The detection of anterior mediastinal masses has significantly increased due to the widespread use of computed tomography screening (1). Surgical intervention remains the primary treatment strategy for these masses. Video-assisted thoracoscopic surgery (VATS) offers several advantages over traditional sternotomy, including reduced trauma, minimal bleeding, decreased pain, quicker recovery, shorter hospital stays, and minimal impact on cardiopulmonary function, all while maintaining comparable recurrence and survival rates (2–4). Among the VATS techniques, the classic lateral intercostal approach (LA) and the subxiphoid approach (SA) are prevalent. Increasing evidence suggests that the SA provides superior exposure, facilitates bilateral phrenic nerve clearance and fat removal, and offers a visual field akin to that of sternotomy, potentially reducing postoperative pain and skin numbness (5, 6). Despite its growing preference among thoracic surgeons, clear indications for choosing between the SA and LA for anterior mediastinal mass resections remain undefined. This retrospective study compares clinical data from patients undergoing LA and SA at our center, aiming to delineate these indications.
Methods Study cohort and clinical dataWe retrospectively reviewed all patients who underwent VATS resection for anterior mediastinal tumors at our center from January 2019 to December 2023. Inclusion criteria included: (I) adults (≥18 years of age); (II) diagnosis of an anterior mediastinal tumor; and (III) complete clinical data and computed tomography scans available. Exclusion criteria were: (I) patients who underwent median sternotomy; (II) lateral position intercostal chest insertion; (III) malignant tumors with apparent organ invasion; (IV) pericardial cysts; and (V) extensive thoracic adhesions. We documented patient characteristics and staged thymomas according to the Masaoka classification (7).
Surgical technique Subxiphoid approach (SA)Patients were placed in a lithotomy position. A 3 cm transverse or vertical incision was made under the xiphoid process for thoracoscope insertion. Additional 0.5 cm incisions were made at the bilateral midclavicular line and costal arch junction for trocar placement. We created an artificial pneumothorax (CO2 pressure: 8–10 mmHg) and performed mediastinal dissection down to the left innominate vein using an ultrasonic knife. Thymic veins were severed and the specimen was removed through the xiphoid incision. Thoracic drainage utilized 15F or 22F negative pressure balls (Figure 1A).
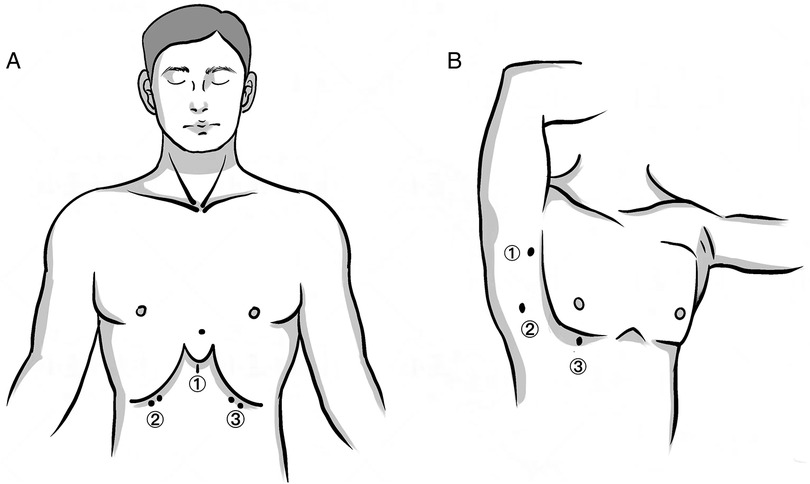
Figure 1. Skin incisions of the two approaches. (A) SA: (1) a 3 cm transverse or vertical incision under the xiphoid process as the observational port; (2, 3) a 0.5–1 cm incision at the intersection of the bilateral midclavicular line and the costal arch as the operating port. (B) LA: (1) a 0.5 cm incision at the anterior axillary line in the third intercostal space as the operating port; (2) a 1 cm incision at the midaxillary line in the fifth intercostal space as the observational port; (3) a 1 cm incision at the mid-clavicular line in the fifth intercostal space as the operating port.
Lateral intercostal approach (LA) (Supplementary Video S1)Patients were positioned supinely. Incisions were made in the third intercostal space at the anterior axillary line, and in the fifth intercostal space at the mid-clavicular and mid-axillary lines. Following pneumothorax induction (CO2 pressure: 8–10 mmHg), resection was performed predominantly from the right side, unless the tumor was left-dominant. The bilateral LA approach facilitated complete adipose tissue removal in patients with myasthenia gravis or positive anti-acetylcholine receptor antibodies (Figure 1B). Based on the size of masses, extended the ③ incision to 1.5–2 cm (Figure 1B), and used a retrieval bag to remove the specimen.
Analgesic strategiesWe employed multimodal analgesia including paravertebral, intercostal nerve block, or incision infiltration. A patient-controlled analgesia (PCIA) pump delivered a mix of 100 μg sufentanil and 100 μg dexmedetomidine in 100 ml saline at 2 ml/h. Additional NSAIDs or opioids were administered as needed, and pain levels were assessed using the visual analogue scale (VAS) on the day of and the day after surgery.
Ethical statementThe study adhered to the ethical standards of the Declaration of Helsinki (2013 revision) and was approved by the Ethics Committee of Huai'an First People's Hospital, Nanjing Medical University. Consent for this retrospective analysis was waived.
Statistical analysisStatistical analyses were performed using SPSS (version 21.0; IBM SPSS Statistics, Chicago, IL, USA). Continuous variables are expressed as mean ± SD, and categorical variables as frequencies and percentages. Differences were evaluated using independent t-tests or Wilcoxon rank-sum tests for continuous variables and chi-square tests for categorical variables. A p-value < 0.05 was considered statistically significant.
Results Patient demographics and baseline characteristicsOur study included 91 patients: 35 underwent the subxiphoid approach (SA) and 56 underwent the lateral intercostal approach (LA). No significant differences were found between the two groups in age, gender, body mass index, and tumor size (all P > 0.05). Thymic cysts were diagnosed in 47.3% of all patients, with a higher incidence in the SA group compared to the LA group, which had a higher proportion of types A, AB, and B1 thymomas (P < 0.05). Myasthenia gravis (MG) was present in 6.6% of the patients (Table 1).
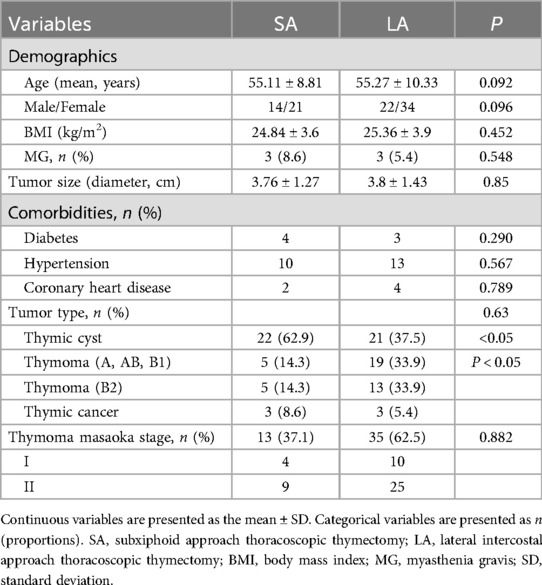
Table 1. Demographic and clinical characteristics of the two groups.
Short-term outcomesOperative outcomes varied significantly between the two approaches. The LA group benefited from shorter operative times (P = 0.00), reduced hospital stays (P = 0.00), and less chest tube drainage (P = 0.007) compared to the SA group. Pain levels assessed by the Visual Analogue Scale (VAS) showed no difference on the day of surgery or the first postoperative day. Additionally, the use of extra analgesics post-surgery did not differ significantly between the groups.
Postoperative complications were minimal and comparable between the groups, including phrenic nerve paralysis, effusions, pneumothoraces requiring thoracocentesis, secondary operations, and dyspnea (all P > 0.05). Notably, one patient in the LA group underwent a second surgery due to persistent hemothorax, and one patient in the SA group developed a pleural effusion. Financial considerations also differed, with the SA group incurring higher hospitalization costs than the LA group (P = 0.024) (Table 2).
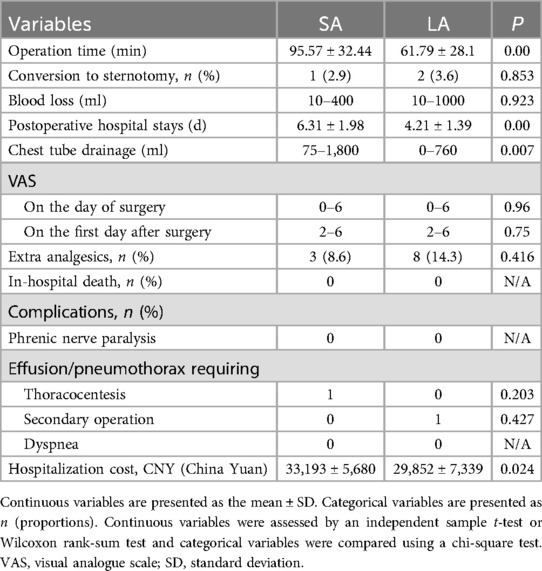
Table 2. Perioperative data and in-hospital outcomes.
DiscussionSurgical resection stands as the primary treatment for anterior mediastinal masses. Since the introduction of VATS thymectomy in 1993 by a team in Boston, MA, USA, the intercostal approach has been globally recognized and adopted (8, 9). Despite its widespread use, the lateral intercostal approach (LA) encounters limitations, particularly in accessing the contralateral phrenic nerve and reaching the superior pole of the thymus. Moreover, intercostal incisions are known to potentially damage nerves, leading to chronic neuralgia and abnormal skin sensations (10). Nevertheless, the five-year survival rates for patients with Masaoka stage I-II thymomas undergoing this procedure align closely with those seen in open surgery (11).
In 1999, Kido introduced the subxiphoid approach (SA) as an alternative for resecting anterior mediastinal masses (12). Presently, both single- and three-port subxiphoid approaches are employed (13), though they come with their challenges, particularly in obese patients or those with mediastinal stenosis where increased chest rigidity complicates the procedure. The SA often necessitates additional surgical instruments and is especially beneficial in larger masses when combined with sternal elevation techniques (14, 15). Mao et al. utilized a sternal elevation device along with a closed incision protection sleeve and CO2 pressurization to enhance the operable space behind the sternum, facilitating the resection of larger or more complex mediastinal tumors (14).
Contrary to earlier findings, our study indicates no significant benefits of SA over LA regarding surgical duration, hospital stay, or drainage volume. While some research shows no difference in operative times between the two approaches (16), others have reported shorter times for SA (17, 18). Contrasting with previous reports that averaged 120 min for LA (13), our findings demonstrate a notably shorter average operating time of 61.79 ± 28.1 min for LA, which might be attributed to the efficient surgical techniques employed, such as supine positioning, single-lumen endotracheal intubation, and a closed incision with a protective sleeve to prevent CO2 leakage. This streamlined process could potentially contribute to incomplete fat clearance and reliance on a unilateral approach differed from the bilateral LA approach in the Madhuri's study (19). Additionally, a shorter operating time inherently reduces the risk of complications (20) and could be influenced by the SA's steep learning curve, as Suda noted it requires 30–40 cases to achieve proficiency (21).
Pain management, evaluated through Visual Analog Scale (VAS) scores, showed no significant differences postoperatively, aligning with findings from Wang et al., where early postoperative pain improvements in SA did not extend beyond the early recovery phase (22). The use of multimodal analgesia and advanced drainage techniques likely contributed to the consistently low pain levels observed.
In our cohort, three patients with Myasthenia Gravis (MG) were treated under each approach, highlighting the importance of removing ectopic thymic tissue to alleviate symptoms and reduce crises (23, 24). Thymectomy and removal of fat alleviates the symptoms of MG and reduces the occurrence of myasthenic crises, which has become an international consensus (10, 25). Studies have shown that minimally invasive procedures can effectively manage MG symptoms as well as traditional sternotomy (26, 27), with SA potentially offering superior outcomes due to its ability to ensure a more complete thymectomy and fat clearance (18, 28).
The incidence of requiring a conversion to sternotomy was similar between the groups, primarily due to injuries to the innominate vein. Both approaches demonstrated a low 30-day postoperative complication rate with no significant differences observed. Cost analysis revealed that LA was more economically advantageous compared to SA, reflecting the latter's requirement for more specialized equipment and potentially longer operating times.
In our single-center study, the high prevalence of thymic cysts (47.3%) underscores the necessity for precise surgical intervention. MRI, which was not utilized in all cases at our center, could provide detailed lesion characterization, potentially preventing unnecessary surgeries (29, 30). In contrast, acquired thymic cysts are much more common, tend to be multilocular, and may arise in association with neoplasms such as thymomas, lymphomas, or germ cell tumors (30).
Our findings suggest that while SA offers a comprehensive solution akin to sternotomy for non-MG thymoma patients, it does not significantly impact long-term survival compared to LA (26, 31–33). The choice between SA and LA should, therefore, be tailored based on individual patient profiles and available resources.
Despite the strengths of this retrospective study, its limitations include potential selection bias, a small sample size, and the single-center design, which might affect the generalizability of the results. Additionally, the lack of long-term follow-up restricts our ability to assess the durability of these surgical outcomes. Future multicenter studies with larger cohorts and longer follow-ups are essential to further elucidate the optimal surgical approach for resecting anterior mediastinal masses.
ConclusionsWhile the SA is favored by many surgeons for its theoretical benefits in visualization and reduced pain, our findings and those of other recent studies suggest that the choice between SA and LA should be guided more by specific patient anatomical considerations, surgeon expertise, and resource availability rather than inherent superiority of one technique over the other. For patients with thymic cysts and early-stage thymomas without MG, LA may offer a more efficient and cost-effective option.
Data availability statementThe original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Ethics statementThe studies involving humans were approved by the study adhered to the ethical standards of the Declaration of Helsinki (2013 revision) and was approved by the Ethics Committee of Huai'an First People's Hospital, Nanjing Medical University. Consent for this retrospective analysis was waived. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributionsXL: Conceptualization, Data curation, Formal Analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. MC: Data curation, Investigation, Writing – original draft. YZ: Investigation, Methodology, Writing – review & editing. JG: Data curation, Writing – review & editing. ZY: Methodology, Writing – review & editing. ZH: Conceptualization, Data curation, Formal Analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing.
FundingThe author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
AcknowledgmentsWe thank International Science Editing (http://www.internationalscienceediting.com) for editing this manuscript.
Conflict of interestThe authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's noteAll claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary materialThe Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fsurg.2024.1463881/full#supplementary-material
References1. Fang W, Xu N, Shen Y, Gu Z, Mao T, Ji C, et al. Management of incidentally detected small anterior mediastinal nodules: which way to go? Lung Cancer. (2022) 168:30–5. doi: 10.1016/j.lungcan.2022.04.007
PubMed Abstract | Crossref Full Text | Google Scholar
2. Agatsuma H, Yoshida K, Yoshino I, et al. Video-assisted thoracic surgery thymectomy versus sternotomy thymectomy in patients with thymoma. Ann Thorac Surg. (2017) 104:1047–53. doi: 10.1016/j.athoracsur.2017.03.054
PubMed Abstract | Crossref Full Text | Google Scholar
3. Seong YW, Kang CH, Choi JW, et al. Early clinical outcomes of robot-assisted surgery for anterior mediastinal mass: its superiority over a conventional sternotomy approach evaluated by propensity score matching†. Eur J Cardiothorac Surg. (2014) 45:e68–73. discussion e73. doi: 10.1093/ejcts/ezt557
PubMed Abstract | Crossref Full Text | Google Scholar
4. Liu TJ, Lin MW, Hsieh MS, et al. Video-assisted thoracoscopic surgical thymectomy to treat early thymoma: a comparison with the conventional transsternal approach. Ann Surg Oncol. (2014) 21:322–8. doi: 10.1245/s10434-013-3228-7
PubMed Abstract | Crossref Full Text | Google Scholar
5. Zhang L, Li M, Jiang F, et al. Subxiphoid versus lateral intercostal approaches thoracoscopic thymectomy for non-myasthenic early-stage thymoma: a propensity score -matched analysis. Int J Surg. (2019) 67:13–7. doi: 10.1016/j.ijsu.2019.01.011
PubMed Abstract | Crossref Full Text | Google Scholar
6. Jiang L, Chen H, Hou Z, et al. Subxiphoid versus unilateral video-assisted thoracoscopic surgery thymectomy for thymomas: a propensity score matching analysis. Ann Thorac Surg. (2022) 113:1656–62. doi: 10.1016/j.athoracsur.2021.05.011
PubMed Abstract | Crossref Full Text | Google Scholar
7. Masaoka A, Monden Y, Nakahara K, Tanioka T. Follow-up study of thymomas with special reference to their clinical stages. Cancer. (1981) 48:2485–92. doi: 10.1002/1097-0142(19811201)48:11%3C2485::AID-CNCR2820481123%3E3.0.CO;2-R
PubMed Abstract | Crossref Full Text | Google Scholar
9. Allakhverdiev A, Davydov M, Allakhverdieva G, et al. Thoracoscopic thymectomy—the method of choise in surgical treatment of non-invasive thymomas. Ann Med Surg (Lond). (2019) 42:29–34. doi: 10.1016/j.amsu.2018.12.005
PubMed Abstract | Crossref Full Text | Google Scholar
11. Xie A, Tjahjono R, Phan K, et al. Video-assisted thoracoscopic surgery versus open thymectomy for thymoma: a systematic review. Ann Cardiothorac Surg. (2015) 4:495–508.26693145
PubMed Abstract | Google Scholar
12. Kido T, Hazama K, Inoue Y, Tanaka Y, Takao T. Resection of anterior mediastinal masses through an infrasternal approach. Ann Thorac Surg. (1999) 67(1):263–5. doi: 10.1016/S0003-4975(98)01210-7
PubMed Abstract | Crossref Full Text | Google Scholar
13. Min C, Qing W, Hang Y, et al. Short-term analysis of uniport video-assisted thoracoscopic surgery via the subxiphoid approach without chest tube drainage for anterior mediastinal tumors: a comparative retrospective study. Ann Transl Med. (2021) 9:1687. doi: 10.21037/atm-21-5790
PubMed Abstract | Crossref Full Text | Google Scholar
14. Mao T, Zhang X, Yang Y, et al. A uniport subxiphoid approach with a modified sternum retractor is safe and feasible for anterior mediastinal tumors. J Thorac Dis. (2023) 15:1364–72. doi: 10.21037/jtd-23-244
PubMed Abstract | Crossref Full Text | Google Scholar
15. Nicky J, Franssen Aimée JPM, Daemen Jean HT, et al.Combining the best of both worlds: sternal elevation for resection of anterior mediastinal tumors through the subxiphoidal uniportal video-assisted thoracoscopic surgery approach.J Thorac Dis. (2023) 15:4573–6. doi: 10.21037/jtd-23-1167
PubMed Abstract | Crossref Full Text | Google Scholar
16. Suda T, Hachimaru A, Tochii D, Maeda R, Tochii S, Takagi Y. Video-assisted thoracoscopic thymectomy versus subxiphoid single-port thymectomy: initial results. Eur J Cardiothorac Surg. (2016) 49(suppl 1):i54–8.26468270
PubMed Abstract | Google Scholar
17. Qiu Z, Chen L, Lin Q, Wu H, Sun H, Zhou X, et al. Perioperative outcomes and mid-term effects in performing video-assisted thoracoscopic extended thymectomy for myasthenia gravis. J Thorac Dis. (2020) 12(4):1529–39. doi: 10.21037/jtd.2020.03.43
PubMed Abstract | Crossref Full Text | Google Scholar
18. Lu Q, Zhao J, Wang J, Chen Z, Han Y, Huang L, et al. Subxiphoid and subcostal arch ‘three ports’ thoracoscopic extended thymectomy for myasthenia gravis. J Thorac Dis. (2018) 10:1711. doi: 10.21037/jtd.2018.02.11
PubMed Abstract | Crossref Full Text | Google Scholar
19. Madhuri R, Aitua S, Alexandria R, et al. Subxiphoid-subcostal versus transthoracic thoracoscopic thymectomy: a safe and feasible approach. JTCVS Tech. (2022) 16:172–81. doi: 10.1016/j.xjtc.2022.08.017
PubMed Abstract | Crossref Full Text | Google Scholar
20. Cheng H, Clymer JW, Po-Han Chen B, Sadeghirad B, Ferko NC, Cameron CG, et al. Prolonged operative duration is associated with complications: a systematic review and meta-analysis. J Surg Res. (2018) 229:134–44. doi: 10.1016/j.jss.2018.03.022
PubMed Abstract | Crossref Full Text | Google Scholar
21. Suda T, Ishizawa H, Nagano H, Negi T, Kawai H, Tochii D, et al. Early outcomes in 147 consecutive cases of subxiphoid single-port thymectomy and evaluation of learning curves. Eur J Cardiothorac Surg. (2020) 58(Suppl 1):i44–9. doi: 10.1093/ejcts/ezaa183
PubMed Abstract | Crossref Full Text | Google Scholar
22. Wang S, Yang X, et al. Subxiphoid versus lateral intercostal thoracoscopic thymectomy for suspected thymoma: results of a randomized controlled trial. J Thorac Cardiovasc Surg. (2024) 168(1):290–8. doi: 10.1016/j.jtcvs.2023.10.040
PubMed Abstract | Crossref Full Text | Google Scholar
23. Masaoka A, Nagaoka Y, Kotake Y. Distribution of thymic tissue at the anterior mediastinum. Current procedures in thymectomy. Thorac Cardiovasc Surg. (1975) 70(4):747–54. doi: 10.1016/S0022-5223(19)40309-7
Crossref Full Text | Google Scholar
25. Sanders DB, Wolfe GI, Benatar M, Evoli A, Gilhus NE, Illa I, et al. International consensus guidance for management of myasthenia gravis: executive summary. Neurology. (2016) 87(4):419–25. doi: 10.1212/WNL.0000000000002790
PubMed Abstract | Crossref Full Text | Google Scholar
26. Zhitao G, Chun C, Yun W, et al. Video-assisted thoracoscopic surgery versus open surgery for stage I thymic epithelial tumours: a propensity score-matched study†. Eur J Cardiothorac Surg. (2018) 54:1037–44. doi: 10.1093/ejcts/ezy239
PubMed Abstract | Crossref Full Text | Google Scholar
28. Zhang S, Chen Z, Li B, Su C, Zhu H, Zhong B, et al. Efficiency of ectopic thymectomy by three surgical approaches in non-thymomatous myasthenia gravis. Updates Surg. (2022) 74(4):1435–43. doi: 10.1007/s13304-022-01295-5
PubMed Abstract | Crossref Full Text | Google Scholar
29. LoCicero J, Feins RH, Colson YL, et al. Shields’ General Thoracic Surgery. 8th ed M. Wolters Kluwer Health (2018).
31. Voulaz E, Veronesi G, Infante M, et al. Radical thymectomy versus conservative thymomectomy in the surgical treatment of thymic malignancies. J Thorac Dis. (2018) 10(7):4127–36. doi: 10.21037/jtd.2018.06.04
PubMed Abstract | Crossref Full Text | Google Scholar
32. Pulle MV, Asaf BB, Puri HV, Bishnoi S, Kumar A. Meta-Analysis of limited thymectomy versus total thymectomy for masaoka stage I and II thymoma. J Chest Surg. (2021) 54(2):127–36. doi: 10.5090/jcs.20.140
PubMed Abstract | Crossref Full Text | Google Scholar
33. Voulaz E, Perroni G, Russo A, Patirelis A, Mangiameli G, Alloisio M, et al. Thymomectomy versus complete thymectomy in early-stage non-myasthenic thymomas: a multicentric propensity score-matched study. Interact Cardiovasc Thorac Surg. (2022) 35(1):ivac167. doi: 10.1093/icvts/ivac167
留言 (0)