The parabrachial nucleus (PBN) is located in the dorsolateral pons and is dissected into lateral and medial subregions by the superior cerebellar peduncle (Palmiter, 2018). The lateral and medial PBN further contain several subnuclei with different biological functions (Chiang et al., 2019). The PBN primarily receives inputs from the nucleus tractus solitarius (Roman et al., 2016), trigeminal spinal subnucleus caudalis (Nakaya et al., 2023), and dorsal horn of the spinal cord (Deng et al., 2020) and sends outputs widely to many brain regions, including the thalamus (Deng et al., 2020; Jarvie et al., 2021; Li et al., 2023), ventral tegmental area (VTA) (Zhang et al., 2021), basal forebrain (BF) (Qiu et al., 2016), hypothalamus (Benevento et al., 2024), insular cortex (Jarvie et al., 2021), periaqueductal gray (Chiang et al., 2020), bed nucleus of the stria terminalis (BNST) (Chiang et al., 2020), central amygdala (CeA) (Chiang et al., 2020), and reticular formation (Barik et al., 2018). The PBN is a well-known wake-promoting region (Qiu et al., 2016; Xing et al., 2024; Chen et al., 2023), and several projections involving the PBN, including the PBN-BF (Qiu et al., 2016; Xu et al., 2021), PBN-lateral hypothalamus (Qiu et al., 2016; Xu et al., 2021), and lateral hypothalamus-PBN (Wang et al., 2021), have been found to regulate sleep–wakefulness behaviors. In addition to regulating sleep–wakefulness behaviors, the PBN is also implicated in many other important physiological and pathological conditions, such as sensory sensation (Ren et al., 2023; Mu et al., 2017), defensive responses (Xie et al., 2022; Yang et al., 2023), feeding (Singh Alvarado et al., 2024), pain (Wu et al., 2023; Zhou et al., 2023), respiratory depression (Liu et al., 2021; Miller et al., 2017), and negative emotions (Wang et al., 2024; Chen et al., 2022).
The majority of neurons in the PBN are glutamatergic neurons, although there is a significant proportion of GABAergic neurons (Sun et al., 2020; Pauli et al., 2022). These two neuron types also coexpress several well-known peptides or receptors, such as calcitonin gene-related peptide (CGRP) (Palmiter, 2018), tachykinin receptor 1 (TACR1) (Deng et al., 2020), substance P (Barik et al., 2018), neurotensin (Chen et al., 2023), cholecystokinin (Yang et al., 2020), neuropeptide S (Xing et al., 2024), prodynorphin (Yang et al., 2020; Norris et al., 2021), and proenkephalin (Norris et al., 2021), and each population is associated with certain functions. The neuropeptide S- (Xing et al., 2024) and neurotensin-expressing neuron (Deng et al., 2020) in the PBN are associated with the regulation of sleep–wake behaviors. General anesthesia shares the classical feature of reversible loss of consciousness with natural sleep (Bao et al., 2023; Yang et al., 2022), and accumulating evidence has demonstrated that the activity of the PBN is also closely related to the actions of general anesthetics (Lu et al., 2023; Liu et al., 2023; Melonakos et al., 2021; Luo et al., 2018; Wang et al., 2019; Muindi et al., 2016). Here, we reviewed the current literature regarding the involvement of PBN in emergence from general anesthesia (Table 1). We also proposed unidentified PBN circuits related to general anesthesia (Figure 1).
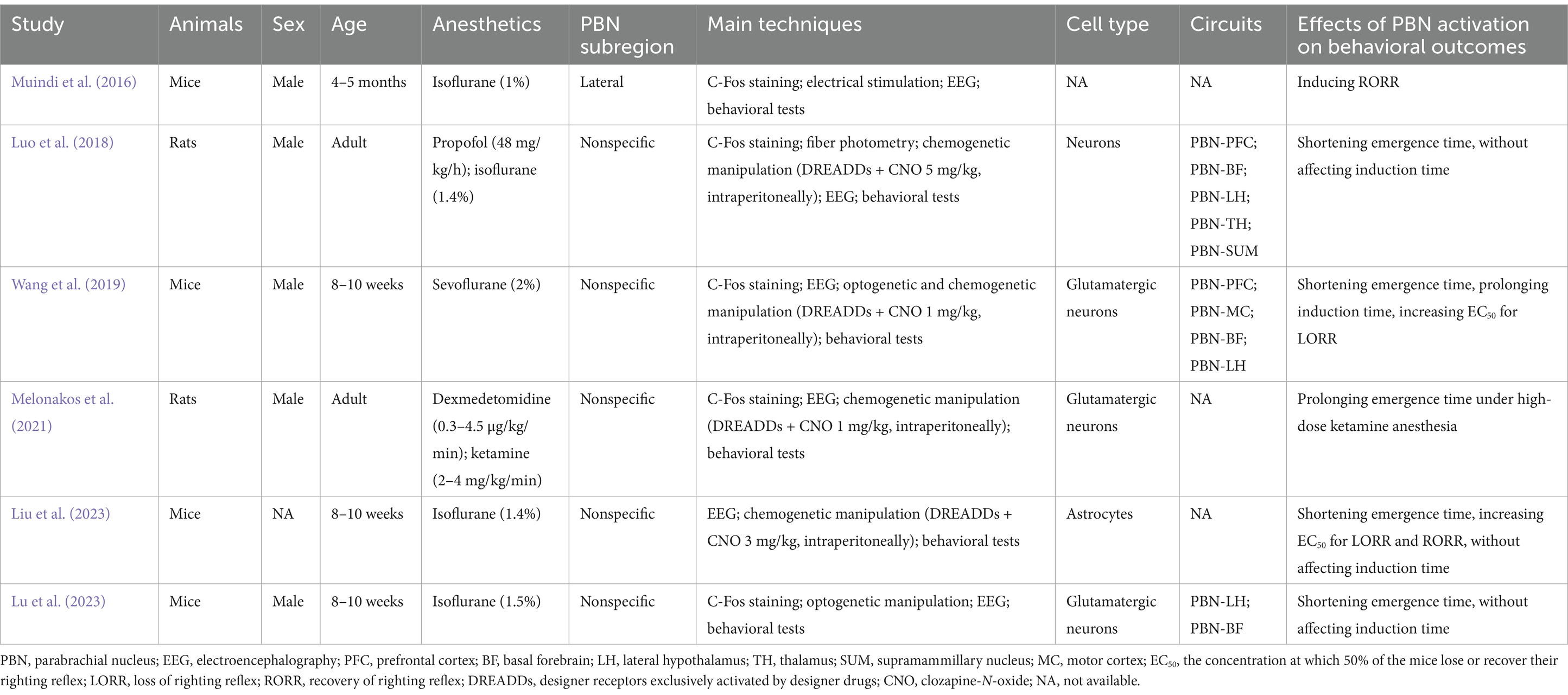
Table 1. Study characteristics.
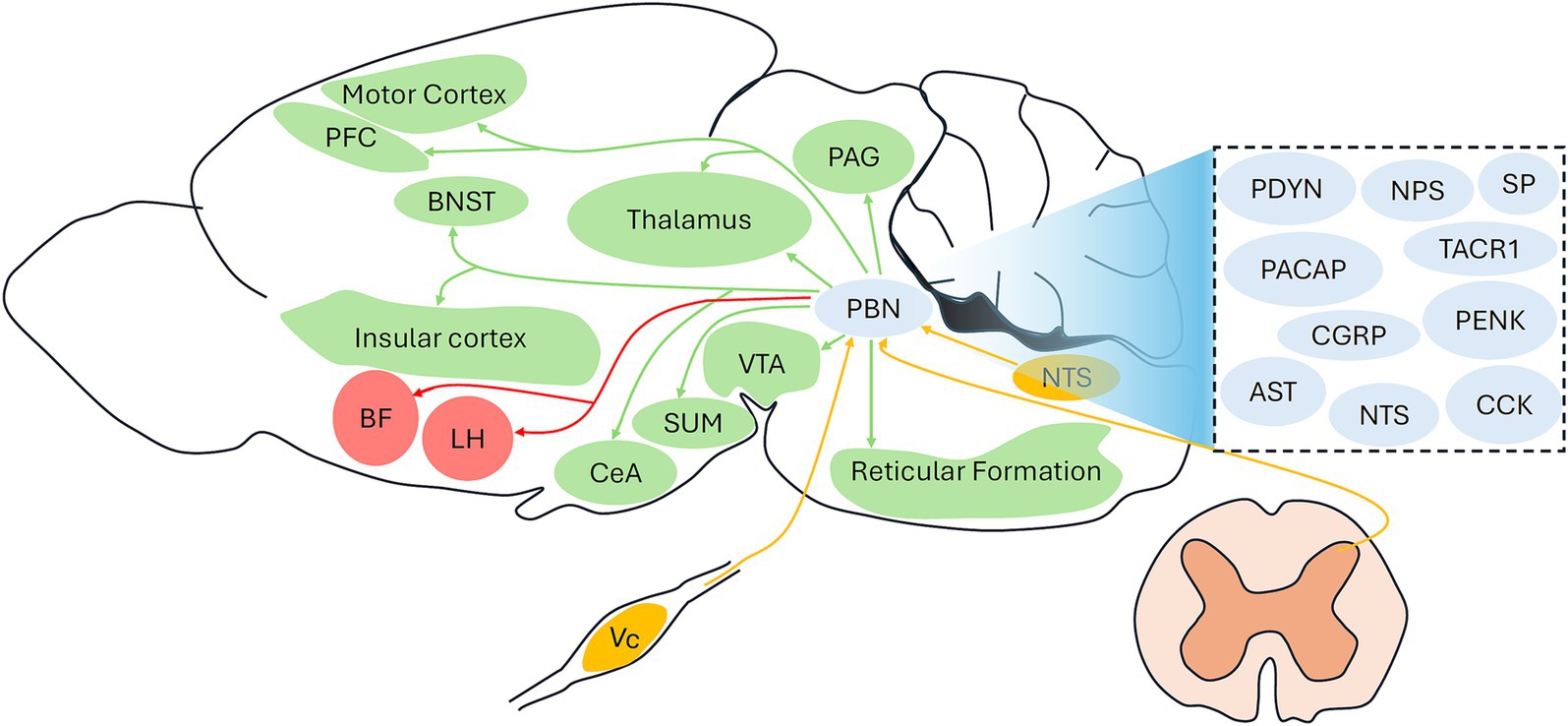
Figure 1. Identified and unidentified circuits involving the PBN under general anesthesia. Red arrows indicate identified PBN projections under general anesthesia; Green arrows indicate unidentified outputs from the PBN under general anesthesia; Yellow arrows indicate unidentified inputs to the PBN under general anesthesia. The dashed box includes the potential neuropeptides types of the PBN. PBN, parabrachial nucleus; PFC, prefrontal cortex; CGRP, calcitonin gene-related peptide; TACR1, tachykinin receptor 1; SP, substance P; CCK, cholecystokinin; NPS, neuropeptide S; PDYN, prodynorphin; PENK, proenkephalin; NTS, neurotensin; Vc, trigeminal spinal subnucleus caudalis; VTA, ventral tegmental area; PAG, periaqueductal gray; BNST, bed nucleus of the stria terminalis: CeA, central amygdala; LH, lateral hypothalamus; BF, basal forebrain; SUM, supramammillary nucleus.
Involvement of PBN in emergence from volatile anesthesia Isoflurane anesthesiaIn 2016, Muindi et al. first reported the involvement of lateral PBN in emergence from isoflurane anesthesia (Muindi et al., 2016). Using c-Fos staining, they found that the activity of lateral PBN neurons increased during the period of emergence from 1% isoflurane anesthesia in mice. Notably, electrical stimulation of the PBN, but not the nearby region, induced cortical activation in the electroencephalogram (EEG), behavioral arousal and recovery of the righting reflex during isoflurane anesthesia, suggesting that the PBN serves as a wakefulness-promoting nucleus during isoflurane anesthesia. However, the cellular type of PBN that responds to isoflurane and whether stimulation with PBN affects isoflurane induction remain unclear. Lu et al. further investigated the involved PBN projections under isoflurane anesthesia (Lu et al., 2023). They showed that optogenetic activation of the PBN-lateral hypothalamus (LH) or PBN-basal forebrain (BF) projection induced cortical arousal on EEG in mice under 1.5% isoflurane anesthesia and promoted emergence from isoflurane anesthesia. Conversely, inhibition of these projections prolonged the emergence time. Interestingly, neither activation nor inhibition of these projections affected the induction time of isoflurane anesthesia. Regrettably, isoflurane sensitivity, i.e., the EC50 of the loss of righting reflex (LORR), was not examined to confirm the role of PBN projections in isoflurane induction.
Astrocytes in the central nervous system are involved in the regulation of sleep–wake behaviors (Chaturvedi et al., 2022; Bojarskaite et al., 2020). Liu et al. explored the contribution of astrocytes in the PBN to the loss of consciousness induced by isoflurane anesthesia in mice (Liu et al., 2023). They found that chemogenetic activation of PBN astrocytes induced cortical arousal (decreased delta power and burst suppression ratio) in EEG, increased the EC50 of loss of the LORR and recovery of the righting reflex (RORR), and shortened the emergence time from 1.4% isoflurane anesthesia in mice. However, the induction time was not affected. Conversely, chemogenetic inhibition of PBN astrocytes decreased the EC50 of the RORR and caused cortical inhibition (increased delta power and burst suppression ratio) on EEG. These findings indicate that PBN astrocytes play an important role in recovery from isoflurane anesthesia. Interestingly, although activation of the PBN-BF neural projection promoted the emergence from general anesthesia, direct activation of astrocytes in the BF facilitated isoflurane induction and delayed recovery time (Lin et al., 2023), suggesting that astrocytes in different arousal-related brain nuclei may have different roles in emergence from anesthesia. The contribution of astrocytes to general anesthesia may be attributed to the expression of many anesthetic targets, such as GABAA receptors, glutamate receptors, sodium, potassium, and calcium ion channels (Mulkey et al., 2022; Chung et al., 2021). Previous studies have indicated that GABAA receptors on astrocytes are targets for commonly used general anesthetics to induce cognitive dysfunction (Chung et al., 2021; Wang et al., 2024). However, the underlying molecular mechanism by which astrocytes modulate emergence from isoflurane anesthesia is not fully understood. It is generally thought that the effects of astrocytes on general anesthesia occur via interactions with neurons (Mulkey et al., 2022). However, several studies have indicated that general anesthetics suppress astrocyte calcium signals independently of neuronal activity, suggesting that the effects of general anesthesia on astrocytes may be generated via a nonneuronal mechanism (Thrane et al., 2012). Whether other glial cells, such as microglia and oligodendrocytes, regulate general anesthesia needs to be investigated because they also contribute to the regulation of sleep–wake cycles (Ma et al., 2024; Rojo et al., 2023).
Sevoflurane anesthesiaThe activity of the PBN is also involved in the general anesthesia induced by sevoflurane (Wang et al., 2019). Wang et al. showed that chemogenetic activation of PBN glutamatergic neurons shortened the emergence time of mice from 2% sevoflurane anesthesia, prolonged the induction time, and increased the EC50 of the LORR. Conversely, chemogenetic inhibition of PBN glutamatergic neurons produced the opposite effects. Optogenetic activation of PBN glutamatergic neurons induced cortical arousal according to EEG during the maintenance stage of sevoflurane anesthesia. They further showed that activation of PBN glutamatergic neurons induced excitation of several cortical and subcortical arousal regions, including the prefrontal cortex, motor cortex, basal forebrain, and lateral hypothalamus, under sevoflurane anesthesia. However, the specific role of these PBN projections under sevoflurane anesthesia has not yet been determined.
Involvement of PBN in emergence from intravenous general anesthesiaIn addition to volatile anesthesia, PBN activity also regulates consciousness under intravenous general anesthesia. Luo et al. used real-time calcium imaging to detect the activity of PBN neurons under general anesthesia induced by propofol and isoflurane (Luo et al., 2018). They found that the activity of PBN neurons was inhibited under propofol (48 mg/kg/h) and 1.4% isoflurane exposure but recovered during the emergence period in rats. The activation of PBN neurons induced cortical activation in EEG signals during the recovery period and shortened the emergence time of propofol and isoflurane anesthesia but had no effect on the induction time or on the EEG signals during the induction period. They further explored PBN projections and found that the prefrontal cortex, basal forebrain, lateral hypothalamus, thalamus, and supramammillary nucleus were activated when activating the PBN. However, the contributions of these PBN projections to general anesthesia were not further examined, nor did they identify the neuronal types of the PBN that respond to general anesthesia.
The modulation of the PBN by other types of intravenous general anesthetics, such as dexmedetomidine and ketamine, was different from that by propofol anesthesia. Melonakos et al. reported the role of PBN during dexmedetomidine and ketamine anesthesia in rats (Melonakos et al., 2021). Their data showed that activation of glutamatergic neurons in the PBN reduced delta power on EEG under low-dose (0.3 μg/kg/min) dexmedetomidine anesthesia but not under high-dose dexmedetomidine (4.5 μg/kg/min) or ketamine anesthesia (4 mg/kg/min), suggesting that these two drugs have distinct effects on delta power generation. They further found that activation of glutamatergic neurons in the PBN did not affect recovery time with dexmedetomidine anesthesia or with low-dose (2 mg/kg/min) ketamine anesthesia but did prolong recovery time with high-dose ketamine anesthesia. These findings suggest that PBN plays a different role in diverse groups of general anesthetics with different molecular targets and that other wake-promoting nuclei or circuits may play a more important role in the emergence of dexmedetomidine and ketamine anesthesia. However, they did not examine the effects of PBN activation on the induction process of dexmedetomidine and ketamine anesthesia. Moreover, additional experiments involving the inhibition of PBN neurons or optogenetic techniques will support these conclusions.
Conclusion and perspectivesRecent studies have consistently revealed that activation of the PBN promotes recovery from general anesthesia induced by general anesthetics, including isoflurane (Lu et al., 2023; Liu et al., 2023), sevoflurane (Wang et al., 2019), and propofol (Luo et al., 2018), suggesting that the PBN plays a pivotal role in the recovery period of general anesthesia. Notably, activation of the PBN did not influence the recovery time of dexmedetomidine anesthesia or low-dose ketamine anesthesia but unexpectedly prolonged the recovery time of high-dose ketamine anesthesia (Melonakos et al., 2021). These findings suggest that the effects of PBN activation on general anesthesia depend on the molecular targets involved. In terms of anesthesia induction, there is still divergence. Manipulation of the activity of PBN did not affect the induction time of isoflurane (Lu et al., 2023) or propofol anesthesia (Luo et al., 2018) but did change anesthesia sensitivity, as evidenced by the altered EC50 of LORR (Liu et al., 2023). Only one study investigated the role of PBN in sevoflurane anesthesia (Wang et al., 2019). Interestingly, activation of PBN prolonged the induction time and increased the EC50 of the LORR under sevoflurane anesthesia (Wang et al., 2019). Future studies are still needed to confirm the involvement of PBN in anesthesia induction.
As the majority population of PBN neurons, glutamatergic neurons are identified as effective targets under general anesthesia (Lu et al., 2023; Wang et al., 2019). However, GABAergic neurons are assumed to constitute a definite proportion of PBN neurons 29, and their contribution to general anesthesia needs to be investigated in future studies. Moreover, glutamatergic neurons can be further divided into several subpopulations, such as CGRP-expressing (Palmiter, 2018) and TACR1-expressing (Deng et al., 2020) neurons, and the specific role of these subpopulations under general anesthesia needs to be determined. The PBN projections involved in general anesthesia remain largely unknown because only the BF and LH have been identified as effective downstream targets of the PBN in isoflurane anesthesia (Lu et al., 2023). Although several regions, including the prefrontal cortex, thalamus, supramammillary nucleus, and motor cortex, are activated by PBN activation (Luo et al., 2018; Wang et al., 2019), whether these projections are associated with the actions of general anesthesia remains unclear. Additionally, it will be interesting to investigate whether other classical projections involving the PBN, such as the PBN-VTA (Zhang et al., 2021), PBN-BNST (Chiang et al., 2020), and PBN-CeA (Chiang et al., 2020), participate in the modulation of consciousness levels under general anesthesia. Figure 1 illustrates the identified PBN involving circuits (PBN-BF and PBN-LH) under general anesthesia and unidentified circuits, including common inputs and outputs of the PBN. Future studies are required to determine whether these circuits are associated with the actions of general anesthetics. As the PBN comprises different functional subregions (Chiang et al., 2019), its specific role in the regulation of general anesthesia should be examined. Notably, current evidence regarding the role of PBN in emergence from general anesthesia has mainly been obtained from male rodents, and whether there is a sex difference should be tested in future studies.
Despite the important involvement of PBN in the regulation of emergence from general anesthesia, limited research has investigated the underlying molecular mechanism by which general anesthetics influence the activities of PBN neurons or astrocytes. Ion channels or receptors distributed on PBN neurons or astrocytes may be molecular targets of general anesthetics. For example, recent studies have revealed that the sodium leak channel in glutamatergic neurons (Wu Y. et al., 2024) and Kir4.1 in astrocytes (Zhao et al., 2024) mediate the effects of sevoflurane on one wake-promoting region, the paraventricular thalamus. Additionally, a previous study showed that the sodium leak channel helps to maintain the neuronal activity of the PBN and regulates respiratory function under sevoflurane anesthesia (Wu L. et al., 2024). Therefore, it will be interesting to explore whether the sodium leak channel participates in the regulation of the emergence-promoting effects of PBN under general anesthesia. Considering that the expression of many peptides or receptors in PBN neurons, such as CGRP (Palmiter, 2018), TACR1 (Deng et al., 2020), substance P (Barik et al., 2018), neurotensin (Chen et al., 2023), cholecystokinin (Yang et al., 2020), neuropeptide S (Xing et al., 2024), prodynorphin (Yang et al., 2020; Norris et al., 2021), and proenkephalin (Norris et al., 2021), and several of them, such as CGRP (Kuroda et al., 2004) and TACR1 (Ishizaki et al., 1997), can be modulated by isoflurane or sevoflurane, future studies are required to determine whether these proteins are molecular targets that mediate the emergence-promoting effects of the PBN under general anesthesia. It is also meaningful to use in-vitro methods to investigate the direct effects of anesthetics on PBN neurons, which will provide the direct evidence for the involvement of PBN neurons in the general anesthesia.
One issue should be noted. Since PBN functions as a physiological relay nucleus for nociceptive information or warning signals (Torres-Rodriguez et al., 2024; Torruella-Suárez et al., 2024; Katagiri and Kato, 2020), which will also stimulate the brain’s arousal circuits and elevate arousal levels (Smith et al., 2023), thereby expediting recovery from general anesthesia. Therefore, sufficient arousal effects can be achieved by simply applying noxious stimuli without resorting to optogenetics, chemogenetics, or ultrasound stimulation methods. For example, the excitation of PBN neurons has already been reported to act as an unconditioned stimulus for fear conditioning and to alter pain sensitivity, leading to nociplastic pain (Palmiter, 2024), which will promote arousal. However, it is difficult to evaluate whether the observed phenomenon resulted from promoting emergence from anesthesia or merely from shifting the arousal-sedation balance. Both factors might contribute to this phenomenon, which is needed to be confirmed by future studies.
Accumulating animal evidence of the involvement of PBN in emergence from general anesthesia will facilitate the clinical translation in many conditions. For example, stimulating PBN may serve as a promising therapy to improve the patients’ recovery from general anesthesia or other unconsciousness conditions, such as coma. Moreover, selective manipulation of the neuronal subtype of PBN might provide more precise treatment and decrease the incidence of side effects. Recent evidence (Bian et al., 2021) indicated that noninvasive ultrasound stimulation of another emergence-promoting region, namely the ventral tegmental area, accelerated the emergence from isoflurane anesthesia in mice, highlighting the possibility of use noninvasive technique to stimulate PBN in clinic. It will be interesting if inhibition of PBN can be combined with the use of anesthetics to maintain the general anesthesia, which will reduce the consumption of general anesthetics as well as their related side effects, especially in patients with unstable respiratory and circulatory functions.
Author contributionsJL: Conceptualization, Funding acquisition, Writing – original draft, Writing – review & editing. QZ: Writing – original draft, Writing – review & editing. JX: Writing – original draft, Writing – review & editing. YW: Writing – original draft, Writing – review & editing, Conceptualization. DZ: Writing – original draft, Writing – review & editing, Conceptualization.
FundingThe author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This work was supported by Grant No. 82204359 from the National Natural Science Foundation of China, Grant No. 2023-JC-QN-0989 from the Natural Science Foundation of Shaanxi Province, Grant No. Y2024004 from the Research Initiation Fund of Longgang District Maternity & Child Healthcare Hospital of Shenzhen City, the Doctor Research Initiation Fund of Zunyi Medical University [(2021)3], and Grant No. 2023MZFS003 from the Research Grant of Key Laboratory of Anesthesiology and Resuscitation (Huazhong University of Science and Technology), Ministry of Education.
Conflict of interestThe authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s noteAll claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
ReferencesBao, W. W., Jiang, S., Qu, W. M., Li, W. X., Miao, C. H., and Huang, Z. L. (2023). Understanding the neural mechanisms of general anesthesia from interaction with sleep-wake state: a decade of discovery. Pharmacol. Rev. 75, 532–553. doi: 10.1124/pharmrev.122.000717
PubMed Abstract | Crossref Full Text | Google Scholar
Barik, A., Thompson, J. H., Seltzer, M., Ghitani, N., and Chesler, A. T. (2018). A brainstem-spinal circuit controlling Nocifensive behavior. Neuron 100, 1491–503.e3. doi: 10.1016/j.neuron.2018.10.037
PubMed Abstract | Crossref Full Text | Google Scholar
Benevento, M., Alpár, A., Gundacker, A., Afjehi, L., Balueva, K., Hevesi, Z., et al. (2024). A brainstem-hypothalamus neuronal circuit reduces feeding upon heat exposure. Nature 628, 826–834. doi: 10.1038/s41586-024-07232-3
PubMed Abstract | Crossref Full Text | Google Scholar
Bian, T., Meng, W., Qiu, M., Zhong, Z., Lin, Z., Zou, J., et al. (2021). Noninvasive ultrasound stimulation of ventral tegmental area induces reanimation from general Anaesthesia in mice. Research 2021:2674692. doi: 10.34133/2021/2674692
Crossref Full Text | Google Scholar
Bojarskaite, L., Bjørnstad, D. M., Pettersen, K. H., Cunen, C., Hermansen, G. H., Åbjørsbråten, K. S., et al. (2020). Astrocytic ca (2+) signaling is reduced during sleep and is involved in the regulation of slow wave sleep. Nat. Commun. 11:3240. doi: 10.1038/s41467-020-17062-2
PubMed Abstract | Crossref Full Text | Google Scholar
Chaturvedi, R., Stork, T., Yuan, C., Freeman, M. R., and Emery, P. (2022). Astrocytic GABA transporter controls sleep by modulating GABAergic signaling in Drosophila circadian neurons. Curr. Biol. 32, 1895–908.e5. doi: 10.1016/j.cub.2022.02.066
PubMed Abstract | Crossref Full Text | Google Scholar
Chen, J., Gannot, N., Li, X., Zhu, R., Zhang, C., and Li, P. (2023). Control of emotion and wakefulness by neurotensinergic neurons in the parabrachial nucleus. Neurosci. Bull. 39, 589–601. doi: 10.1007/s12264-022-00994-8
PubMed Abstract | Crossref Full Text | Google Scholar
Chiang, M. C., Bowen, A., Schier, L. A., Tupone, D., Uddin, O., and Heinricher, M. M. (2019). Parabrachial complex: a hub for pain and aversion. J. Neurosci. 39, 8225–8230. doi: 10.1523/JNEUROSCI.1162-19.2019
PubMed Abstract | Crossref Full Text | Google Scholar
Chiang, M. C., Nguyen, E. K., Canto-Bustos, M., Papale, A. E., Oswald, A. M. M., and Ross, S. E. (2020). Divergent neural pathways emanating from the lateral parabrachial nucleus mediate distinct components of the pain response. Neuron 106, 927–39.e5. doi: 10.1016/j.neuron.2020.03.014
PubMed Abstract | Crossref Full Text | Google Scholar
Chung, W., Wang, D. S., Khodaei, S., Pinguelo, A., and Orser, B. A. (2021). GABA (a) receptors in astrocytes are targets for commonly used intravenous and inhalational general anesthetic drugs. Front. Aging Neurosci. 13:802582. doi: 10.3389/fnagi.2021.802582
Crossref Full Text | Google Scholar
Deng, J., Zhou, H., Lin, J. K., Shen, Z. X., Chen, W. Z., Wang, L. H., et al. (2020). The parabrachial nucleus directly channels spinal nociceptive signals to the Intralaminar thalamic nuclei, but not the amygdala. Neuron 107, 909–23.e6. doi: 10.1016/j.neuron.2020.06.017
PubMed Abstract | Crossref Full Text | Google Scholar
Ishizaki, K., Karasawa, S., Takahashi, K., Hasegawa, M., and Goto, F. (1997). Intrathecal neurokinin-1 receptor antagonist reduces isoflurane MAC in rats. Can. J. Anaesth. 44, 543–549. doi: 10.1007/BF03011945
PubMed Abstract | Crossref Full Text | Google Scholar
Jarvie, B. C., Chen, J. Y., King, H. O., and Palmiter, R. D. (2021). Satb 2 neurons in the parabrachial nucleus mediate taste perception. Nat. Commun. 12:224. doi: 10.1038/s41467-020-20100-8
Crossref Full Text | Google Scholar
Katagiri, A., and Kato, T. (2020). Multi-dimensional role of the parabrachial nucleus in regulating pain-related affective disturbances in trigeminal neuropathic pain. J. Oral Sci. 62, 160–164. doi: 10.2334/josnusd.19-0432
PubMed Abstract | Crossref Full Text | Google Scholar
Kuroda, M., Yoshikawa, D., Nishikawa, K., Saito, S., and Goto, F. (2004). Volatile anesthetics inhibit calcitonin gene-related peptide receptor-mediated responses in pithed rats and human neuroblastoma cells. J. Pharmacol. Exp. Ther. 311, 1016–1022. doi: 10.1124/jpet.104.071936
PubMed Abstract | Crossref Full Text | Google Scholar
Li, J. N., Wu, X. M., Zhao, L. J., Sun, H. X., Hong, J., Wu, F. L., et al. (2023). Central medial thalamic nucleus dynamically participates in acute itch sensation and chronic itch-induced anxiety-like behavior in male mice. Nat. Commun. 14:2539. doi: 10.1038/s41467-023-38264-4
PubMed Abstract | Crossref Full Text | Google Scholar
Lin, J., Cheng, X., Wang, H., du, L., Li, X., Zhao, G., et al. (2023). Activation of astrocytes in the basal forebrain in mice facilitates isoflurane-induced loss of consciousness and prolongs recovery. BMC Anesthesiol. 23:213. doi: 10.1186/s12871-023-02166-1
PubMed Abstract | Crossref Full Text | Google Scholar
Liu, S., Kim, D. I., Oh, T. G., Pao, G. M., Kim, J. H., Palmiter, R. D., et al. (2021). Neural basis of opioid-induced respiratory depression and its rescue. Proc. Natl. Acad. Sci. USA 118:118. doi: 10.1073/pnas.2022134118
Crossref Full Text | Google Scholar
Liu, P. C., Yao, W., Chen, X. Y., Su, W. K., Zheng, Z. H., Yan, X. B., et al. (2023). Parabrachial nucleus astrocytes regulate wakefulness and isoflurane anesthesia in mice. Front. Pharmacol. 13:991238. doi: 10.3389/fphar.2022.991238
Crossref Full Text | Google Scholar
Lu, K., Wang, Z., Bai, N., Zhao, Z., Zhao, X., and He, Y. (2023). Selective optogenetic modulation of the PBN terminals in the lateral hypothalamic area and basal forebrain regulates emergence from isoflurane anesthesia in mice. BMC Anesthesiol. 23:328. doi: 10.1186/s12871-023-02294-8
PubMed Abstract | Crossref Full Text | Google Scholar
Luo, T., Yu, S., Cai, S., Zhang, Y., Jiao, Y., Yu, T., et al. (2018). Parabrachial neurons promote behavior and electroencephalographic arousal from general anesthesia. Front. Mol. Neurosci. 11:420. doi: 10.3389/fnmol.2018.00420
PubMed Abstract | Crossref Full Text | Google Scholar
Ma, C., Li, B., Silverman, D., Ding, X., Li, A., Xiao, C., et al. (2024). Microglia regulate sleep through calcium-dependent modulation of norepinephrine transmission. Nat. Neurosci. 27, 249–258. doi: 10.1038/s41593-023-01548-5
PubMed Abstract | Crossref Full Text | Google Scholar
Melonakos, E. D., Siegmann, M. J., Rey, C., O’Brien, C., Nikolaeva, K. K., Solt, K., et al. (2021). Excitation of putative glutamatergic neurons in the rat parabrachial nucleus region Reduces Delta power during Dexmedetomidine but not ketamine anesthesia. Anesthesiology 135, 633–648. doi: 10.1097/ALN.0000000000003883
PubMed Abstract | Crossref Full Text | Google Scholar
Miller, J. R., Zuperku, E. J., Stuth, E. A. E., Banerjee, A., Hopp, F. A., and Stucke, A. G. (2017). A subregion of the parabrachial nucleus partially mediates respiratory rate depression from intravenous remifentanil in young and adult rabbits. Anesthesiology 127, 502–514. doi: 10.1097/ALN.0000000000001719
PubMed Abstract | Crossref Full Text | Google Scholar
Mu, D., Deng, J., Liu, K. F., Wu, Z. Y., Shi, Y. F., Guo, W. M., et al. (2017). A central neural circuit for itch sensation. Science 357, 695–699. doi: 10.1126/science.aaf4918
PubMed Abstract | Crossref Full Text | Google Scholar
Muindi, F., Kenny, J. D., Taylor, N. E., Solt, K., Wilson, M. A., Brown, E. N., et al. (2016). Electrical stimulation of the parabrachial nucleus induces reanimation from isoflurane general anesthesia. Behav. Brain Res. 306, 20–25. doi: 10.1016/j.bbr.2016.03.021
PubMed Abstract | Crossref Full Text | Google Scholar
Mulkey, D. K., Olsen, M. L., Ou, M., Cleary, C. M., and du, G. (2022). Putative roles of astrocytes in general anesthesia. Curr. Neuropharmacol. 20, 5–15. doi: 10.2174/1570159X19666210215120755
PubMed Abstract | Crossref Full Text | Google Scholar
Nakaya, Y., Yamamoto, K., and Kobayashi, M. (2023). Descending projections from the insular cortex to the trigeminal spinal subnucleus caudalis facilitate excitatory outputs to the parabrachial nucleus in rats. Pain 164, e157–e173. doi: 10.1097/j.pain.0000000000002755
PubMed Abstract | Crossref Full Text | Google Scholar
Norris, A. J., Shaker, J. R., Cone, A. L., Ndiokho, I. B., and Bruchas, M. R. (2021). Parabrachial opioidergic projections to preoptic hypothalamus mediate behavioral and physiological thermal defenses. eLife 10:e60779. doi: 10.7554/eLife.60779
PubMed Abstract | Crossref Full Text | Google Scholar
Pauli, J. L., Chen, J. Y., Basiri, M. L., Park, S., Carter, M. E., Sanz, E., et al. (2022). Molecular and anatomical characterization of parabrachial neurons and their axonal projections. eLife 11:11. doi: 10.7554/eLife.81868
PubMed Abstract | Crossref Full Text | Google Scholar
Qiu, M. H., Chen, M. C., Fuller, P. M., and Lu, J. (2016). Stimulation of the pontine parabrachial nucleus promotes wakefulness via extra-thalamic forebrain circuit nodes. Curr. Biol. 26, 2301–2312. doi: 10.1016/j.cub.2016.07.054
PubMed Abstract | Crossref Full Text | Google Scholar
Ren, X., Liu, S., Virlogeux, A., Kang, S. J., Brusch, J., Liu, Y., et al. (2023). Identification of an essential spinoparabrachial pathway for mechanical itch. Neuron 111, 1812–29.e6. doi: 10.1016/j.neuron.2023.03.013
PubMed Abstract | Crossref Full Text | Google Scholar
Rojo, D., Dal Cengio, L., Badner, A., Kim, S., Sakai, N., Greene, J., et al. (2023). BMAL1 loss in oligodendroglia contributes to abnormal myelination and sleep. Neuron 111, 3604–18.e11. doi: 10.1016/j.neuron.2023.08.002
PubMed Abstract | Crossref Full Text | Google Scholar
Roman, C. W., Derkach, V. A., and Palmiter, R. D. (2016). Genetically and functionally defined NTS to PBN brain circuits mediating anorexia. Nat. Commun. 7:11905. doi: 10.1038/ncomms11905
PubMed Abstract | Crossref Full Text | Google Scholar
Singh Alvarado, J., Lutas, A., Madara, J. C., Isaac, J., Lommer, C., Massengill, C., et al. (2024). Transient cAMP production drives rapid and sustained spiking in brainstem parabrachial neurons to suppress feeding. Neuron 112, 1416–1425.e5. doi: 10.1016/j.neuron.2024.02.002
PubMed Abstract | Crossref Full Text | Google Scholar
Smith, J. A., Ji, Y., Lorsung, R., Breault, M. S., Koenig, J., Cramer, N., et al. (2023). Parabrachial nucleus activity in nociception and pain in awake mice. J. Neurosci. 43, 5656–5667. doi: 10.1523/JNEUROSCI.0587-23.2023
PubMed Abstract | Crossref Full Text | Google Scholar
Sun, L., Liu, R., Guo, F., Wen, M. Q., Ma, X. L., Li, K. Y., et al. (2020). Parabrachial nucleus circuit governs neuropathic pain-like behavior. Nat. Commun. 11:5974. doi: 10.1038/s41467-020-19767-w
PubMed Abstract | Crossref Full Text | Google Scholar
Thrane, A. S., Rangroo Thrane, V., Zeppenfeld, D., Lou, N., Xu, Q., Nagelhus, E. A., et al. (2012). General anesthesia selectively disrupts astrocyte calcium signaling in the awake mouse cortex. Proc. Natl. Acad. Sci. U. S. A. 109, 18974–18979. doi: 10.1073/pnas.1209448109
PubMed Abstract | Crossref Full Text | Google Scholar
Torres-Rodriguez, J. M., Wilson, T. D., Singh, S., Torruella-Suárez, M. L., Chaudhry, S., Adke, A. P., et al. (2024). The parabrachial to central amygdala pathway is critical to injury-induced pain sensitization in mice. Neuropsychopharmacology 49, 508–520. doi: 10.1038/s41386-023-01673-6
PubMed Abstract | Crossref Full Text | Google Scholar
Torruella-Suárez, M. L., Neugebauer, B., Flores-Felix, K., Keller, A., Carrasquillo, Y., and Cramer, N. (2024). Divergent changes in PBN excitability in a mouse model of neuropathic pain. eNeuro 11, ENEURO.0416–ENEU23.2024. doi: 10.1523/eneuro.0416-23.2024
留言 (0)