Gastric mixed adenoneuroendocrine carcinoma (MANEC) is a rare type of gastric tumor characterized by both exocrine and neuroendocrine components, each comprising more than 30% of the tumor (1). In 2019, the World Health Organization (WHO) classified MANEC as part of the spectrum of gastric mixed neuroendocrine-non-neuroendocrine neoplasms (2). Gastric MANEC is more aggressive and more likely to metastasize distantly compared to gastric adenocarcinoma, making its treatment more challenging (3). Despite the lack of effective treatments for distant metastatic gastric MANEC, clinicians commonly employ chemotherapy and immunotherapy. However, this carcinoma typically shows poor responsiveness to chemotherapy (4). Retrospective studies have suggested that conversion surgery may confer a survival benefit for stage IV gastric adenocarcinoma patients (5–7). However, there is ongoing controversy regarding whether conversion surgery offers similar benefits for patients with stage IV gastric neuroendocrine carcinoma or gastric MANEC (8, 9).
In this report, we present a unique case of a patient with distant metastatic gastric MANEC exhibiting microsatellite instability-high (MSI-H) but proficient mismatch repair (pMMR). This patient underwent conversion surgery following a regimen of targeted therapy, immunotherapy, and chemotherapy. The conversion surgery was successful, achieving both a D2 gastrectomy and an R0 resection. Our aim is to contribute to a deeper understanding of the potential role of conversion surgery in the treatment of metastatic gastric MANEC.
Case presentationIn March 2023, a 68-year-old male presented to a local hospital with intermittent upper abdominal pain for three months. The patient reported a weight loss of 3 kg over the past three months and mentioned occasional alcohol consumption and smoking for over forty years. The patient’s BMI at the time of presentation was 20.1. Gastroscopy revealed a large irregular lesion on the lesser curvature side of the gastric cardia and body, with pathology indicating moderately differentiated adenocarcinoma. The patient had no significant medical history and no comorbidities such as diabetes or hypertension. Moreover, the patient has no family history of tumors or hereditary diseases. For further evaluation and treatment, the patient was referred to our center.
At our center, a thorough physical examination was performed on the patient. The patient’s abdomen was flat, with mild tenderness in the left upper quadrant. No abnormalities were found in the physical examination of the rest of the body. Moreover, enhanced abdominal computed tomography (CT), gastric endoscopic ultrasonography, and immunohistochemical examination of gastric biopsies were performed (Figure 1). CT revealed thickening of the gastric wall at the cardia and fundus, extending into the lower esophagus, along with retroperitoneal lymph node metastasis. Additionally, multiple low-density nodules were identified in the liver, suggesting gastric cancer liver metastases. Pathological and immunohistochemical results suggested that the gastric tumor was a gastric mixed adenoneuroendocrine carcinoma. Additionally, the immunohistochemistry for PD-L1 of the gastric tumor cells indicated focal positivity. The gastric MANEC was classified as stage IV (cT4N3M1).
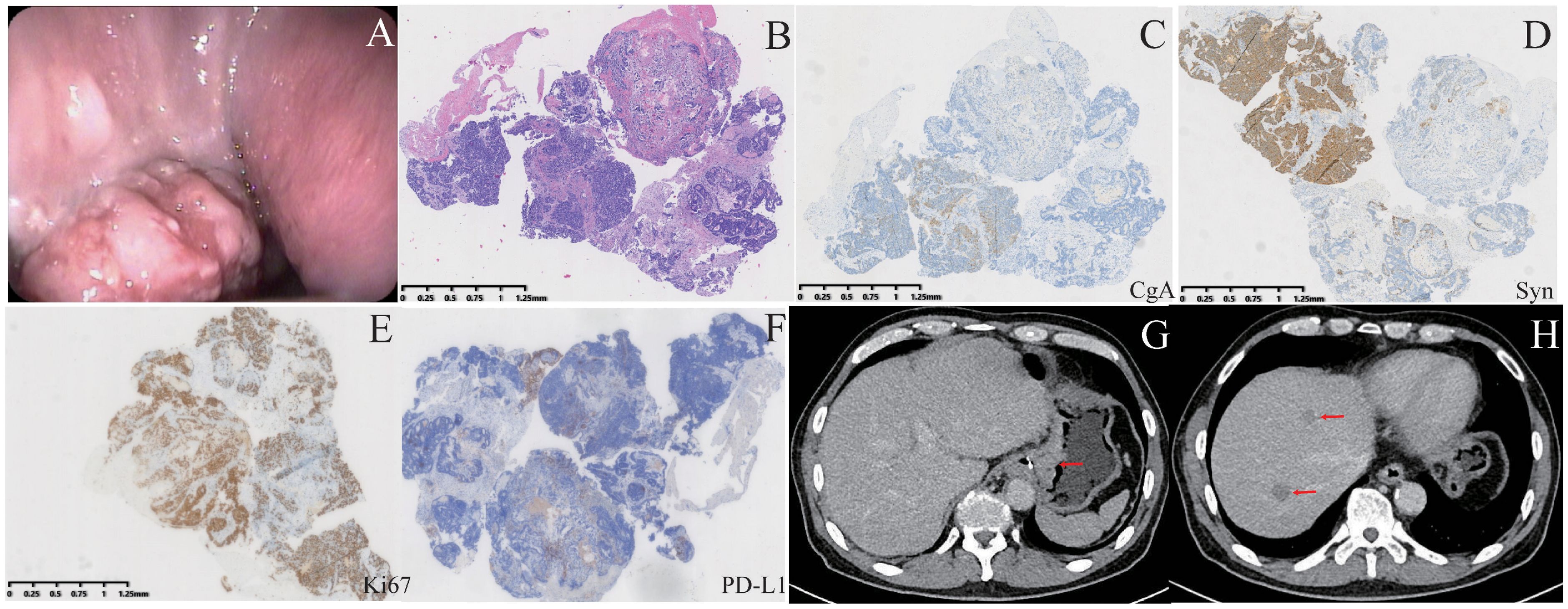
Figure 1. Patient examinations upon first admission. (A) A tumor is visible during gastroscopy. (B) HE staining of gastroscopic biopsy samples. (C–F) Immunohistochemical staining for CgA, Syn, Ki67, and PD-L1. CgA and PD-L1 exhibited focal positivity, whereas Syn and Ki67 showed strong positivity. (G, H) CT revealed gastric wall thickening and liver metastases.
Due to liver metastasis from the tumor, a multidisciplinary team, including oncologists, gastroenterological surgeons, and radiologists, was convened to discuss the treatment for this patient. Ultimately, they decided on a treatment regimen comprising S-1 (60 mg, orally, twice daily on days 1 to 14), apatinib (0.25 g, orally, once daily on days 1 to 21), cadonilimab (720 mg, intravenously, on day 1), and paclitaxel (210 mg, intravenously, on day 1), with each treatment cycle lasting three weeks. After six treatment cycles, the primary tumor achieved partial remission, and the liver metastases disappeared (Figure 2). In the seventh cycle, apatinib was discontinued, and the patient continued with chemotherapy and immunotherapy, followed by conversion surgery. No comorbidities were found during the preoperative examination.
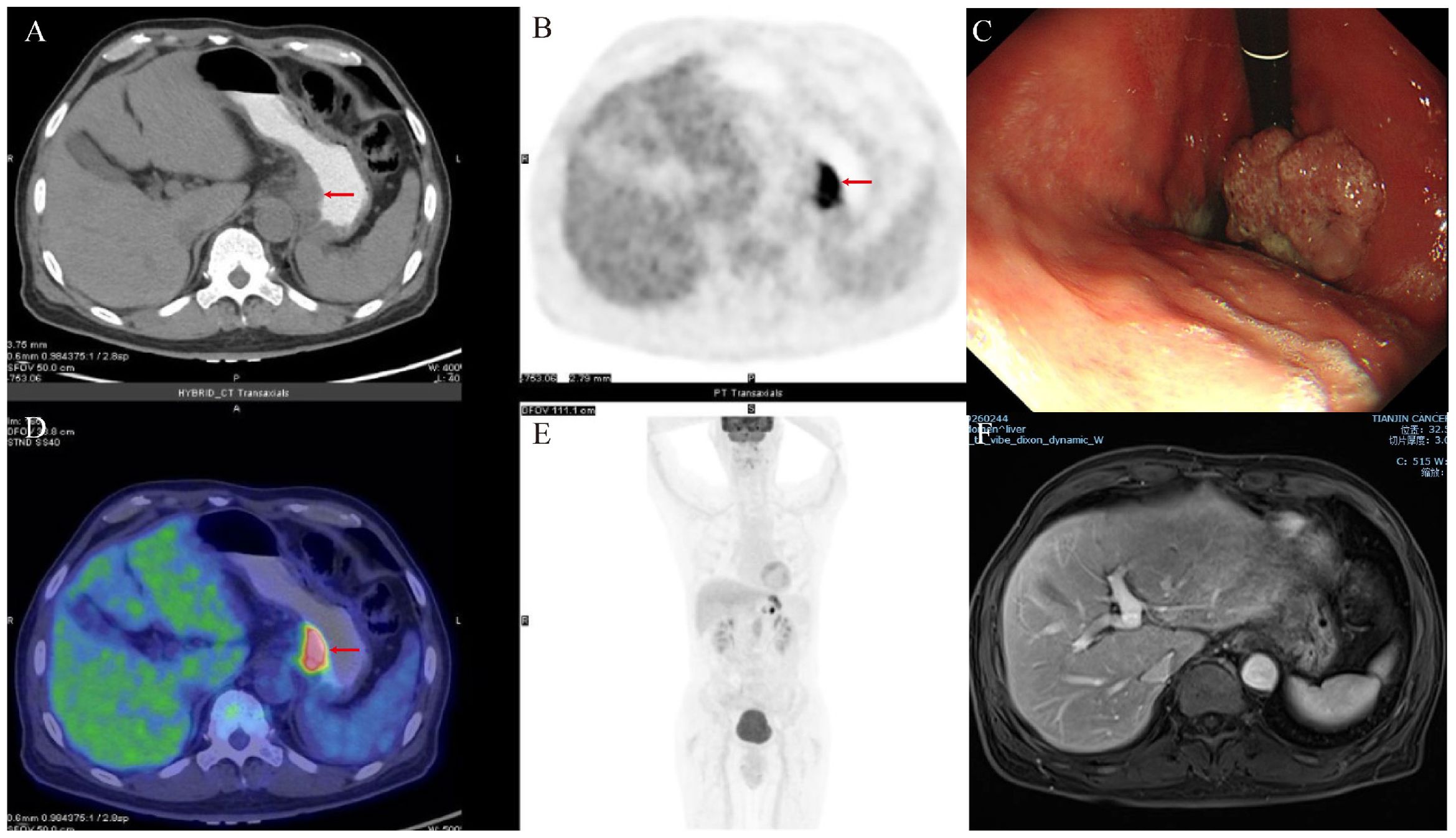
Figure 2. Examinations of the patient after comprehensive treatment. (A, B, D, E) Gastric tumors shrank, and liver metastases disappeared on PET−CT after neoadjuvant therapy. (C) The tumor volume showed reduction during gastroscopy. (F) MRI revealed the absence of liver metastases.
The surgery was performed via an open approach, lasting 312 minutes with a blood loss of 328ml. The procedure went smoothly, with no intraoperative or postoperative complications. The surgery involved proximal gastrectomy, splenectomy, omentectomy, and esophagogastric anastomosis, along with intraoperative frozen pathology assessment and radiofrequency ablation of the liver metastases. R0 resection was successfully achieved, and the patient recovered well, being discharged seven days after the operation. Intraoperative frozen pathology evaluation revealed inflammatory cells in the liver metastases. Postoperative pathological examination revealed a substantial amount of residual adenocarcinoma tissue in the primary gastric lesion, with a tumor regression grade (TRG AJCC 8th) of three (Figure 3). A total of 41 lymph nodes were retrieved during surgery, and two positive lymph nodes were detected. The pathological and immunohistochemical results of the gastric lesion confirmed it as gastric MANEC with pMMR. Additionally, the tumor was negative for both Epstein–Barr virus (EBV) and HER2. However, the polymerase chain reaction (PCR)-based genetic testing revealed that the gastric MANEC exhibited MSI-H. Subsequent genetic testing of multiple gastric lesions consistently indicated MSI-H status. After surgery, the gastric MANEC was classified as stage IIa (ypT2N1M0). The patient declined further adjuvant treatment due to financial reasons and opted for regular follow-up instead.

Figure 3. Treatment procedure and postoperative pathological examination of the gastric tumor. (A) HE staining of postoperative gastric tumor. (B, C) Immunohistochemical staining for CgA and Syn exhibited focal positivity. (D) The patient’s treatment course.
Five months after surgery, the patient returned for follow-up. Abdominal CT revealed multiple new low-density lesions in the liver (Figure 4). Subsequently, a biopsy of the lesions confirmed poorly differentiated carcinoma with a neuroendocrine component. Based on the patient’s medical history, these findings were suggestive of tumor recurrence. Due to the patient’s poor general condition and advanced age following tumor recurrence, S-1 monotherapy was chosen for chemotherapy. Additionally, as the tumor was MSI-H, which tends to respond well to immunotherapy (PD-1 inhibitor), pembrolizumab was selected. Despite treatment with S-1 and pembrolizumab, the disease continued to progress. Given the patient’s poor general condition and severely impaired liver function, palliative care was chosen for subsequent management. The patient passed away in August of this year, with an overall survival of 17 months.
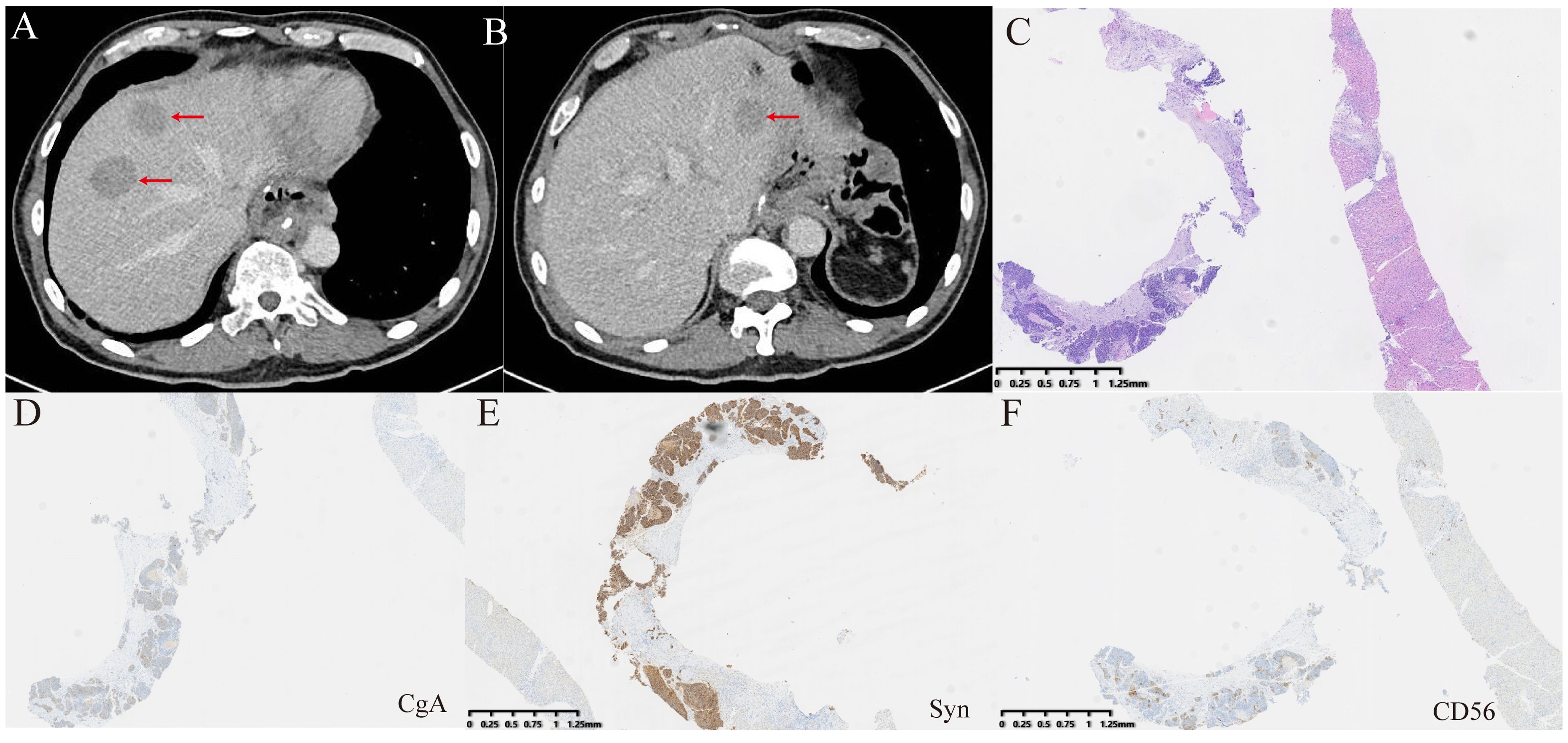
Figure 4. Examinations of liver metastases after surgery. (A, B) CT revealed new liver metastatic lesions. (C) HE staining was performed on the biopsy tissue from the new liver metastases. (D–F) Immunohistochemical staining for CgA, Syn, and CD56. CgA and CD56 exhibited focal positivity, while Syn showed strong positivity.
DiscussionGastric MANEC, as a rare malignant tumor, exhibits biological behavior different from that of gastric adenocarcinoma and tends to metastasize distantly at an early stage (10). A study has found that when the neuroendocrine component in gastric cancer exceeds 10%, it becomes an unfavorable prognostic factor (11). For early-stage gastric neuroendocrine carcinoma and gastric MANEC, surgery is the primary curative approach. However, once gastric MANEC metastasizes, chemotherapy becomes the mainstay of treatment, and surgery is generally not considered. In cases of severe complications such as bleeding or pyloric obstruction, palliative resection may be performed.
Conversion surgery involves performing radical surgery on both primary and metastatic lesions following partial or complete tumor remission achieved through chemotherapy, targeted therapy, immunotherapy, or other treatments. Patients with initially metastatic gastric adenocarcinoma may experience improved survival outcomes if they undergo conversion surgery and achieve R0 resection following chemotherapy. Furthermore, several case reports document successful conversion surgery to achieve a pathological complete response in metastatic gastric adenocarcinoma through the combination of chemotherapy and immunotherapy (12, 13). Indeed, some retrospective studies have indicated that the primary tumor surgery might offer survival advantages to patients with metastatic gastric cancer (14–16). However, it is important to note that these studies included various types of gastric cancer and did not specifically focus on subgroups such as gastric neuroendocrine carcinoma or gastric MANEC. Research on the application of conversion surgery for metastatic gastric MANEC is very limited. As treatment options for metastatic gastric MANEC expand, the feasibility of conversion surgery remains uncertain when tumors achieve partial or complete remission following various systemic treatments.
Microsatellite instability (MSI) is a crucial molecular subtype of gastric cancer that can guide therapeutic decisions. Typically, MSI-H in gastric cancer is closely associated with deficient mismatch repair (dMMR) (17, 18). Interestingly, genetic testing revealed that the gastric MANEC in this patient exhibited MSI-H despite the tumor’s pMMR status. Additional testing is recommended when results inconsistent with MSI-H and dMMR are found in gastric cancer. Therefore, we conducted genetic testing on multiple gastric lesions post-surgery, as well as on the lesions obtained during the initial gastroscopy. The results consistently indicated MSI-H status. The interpretation of the immunohistochemistry results for MMR proteins may also be the reason for this discrepancy (19). Independent reviews by different pathologists consistently confirmed that the tumor exhibited pMMR based on immunohistochemical findings. In a study comparing MSI testing and MMR protein immunohistochemistry, which analyzed over 5,000 gastric cancer tissue samples, the occurrence rate of MSI-H with pMMR was only 0.05% (20). Rare missense mutations in MMR proteins, such as MLH1 and MSH6, that impact protein function without affecting protein translation or antigenicity, may contribute to this discrepancy (21, 22). Next-generation sequencing can be employed to detect these missense mutations. Moreover, it is probably due to alterations in other pathways, mutations in specific regions of the genome that cause microsatellite instability without directly altering the proteins responsible for mismatch repair. Finally, the technical limitations of immunohistochemistry tests may also be one of the reasons.
The survival benefit of chemotherapy in patients with dMMR/MSI-H gastric cancer remains controversial (23). The large number of mutations in MSI-H tumors generate neoantigens, which activate the immune system, resulting in a better response to immunotherapy (24, 25). Notably, research has demonstrated that dual immunotherapy (nivolumab plus ipilimumab) targeting PD-1 and CTLA-4 in locally advanced dMMR/MSI-H gastric or gastroesophageal adenocarcinoma cases can achieve a pathological complete response (pCR) in over half of the patients (24). In our study, the immunohistochemistry for PD-L1 in gastric tumor cells revealed focal positivity, suggesting that the patient is likely to benefit from immunotherapy. We employed a novel immunotherapy drug called cadonilimab. Cadonilimab represents the world’s first bispecific antibody targeting both PD-1 and CTLA-4 (26). In 2017, pembrolizumab received approval from the U.S. Food and Drug Administration (FDA) for unresectable or metastatic dMMR/MSI-H solid tumors (27). Postoperatively, with the emergence of new liver metastases, the patient received combination therapy with pembrolizumab and S-1.
Before the surgery, we adopted a multi-drug regimen based on the recommendations from the multidisciplinary team. The oncologists proposed the use of apatinib for anti-angiogenesis, as it is a targeted therapy approved for gastric cancer, alongside trastuzumab. Cadonilimab has been shown in studies to improve survival outcomes in patients with advanced gastric cancer, including those with low PD-L1 expression. Given the significant side effects associated with platinum-based chemotherapy, along with the patient’s age and the use of multiple medications, we opted for a chemotherapy regimen combining S-1 and paclitaxel. The radiologists recommended against radiotherapy due to the presence of multiple metastatic lesions in the liver, which made it unsuitable for treatment at this stage. The treatment plan was adjusted based on the patient’s response, and due to the favorable outcome observed prior to surgery, no alterations were made to the treatment strategy. Moreover, the patient did not experience significant symptoms from apatinib. Regarding chemotherapy with S-1 and paclitaxel, the patient reported mild fatigue and occasional nausea, which were managed with symptomatic treatment. As for immunotherapy with cadonilimab, the patient did not experience any significant immune-related adverse events.
Throughout the course of treatment, the patient demonstrated excellent cooperation. However, upon initially being informed of the liver metastasis, the patient lost confidence in the treatment. With encouragement from both the family and the medical team, along with the positive effects of comprehensive treatment, the patient regained motivation for therapy. We fully understand the patient’s decision to forgo adjuvant therapy after surgery due to financial constraints. Following postoperative recurrence and progression, with limited efficacy from further treatment and considering the patient’s suffering, we recommended palliative care to alleviate the patient’s discomfort.
Although the liver metastasis probably disappeared after preoperative treatment, it is still possible that cancer cells have metastasized to other parts of the body but were not detected. The early postoperative liver lesions may have originated from cancer cells that had metastasized to other sites before surgery. Notably, both lymph node and liver metastases following systemic therapy were composed of neuroendocrine components. This observation indicates that neuroendocrine components in gastric cancer exhibit greater malignancy and a higher likelihood of metastasis. Research has found that the neuroendocrine component in gastric MANEC originates from adenocarcinoma, leading to increased malignancy (28). In the few reported cases of metastatic gastric MANEC undergoing surgery, new liver metastases often developed shortly afterward (Table 1). In this study, new liver metastases were discovered five months post-surgery and identified as neuroendocrine carcinoma. Although previous reports did not biopsy the new liver metastases, it is highly likely they were also neuroendocrine carcinoma, given the tumor’s propensity for metastasis and recurrence. Despite achieving R0 resection, conversion surgery did not improve survival in this patient. This may be closely related to the neuroendocrine component of the tumor.
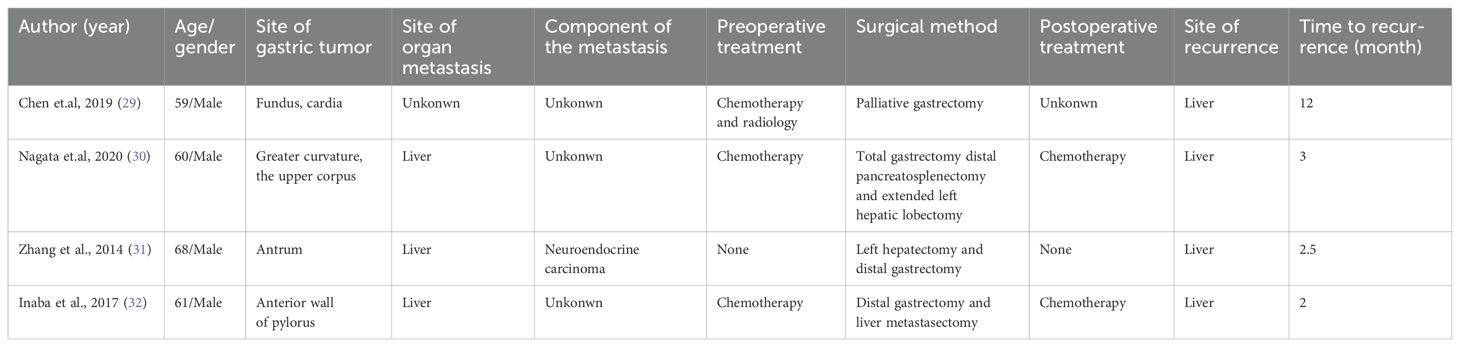
Table 1. Summary of surgical treatment cases for metastatic gastric mixed adenoneuroendocrine carcinoma.
These findings highlight the significant heterogeneity of gastric cancer. While conversion surgery can yield favorable outcomes for advanced gastric adenocarcinoma in the era of targeted and immunotherapy, caution should be exercised when considering conversion surgery for advanced gastric neuroendocrine carcinoma and mixed adenoneuroendocrine carcinoma. Further research is needed to evaluate the long-term benefits of conversion surgery in metastatic gastric MANEC and to develop tailored therapeutic strategies.
ConclusionThis case demonstrates the potential benefit of conversion surgery for metastatic gastric MANEC following systemic therapy, including targeted therapy, immunotherapy, and chemotherapy. Although the conversion surgery achieved an R0 resection, the recurrence of neuroendocrine-differentiated liver metastases highlights the aggressive nature of the disease. These findings suggest that while conversion surgery can be effective for advanced gastric adenocarcinoma, it requires careful consideration for gastric MANEC. Further research is essential to evaluate the long-term outcomes and develop tailored therapeutic strategies for metastatic gastric MANEC.
Data availability statementThe original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Ethics statementWritten informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributionsJH: Writing – original draft. LW: Data curation, Methodology, Writing – review & editing. CT: Data curation, Methodology, Writing – review & editing. WG: Writing – review & editing. YL: Writing – review & editing.
FundingThe author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
AcknowledgmentsWe thank all the authors and the patient who participated in this study.
Conflict of interestThe authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s noteAll claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References2. Nagtegaal ID, Odze RD, Klimstra D, Paradis V, Rugge M, Schirmacher P, et al. The 2019 WHO classification of tumours of the digestive system. Histopathology. (2020) 76:182–8. doi: 10.1111/his.13975
PubMed Abstract | Crossref Full Text | Google Scholar
3. Lin J, Zhao Y, Zhou Y, Tian Y, He Q, Lin J, et al. Comparison of survival and patterns of recurrence in gastric neuroendocrine carcinoma, mixed adenoneuroendocrine carcinoma, and adenocarcinoma. JAMA Netw Open. (2021) 4:e2114180. doi: 10.1001/jamanetworkopen.2021.14180
PubMed Abstract | Crossref Full Text | Google Scholar
4. Lin Z, Kwok HF. RUNDC3A/SNAP25/Akt signaling mediates tumor progression and chemoresistance in gastric neuroendocrine carcinoma. Cell Death Dis. (2022) 13:840. doi: 10.1038/s41419-022-05294-7
PubMed Abstract | Crossref Full Text | Google Scholar
5. Choe HJ, Kim JW, Han SH, Lee JH, Ahn SH, Park DJ, et al. Conversion surgery in metastatic gastric cancer and cancer dormancy as a prognostic biomarker. Cancers (Basel). (2019) 12. doi: 10.3390/cancers12010086
PubMed Abstract | Crossref Full Text | Google Scholar
6. Morgagni P, Solaini L, Framarini M, Vittimberga G, Gardini A, Tringali D, et al. Conversion surgery for gastric cancer: A cohort study from a western center. Int J Surg. (2018) 53:360–5. doi: 10.1016/j.ijsu.2018.04.016
PubMed Abstract | Crossref Full Text | Google Scholar
7. Yoshida K, Yamaguchi K, Okumura N, Tanahashi T, Kodera Y. Is conversion therapy possible in stage IV gastric cancer: the proposal of new biological categories of classification. Gastric Cancer. (2016) 19:329–38. doi: 10.1007/s10120-015-0575-z
PubMed Abstract | Crossref Full Text | Google Scholar
8. Li Z, Ren H, Wang T, Zhang X, Zhao L, Sun C, et al. Resection of the primary tumor improves the survival of patients with stage IV gastric neuroendocrine carcinoma. Front Oncol. (2022) 12:930491. doi: 10.3389/fonc.2022.930491
PubMed Abstract | Crossref Full Text | Google Scholar
9. Zheng M, Li Y, Li T, Zhang L, Zhou L. Resection of the primary tumor improves survival in patients with gastro-entero-pancreatic neuroendocrine neoplasms with liver metastases: A SEER-based analysis. Cancer Med. (2019) 8:5128–36. doi: 10.1002/cam4.v8.11
PubMed Abstract | Crossref Full Text | Google Scholar
10. Lin JP, Zhao YJ, He QL, Hao HK, Tian YT, Zou BB, et al. Adjuvant chemotherapy for patients with gastric neuroendocrine carcinomas or mixed adenoneuroendocrine carcinomas. Br J Surg. (2020) 107:1163–70. doi: 10.1002/bjs.11608
PubMed Abstract | Crossref Full Text | Google Scholar
11. Park JY, Ryu MH, Park YS, Park HJ, Ryoo BY, Kim MG, et al. Prognostic significance of neuroendocrine components in gastric carcinomas. Eur J Cancer. (2014) 50:2802–9. doi: 10.1016/j.ejca.2014.08.004
PubMed Abstract | Crossref Full Text | Google Scholar
12. Matsumoto R, Arigami T, Matsushita D, Okubo K, Tanaka T, Yanagita S, et al. Conversion surgery for stage IV gastric cancer with a complete pathological response to nivolumab: a case report. World J Surg Oncol. (2020) 18:179. doi: 10.1186/s12957-020-01954-0
PubMed Abstract | Crossref Full Text | Google Scholar
13. Izumo W, Hosoda K, Kuramochi H, Nakajima G, Maeda S, Ito S, et al. A case of pathologically complete response after nivolumab combined with chemotherapy in a gastric cancer patient with Virchow's lymph node metastasis. Clin Exp Gastroenterol. (2023) 16:107–15. doi: 10.2147/CEG.S417644
PubMed Abstract | Crossref Full Text | Google Scholar
14. Park JY, Yu B, Park KB, Kwon OK, Lee SS, Chung HY. Impact of palliative gastrectomy in patients with incurable gastric cancer. Medicina (Kaunas). (2021) 57. doi: 10.3390/medicina57030198
PubMed Abstract | Crossref Full Text | Google Scholar
15. Ren B, Yang Y, Lv Y, Liu K. Survival benefits of palliative gastrectomy for gastric cancer patients with liver metastasis: a population-based propensity score-matched cohort analysis. Front Oncol. (2023) 13:1309699. doi: 10.3389/fonc.2023.1309699
PubMed Abstract | Crossref Full Text | Google Scholar
16. Wu J, Yu J, Chen Z, Zhu H, Zhong C, Liang Y, et al. Survival benefit of primary tumor resection for gastric cancer with liver metastasis: A propensity score-matched, population-based study. Front Oncol. (2022) 12:1039086. doi: 10.3389/fonc.2022.1039086
PubMed Abstract | Crossref Full Text | Google Scholar
17. Kim HS, Lee BL, Woo DK, Bae SI, Kim WH. Assessment of markers for the identification of microsatellite instability phenotype in gastric neoplasms. Cancer Lett. (2001) 164:61–8. doi: 10.1016/S0304-3835(00)00719-9
PubMed Abstract | Crossref Full Text | Google Scholar
18. Smyth EC, Wotherspoon A, Peckitt C, Gonzalez D, Hulkki-Wilson S, Eltahir Z, et al. Mismatch repair deficiency, microsatellite instability, and survival: an exploratory analysis of the medical research council adjuvant gastric infusional chemotherapy (MAGIC) trial. JAMA Oncol. (2017) 3:1197–203. doi: 10.1001/jamaoncol.2016.6762
PubMed Abstract | Crossref Full Text | Google Scholar
19. Sari A, Pollett A, Eiriksson LR, Lumsden-Johanson B, Van de Laar E, Kazerouni H, et al. Interobserver agreement for mismatch repair protein immunohistochemistry in endometrial and nonserous, nonmucinous ovarian carcinomas. Am J Surg Pathol. (2019) 43:591–600. doi: 10.1097/PAS.0000000000001220
PubMed Abstract | Crossref Full Text | Google Scholar
20. Park Y, Nam SK, Seo SH, Park KU, Oh HJ, Park YS, et al. Comprehensive study of microsatellite instability testing and its comparison with immunohistochemistry in gastric cancers. J Gastric Cancer. (2023) 23:264–74. doi: 10.5230/jgc.2023.23.e5
PubMed Abstract | Crossref Full Text | Google Scholar
21. Klarskov L, Holck S, Bernstein I, Okkels H, Rambech E, Baldetorp B, et al. Challenges in the identification of MSH6-associated colorectal cancer: rectal location, less typical histology, and a subset with retained mismatch repair function. Am J Surg Pathol. (2011) 35:1391–9. doi: 10.1097/PAS.0b013e318225c3f0
PubMed Abstract | Crossref Full Text | Google Scholar
23. Wu H, Ma W, Jiang C, Li N, Xu X, Ding Y, et al. Heterogeneity and adjuvant therapeutic approaches in MSI-H/dMMR resectable gastric cancer: emerging trends in immunotherapy. Ann Surg Oncol. (2023) 30:8572–87. doi: 10.1245/s10434-023-14103-0
PubMed Abstract | Crossref Full Text | Google Scholar
24. André T, Tougeron D, Piessen G, de la Fouchardière C, Louvet C, Adenis A, et al. Neoadjuvant nivolumab plus ipilimumab and adjuvant nivolumab in localized deficient mismatch repair/microsatellite instability-high gastric or esophagogastric junction adenocarcinoma: the GERCOR NEONIPIGA phase II study. J Clin Oncol. (2023) 41:255–65. doi: 10.1200/jco.22.00686
PubMed Abstract | Crossref Full Text | Google Scholar
25. Lei X, Wang Y, Shan F, Li S, Jia Y, Miao R, et al. Efficacy and safety of preoperative immunotherapy alone followed by surgery in the treatment of advanced gastric cancer with MSI-H/dMMR or EBV-positive. J Chin Med Assoc. (2023) 86:717–24. doi: 10.1097/JCMA.0000000000000944
PubMed Abstract | Crossref Full Text | Google Scholar
27. Hargadon KM, Johnson CE, Williams CJ. Immune checkpoint blockade therapy for cancer: An overview of FDA-approved immune checkpoint inhibitors. Int Immunopharmacol. (2018) 62:29–39. doi: 10.1016/j.intimp.2018.06.001
PubMed Abstract | Crossref Full Text | Google Scholar
28. Qiu MZ, Chen Q, Zheng DY, Zhao Q, Wu QN, Zhou ZW, et al. Precise microdissection of gastric mixed adeno-neuroendocrine carcinoma dissects its genomic landscape and evolutionary clonal origins. Cell Rep. (2023) 42:112576. doi: 10.1016/j.celrep.2023.112576
PubMed Abstract | Crossref Full Text | Google Scholar
29. Chen H, Shu M, Chen S, Xue L, Lin Y. Clinicopathological features and lymph node metastatic patterns of gastric mixed adenoneuroendocrine carcinoma. Histol Histopathol. (2019) 34:373–9. doi: 10.14670/hh-18-045
PubMed Abstract | Crossref Full Text | Google Scholar
30. Nagata H, Tsujimoto H, Yaguchi Y, Kouzu K, Itazaki Y, Ishibashi Y, et al. Mixed adenoneuroendocrine carcinoma with loss of HER2 positivity after trastuzumab-based chemotherapy for HER2-positive gastric cancer: a case report. Surg Case Rep. (2020) 6:5. doi: 10.1186/s40792-020-0774-7
PubMed Abstract | Crossref Full Text | Google Scholar
31. Zhang W, Xiao W, Ma H, Sun M, Chen H, Zheng S. Neuroendocrine liver metastasis in gastric mixed adenoneuroendocrine carcinoma with trilineage cell differentiation: a case report. Int J Clin Exp Pathol. (2014) 7:6333–8.
PubMed Abstract | Google Scholar
32. Inaba Y, Fujita M, Ninomiya R, Hashimoto D. Conversion therapy using etoposide and cisplatin chemotherapy for liver metastases from advanced gastric mixed adenoneuroendocrine carcinoma - A case report. Gan To Kagaku Ryoho. (2017) 44:1488–90.
留言 (0)