders: A valuable target to stimulate or suppress? Int J Neuropsychopharmacol. (2024) 27(2):pyae010. doi: 10.1093/ijnp/pyae010
PubMed Abstract | Crossref Full Text | Google Scholar
10. Cao KX, Ma ML, Wang CZ, Iqbal J, Si JJ, Xue YX, et al. Tms-eeg: an emerging tool to study the neurophysiologic biomarkers of psychiatric disorders. Neuropharmacology. (2021) 197:108574. doi: 10.1016/j.neuropharm.2021.108574
PubMed Abstract | Crossref Full Text | Google Scholar
13. Harsanyi S, Kupcova I, Danisovic L, Klein M. Selected biomarkers of depression: what are the effects of cytokines and inflammation? Int J Mol Sci. (2022) 24:578. doi: 10.3390/ijms24010578
PubMed Abstract | Crossref Full Text | Google Scholar
14. Song Y, Zhao Y, Shu Y, Zhang L, Cheng W, Wang L, et al. Combination model of neutrophil to high-density lipoprotein ratio and system inflammation response index is more valuable for predicting peripheral arterial disease in type 2 diabetic patients: A cross-sectional study. Front Endocrinol. (2023) 14:1100453. doi: 10.3389/fendo.2023.1100453
PubMed Abstract | Crossref Full Text | Google Scholar
15. Jin Z, Wu Q, Chen S, Gao J, Li X, Zhang X, et al. The associations of two novel inflammation indexes, sii and siri with the risks for cardiovascular diseases and all-cause mortality: A ten-year follow-up study in 85,154 individuals. J Inflammation Res. (2021) 14:131–40. doi: 10.2147/jir.S283835
PubMed Abstract | Crossref Full Text | Google Scholar
16. Bulut NS, Yorguner N, Çarkaxhiu Bulut G. The severity of inflammation in major neuropsychiatric disorders: comparison of neutrophil-lymphocyte and platelet-lymphocyte ratios between schizophrenia, bipolar mania, bipolar depression, major depressive disorder, and obsessive compulsive disorder. Nordic J Psychiatry. (2021) 75:624–32. doi: 10.1080/08039488.2021.1919201
PubMed Abstract | Crossref Full Text | Google Scholar
17. Sandberg AA, Steen VM, Torsvik A. Is elevated neutrophil count and neutrophil-to-lymphocyte ratio a cause or consequence of schizophrenia?-a scoping review. Front Psychiatry. (2021) 12:728990. doi: 10.3389/fpsyt.2021.728990
PubMed Abstract | Crossref Full Text | Google Scholar
18. Zhou X, Wang X, Li R, Yan J, Xiao Y, Li W, et al. Neutrophil-to-lymphocyte ratio is independently associated with severe psychopathology in schizophrenia and is changed by antipsychotic administration: A large-scale cross-sectional retrospective study. Front Psychiatry. (2020) 11:581061. doi: 10.3389/fpsyt.2020.581061
PubMed Abstract | Crossref Full Text | Google Scholar
19. Mahemuti N, Jing X, Zhang N, Liu C, Li C, Cui Z, et al. Association between systemic immunity-inflammation index and hyperlipidemia: A population-based study from the nhanes (2015-2020). Nutrients. (2023) 15(5):1177. doi: 10.3390/nu15051177
PubMed Abstract | Crossref Full Text | Google Scholar
20. Wei Y, Wang T, Li G, Feng J, Deng L, Xu H, et al. Investigation of systemic immune-inflammation index, neutrophil/high-density lipoprotein ratio, lymphocyte/high-density lipoprotein ratio, and monocyte/high-density lipoprotein ratio as indicators of inflammation in patients with schizophrenia and bipolar disorder. Front Psychiatry. (2022) 13:941728. doi: 10.3389/fpsyt.2022.941728
PubMed Abstract | Crossref Full Text | Google Scholar
21. Wei Y, Gao H, Luo Y, Feng J, Li G, Wang T, et al. Systemic inflammation and oxidative stress markers in patients with unipolar and bipolar depression: A large-scale study. J Affect Disord. (2024) 346:154–66. doi: 10.1016/j.jad.2023.10.156
PubMed Abstract | Crossref Full Text | Google Scholar
22. Smith CK, Vivekanandan-Giri A, Tang C, Knight JS, Mathew A, Padilla RL, et al. Neutrophil extracellular trap-derived enzymes oxidize high-density lipoprotein: an additional proatherogenic mechanism in systemic lupus erythematosus. Arthritis Rheumatol (Hoboken NJ). (2014) 66:2532–44. doi: 10.1002/art.38703
PubMed Abstract | Crossref Full Text | Google Scholar
23. Murphy AJ, Woollard KJ, Suhartoyo A, Stirzaker RA, Shaw J, Sviridov D, et al. Neutrophil Activation Is Attenuated by High-Density Lipoprotein and Apolipoprotein a-I in in Vitro and in Vivo Models of Inflammation. Arteriosclerosis thrombosis Vasc Biol. (2011) 31:1333–41. doi: 10.1161/atvbaha.111.226258
PubMed Abstract | Crossref Full Text | Google Scholar
24. Gao Z, Li B, Guo X, Bai W, Kou C. The association between schizophrenia and white blood cells count: A bidirectional two-sample mendelian randomization study. BMC Psychiatry. (2023) 23:271. doi: 10.1186/s12888-023-04760-6
PubMed Abstract | Crossref Full Text | Google Scholar
25. Torsvik A, Brattbakk HR, Trentani A, Holdhus R, Stansberg C, Bartz-Johannessen CA, et al. Patients with schizophrenia and bipolar disorder display a similar global gene expression signature in whole blood that reflects elevated proportion of immature neutrophil cells with association to lipid changes. Trans Psychiatry. (2023) 13:147. doi: 10.1038/s41398-023-02442-1
PubMed Abstract | Crossref Full Text | Google Scholar
26. Yu S, Qu Y, Du Z, Ou M, Lu R, Yuan J, et al. The expression of immune related genes and potential regulatory mechanisms in schizophrenia. Schizophr Res. (2024) 267:507–18. doi: 10.1016/j.schres.2023.11.007
PubMed Abstract | Crossref Full Text | Google Scholar
27. Zhu X, Zhou J, Zhu Y, Yan F, Han X, Tan Y, et al. Neutrophil/lymphocyte, platelet/lymphocyte and monocyte/lymphocyte ratios in schizophrenia. Australas psychiatry: Bull R Aust New Z Coll Psychiatrists. (2022) 30:95–9. doi: 10.1177/10398562211022753
PubMed Abstract | Crossref Full Text | Google Scholar
28. Korkmaz ŞA, Kızgın S. Neutrophil/high-density lipoprotein cholesterol (Hdl), monocyte/hdl and platelet/hdl ratios are increased in acute mania as markers of inflammation, even after controlling for confounding factors. Curr Med Res Opin. (2023) 39:1383–90. doi: 10.1080/03007995.2023.2260302
PubMed Abstract | Crossref Full Text | Google Scholar
29. Zorkina Y, Ushakova V, Ochneva A, Tsurina A, Abramova O, Savenkova V, et al. Lipids in psychiatric disorders: functional and potential diagnostic role as blood biomarkers. Metabolites. (2024) 14(2):80. doi: 10.3390/metabo14020080
PubMed Abstract | Crossref Full Text | Google Scholar
30. Zhang G, Ye X, Wang X, Lin Y, Zhu C, Pan J, et al. Serum total cholesterol levels associated with immediate memory performance in patients with chronic schizophrenia. Schizophr Res. (2023) 255:256–60. doi: 10.1016/j.schres.2023.03.046
PubMed Abstract | Crossref Full Text | Google Scholar
31. Nandeesha H, Keshri N, Rajappa M, Menon V. Association of hyperglycaemia and hyperlipidaemia with cognitive dysfunction in schizophrenia spectrum disorder. Arch Physiol Biochem. (2023) 129:497–504. doi: 10.1080/13813455.2020.1839500
PubMed Abstract | Crossref Full Text | Google Scholar
33. Xu K, Zheng P, Zhao S, Wang M, Tu D, Wei Q, et al. Manf/ewsr1/anxa6 pathway might as the bridge between hypolipidemia and major depressive disorder. Trans Psychiatry. (2022) 12:527. doi: 10.1038/s41398-022-02287-0
PubMed Abstract | Crossref Full Text | Google Scholar
34. Goh XX, Tang PY, Tee SF. Blood-based oxidation markers in medicated and unmedicated schizophrenia patients: A meta-analysis. Asian J Psychiatry. (2022) 67:102932. doi: 10.1016/j.ajp.2021.102932
PubMed Abstract | Crossref Full Text | Google Scholar
35. Pillinger T, McCutcheon RA, Vano L, Mizuno Y, Arumuham A, Hindley G, et al. Comparative effects of 18 antipsychotics on metabolic function in patients with schizophrenia, predictors of metabolic dysregulation, and association with psychopathology: A systematic review and network meta-analysis. Lancet Psychiatry. (2020) 7:64–77. doi: 10.1016/s2215-0366(19)30416-x
留言 (0)