IBS, or irritable bowel syndrome, is a prevalent illness that affects the relationship between the brain and the gut. Its prevalence rates range from 1.1 to 45% worldwide, with most Western countries and Asian populations seeing rates between 5 and 10% (Lovell and Ford, 2012). IBS is characterized by persistent stomach pain that occurs regularly and is accompanied by changes in bowel movements without any identifiable physical illness (Simrén et al., 2019). The symptoms of IBS can be incapacitating in a minority of patients but are generally mild to moderate in the majority of affected persons. According to this description, additional commonly linked physical or internal pain and suffering, together with anxiety and depression, are referred to as comorbid illnesses (Drossman, 2016).
IBS has been attributed to altered gastrointestinal motility, visceral hypersensitivity, and psychosocial factors, but recent studies suggest that there is a dysregulation of the brain-gut axis in IBS (Öhman and Simren, 2007). In particular, functional MRI (fMRI) studies (Kwan et al., 2005) and recent studies of cortical gray matter point to dysfunction of emotional and attentional processing of pain in IBS. Furthermore, the subjective nature of pain perception underlines the importance of individual differences such as personality and coping strategy.
Understanding that brain areas do not function independently but as a complex network is essential. The perception of pain results from the integrated activity within this network. The anterior/midcingulate cortex (ACC/MCC), primary and secondary somatosensory cortex (S1, S2), insular cortex (IC), thalamus (Th), and prefrontal cortex (PFC) are the six cortical areas most frequently associated with pain-evoked activity during acute stimulation in humans (Chen et al., 2011).
These identical areas have exhibited distinct responses in patients as opposed to healthy individuals in investigations of persistent pain syndromes, such as migraine, heart pain, fibromyalgia (FM), chronic back pain, temporomandibular disorder, and IBS.
Multiple studies have also documented anomalies in the gray and white matter structure within these specific brain regions in individuals with chronic pain syndromes, including chronic back pain, fibromyalgia, chronic tension-type headache, temporomandibular dysfunction, and irritable bowel syndrome. Consequently, it seems that there are irregularities in the functioning of chronic pain, which are accompanied by abnormalities in the structure of gray and white matter. As a result, developing novel methods to assess the integrity of white matter is stimulating a new area of research in chronic pain of IBS patients. Diffusion tensor imaging (DTI) has gained popularity as a method for evaluating the integrity and connectivity of the brain. One specific value produced from DTI, fractional anisotropy (FA), is frequently used to estimate the microstructural integrity of white matter (Chen et al., 2011).
The presence of persistent symptoms in people with IBS and the lack of effective treatments necessitate continuous attempts to comprehend the causes and perpetuation of symptoms in these individuals. Neuroimaging is a method used to examine the central mechanisms in patients with IBS, which can provide insights into the functioning of the brain-gut axis and its connection to the expression of symptoms. Prior investigations have produced essential discoveries, but ongoing research and technology advancements necessitate a reevaluation of the progress achieved in the sector. However, little is known about white matter abnormalities in patients with IBS, and the current literature does not agree with these changes in the WM tract. Therefore, in this study, we aimed to assess the integrity of white matter in IBS patients through a systematic review and meta-analysis approach.
MethodsThe current study is a systematic review and meta-analysis that adheres to the principles outlined in the PRISMA checklist (Page et al., 2020). The study protocol has been registered within the Open Science Framework (OSF) (DOI 10.17605/OSF.IO/NAJ7Y).
Search strategyTwo researchers independently searched PubMed, Scopus, and Web of Science for articles published up to April 2024. They used specific search terms including (“irritable bowel* “) OR (IBD) OR (IBS) OR (“Colitis”) AND (“White Matter”) OR (tract) OR (“tract alteration”) OR (“tract change”) OR (“brain connectivity”) OR (“fractional anisotropy”) AND ((((DTI OR (“Diffusion Tensor Imaging”) OR (“Diffusion Tensor Magnetic Resonance Imaging”) OR (Tractography). This search strategy included a mix of Medical Subject Headings (MeSH) and text terms. Additionally, they checked the reference lists of the included articles and relevant reviews and meta-analyses for any additional relevant publications (Table 1).

Table 1. Search strategies and the result of the search procedure.
The inclusion and exclusion criteriaThe selection of eligible articles was based on specific criteria. Inclusion criteria comprised an original, peer-reviewed research paper, a human observational study, the provision of sample size, and fractional anisotropy in IBS cases and healthy controls; it was written in English. Exclusion criteria involved: repeated or duplicated publications; animal studies; disregarding reviews, abstracts, letters, case reports, or conference abstracts lacking original data; studies that did not FA for case and healthy controls; and studies with outcomes related to neuropsychological dysfunction and studies with a sample size having neuropsychological comorbidities were excluded due to confounding DTI results.
Study selection and data extractionWe used the RAYYAN intelligent tool for systematic reviews to screen the search results (Ouzzani et al., 2016). Titles and abstracts from 7,069 articles obtained from our search strategy were independently and mindlessly screened by two reviewers (MAA, SSRS.). The duplicate records were removed using the same tool. The conflicts were resolved by a third reviewer (FN) using RAYYAN’s compute rating feature.
Quality assessment of studiesTwo authors individually evaluated each candidate article and extracted the relevant information, including the surname of the first author, publication year, country or region, sample size, age and gender distribution of participants, Region of Interest (ROI), fractional anisotropy, BMI, Education years duration of disease, and DTI metrics.
Risk of bias assessmentThe JBI critical appraisal tool evaluated the articles’ methodological quality. Two reviewers independently conducted the quality assessment of all included articles. Any discrepancies were deliberated between the two reviewers, and if a consensus could not be reached, a third reviewer intervened to resolve the disagreement.
Statistical analysisSTATA ver18 was used to conduct the study analysis. A meta-analysis used Fractional Anisotropy (FA) data as mean ± SD. A random effects model calculated the mean difference and 95% confidence intervals (CIs). A random effects model was also used to combine the study-specific Standardized Mean Difference (SMD) to determine the pooled estimate of the difference in FA of different tracts between IBS cases and control groups. Heterogeneity was assessed using the Chi-square and I-square tests. A subgroup analysis was performed to investigate the factors contributing to heterogeneity. Data points from graphical representations in studies were extracted using WebPlot Digitizer (Automeris LLC, Frisco, Texas). All statistical analyses were two-tailed, with significance at a p value <0.05.
Publication bias assessmentThe study examined publication bias using Egger’s regression, and when Egger’s regression identified significant bias (p < 0.05), a trim and fill analysis was used to estimate the potential missing effect sizes and to determine a revised overall effect.
Results Study selection and characteristicsThe curated search strategies yielded a total number of 62 studies across chosen databases (Figure 1). After removing 29 duplicates, the remaining 33 articles were screened by their title and abstracts. Finally, 22 articles were excluded, and 11 studies were incorporated in the systematic synthesis of our study; of these 11 studies, three were assessed analytically appropriate for the meta-analysis.
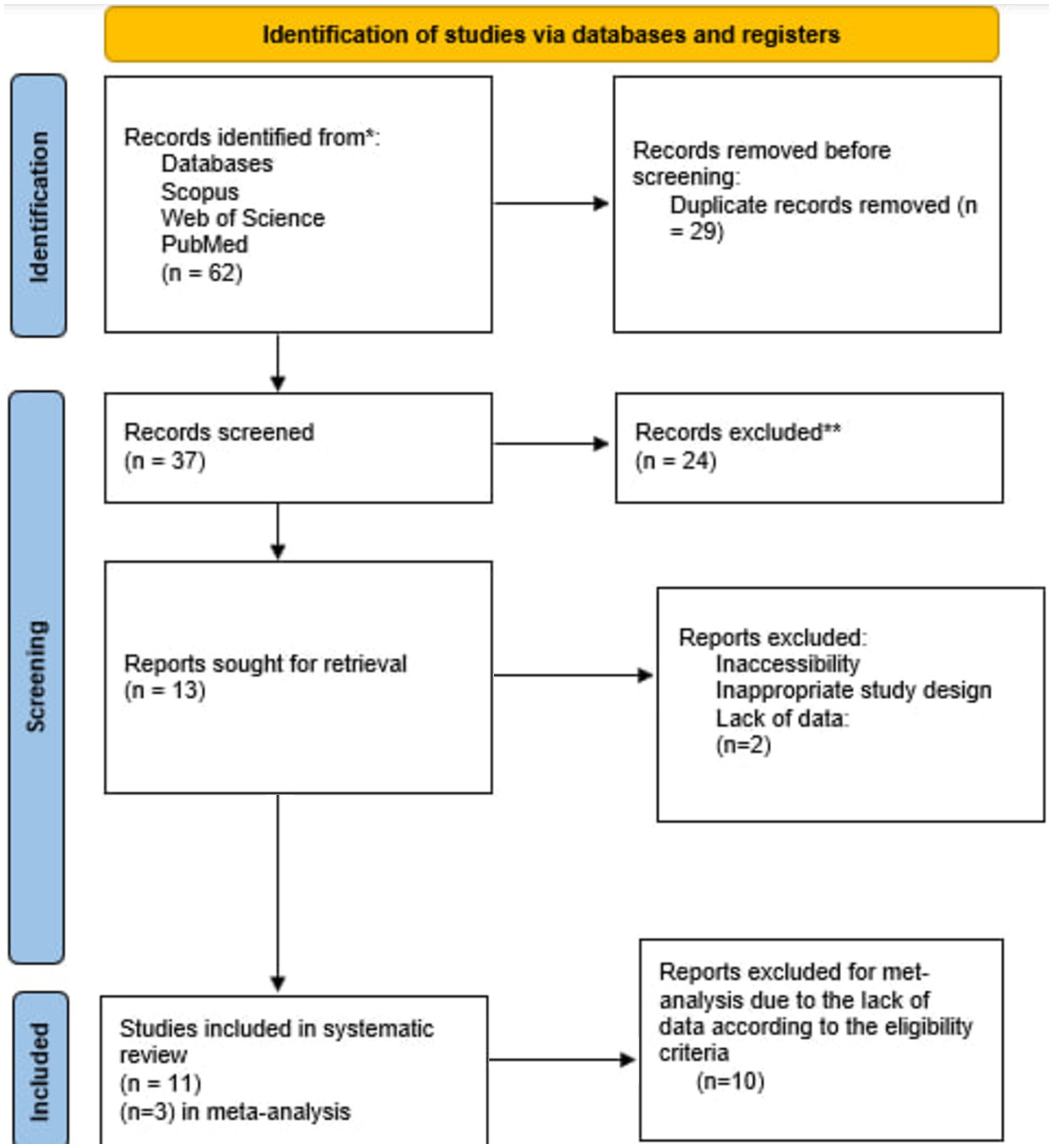
Figure 1. Flow diagram of the study selection procedure.
Findings543 IBS cases and 472 healthy controls were included in this systematic review. The mean age of the case and control group was 35.2 ± 17.4 and 33.6 ± 15.8 (mean ± SD), respectively. There was no statistically significant difference in age between groups (p > 0.05). The geographical distribution of included studies revealed four studies in China (Fang et al., 2017; Qi et al., 2016; Nan et al., 2018; Zhao et al., 2018), three studies in the USA (Hubbard et al., 2018; Irimia et al., 2015; Ellingson et al., 2013), and the rest were conducted in Canada (Chen et al., 2011), Japan (Chiba et al., 2020), Greece (Zikou et al., 2014), and Sweden (Grinsvall et al., 2021). The summary findings and characteristics of the included studies are demonstrated in Table 2.
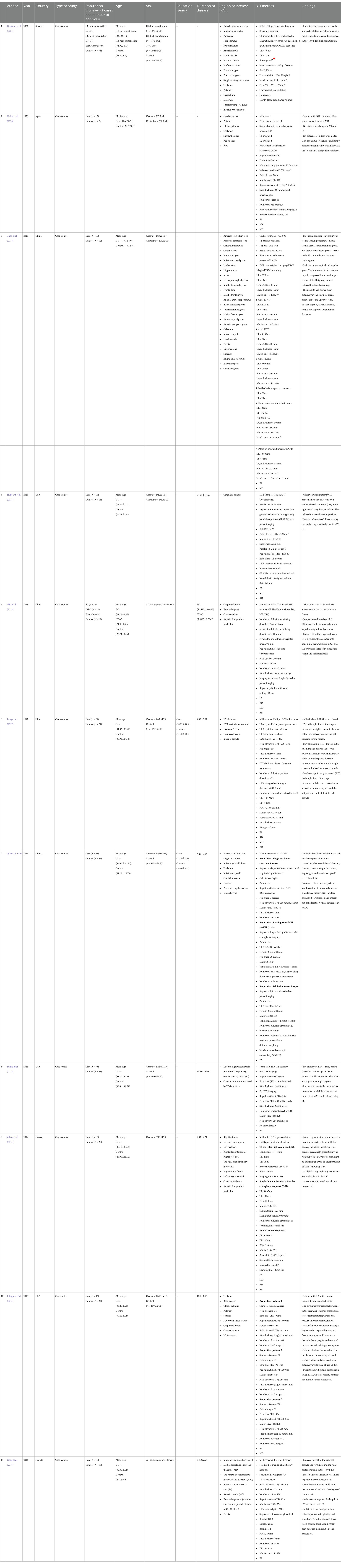
Table 2. Summary characteristics and findings of the included studies.
Of the 11 included studies, all reported changed FA of ROIs in IBS groups compared to healthy controls except for one study that showed no changed FA.
Chiba et al. (2020) reported that Patients with IBS showed diffuse white matter decreased MD, no discernible changes in MK and FA, and no differences in deep gray matter.
Both the supramarginal and angular gyrus, The brainstem, fornix, internal capsule, corpus callosum, and upper corona of the IBS group showed reduced fractional anisotropy, according to diffusion tensor imaging in the study by Zhao et al. (2018).
Hubbard et al. (2018) found white matter (WM) abnormalities in adolescents with irritable bowel syndrome (IBS) In contrast to a healthy cohort in the right dorsal cingulum, as indicated by reduced fractional anisotropy (FA). IBS patients showed FA and RD aberrations in the corpus callosum in Nan et al.’s study (Nan et al., 2018).
In Qi et al.’s research, individuals with IBS exhibit increased interhemispheric functional connectivity between bilateral thalami, cuneus, posterior cingulate cortices, lingual gyri, and inferior occipital/cerebellum lobes (Qi et al., 2016). According to Ellingson et al.’s findings, Patients’ fractional anisotropy (FA) is higher in the corpus callosum and frontal lobe areas and lower in the thalamic, basal ganglia, and sensory/motor association/integration regions (Ellingson et al., 2013).
According to the study by Chen et al., there was an increase in (FA) in the external capsule and fornix around the right posterior insula in those with IBS. The left anterior insula FA was linked to pain unpleasantness, but the bilateral anterior insula and lateral thalamus correlated with the degree of chronic pain. In the exterior capsule, the length of IBS was linked with FA. In IBS, there was a negative link between pain catastrophizing and cingulum FA, but in controls, there was a positive correlation between pain catastrophizing and external capsule FA (Chen et al., 2011).
Meta-analysisAnalyzed Standard mean difference using a fixed model for Fractional anisotropy of regions of interest (ROI) associated with sensory processing such as thalamus, insula, primary somatosensory cortex, dorsal cingulum and the fornix in selected studies showcased decreased white matter interactivity in case group however this decrease was not statistically different (SMD −88, 95%CI (−1.32, −0.44), p > 0.05) (Figures 2, 3). The I-squared test revealed no significant heterogeneity among studies (I2:0.00%) (Figure 4). Results of Egger’s test and funnel plot demonstrated no publication bias (p > 0.05, symmetric plot) (Figure 5).
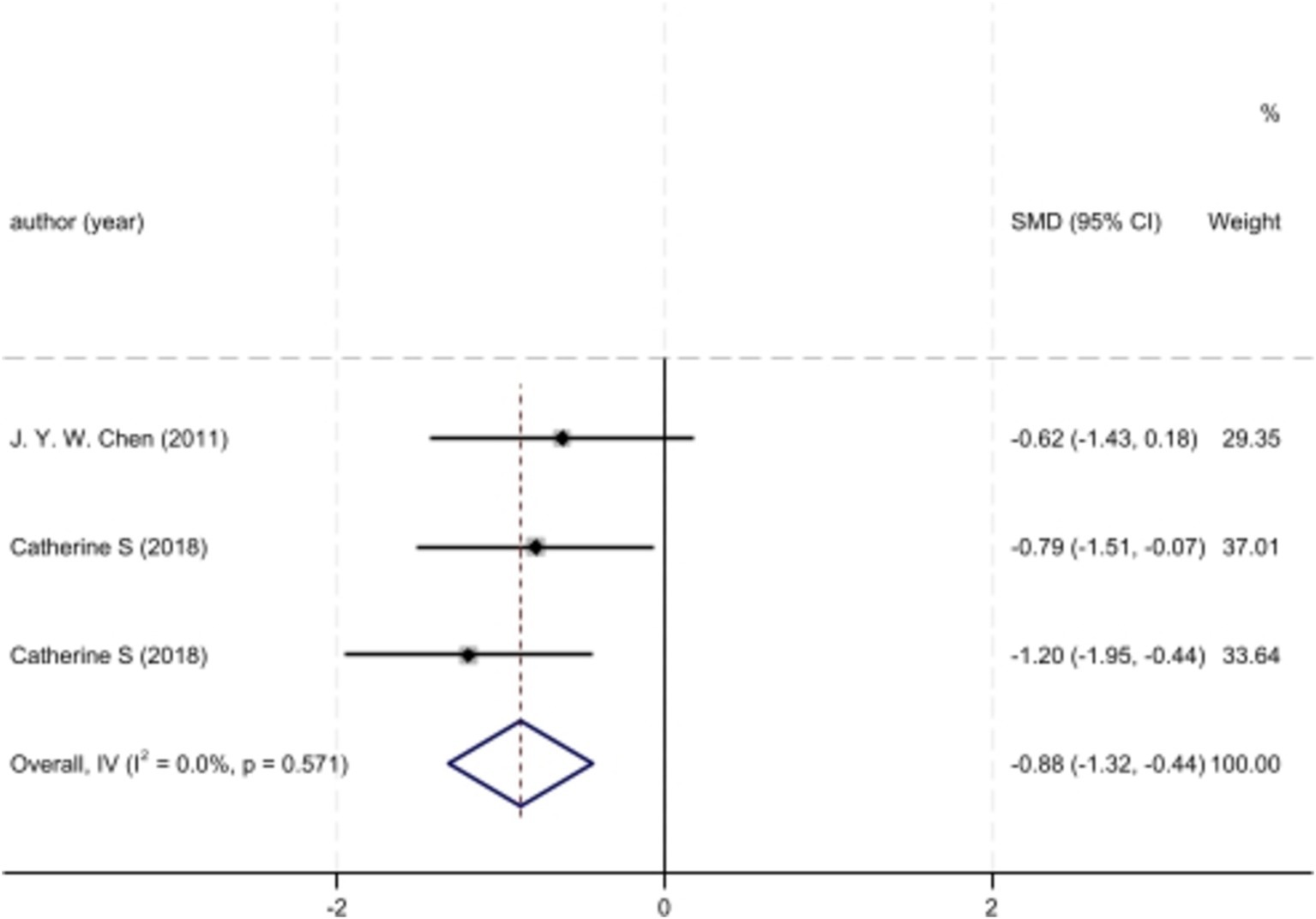
Figure 2. Forest plot of fractional anisotropy alteration analysis across IBS cases and controls of chosen studies.
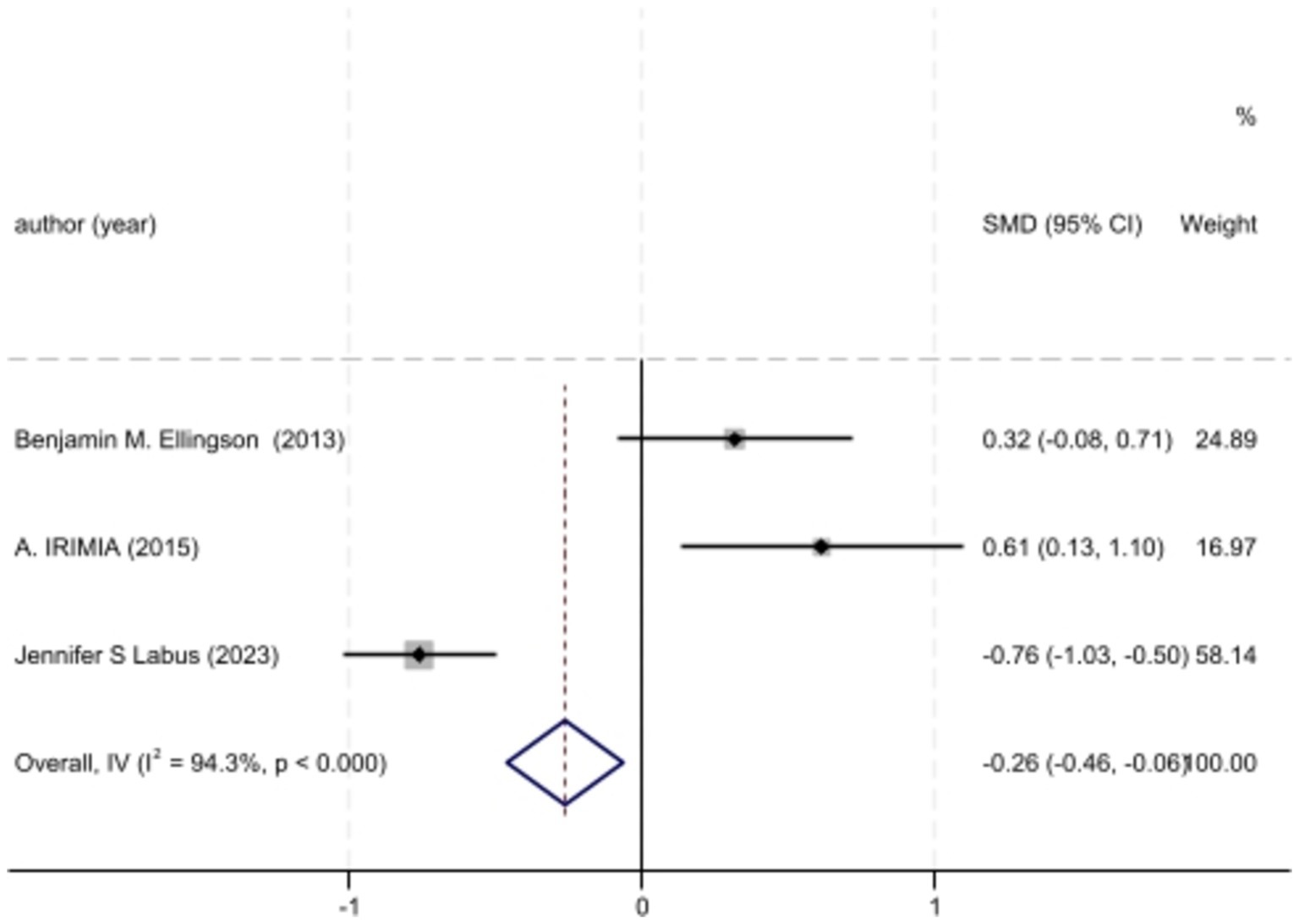
Figure 3. Forest plot of BMI differences across IBS cases and controls of chosen studies.
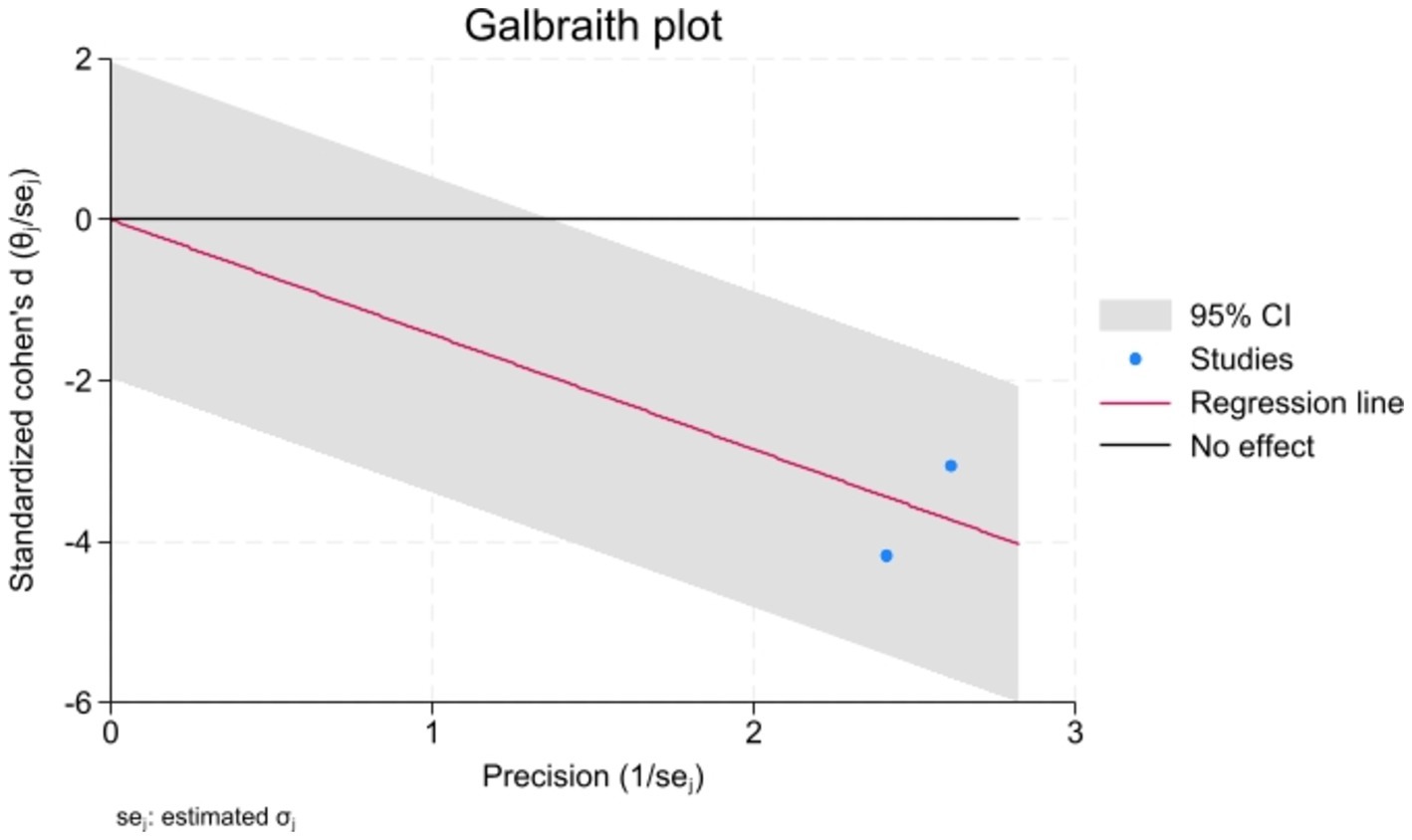
Figure 4. The Galbraith plot for heterogeneity assessment indicated no heterogeneity, further confirmed with the I-squared test (I2 < 50%).
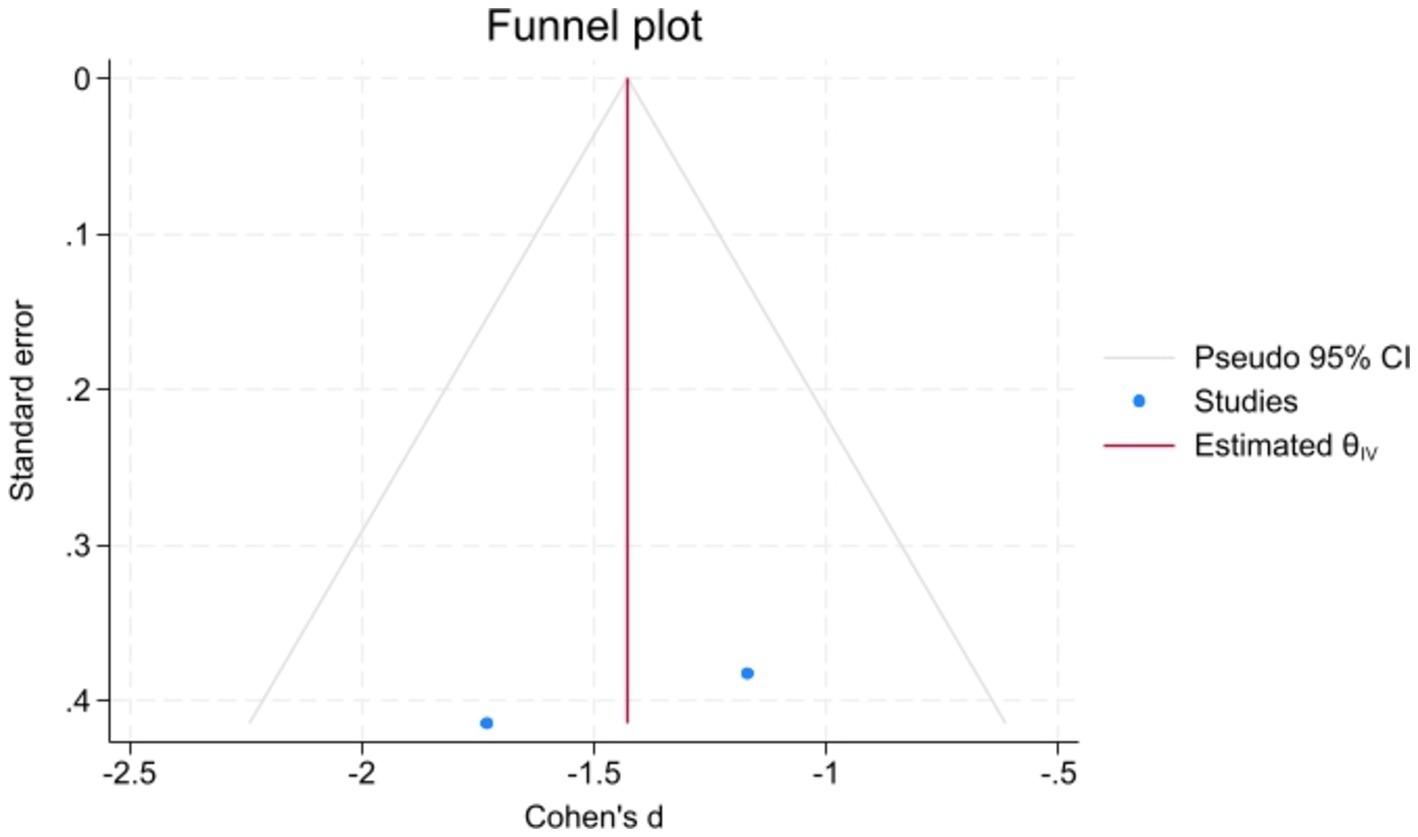
Figure 5. Symmetric demonstration of the funnel plot indicated no publication bias, further confirmed by Egger’s test for publication bias (p > 0.05).
DiscussionOur research showed that although FA is decreased in IBS patients, it does not differ substantially from the control group’s decline.
Consistent with the findings of this investigation, the great majority of neuropathologies had lower FA with increased MD of WM. However, the FA evaluation is inadequate because it does not account for specific causes of WM integrity degradation, such as changed axon density or diameter and myelination level. To some extent, AD and RD might be indicators of these alterations. AD reflects axons’ width and density and represents axial diffusion’s direction. Decreased AD may indicate damage to specific pathways or axonal degeneration. RD is a measure of myelination degree and indicates the direction of radial diffusion. The RD value is raised by demyelination and hypomyelination and decreased by myelination. Generally speaking, a drop in FA corresponds with either an increase in RD or a decrease in AD. Thus, variations in AD and RD may indicate possible reasons why the integrity of the WM tract may be harmed (Fang et al., 2017; Hubbard et al., 2018). However, we could not expand our analysis to incorporate these divisibility metrics due to a significant lack of data on the MD, RD, and AD of IBS patients.
Similar to numerous other chronic pain diseases and mood disorders, IBS is more prevalent in women, and sex-related disparities in the autonomic, perceptual, and emotional responses of IBS patients to aversive visceral stimuli have been documented. Sex variations in the anomalies in brain function and structure associated with chronic pain are inadequately characterized. The observed sex differences in FA and MD within the IBS cohort indicate more significant white matter alterations in female patients, however these changes are confined to the same locations that differ between healthy controls and IBS patients (Labus et al., 2014). Our study further confirmed these by showing statistically significant difference between white matter integrity alteration between female and male subject (p < 0.05).
It is essential to note the technical limits of tractography and DTI, especially concerning the tendency for false positives when employing probabilistic approaches and crossing fibers. To completely understand the differences in tractography between IBS patients and healthy controls, as well as potential confounding factors such psychological distress imposed by the disease itself, future studies are required to use a combination of techniques to alleviate these limitations.
To address these limitations, the implementation and development of a high-efficiency, high-resolution 3D imaging technique for the simultaneous mapping of multiple essential tissue parameters in routine brain imaging, including T1, T2, proton density (PD), ADC, and fractional anisotropy (FA), is crucial. Cao et al.’s suggested DTI-MR fingerprinting method can be used in this regard to advance routine clinical brain imaging from weighted to quantitative imaging, and it is especially beneficial for diffusion investigations, which often have prolonged acquisition times (Cao et al., 2024).
IBS is a complex condition with multiple causes that significantly impact society’s financial and human resources. IBS symptoms might appear at any point along the Brain Gut Axis (BGA) spectrum and have not yet responded to curative medical treatment. Despite significant progress in studying BGA dysfunction in people with IBS, we still do not fully understand how symptoms emerge. Neuroimaging has revealed the physiological distinctions between people with IBS and healthy individuals. Examining variations in neurotransmitter levels, disparities in overall and functional anatomical structure, and the advancing elucidation of a network associated with discomfort caused by rectal distention are crucial scientific advancements in comprehending the pathophysiology of IBS. In order to enhance our comprehension, it will be crucial to utilize appropriate comparator groups, such as individuals with inflammatory bowel disease and psychological illnesses.
The initial results of this study may illustrate the relationship between IBS and brain structure in the examined sample, highlighting that IBS diagnosis in these patients correlates with structural brain differences that may be significant for clinicians. This underscores the potential connection between gastrointestinal diseases, specifically irritable bowel syndrome, and the viscerotropic circuitry of the cerebral cortex.
These studies demonstrate evidence of changes in the brain-gut axis and its potential modulation for therapeutic purposes in patients with IBS.
The importance of this work should be mainly seen as methodological for some reasons. First, altered diffusivity and connection measures will probably need to be addressed in later research with larger samples. A bigger sample size would also aid in performing stronger meta-analyses with higher statistical power in identifying relevant differences between the two groups. Ultimately, more research is required to determine whether the altered structural connectivity described here is a cause of IBS, a result of the condition, a risk factor for it, or, more likely, the result of a reciprocally modulatory relationship between the alterations described in the white matter tract and IBS symptoms.
Data availability statementThe original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Author contributionsMA: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. AH: Writing – original draft, Writing – review & editing. SP: Writing – original draft, Writing – review & editing. AI: Writing – original draft, Writing – review & editing. SR: Writing – original draft, Writing – review & editing. FN: Writing – original draft, Writing – review & editing. YP: Writing – original draft, Writing – review & editing. AR: Writing – original draft, Writing – review & editing. SK: Writing – original draft, Writing – review & editing.
FundingThe author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interestThe authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s noteAll claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
ReferencesCao, X., Liao, C., Zhou, Z., Zhong, Z., Li, Z., Dai, E., et al. (2024). DTI-MR fingerprinting for rapid high-resolution whole-brain T(1), T(2), proton density, ADC, and fractional anisotropy mapping. Magn. Reson. Med. 91, 987–1001. doi: 10.1002/mrm.29916
PubMed Abstract | Crossref Full Text | Google Scholar
Chen, J. Y. W., Blankstein, U., Diamant, N. E., and Davis, K. D. (2011). White matter abnormalities in irritable bowel syndrome and relation to individual factors. Brain Res. 1392, 121–131. doi: 10.1016/j.brainres.2011.03.069
PubMed Abstract | Crossref Full Text | Google Scholar
Chiba, T., Ito, K., Mori, F., Sasaki, M., and Matsumoto, T. (2020). Detection of microstructural white matter alterations in functional gastrointestinal disorders assessed by diffusion kurtosis imaging. JGH Open. 4, 958–963. doi: 10.1002/jgh3.12375
PubMed Abstract | Crossref Full Text | Google Scholar
Drossman, D. A. (2016). Functional gastrointestinal disorders: history, pathophysiology, clinical features, and Rome IV. Gastroenterology 150, 1262–1279.e2. e2. doi: 10.1053/j.gastro.2016.02.032
PubMed Abstract | Crossref Full Text | Google Scholar
Ellingson, B. M., Mayer, E., Harris, R. J., Ashe-McNally, C., Naliboff, B. D., Labus, J. S., et al. (2013). Diffusion tensor imaging detects microstructural reorganization in the brain associated with chronic irritable bowel syndrome. Pain 154, 1528–1541. doi: 10.1016/j.pain.2013.04.010
PubMed Abstract | Crossref Full Text | Google Scholar
Fang, J., Li, S., Li, M., Chan, Q., Ma, X., Su, H., et al. (2017). Altered white matter microstructure identified with tract-based spatial statistics in irritable bowel syndrome: a diffusion tensor imaging study. Brain Imaging Behav. 11, 1110–1116. doi: 10.1007/s11682-016-9573-y
PubMed Abstract | Crossref Full Text | Google Scholar
Grinsvall, C., Van Oudenhove, L., Dupont, P., Ryu, H. J., Ljungberg, M., Labus, J. S., et al. (2021). Altered structural covariance of insula, cerebellum and prefrontal cortex is associated with somatic symptom levels in irritable bowel syndrome (IBS). Brain Sci. 11:1580. doi: 10.3390/brainsci11121580
PubMed Abstract | Crossref Full Text | Google Scholar
Hubbard, C. S., Becerra, L., Heinz, N., Ludwick, A., Rasooly, T., Yendiki, A., et al. (2018). Microstructural white matter abnormalities in the dorsal cingulum of adolescents with IBS. eNeuro 5, ENEURO.0354–ENEU17.2018. doi: 10.1523/ENEURO.0354-17.2018
PubMed Abstract | Crossref Full Text | Google Scholar
Irimia, A., Labus, J. S., Torgerson, C. M., Van Horn, J. D., and Mayer, E. A. (2015). Altered viscerotopic cortical innervation in patients with irritable bowel syndrome. Neurogastroenterol. Motil. 27, 1075–1081. doi: 10.1111/nmo.12586
PubMed Abstract | Crossref Full Text | Google Scholar
Kwan, C., Diamant, N., Pope, G., Mikula, K., Mikulis, D., and Davis, K. (2005). Abnormal forebrain activity in functional bowel disorder patients with chronic pain. Neurology 65, 1268–1277. doi: 10.1212/01.wnl.0000180971.95473.cc
PubMed Abstract | Crossref Full Text | Google Scholar
Labus, J. S., Dinov, I. D., Jiang, Z., Ashe-McNalley, C., Zamanyan, A., Shi, Y., et al. (2014). Irritable bowel syndrome in female patients is associated with alterations in structural brain networks. Pain 155, 137–149. doi: 10.1016/j.pain.2013.09.020
PubMed Abstract | Crossref Full Text | Google Scholar
Lovell, R. M., and Ford, A. C. (2012). Global prevalence of and risk factors for irritable bowel syndrome: a meta-analysis. Clin. Gastroenterol. Hepatol. 10, 712–721.e4. doi: 10.1016/j.cgh.2012.02.029
PubMed Abstract | Crossref Full Text | Google Scholar
Nan, J. F., Zhang, L. L., Chen, Q. Q., Zong, N. N., Zhang, P. Y., Ji, X., et al. (2018). White matter microstructural similarity and diversity of functional constipation and constipation-predominant irritable bowel syndrome. J. Neurogastroenterol. Motil. 24, 107–118. doi: 10.5056/jnm17038
PubMed Abstract | Crossref Full Text | Google Scholar
Öhman, L., and Simren, M. (2007). New insights into the pathogenesis and pathophysiology of irritable bowel syndrome. Dig. Liver Dis. 39, 201–215. doi: 10.1016/j.dld.2006.10.014
Crossref Full Text | Google Scholar
Ouzzani, M., Hammady, H., Fedorowicz, Z., and Elmagarmid, A. (2016). Rayyan—a web and mobile app for systematic reviews. Syst. Rev. 5, 1–10. doi: 10.1186/s13643-016-0384-4
Crossref Full Text | Google Scholar
Page, M. J., McKenzie, J. E., Bossuyt, P. M., Boutron, I., Hoffmann, T. C., Mulrow, C. D., et al. (2020). Statement: an updated guideline for reporting systematic reviews. BMJ 2021:372. doi: 10.1136/bmj.n71
Crossref Full Text | Google Scholar
Qi, R. F., Liu, C., Weng, Y. F., Xu, Q., Chen, L. Y., Wang, F. Y., et al. (2016). Disturbed interhemispheric functional connectivity rather than structural connectivity in irritable bowel syndrome. Front. Mol. Neurosci. 9:141. doi: 10.3389/fnmol.2016.00141
PubMed Abstract | Crossref Full Text | Google Scholar
Simrén, M., Törnblom, H., Palsson, O. S., Van Oudenhove, L., Whitehead, W. E., and Tack, J. (2019). Cumulative effects of psychologic distress, visceral hypersensitivity, and abnormal transit on patient-reported outcomes in irritable bowel syndrome. Gastroenterology 157, 391–402.e2. doi: 10.1053/j.gastro.2019.04.019
PubMed Abstract | Crossref Full Text | Google Scholar
Zikou, A. K., Kosmidou, M., Astrakas, L. G., Tzarouchi, L. C., Tsianos, E., and Argyropoulou, M. I. (2014). Brain involvement in patients with inflammatory bowel disease: a voxel-based morphometry and diffusion tensor imaging study. Eur. Radiol. 24, 2499–2506. doi: 10.1007/s00330-014-3242-6
留言 (0)