Colorectal cancer (CRC), a malignant neoplasm, represents a significant global health challenge, with over 1.85 million new cases and 850,000 deaths annually, ranking it as the third leading cause of cancer mortality worldwide. Nearly 20% of CRC patients exhibit metastasis at the time of diagnosis, and metastatic CRC (mCRC) is often an incurable disease. Due to the high frequency of metastasis and drug resistance, colorectal cancer remains one of the most difficult cancers to treat despite all the advances in biological knowledge and treatment improvements (1–3). Approximately 70% to 75% of patients with metastatic colorectal cancer survive beyond one year, 30% to 35% beyond three years, and fewer than 20% beyond five years. The primary treatment for inoperable metastatic colorectal cancer involves systemic therapy, encompassing chemotherapy, targeted therapy, and immunotherapy. Colorectal cancer is caused by the activation of oncogene mutations and the inactivation of tumor suppressor genes, with the latter being the main cause (4, 5). Different genetic characteristics lead to different prognoses (6, 7). Genomic analyses, focusing on somatic mutations in genes such as KRAS, NRAS, and BRAF, aid in selecting targeted medications and predicting future survival outcomes. Based on numerous seminal studies, guidelines for patients with refractory metastatic colorectal adenocarcinoma that is microsatellite stable recommend a standard chemotherapy regimen of fluorouracil, oxaliplatin, and irinotecan for the first and second lines of treatment. This regimen is to be used in conjunction with targeted drugs against vascular endothelial growth factor (VEGF) and its receptor (VEGFR), and epidermal growth factor receptor (EGFR), such as bevacizumab or cetuximab, based on genetic testing (8). Regorafenib, a novel oral multi-kinase inhibitor, disrupts kinases involved in tumor angiogenesis (VEGFR1, VEGFR2, VEGFR3, TIE2), tumorigenesis (KIT, RET, RAF1, BRAF, and BRAFV600E), and the tumor microenvironment (PDGFR and FGFR) (9). According to the CORRECT study (10), regorafenib has been approved for treating metastatic colorectal cancer after failure of all standard therapies, marking it as the first small molecule multi-kinase inhibitor to demonstrate a survival advantage in this setting (11). Fruquintinib, a novel VEGFR inhibitor, has received approval from the China National Medical Products Administration for treating advanced colorectal cancer patients who have undergone at least two standard anticancer treatments, as evidenced by the FRESCO study (12). Trifluridine/tipiracil, an innovative oral antimetabolic agent comprising trifluridine (a thymidine nucleoside analog) and tipiracil (a potent thymidine phosphorylase inhibitor), is currently approved for patients with refractory metastatic colorectal cancer after standard chemotherapy (13). An amalgamation of results from multiple related meta-analyses indicates that the differences in overall survival (OS) and progression-free survival (PFS) among these three drugs in third-line treatment are generally insignificant (14–16). According to the C-TASK-FORCE study and Danish studies, trifluridine/tipiracil combined with bevacizumab has demonstrated promising activity and manageable safety (17, 18). In metastatic colorectal cancer, the clinical efficacy of anti-PD-1 therapy is typically limited to tumors with high microsatellite instability (MSI-H), which constitute only 4-6% of cases (19). However, the significant progress of the REGONIVO study (20) have led to the increasing researchers trying to use the combination therapy of TKIs and PD-1 inhibitors, and has shown efficacy to a certain extent, indicating that the combination therapy of targeted and immune for advanced colorectal cancer may be about to enter a new era. Nonetheless, many ongoing studies on targeted and immune combination therapy remain placebo-controlled, without direct comparisons. Hence, selecting the optimal third-line combination treatment for advanced colorectal cancer post-standard treatment continues to be a key focus of research.
Materials and methodsEthical approval and informed consentThe Ethics Committee of Henan Cancer Hospital reviewed and approved the study protocol, assigning it the ethical approval number: 2023-187-002. Given the study’s retrospective and non-interventional nature, the committee waived the requirement for informed consent.
Patient populationThis study is a retrospective cohort analysis. It collected clinical data from patients treated at Henan Cancer Hospital from May 2019 to April 2023, who were diagnosed with histopathologically confirmed microsatellite-stable advanced colorectal adenocarcinoma and who underwent third-line treatment. These individuals had previously received standard first- and second-line treatments, predominantly involving fluorouracil, irinotecan, oxaliplatin, bevacizumab, and cetuximab, until experiencing progression or intolerance. The patients were in generally good condition, with ECOG performance status scores of 0-2.
Treatment regimenPatients received third-line treatment with either fruquintinib (Elunate, Hutchison Whampoa Pharmaceuticals) combined with a PD-1 inhibitor, regorafenib (Stivarga, Bayer) combined with a PD-1 inhibitor, or trifluridine/tipiracil (Lonsurf, Taiho Pharmaceutical Co., Ltd.; Suhoo, Qilu Pharmaceutical; Qilu, Chia Tai Tianqing) combined with bevacizumab (Avastin, Roche Pharma (Switzerland) Ltd.; Eritu, Jiangsu Hengrui Medicine Co., Ltd.; Ankada, Qilu Pharmaceutical; Dayutong, Cinda Biopharmaceutical). PD-1 inhibitors include, but are not limited to, sintilimab (Tyvyt, Innovent Biologics), camrelizumab (AiRuiKa, Jiangsu Hengrui Medicine Co., Ltd.), tislelizumab (BaiZeAn, BeiGene Ltd.), toripalimab (Tuoyi, Suzhou Zelgen Biopharmaceuticals), nivolumab (Opdivo, Bristol-Myers Squibb), and pembrolizumab (Keytruda, Merck), all of which were administered in accordance with routine clinical treatment protocols.
Efficacy and safetyPatients were monitored according to the routine protocols of Henan Cancer Hospital, with survival data obtained from the hospital’s follow-up center through April 28, 2023. Clinical efficacy was assessed through imaging examinations according to the Response Evaluation Criteria in Solid Tumors (RECIST version 1.1), encompassing complete response (CR), partial response (PR), stable disease (SD), and progressive disease (PD). The objective response rate (ORR) is calculated as the sum of CR and PR, while the disease control rate (DCR) includes CR, PR, and SD. The Common Terminology Criteria for Adverse Events (version 5.0) were utilized to evaluate drug toxicity. Progression-free survival (PFS) was determined from the initiation of combined treatment to disease progression (either clinical or radiological) or death from any cause, whichever came first. Overall survival (OS) was calculated from the beginning of treatment until death from any cause.
Statistical analysisPFS is characterized as the duration from the onset of third-line treatment to disease progression or death, while OS refers to the time from the start of third-line treatment to the patient’s death or the last follow-up. The Kaplan-Meier method is employed for survival analysis. The Pearson’s chi-square test or Fisher’s exact test are applied to evaluate differences between groups. Univariate analysis identifies predictive factors for PFS and OS. Statistical analysis and visualization are conducted using SPSS 27 and PRISM 9.0. The significance level (α) is established at 0.05.
ResultsPatient characteristics and treatmentBased on the inclusion and exclusion criteria, clinical data from 60 patients with microsatellite-stable metastatic colorectal adenocarcinoma who underwent third-line treatment were analyzed. Table 1 presents the demographic characteristics, disease status, and treatment details of these patients. The median age was 56 years, ranging from 31 to 75 years, with a majority (56.7%) being male. The rectum and sigmoid colon were the most common sites of the primary tumor, with 36 patients (60.0%) having tumors in the rectum and 9 patients (15.0%) in the sigmoid colon. A significant proportion of patients (51.7%) were diagnosed at stage IV. During the third-line treatment period, 12 patients (20.0%) received local combined therapy, including local radiotherapy, particle implantation, interventional embolization, and local surgery. Gene mutations were prevalent among the patients, with 37 individuals (61.7%) exhibiting KRAS mutations. Treatment regimens varied; 29 patients (48.3%) received fruquintinib combined with a PD-1 inhibitor, surpassing the numbers treated with regorafenib combined with a PD-1 inhibitor (15 patients, 25.0%) and trifluridine/tipiracil combined with bevacizumab (16 patients, 26.7%).
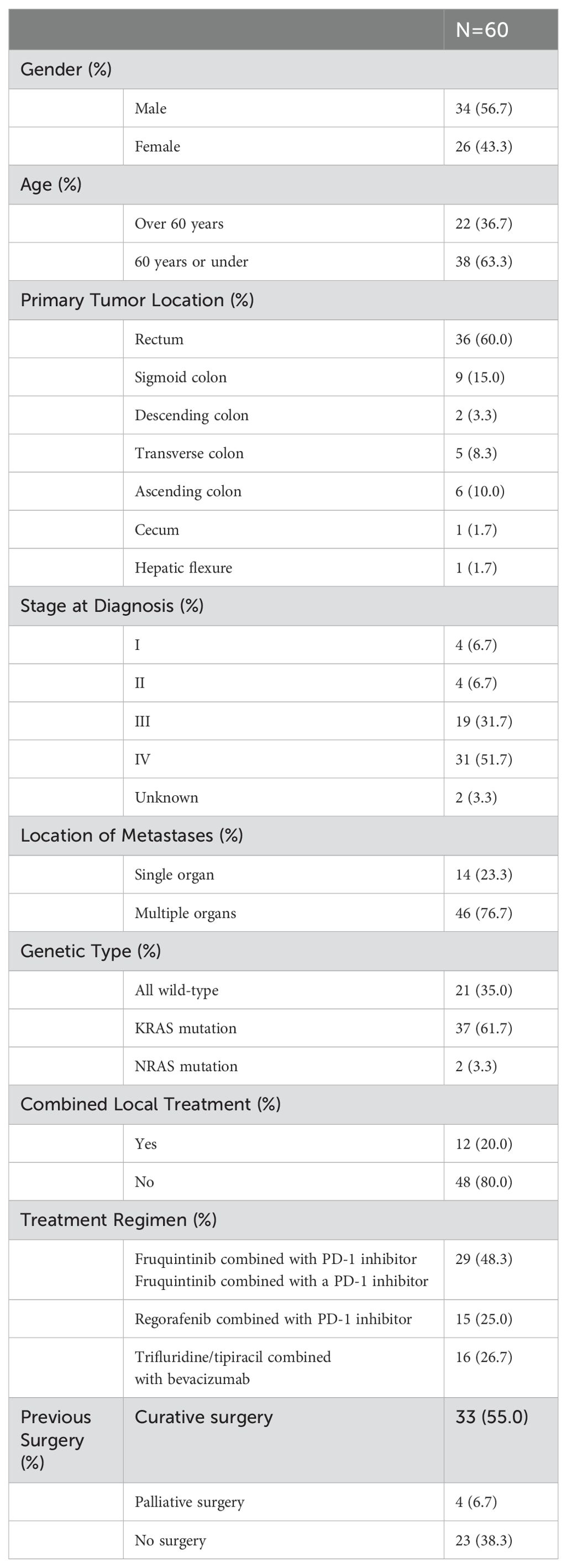
Table 1. Demographic and baseline characteristics.
EfficacyThe average follow-up period was 12.6 months (2.3-37.6 months). Following third-line treatment, 2 patients did not provide evaluation results, 0 patients achieved CR, 5 patients achieved PR, 32 patients exhibited SD, and 21 patients experienced PD. Within the regorafenib combination group, there were 3 PRs, 5 SDs, and 7 PDs; in the fruquintinib combination group, there were 2 PRs, 16 SDs, and 10 PDs; and in the trifluridine/tipiracil combination group, there were 11 SDs and 3 PDs (Table 2; Figure 1). The overall objective response rate (ORR) was 8.6%, and the disease control rate (DCR) was 63.8%. Specifically, the ORR and DCR in the regorafenib combination group were 20% and 53.3%, respectively; 6.9% and 62.1% in the fruquintinib combination group; and 0% and 78.6% in the trifluridine/tipiracil combination group.
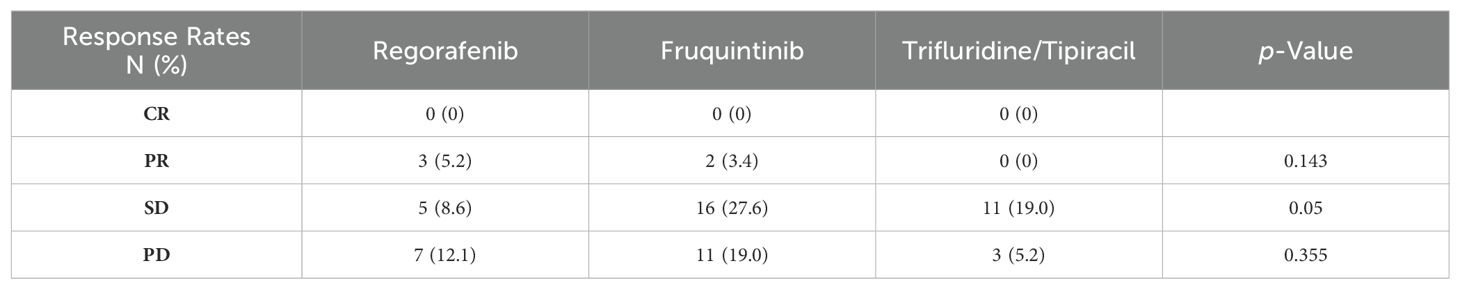
Table 2. The antitumor response of each treatment group assessed by response evaluation criteria in solid tumor Version 1.1.
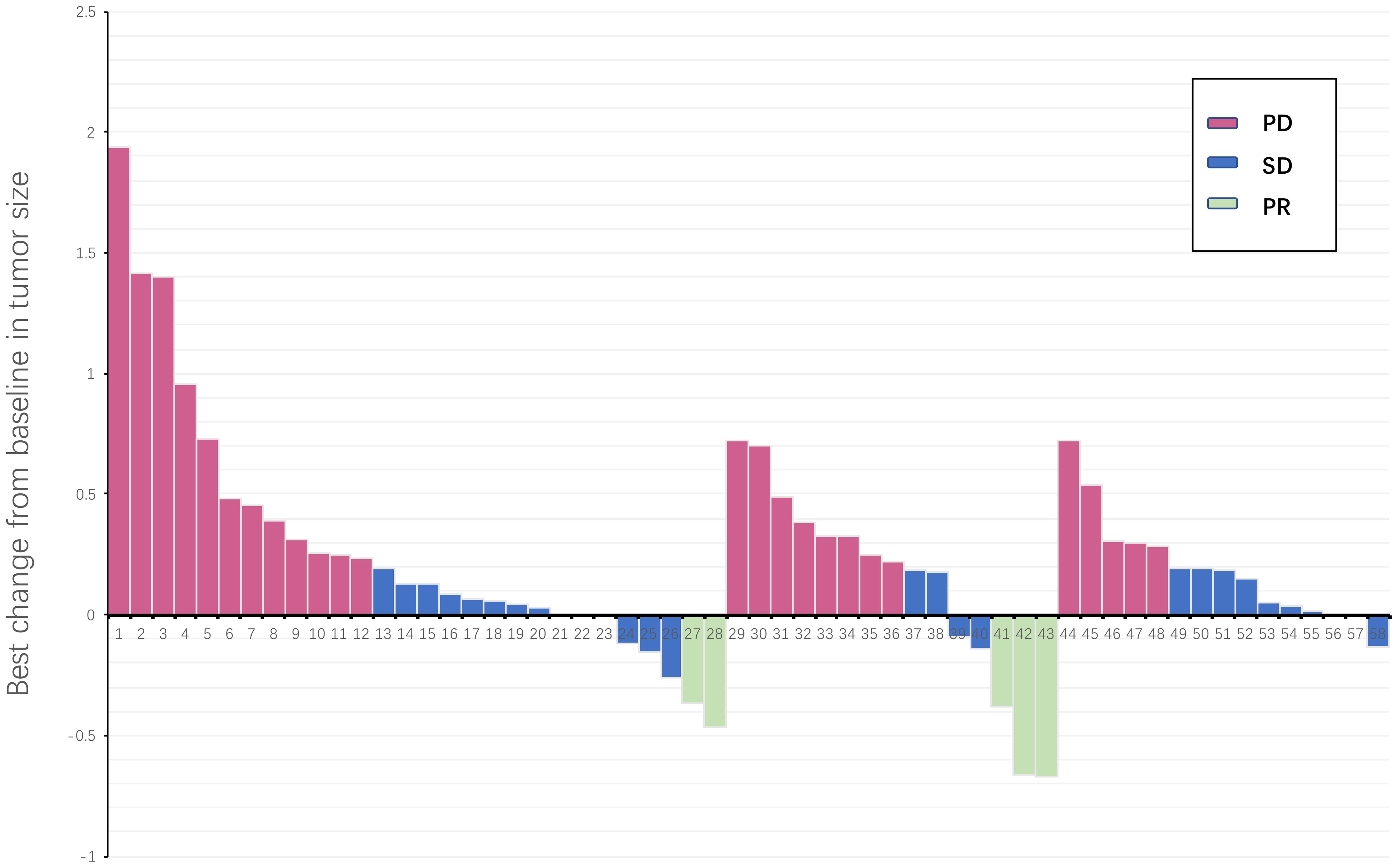
Figure 1. Waterfall plot illustrating maximum change in target lesion size for all treatment line patients (N = 58). The horizontal axis represents all patients included in the study, and the vertical axis represents the proportion of tumor size changes in patients. PD, progressive disease; PR, partial response; SD, stable disease.
In the fruquintinib group, the median OS was 16.0 months (95% CI, 10.2-21.8 months), in the regorafenib group, the median OS was 19.2 months (95% CI, 9.1-29.3 months), and in the trifluridine/tipiracil group, the median OS was 14.2 months (95% CI, 6.4-22.0 months). Statistical analysis revealed no significant differences in OS among the three groups, with a p-value of 0.4814 (Figure 2A). Similarly, no significant differences in OS were observed between the fruquintinib and regorafenib groups, with a p-value of 0.2475(HR 0.61) (Figure 2B); between the regorafenib and trifluridine/tipiracil groups, with a p-value of 0.2994 (HR 0.50)(Figure 2C); and between the fruquintinib and trifluridine/tipiracil groups, with a p-value of 0.6646 (HR 0.89)(Figure 2D).
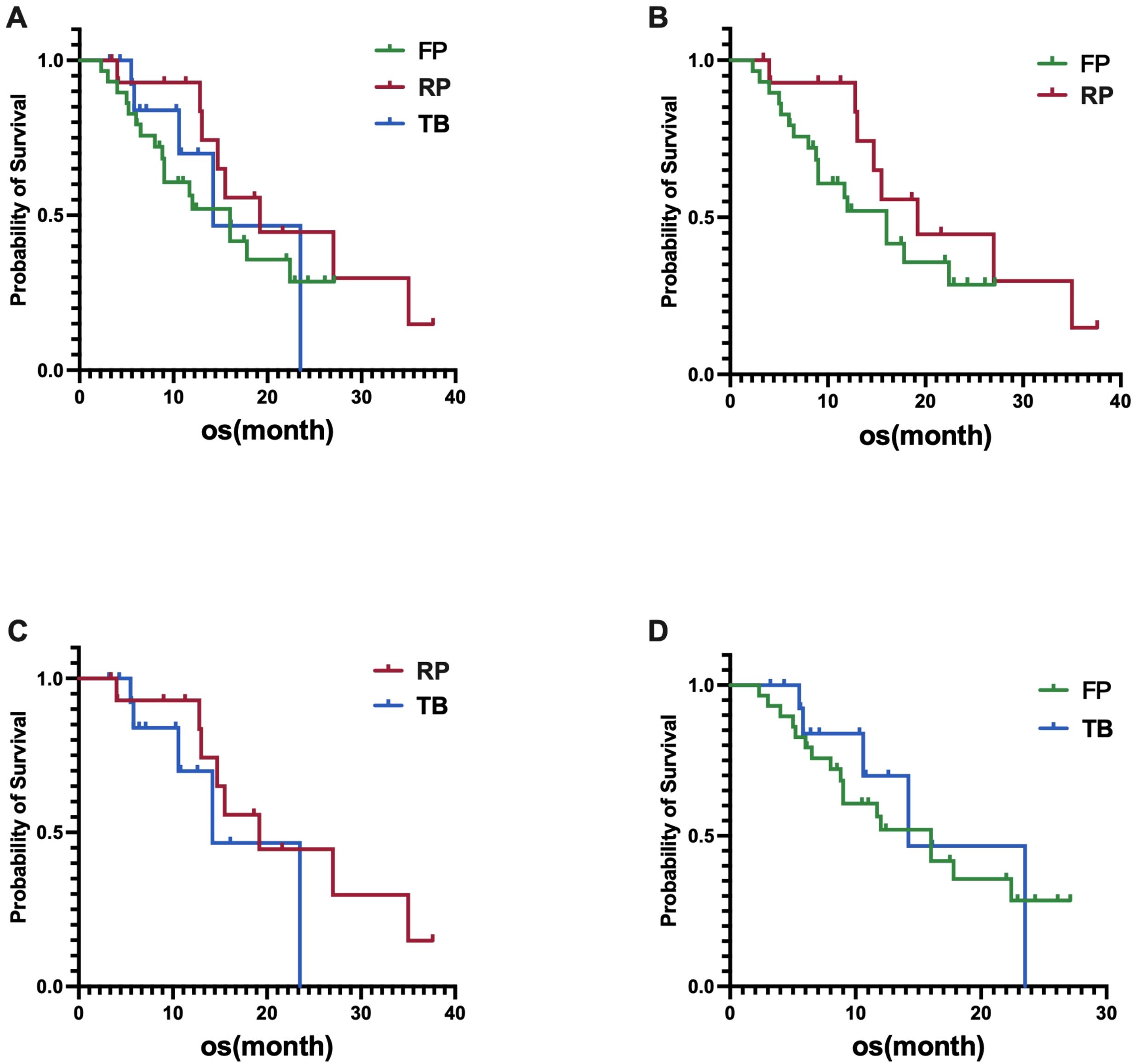
Figure 2. (A) Difference in OS among the three groups; (B) Difference in OS between FP (fruquintinib plus PD-1 inhibitor) and RP (regorafenib plus PD-1 inhibitor); (C) Difference in OS between RP (regorafenib plus PD-1 inhibitor) and TB (trifluridine/tipiracil plus bevacizumab); (D) Difference in OS between FP (fruquintinib plus PD-1 inhibitor) and TB (trifluridine/tipiracil plus bevacizumab).
In terms of PFS, the fruquintinib group had a median PFS of 4.2 months (95% CI, 3.2-5.2 months), the regorafenib group had a median PFS of 6.3 months (95% CI, 3.6-9.0 months), and the trifluridine/tipiracil group had a median PFS of 5.4 months (95% CI, 0-12.5 months). A statistically significant difference in PFS was found among the three groups, with a p-value of 0.0157 (Figure 3A). A significant difference in PFS was noted between the fruquintinib and regorafenib groups, with a p-value of 0.0316 (HR 0.48)(Figure 3B); however, no significant difference in PFS was found between the regorafenib and trifluridine/tipiracil groups, with a p-value of 0.6402(HR 0.82) (Figure 3C); and a significant difference in PFS was observed between the fruquintinib and trifluridine/tipiracil groups, with a p-value of 0.0113(HR 0.41) (Figure 3D).
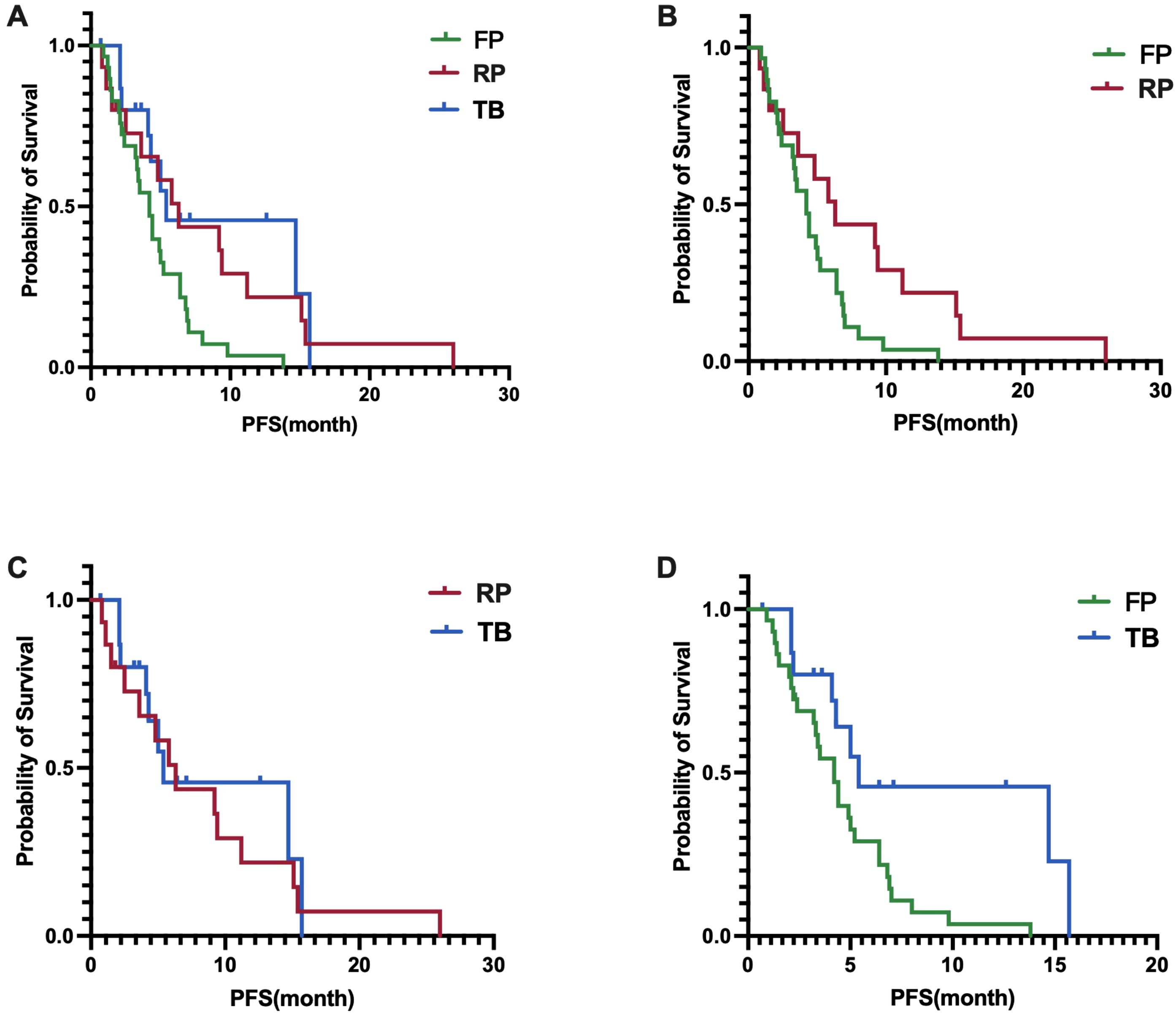
Figure 3. (A) Difference in PFS among the three groups; (B) Difference in PFS between FP and RP; (C) Difference in PFS between RP and TB; (D) Difference in PFS between FP and TB.
Factor analysisUnivariate Cox regression analysis indicated that for all participants, OS was not associated with baseline characteristics (age, gender, lesion location, stage at diagnosis, genotype, number of metastatic sites, and the presence or absence of liver, lung, and peritoneal metastases, combined local treatment, and surgery) (Table 3). However, the presence of liver and peritoneal metastases was associated with PFS in third-line treatment, while age, gender, lesion location, stage at diagnosis, genotype, number of metastatic sites, the presence or absence of lung metastasis, combined local treatment, and surgery were not associated with PFS (Figure 4).
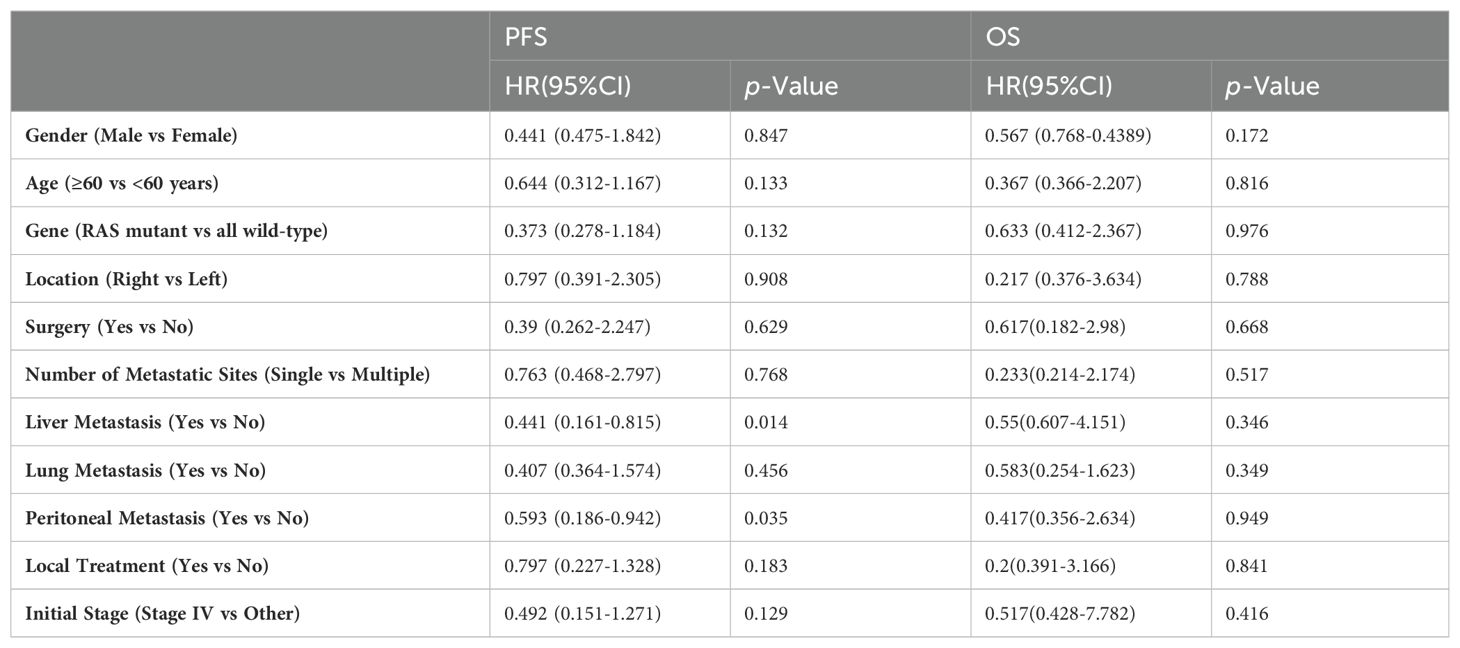
Table 3. Univariate COX regression to determine factors associated with PFS and OS.
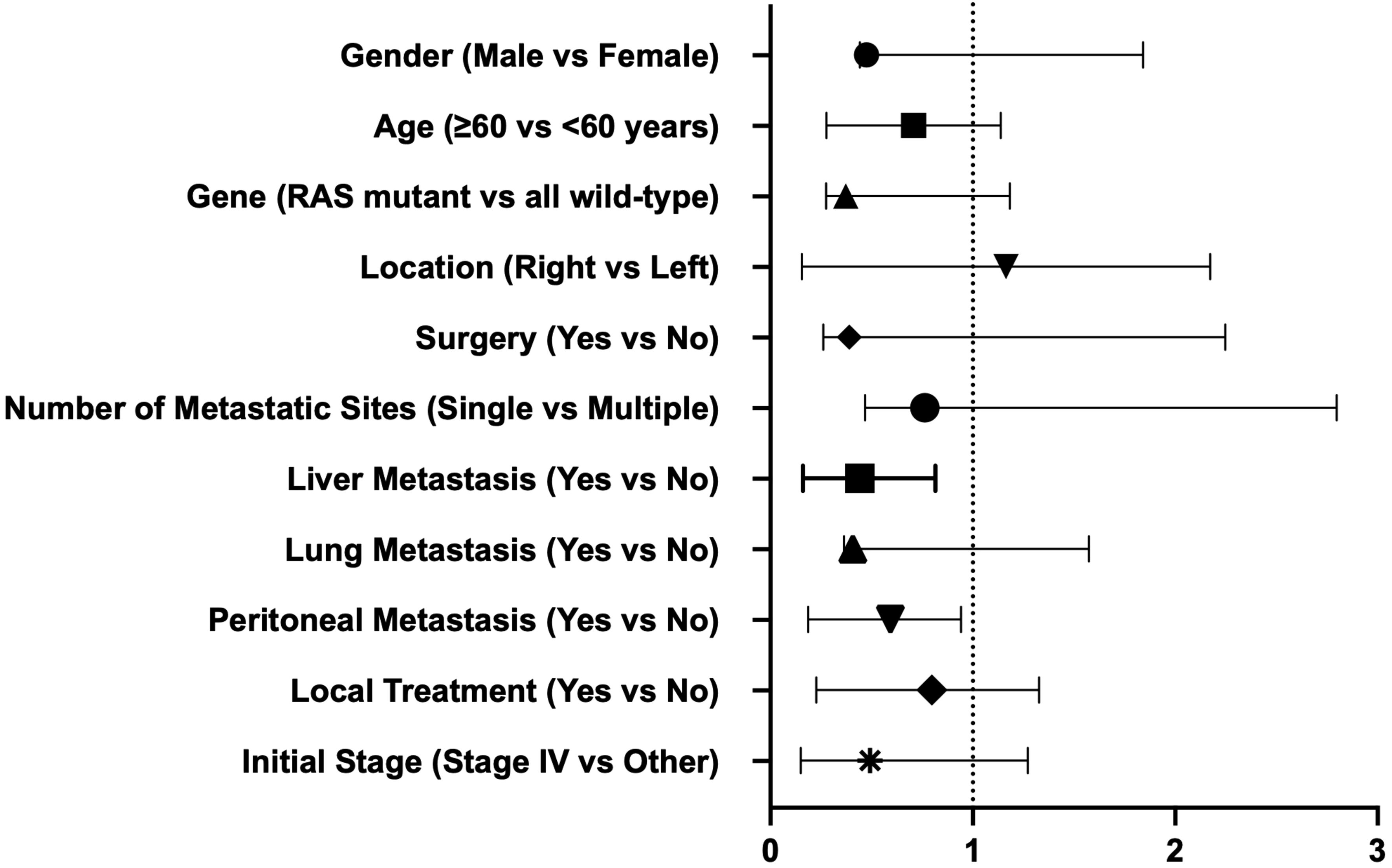
Figure 4. Univariate COX regression to determine factors associated with PFS.
SafetyOf all participants, twenty-eight patients reported treatment-related adverse events (TRAEs), with four cases (14.3%) experiencing grade 3-4 TRAEs, including two cases of myelosuppression, one case of myocarditis, and one case of rash. Specifically, seven patients (25.0%) experienced myelosuppression, four (14.3%) reported poor appetite, five (17.9%) developed rashes, four (14.3%) experienced fatigue, three (10.7%) had myocarditis, four (14.3%) developed hypertension, two (7.1%) experienced vomiting, and there was one case (3.2%) each of incomplete intestinal obstruction, abdominal pain, abdominal distension, intestinal fistula, vaginal bleeding, ventricular premature beats, chest pain, and abnormal transaminase levels.
Among patients receiving fruquintinib combination therapy, 14 reported treatment-related adverse events, with no grade 3-4 TRAEs observed. Adverse events included hypertension (two patients), poor appetite (three), rashes (two), fatigue (three), and myocarditis (two). In the regorafenib combination therapy group, seven patients experienced treatment-related adverse events, including two cases of grade 3-4 TRAEs (myocarditis and rash), along with three instances of rash and two of hypertension. For those undergoing trifluridine/tipiracil combination therapy, seven reported treatment-related adverse events, with two experiencing grade 3-4 TRAEs (grade 4 myelosuppression); the remainder comprised myelosuppression (five cases) and vomiting (two cases). Clinicians successfully managed all adverse events, resulting in no TRAE-related fatalities (Table 4).
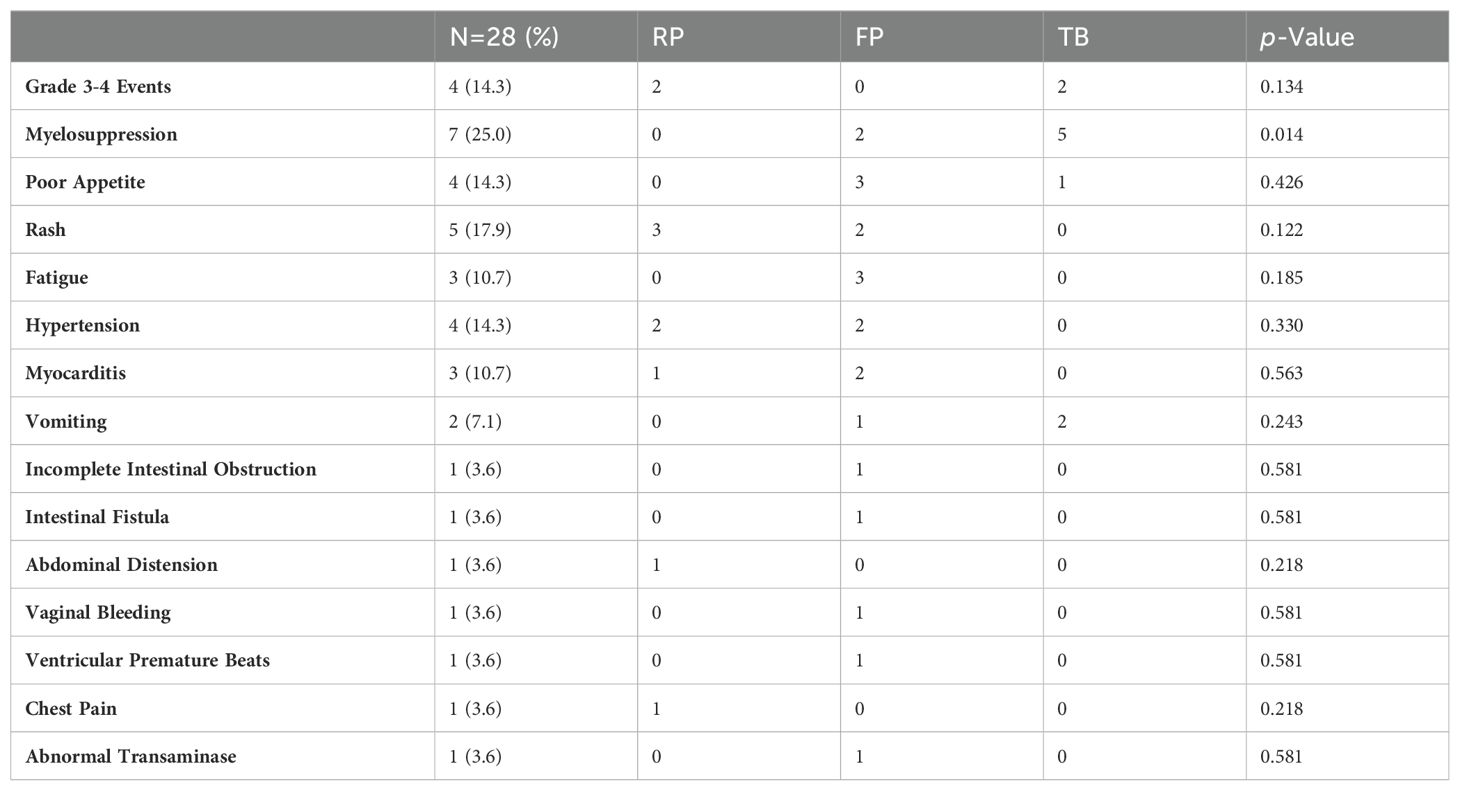
Table 4. Treatment-related adverse reactions.
DiscussionCurrently, the third-line treatment for microsatellite stabilized colorectal adenocarcinoma includes multiple options, including chemotherapy and targeted therapy (8, 21). Cetuximab and bevacizumab have been received since most are in first - and second-line standard therapy. Based on CSCO guidelines in China and relevant studies, third-line therapies typically include rifenib, fruquininib and trifluridine/tipiracil monotherapy and trifluridine/tipiracil and bevacizumab combination therapy (21). For example, an open-label, randomized Phase II trial involving advanced colorectal cancer patients from four Danish centers, who had not responded to standard treatments, demonstrated that the combination of trifluridine/tipiracil and bevacizumab achieved a median PFS of 4.6 months, compared to 2.6 months for trifluridine/tipiracil alone (p=0.0015) (17). The REGONIVO study further revealed that regorafenib combined with a PD-1 inhibitor in advanced MSS colorectal cancer patients yielded an ORR of 33%, significantly influencing patient treatment and underscoring the effective synergy between PD-1 inhibitors and TKIs in this cancer type. Although the REGONIVO study had advanced the development of immunocombination targeting in colon cancer, it was not recommended in the guidelines because follow-up studies have failed to replicate the previous superior efficacy. Subsequent investigations reported that combining fruquintinib with PD-1 inhibitors led to a median PFS of 3.8-6.4 months, median OS of 11.1-14.9 months, ORR of 7.1%-21.05%, and DCR of 62.2%-89.3% (22–25), signifying an improvement from the median OS of 9.3 months and PFS of 3.7 months observed with fruquintinib monotherapy in mCRC patients as outlined in the FRESCO-2 study (26). In the pMMR population, with the combination of regorafenib and PD-1 inhibitors, PFS ranged from 4.0-7.9 months, OS from 11.1-15.03 months, and the highest DCR reached 70.8% (27, 28). Nevertheless, in the realm of third-line treatment for advanced MSS colorectal cancer patients, there remains a notable absence of direct comparison studies to more effectively guide the selection of combination therapies.
In our study, the fruquintinib combination group exhibited an ORR of 6.9%, a DCR of 62.1%, a median OS of 16.0 months, and a median PFS of 4.2 months. These outcomes are in line with those reported by Sun et al. (24) for the fruquintinib plus PD-1 inhibitor group in terms of ORR and DCR and are comparable to the DCR noted by GOU et al. (23), albeit with a marginally lower ORR. The regorafenib combination group demonstrated an ORR of 20%, DCR of 53.3%, median OS of 19.2 months, and median PFS of 6.3 months. These figures surpass the ORR of 7.1% reported by Sun et al. (24) and align closely with their reported DCR of 56.5%; both the OS and PFS exceed the findings from Chen et al.’s study (27) on elderly colorectal cancer patients. Despite regorafenib and fruquintinib both being oral anti-angiogenesis medications, regorafenib’s multi-target capabilities contrast with fruquintinib’s high selectivity for VEGFR1, VEGFR2, and VEGFR3 (29), potentially explaining the varied treatment outcomes. Nonetheless, the ORR and PFS for TKI drugs combined with immunotherapy were lower than the 33% ORR and 7.9 months PFS observed in the REGONIVO study (20). This discrepancy may be attributed to the phase 1b nature of the REGONIVO trial, which focused on dose exploration and expansion to establish safety and recommend dosages. Additionally, the REGONIVO study’s results, primarily from Japan, might reflect ethnic differences and distinct disease management practices, contributing to the variation in outcomes. The diverse PD-1 inhibitors used in combination could also influence the results. In addition, due to the small sample size of our study, there may be biases. In our study, patients had prolonged OS compared to other studies. We considered the following aspects: First, because the retrospective analysis did not exclude patients receiving local treatment, some patients may benefit from local treatment; Secondly, whether it is related to the long-term benefits obtained by some patients from immunotherapy needs further exploration and research. In addition, some patients with good ECOG score received 1-2 lines of follow-up treatment, and some may benefit from follow-up treatment. Finally, due to our limited sample size and the limitations of retrospective study follow-up, there are some deleted data, which may affect the OS data due to follow-up. In our analysis, the trifluridine/tipiracil combination group recorded an ORR of 0%, DCR of 78.6%, median OS of 14.2 months, and median PFS of 5.4 months. These results surpass those of studies such as TAS-CC3 and TAS-CC4 (25, 30, 31) in terms of OS and PFS, which may be due to racial differences, the employment of biweekly versus weekly dosing schedules, and our adoption of a three-week regimen. Furthermore, clinical trials commonly set Eastern Cooperative Oncology Group (ECOG) performance scores at 0-1, a standard challenging to rigorously maintain in clinical practice; these were phase II studies, necessitating further validation. Although there were patients with both wild-type and mutant RAS in our study, due to the relatively standardized early treatment, treatment options such as the rechallenge of cetuxib were not considered in third-line treatment, and the type of RAS had nothing to do with the benefits of third-line treatment options.
Regarding adverse reactions, our study documented a 14.3% incidence rate of grade 3-4 adverse events, with two instances of myelosuppression identified in the trifluridine/tipiracil group and immunological inflammation and rashes predominantly observed in the TKI plus PD-1 inhibitor groups. Overall, the incidence rate of myelosuppression exhibited variation among the three groups, while other adverse reactions were not significantly different, aligning with findings from other studies. This suggests that the tolerance for the combined treatments in advanced colorectal adenocarcinoma is generally favorable, with manageable adverse reactions. Upon reviewing the patients’ baseline conditions, we discovered that peritoneal or liver metastasis could impact PFS; however, the limited number of participants underscores the necessity for further investigation with a larger cohort.
In summary, our study represents the first comparative analysis of combination treatments involving regorafenib, fruquintinib, and trifluridine/tipiracil. The findings suggest that, with respect to PFS, both the regorafenib and trifluridine/tipiracil groups surpassed the fruquintinib group, yet there were no significant differences in OS among the three groups. A more holistic approach to selecting third-line treatment options should incorporate economic considerations and the specific needs of the patient. Considering our study’s retrospective nature and the limited sample size, the efficacy and safety of these treatment options require further clinical investigation in the future.
ConclusionIn this third-line treatment for colorectal cancer in the real world, although rifenil combined with PD-1 inhibitor and trefludine/tipirizumab combined with bevacizumab were superior to fruquintinib combined with PD-1 inhibitor in PFS, there was no significant difference in OS among the three groups. In third-line treatments, prospective studies are needed to confirm current findings and make better choices.
Data availability statementThe raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statementThe studies involving humans were approved by The Ethics Committee of Henan Cancer Hospital belongs to Henan Cancer Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and institutional requirements. Written informed consent was not obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article because Given the study’s retrospective and non-interventional nature, the committee waived the requirement for informed consent.
Author contributionsCW: Formal analysis, Software, Supervision, Writing – original draft, Writing – review & editing. SL: Investigation, Supervision, Writing – review & editing. XH: Formal analysis, Methodology, Project administration, Resources, Supervision, Writing – review & editing.
FundingThe author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interestThe authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s noteAll claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References2. Coradduzza D, Arru C, Culeddu N, Congiargiu A, Azara EG, Scanu AM, et al. Quantitative metabolomics to explore the role of plasma polyamines in colorectal cancer. Int J Mol Sci. (2023) 24:101. doi: 10.3390/ijms24010101
PubMed Abstract | Crossref Full Text | Google Scholar
3. Zheng J, Wang X, Yu J, Zhan Z, Guo Z. IL-6, TNF-α and IL-12p70 levels in patients with colorectal cancer and their predictive value in anti-vascular therapy. Front Oncol. (2022) 12:997665. doi: 10.3389/fonc.2022.997665
PubMed Abstract | Crossref Full Text | Google Scholar
4. Pan P, Li J, Wang B, Tan X, Yin H, Han Y, et al. Molecular characterization of colorectal adenoma and colorectal cancer via integrated genomic transcriptomic analysis. Front Oncol. (2023) 13:1067849. doi: 10.3389/fonc.2023.1067849
PubMed Abstract | Crossref Full Text | Google Scholar
5. Ning S, Chen Y, Wang G, Liu Y, Yang Y, Zhang Z. Ring finger protein 128 promotes, rather than inhibits, colorectal cancer progression by regulating the Hippo signaling pathway. Front Oncol. (2022) 12:1031160. doi: 10.3389/fonc.2022.1031160
PubMed Abstract | Crossref Full Text | Google Scholar
6. Li P, Meng Q, Xue Y, Teng Z, Chen H, Zhang J, et al. Comprehensive genomic profiling of colorectal cancer patients reveals differences in mutational landscapes among clinical and pathological subgroups. Front Oncol. (2022) 12:1000146. doi: 10.3389/fonc.2022.1000146
PubMed Abstract | Crossref Full Text | Google Scholar
7. Tang GH, Chen X, Ding JC, Du J, Lin XT, Xia L, et al. LncRNA LUCRC regulates colorectal cancer cell growth and tumorigenesis by targeting endoplasmic reticulum stress response. Front Genet. (2020) 10:1409. doi: 10.3389/fgene.2019.01409
PubMed Abstract | Crossref Full Text | Google Scholar
8. Benson AB, Venook AP, Adam M, Chang G, Chen YJ, Ciombor KK, et al. Colon Cancer, Version 3.2024, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. (2024) 22(2D):e240029. doi: 10.6004/jnccn.2024.0029
PubMed Abstract | Crossref Full Text | Google Scholar
10. Grothey A, Van Cutsem E, Sobrero A, Siena S, Falcone A, Ychou M, et al. CORRECT Study Group, Regorafenib monotherapy for previously treated metastatic colorectal cancer (CORRECT): an international, multicentre, randomised, placebo-controlled, phase 3 trial. Lancet. (2013) 381:303–12. doi: 10.1016/S0140-6736(12)61900-X
PubMed Abstract | Crossref Full Text | Google Scholar
12. Li J, Qin S, Xu RH, Shen L, Xu J, Bai Y, et al. Effect of fruquintinib vs placebo on overall survival in patients with previously treated metastatic colorectal cancer: the FRESCO randomized clinical trial. JAMA. (2018) 319:2486–96. doi: 10.1001/jama.2018.7855
PubMed Abstract | Crossref Full Text | Google Scholar
14. Wang C, Chevalier D, Saluja J, Sandhu J, Lau C, Fakih M. Regorafenib and nivolumab or pembrolizumab combination and circulating tumor DNA response assessment in refractory microsatellite stable colorectal cancer. Oncologist. (2020) 25:e1188–94. doi: 10.1634/theoncologist.2020-0161
PubMed Abstract | Crossref Full Text | Google Scholar
15. Su G, Wang YY, Wang JC, Liu H. A meta-analysis comparing regorafenib with TAS-102 for treating refractory metastatic colorectal cancer. J Int Med Res. (2020) 48:300060520926408. doi: 10.1177/0300060520926408
PubMed Abstract | Crossref Full Text | Google Scholar
16. Gao Z, Cao C, Bao Y, Fan Y, Chen G, Fu P. Systematic review and meta-analysis of multitargeted tyrosine kinase inhibitors in patients with intractable metastatic colorectal cancer. Technol Cancer Res Treat. (2020) 19:1533033820943241. doi: 10.1177/1533033820943241
PubMed Abstract | Crossref Full Text | Google Scholar
17. Pfeiffer P, Yilmaz M, Möller S, Zitnjak D, Krogh M, Petersen LN, et al. TAS-102 with or without bevacizumab in patients with chemorefractory metastatic colorectal cancer: an investigator-initiated, open-label, randomised, phase 2 trial. Lancet Oncol. (2020) 21:412–20. doi: 10.1016/S1470-2045(19)30827-7
PubMed Abstract | Crossref Full Text | Google Scholar
18. Kuboki Y, Nishina T, Shinozaki E, Yamazaki K, Shitara K, Okamoto W, et al. TAS-102 plus bevacizumab for patients with metastatic colorectal cancer refractory to standard therapies (C-TASK FORCE): an investigator-initiated, open-label, single-arm, multicentre, phase 1/2 study. Lancet Oncol. (2017) 18:1172–81. doi: 10.1016/S1470-2045(17)30425-4
PubMed Abstract | Crossref Full Text | Google Scholar
19. Le D, Uram JN, Wang H, Bartlett BR, Kemberling H, Eyring AD, et al. PMID: 26028255; PMCID: PMC4481136., PD-1 blockade in tumors with mismatch-repair deficiency. N Engl J Med. (2015) 372:2509–20. doi: 10.1056/NEJMoa1500596
PubMed Abstract | Crossref Full Text | Google Scholar
20. Fukuoka S, Hara H, Takahashi N, Kojima T, Kawazoe A, Asayama M, et al. Regorafenib plus nivolumab in patients with advanced gastric or colorectal cancer: an open-label, dose-escalation, and dose-expansion phase Ib trial (REGONIVO, EPOC1603). J Clin Oncol. (2020) 38:2053–61. doi: 10.1200/JCO.19.03296
PubMed Abstract | Crossref Full Text | Google Scholar
21. Ruihua X, Jin L, Yin C, Jia F, Jun G, Zefei J, et al. Chinese Society of Clinical Oncology Guidelines Working Committee. Guidelines of chinese society of clinical oncology(CSCO) Colorectal cancer. People`s health publishing house (2024).
22. Guo Y, Zhang W, Ying J, Zhang Y, Pan Y, Qiu W, et al. Phase 1b/2 trial of fruquintinib plus sintilimab in treating advanced solid tumours: The dose-escalation and metastatic colorectal cancer cohort in the dose-expansion phases. Eur J Cancer. (2023) 181:26–37. doi: 10.1016/j.ejca.2022.12.004
PubMed Abstract | Crossref Full Text | Google Scholar
23. Gou M, Qian N, Zhang Y, Yan H, Si H, Wang Z, et al. Fruquintinib in combination with PD-1 inhibitors in patients with refractory non-MSI-H/pMMR metastatic colorectal cancer: A real-world study in China. Front Oncol. (2022) 12:851756. doi: 10.3389/fonc.2022.851756
PubMed Abstract | Crossref Full Text | Google Scholar
24. Sun L, Huang S, Li D, Mao Y, Wang Y, Wu J. Efficacy and safety of fruquintinib plus PD-1 inhibitors versus regorafenib plus PD-1 inhibitors in refractory microsatellite stable metastatic colorectal cancer. Front Oncol. (2021) 11:754881. doi: 10.3389/fonc.2021.754881
PubMed Abstract | Crossref Full Text | Google Scholar
25. Matsuoka H, Yamada T, Ohta R, Yoshida Y, Watanabe T, Takahashi M, et al. TAS CC4 Study Group, Biweekly TAS-102 and bevacizumab as third-line chemotherapy for advanced or recurrent colorectal cancer: a phase II, multicenter, clinical trial (TAS- CC4 study). Int J Clin Oncol. (2022) 27:1859–66. doi: 10.1007/s10147-022-02243-4
PubMed Abstract | Crossref Full Text | Google Scholar
26. Dasari A, Lonardi S, Garcia-Carbonero R, Elez E, Yoshino T, Sobrero A, et al. FRESCO-2 Study Investigators, Fruquintinib versus placebo in patients with refractory metastatic colorectal cancer (FRESCO-2): an international, multicentre, randomised, double-blind, phase 3 study. Lancet. (2023) 402:41–53. doi: 10.1016/S0140-6736(23)00772-9
PubMed Abstract | Crossref Full Text | Google Scholar
27. Chen B, Zhao H, Huang J, Lv H, Xu W, Nie C, et al. Efficacy of regorafenib combined with PD-1 inhibitors in elderly patients with advanced metastatic colorectal cancer. BMC Geriatr. (2022) 22:987. doi: 10.1186/s12877-022-03637-9
PubMed Abstract | Crossref Full Text | Google Scholar
28. Kim R, Kovari BP, Martinez M, Xie H, Sahin IH, Mehta R, et al. A phase I/Ib study of regorafenib and nivolumab in mismatch repair proficient advanced refractory colorectal cancer. Eur J Cancer. (2022) 169:93–102. doi: 10.1016/j.ejca.2022.03.026
PubMed Abstract | Crossref Full Text | Google Scholar
30. Yoshida Y, Yamada T, Kamiyama H, Kosugi C, Ishibashi K, Yoshida H, et al. TAS CC3 Study Group, Combination of TAS-102 and bevacizumab as third-line treatment for metastatic colorectal cancer: TAS-CC3 study. Int J Clin Oncol. (2021) 26:111–7. doi: 10.1007/s10147-020-01794-8
PubMed Abstract | Crossref Full Text | Google Scholar
31. Takahashi T, Yamazaki K, Oki E, Shiozawa M, Mitsugi K, Makiyama A, et al. Phase II study of trifluridine/tipiracil plus bevacizumab by RAS mutation status in patients with metastatic colorectal cancer refractory to standard therapies: JFMC51-1702-C7. ESMO Open. (2021) 6:100093. doi: 10.1016/j.esmoop.2021.100093
留言 (0)