Stroke is a predominant cause of mortality and disability globally. A 2019 survey conducted by the World Health Organization (WHO) identifies stroke as the second leading cause of death, responsible for 11% of total fatalities, with a significant proportion of survivors experiencing various functional disabilities (Feigin et al., 2022). Post-stroke cognitive impairment (PSCI) encompasses a broad spectrum of neurological disorders characterized by varying degrees of cognitive dysfunction that may persist for 3 to 6 months following a stroke (Wang and Dong, 2021). The prevalence of post-stroke cognitive impairment (PSCI) ranges from approximately 17 to 66%, with variations influenced by factors such as country, race, and diagnostic criteria (Li M. et al., 2023). PSCI primarily manifests as impairments in higher-level cognitive functions, including learning, memory, executive function, and visuospatial abilities following a stroke. Common symptoms encompass attention deficits, memory impairments, and difficulties in learning (Levine et al., 2015). These cognitive challenges significantly diminish the patient’s quality of life and impose substantial financial and psychological burdens on the patient and their family. Consequently, the pursuit of a safe, effective, affordable, and acceptable treatment has become an urgent priority.
Current effective methods for enhancing cognitive function in stroke patients primarily include pharmacological treatments such as nimodipine, donepezil, and piracetam, as well as cognitive function training (CFT), the use of virtual environments and nursing care (Chang et al., 2011; Rao et al., 2013; Li Y. et al., 2023). Furthermore, research has demonstrated that several other neuroprotective agents can safely and effectively ameliorate cognitive impairment to some degree (Zhang et al., 2014). Nevertheless, long-term treatment presents several challenges that warrant attention, including potential liver and kidney toxicity, gastrointestinal issues, high costs, and concerns regarding patient compliance (Marucci et al., 2021).
Acupuncture has been widely employed in the treatment of stroke patients, addressing cognitive impairment (Liu Y. et al., 2023), dysphagia (Zhu et al., 2023), and motor functions (Bao et al., 2021). Systematic reviews and meta-analyses suggest that acupuncture may enhance post-stroke cognitive impairment (PSCI); however, the considerable heterogeneity among studies raises concerns regarding the reliability of these findings (Wu et al., 2024). Scalp acupuncture (SA), a novel approach distinct from traditional acupuncture, targets specific reflex areas of the brain that correspond to anatomical structures on the surface of the head. This technique stimulates particular regions of the scalp, which can exert direct or indirect effects on the associated brain functional areas, thereby improving cerebral blood circulation and regulating neurotransmitter release, ultimately facilitating neurological recovery. Research has also confirmed its efficacy in ameliorating the condition of patients with PSCI (Du et al., 2018; Chen J. et al., 2020; Xiong et al., 2020). Furthermore, studies suggest that the mechanisms by which acupuncture enhances PSCI involve the inhibition of nuclear factor (NF)-κB and its downstream target gene P53, as well as the modulation of neuronal apoptosis and hippocampal synaptic plasticity (Feng et al., 2013; Yang J. W. et al., 2019; Zhang et al., 2023). Additionally, some research indicates that electroacupuncture can reduce the expression and activity of calmodulin (CaM) while simultaneously increasing calmodulin-dependent protein kinase type IV (CaMKIV) and cyclic adenosine monophosphate response element-binding protein (CREB), along with their related phosphorylation, thereby improving cognitive function (Zhang et al., 2016).
Current research is deficient in data analysis concerning the efficacy of scalp acupuncture treatment for PSCI, which limits the availability of credible evidence. To scientifically assess the effectiveness of scalp acupuncture in treating PSCI, this study conducts a comprehensive analysis of randomized controlled trial data from the establishment of the database up to October 24, 2024. By evaluating multiple outcome indicators, this research aims to provide more robust evidence to support clinical practice.
2 Methods 2.1 Protocol and registrationThis study was conducted as a meta-analysis by the Cochrane Handbook for Systematic Reviews of Interventions and has been registered with PROSPERO under registration number CRD 42024519200. Our research strictly adhered to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) reporting guidelines (Page et al., 2021). The PRISMA checklist is provided in the Appendix 1.
2.2 Literature searchWe conducted a comprehensive search across eight databases, which included four English databases—PubMed, Cochrane Library, Embase, and Web of Science (WoS)—and four Chinese databases—China Biomedicine (CBM), China National Knowledge Infrastructure (CNKI), Chinese Science and Technology Journals (VIP), and WanFang Database. The search covered the period from the establishment of each database up to October 24, 2024, without restrictions on country, language, or publication status. We utilized the MeSH subject headings “scalp acupuncture,” “stroke,” and “cognitive impairment,” with a focus on randomized controlled trials (RCTs) to develop our search strategy. This strategy was specifically tailored to accommodate the unique characteristics of each database. Detailed search terms and strategies for each database are provided in Appendix 2. Additionally, we manually screened the reference lists of all included articles to identify potentially relevant RCTs.
2.3 Inclusion and exclusion criteriaThe studies included in this analysis were not restricted by age, gender, race, or ethnicity. The inclusion is as follows: (1) research subjects must meet the diagnostic criteria for stroke patients; (2) the intervention involves scalp acupuncture; (3) the control group consists of sham acupuncture or other treatment methods such as primary treatment or blank control; (4) the study design must be a RCTs; (5) the research must include complete data recording and analysis.
The exclusion criteria are (1) non-randomized controlled trials; (2) exclusion of animal studies; (3) dissertations, conference papers, and case reports; (4) unavailability of original text or full-text data; (5) review articles.
2.4 Study selection and data extractionTwo researchers independently screened the literature included in this study based on established inclusion and exclusion criteria. They selected the titles and abstracts of relevant literature to identify qualified studies for retention. A third researcher assessed any controversial literature. Basic information from the included studies was extracted, including the first author’s name, year of publication, subject status, sample size, intervention measures, and outcome indicators. For articles with incomplete data, the original authors were contacted via email or telephone to obtain any missing or unclear information.
2.5 Risk of bias in individual studiesAll included studies were RCTs, and the Cochrane Risk of Bias Tool 2.0 (RoB 2) was utilized to assess the risk of bias within these trials. This comprehensive tool evaluates potential biases across five key domains: (1) the randomization process, (2) deviations from the intended intervention, (3) instances of missing outcome data, (4) methods of outcome measurement, and (5) criteria for the selection of reported outcomes. Each domain is classified according to the risk of bias as low risk, high risk, or some concern. To maintain rigorous methodological standards, the risk of bias for each included study was independently assessed by two researchers. A third researcher addressed any discrepancies or inconsistencies identified during the independent assessment to ensure an unbiased evaluation.
2.6 Data analysisThis study utilized RevMan 5.4 software to perform a meta-analysis. The mean difference (MD) is reported as the effect size for continuous outcome measures. In the case of binary variables, the relative risk (RR) and the corresponding 95% confidence interval (CI) represent the effect size. We employed the χ2 test with I2 quantitative analysis to assess inter-study heterogeneity. If p > 0.1 and I2 < 50%, it is deemed that there is no significant heterogeneity among the included studies, and a fixed effects model is applied for the meta-analysis. Conversely, if p < 0.1 and I2 > 50%, it indicates a significant heterogeneity among the studies. Lastly, we conducted subgroup and sensitivity analyses to investigate the sources of heterogeneity and gain a deeper understanding of the intervention’s effects. Publication bias was evaluated using funnel plots and Egger’s bias test.
3 Results 3.1 Study selectionAccording to the search strategy, we retrieved 398 documents. Our preliminary screening excluded 119 duplicate documents, 82 conference papers and theses, and 49 reviews, systematic reviews, meta-analyses, and animal experiments. Reviewing the titles, abstracts, and keywords, we identified 97 documents that did not align with the research content. Upon examining the complete texts, we discovered that 23 articles contained duplicate or incomplete data. Ultimately, 28 articles were included in this study (Figure 1).
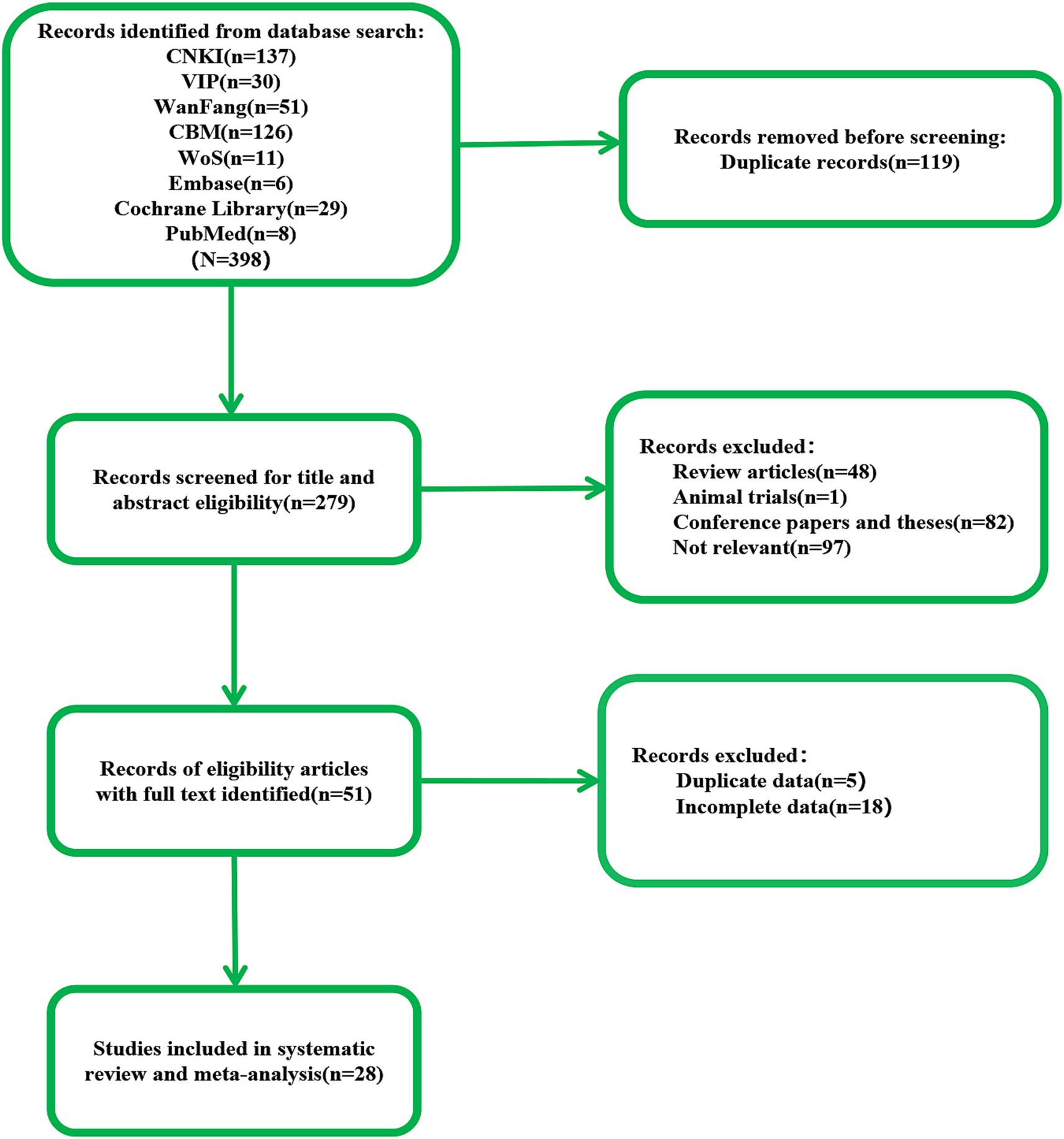
Figure 1. The flow of literature search and selection.
3.2 Study characteristicsAll studies included in this analysis were RCTs. A total of 28 studies involving 1995 participants were examined, with 998 in the treatment group and 997 in the control group. Among the included studies, four were conducted in English (Xie et al., 2012; Du et al., 2018; Chen J. et al., 2020; Xiong et al., 2020), while the remaining 24 were conducted in Chinese (Xie et al., 2007; Fu et al., 2010; Wang et al., 2012; Qin, 2015; Ding and Zhang, 2016; Wang, 2016; Huang et al., 2017; Wang et al., 2017; Han et al., 2018; Wang W. et al., 2018; Wang Y. X. et al., 2018; Zhang and An, 2018; Yang L. et al., 2019; Liu et al., 2019; Mao and Cheng, 2019; Zhang et al., 2019; Chen A. Z. et al., 2020; Zhang et al., 2020; Cai et al., 2022; Zhai et al., 2022; Li and Pan, 2023; Wei et al., 2023; Liu Y. F. et al., 2023; Liu et al., 2024), all within China. There was no statistically significant difference in the baseline characteristics of the treatment and control groups regarding age, disease duration, education level, outcome indicators, and other basic information before enrollment. The intervention methods varied across studies. One study exclusively utilized scalp acupuncture combined with basic treatment (B) (Wang et al., 2012), while the other 27 studies incorporated additional intervention methods, including rehabilitation therapy (R) (Xie et al., 2007; Fu et al., 2010; Xie et al., 2012; Qin, 2015; Ding and Zhang, 2016; Huang et al., 2017; Wang W. et al., 2018; Wang Y. X. et al., 2018; Mao and Cheng, 2019; Zhang et al., 2019; Cai et al., 2022; Wei et al., 2023; Liu et al., 2024), computer-assisted cognitive training (CACT) (Wang, 2016; Zhang et al., 2020) hyperbaric oxygen therapy (H) (Wang et al., 2017; Yang L. et al., 2019), repetitive transcranial magnetic stimulation (rTMS) (Han et al., 2018; Liu et al., 2019), cognitive training (CT) (Du et al., 2018; Chen A. Z. et al., 2020; Xiong et al., 2020; Zhai et al., 2022; Li and Pan, 2023), the donepezil hydrochloride (Chen J. et al., 2020; Liu Y. et al., 2023) and the drug idebenone (Zhang and An, 2018). The control group interventions included nimodipine, CT, donepezil hydrochloride, R, CACT, H, rTMS, idebenone, and conventional symptomatic treatment.
The 28 studies included in this analysis employed four evaluation tools—MoCA, MMSE, LOTCA, and P300—as outcome indicators for scalp acupuncture treatment of post-stroke cognitive impairment (PSCI). Among these, 13 studies (Wang et al., 2017; Wang W. et al., 2018; Yang L. et al., 2019; Liu et al., 2019; Mao and Cheng, 2019; Chen A. Z. et al., 2020; Chen J. et al., 2020; Zhang et al., 2020; Cai et al., 2022; Li and Pan, 2023; Wei et al., 2023; Liu Y. F. et al., 2023; Liu et al., 2024) utilized the MoCA scale as their primary outcome measure. In contrast, 14 studies (Ding and Zhang, 2016; Han et al., 2018; Wang W. et al., 2018; Wang Y. X. et al., 2018; Zhang and An, 2018; Yang L. et al., 2019; Liu et al., 2019; Zhang et al., 2019; Xiong et al., 2020; Cai et al., 2022; Zhai et al., 2022; Wei et al., 2023; Liu Y. et al., 2023; Liu et al., 2024) selected the MMSE scale as their outcome measure, while seven studies (Wang W. et al., 2018; Yang L. et al., 2019; Liu et al., 2019; Cai et al., 2022; Wei et al., 2023; Liu Y. F. et al., 2023; Liu et al., 2024) employed both the MoCA and MMSE scales as outcome measures. Additionally, six studies (Fu et al., 2010; Wang et al., 2012; Wang, 2016; Huang et al., 2017; Du et al., 2018; Xiong et al., 2020) utilized the LOTCA score as an outcome measure. Among the 28 studies, seven studies (Xie et al., 2012; Zhang and An, 2018; Yang L. et al., 2019; Mao and Cheng, 2019; Zhai et al., 2022; Wei et al., 2023; Liu Y. F. et al., 2023) indicated total effectiveness as an outcome indicator. Furthermore, five studies (Xie et al., 2007; Xie et al., 2012; Qin, 2015; Wang Y. X. et al., 2018; Wei et al., 2023) assessed P300’s latency and amplitude as evaluation tools. The included studies primarily employed the Barthel index (BI) and modified Barthel index (MBI) scores to evaluate daily activities. Notably, one study reported an adverse event involving participant withdrawal during the investigation. The characteristics of all 28 studies are summarized in Table 1.
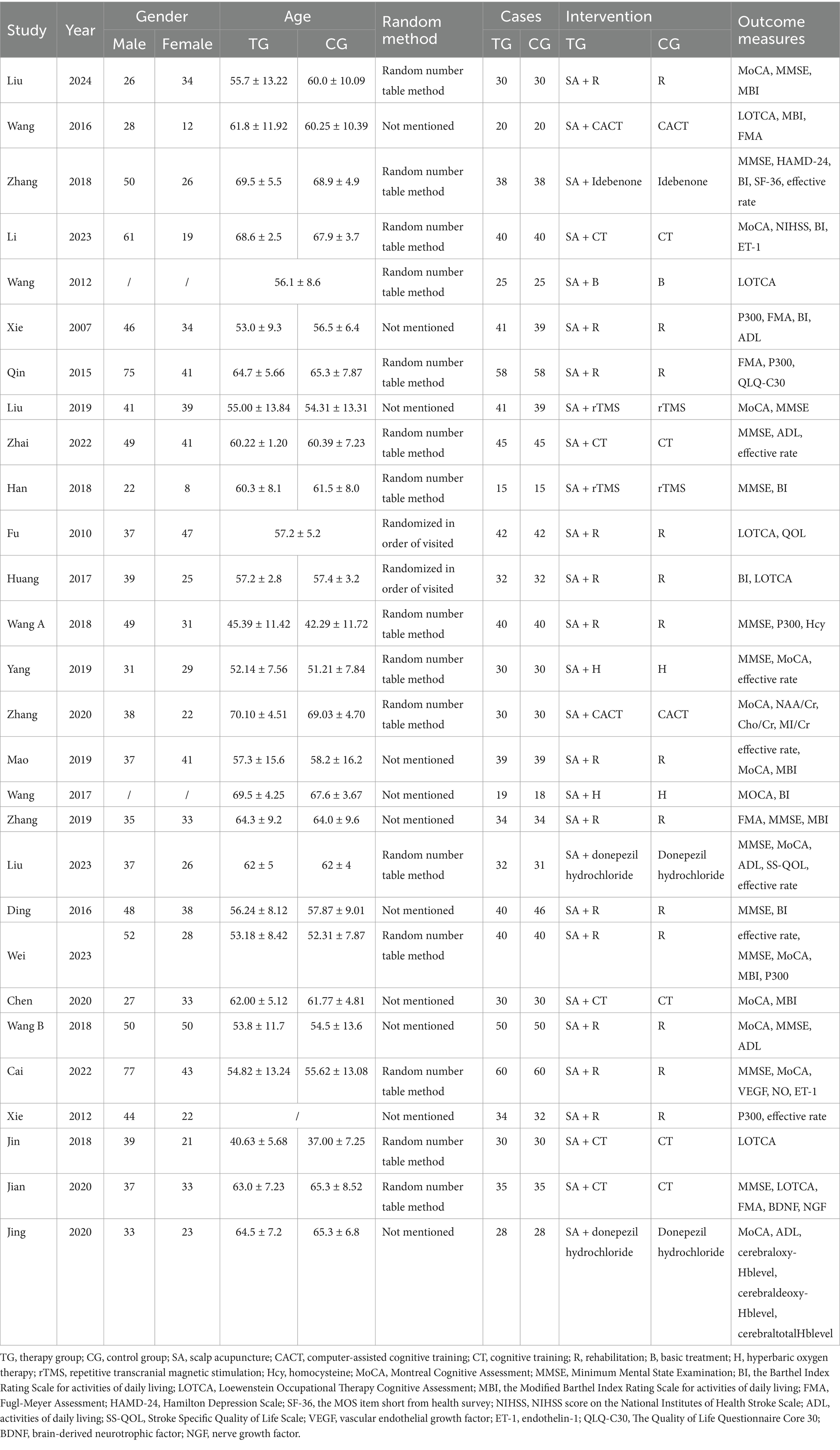
Table 1. Characteristics of included studies.
The characteristics of SA parameters vary across the 28 studies examined. Regarding SA type, 19 studies employed International SA (Xie et al., 2007; Fu et al., 2010; Wang et al., 2012; Xie et al., 2012; Qin, 2015; Ding and Zhang, 2016; Wang, 2016; Huang et al., 2017; Wang et al., 2017; Du et al., 2018; Wang W. et al., 2018; Yang L. et al., 2019; Liu et al., 2019; Mao and Cheng, 2019; Zhang et al., 2019; Zhang et al., 2020; Cai et al., 2022; Liu et al., 2024), while two studies utilized Fang SA (Zhang and An, 2018; Liu Y. F. et al., 2023), and another two studies employed Yu SA (Wang Y. X. et al., 2018; Wei et al., 2023). Additionally, 1 study focused on Lingnan SA (Li and Pan, 2023). In terms of treatment duration, 17 studies administered sessions lasting 30 min each (Xie et al., 2007; Wang et al., 2012; Qin, 2015; Wang, 2016; Wang et al., 2017; Du et al., 2018; Han et al., 2018; Wang W. et al., 2018; Zhang and An, 2018; Yang L. et al., 2019; Liu et al., 2019; Chen A. Z. et al., 2020; Zhang et al., 2020; Cai et al., 2022; Li and Pan, 2023; Liu Y. F. et al., 2023; Liu et al., 2024), whereas 1 study lasted 40 min (Ding and Zhang, 2016), another lasted 60 min (Wei et al., 2023), and 1 study had a duration of 120 min (Wang et al., 2012), furthermore, five studies reported sessions lasting 360 min each (Fu et al., 2010; Huang et al., 2017; Wang Y. X. et al., 2018; Mao and Cheng, 2019; Chen J. et al., 2020), while 1 study varied between 15 to 30 min (Zhai et al., 2022), and another ranged from 180 to 240 min (Xiong et al., 2020). The SA parameter characteristics are detailed in Table 2.
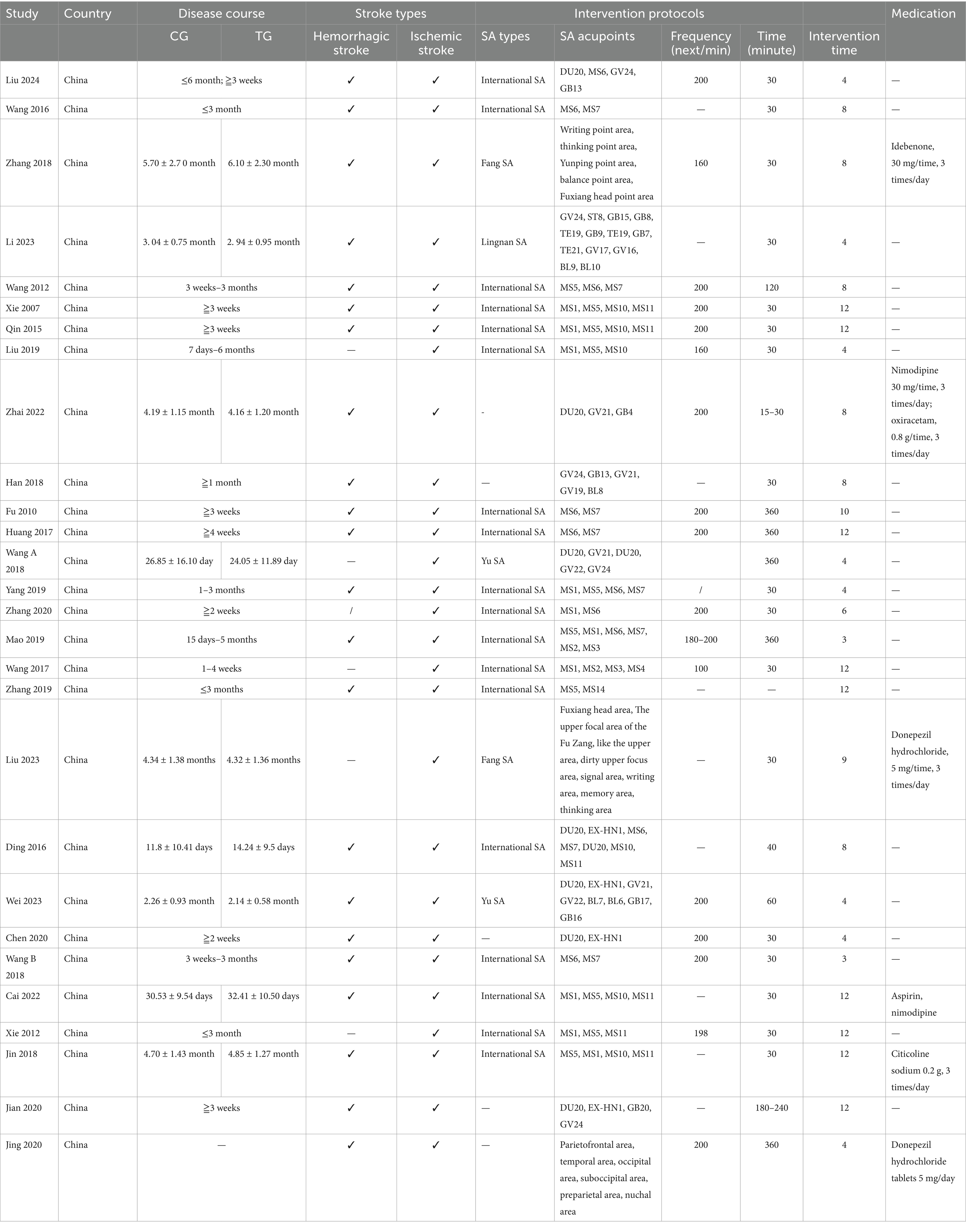
Table 2. The SA parameter characteristics.
3.3 Study design and risk of biasThe quality of the 28 included randomized controlled trials was generally classified as “low to moderate.” It is important to note that due to the unique nature of scalp acupuncture, all studies were unable to blind operators and participants. Two studies were assessed as having a risk of “some concern” (Fu et al., 2010; Wang, 2016), while 19 studies were categorized as having a low risk (Wang et al., 2012; Qin, 2015; Huang et al., 2017; Wang et al., 2017; Du et al., 2018; Han et al., 2018; Wang W. et al., 2018; Zhang and An, 2018; Yang L. et al., 2019; Zhang et al., 2019; Chen A. Z. et al., 2020; Chen J. et al., 2020; Xiong et al., 2020; Zhang et al., 2020; Cai et al., 2022; Zhai et al., 2022; Li and Pan, 2023; Wei et al., 2023; Liu et al., 2024), and seven studies were identified as having a high risk of bias (Xie et al., 2007; Xie et al., 2012; Ding and Zhang, 2016; Wang Y. X. et al., 2018; Liu et al., 2019; Mao and Cheng, 2019; Liu Y. F. et al., 2023). Regarding the randomization process, most studies were deemed to have a low risk, as the randomization methods were reported in detail, with the random number table method being the most commonly employed technique. Additionally, there was a possibility of selective reporting of results in one study (Wang W. et al., 2018). Detailed quality assessments are presented in Figures 2, 3.
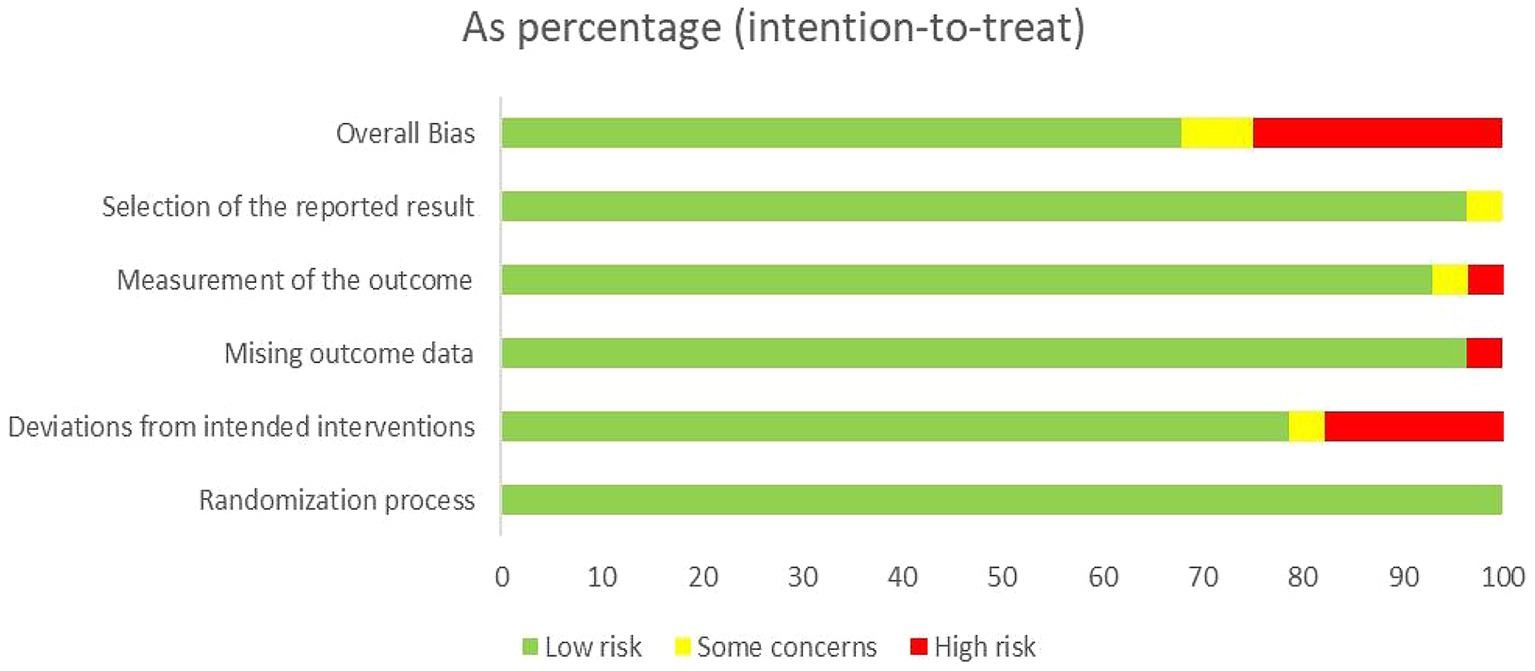
Figure 2. Assessment of risk of bias summary of included studies using the Cochrane tool.
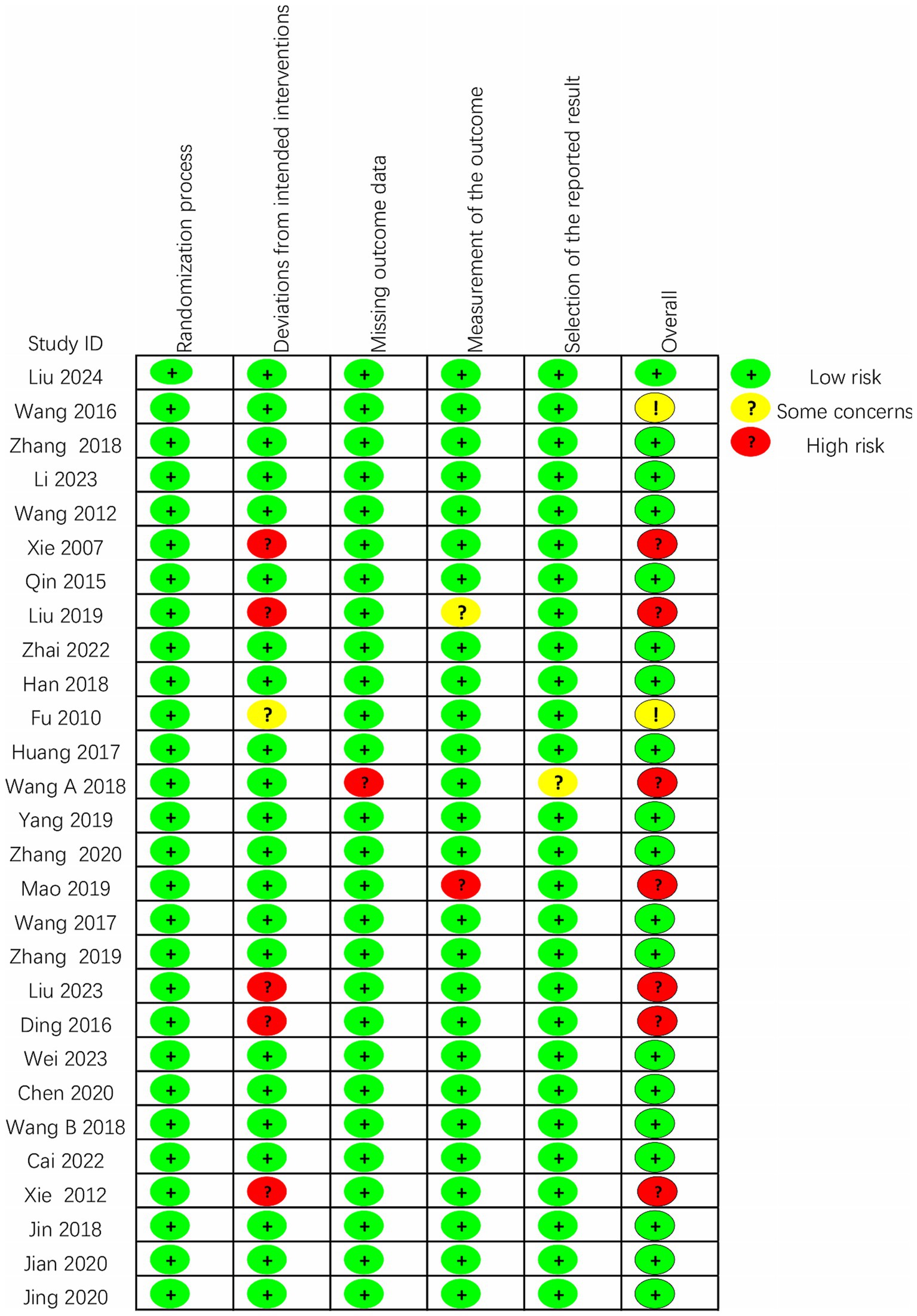
Figure 3. Assessment of risk of bias graph of included studies using the Cochrane tool.
3.4 Meta-analysis 3.4.1 The total effective rateThis study encompassed 28 individual studies, of which seven reported on this specific outcome. A subsequent meta-analysis employing a random effects model revealed that the efficacy of the combined scalp acupuncture treatment group was significantly superior to that of the control group (RR = 1.28, 95% CI: 1.14 to 1.45, I2 = 51%, 7 studies, 513 participants). Additionally, the results of the heterogeneity analysis suggest that these findings are robust (Figure 4).
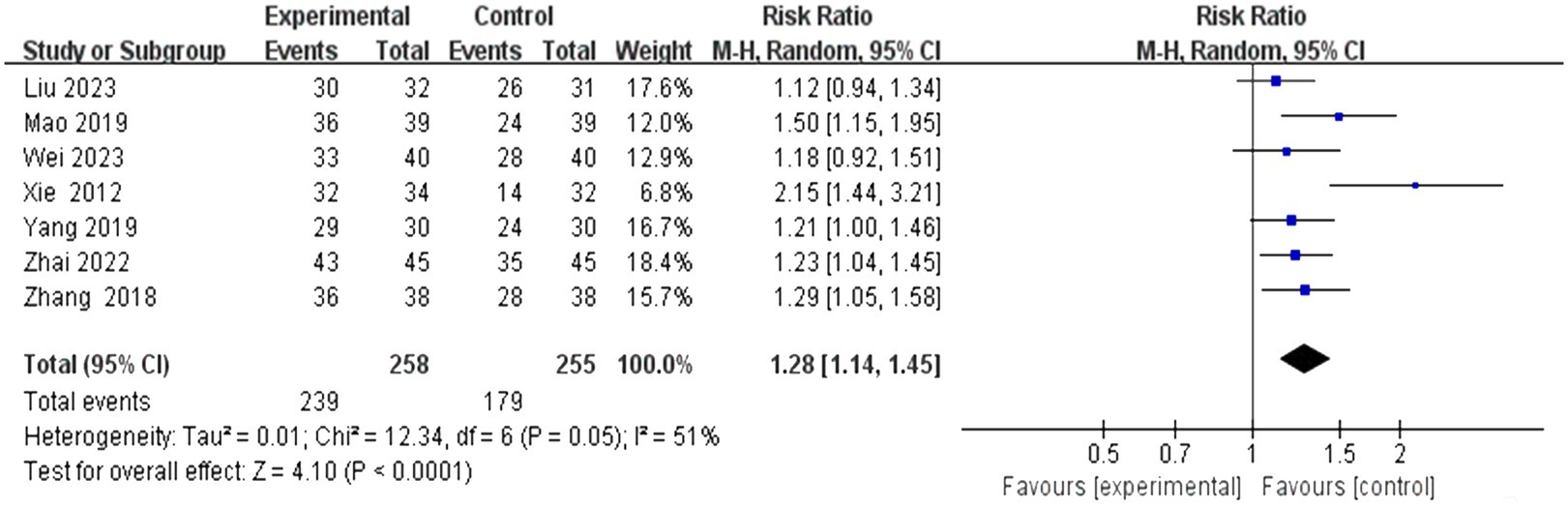
Figure 4. Forest plots of the total effective rate.
3.4.2 The MoCA scoresIn this study, 13 investigations reported MoCA scores. A meta-analysis of the included studies was conducted using a random effects model. The results indicated that the combination of the combined scalp acupuncture treatment was more effective than the control group in enhancing the MoCA scores of PSCI patients (MD = 3.55, 95% CI: 2.68–4.41, I2 = 93%, 13 studies, 944 participants) (Figure 5A). However, there was a high level of heterogeneity across the studies.
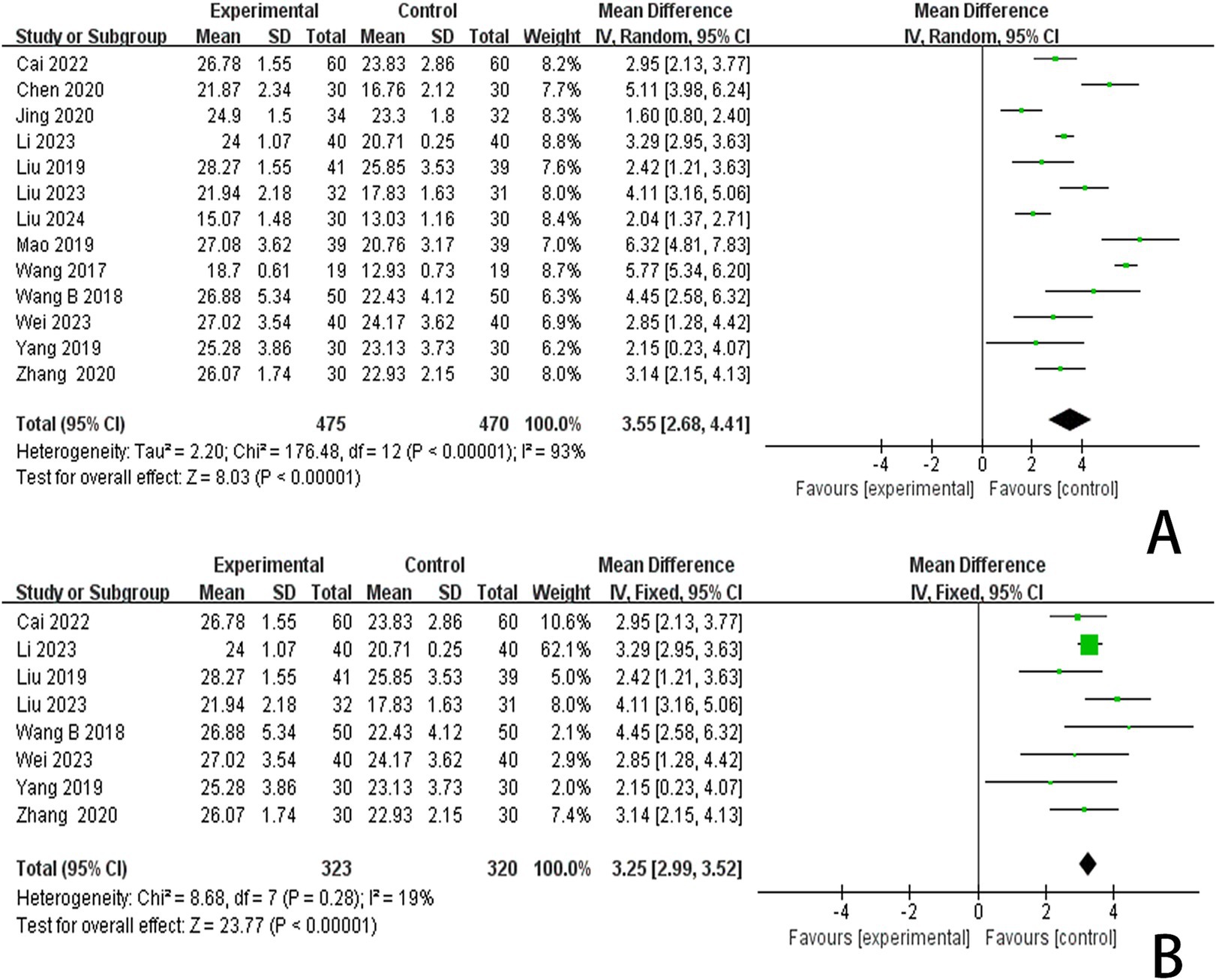
Figure 5. (A) Forest plots of the MoCA scores. (B) Forest plots of the MoCA scores after removing four studies.
3.4.3 The MMSE scoresIn this study, 13 investigations reported MoCA scores. A meta-analysis of the included studies was conducted using a random effects model. The results indicated that the combination of scalp acupuncture treatment was more effective than the control group in enhancing the MoCA scores of PSCI patients (MD = 3.78, 95% CI: 2.83–4.73, I2 = 94%, 14 studies, 1,063 participants) (Figure 6A). However, there was a high level of heterogeneity across the studies.
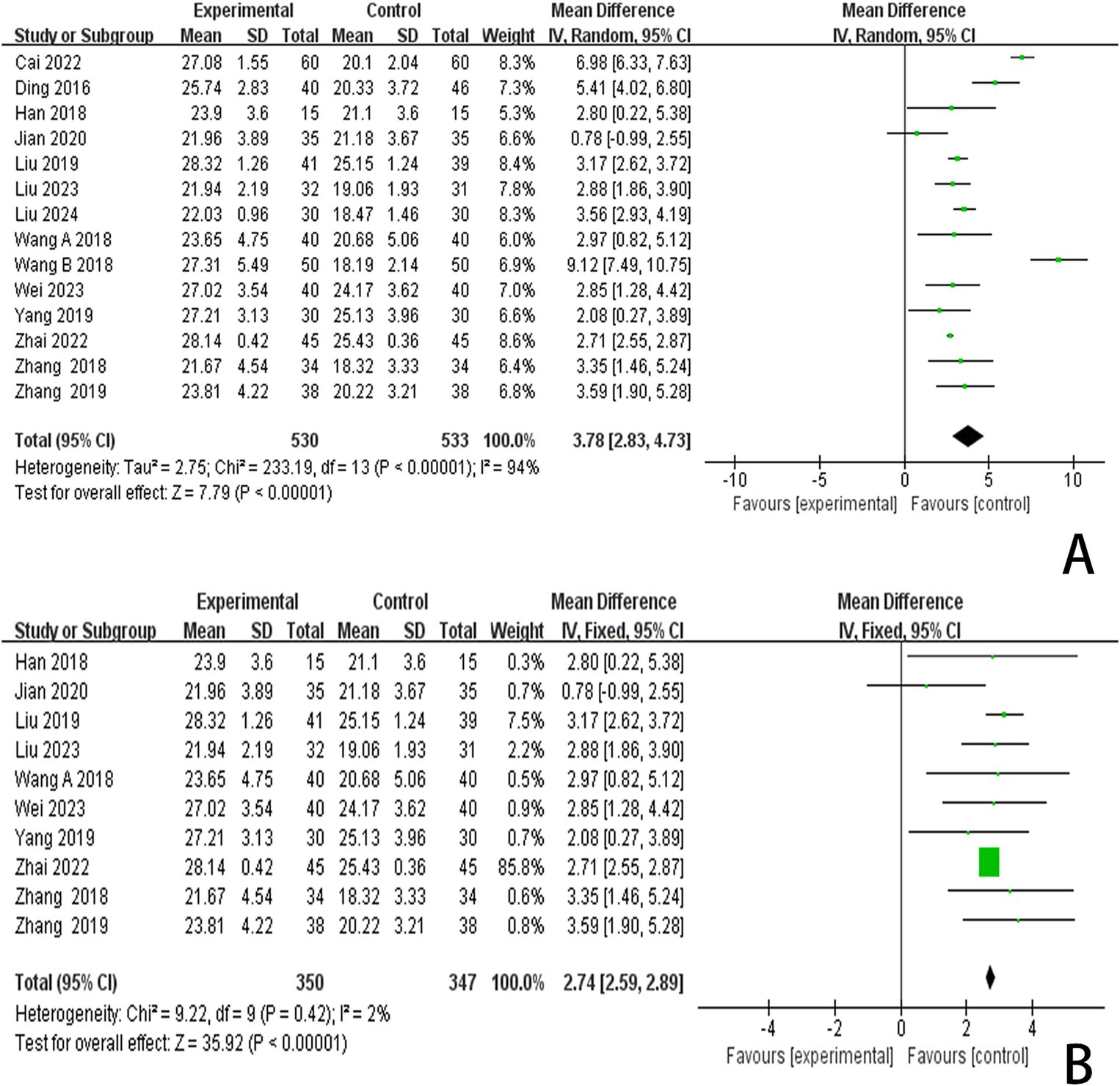
Figure 6. (A) Forest plots of the MMSE scores. (B) Forest plots of the MMSE scores after removing four studies.
3.4.4 The LOTCA scoresSix studies reported LOTCA scores and a random effects model was employed to conduct a meta-analysis of the data from each study. The results indicated that combined scalp acupuncture treatment significantly improves the LOTCA scores of PSCI patients compared to the control group (MD = 9.70, 95% CI: 7.72–11.69, I2 = 57%, 6 studies, 368 participants) (Figure 7A), with moderate heterogeneity observed between the studies.
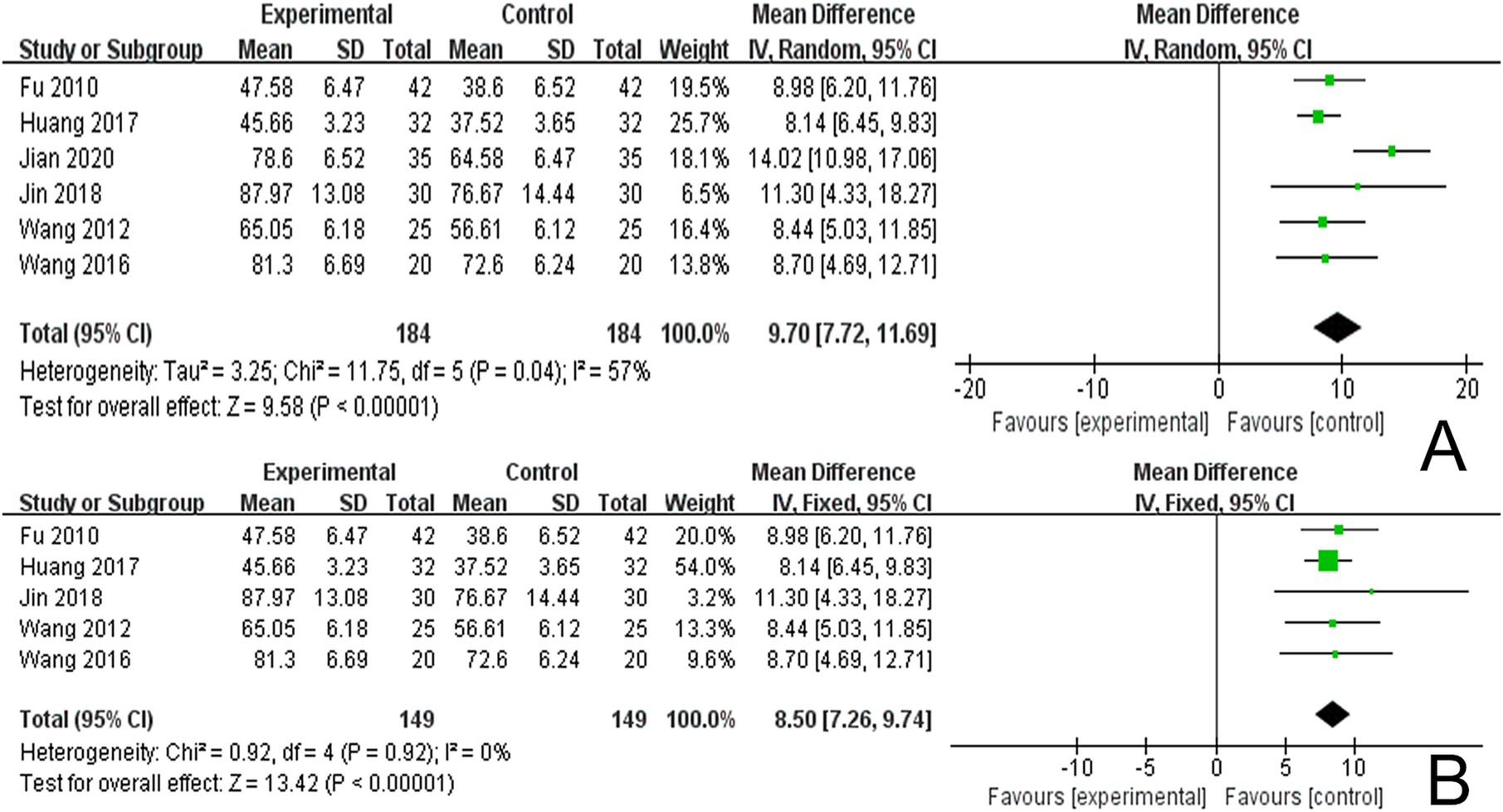
Figure 7. (A) Forest plots of the LOTCA scores. (B) Forest plots of the LOTCA scores after removing one study.
3.4.5 The P300 scoresIn reviewing the 28 studies included in this analysis, 5 reported P300 latency, while 4 studies reported P300 amplitude. We employed a random effects model to meta-analyze the latency results and found that scalp acupuncture combined with treatment significantly reduced P300 latency in patients with PSCI compared to the control group (MD = −21.83, 95% CI: −26.31 to −17.35, I2 = 55%, 5 studies, 422 participants) (Figure 8A). Additionally, a meta-analysis of the 4 studies reporting P300 amplitude, utilizing a fixed effects model, indicated that scalp acupuncture combined with treatment could enhance P300 amplitude in PSCI patients relative to the control group (MD = 1.05, 95% CI: 0.76–1.34, I2 = 0%, 4 studies, 342 participants) (Figure 8B).
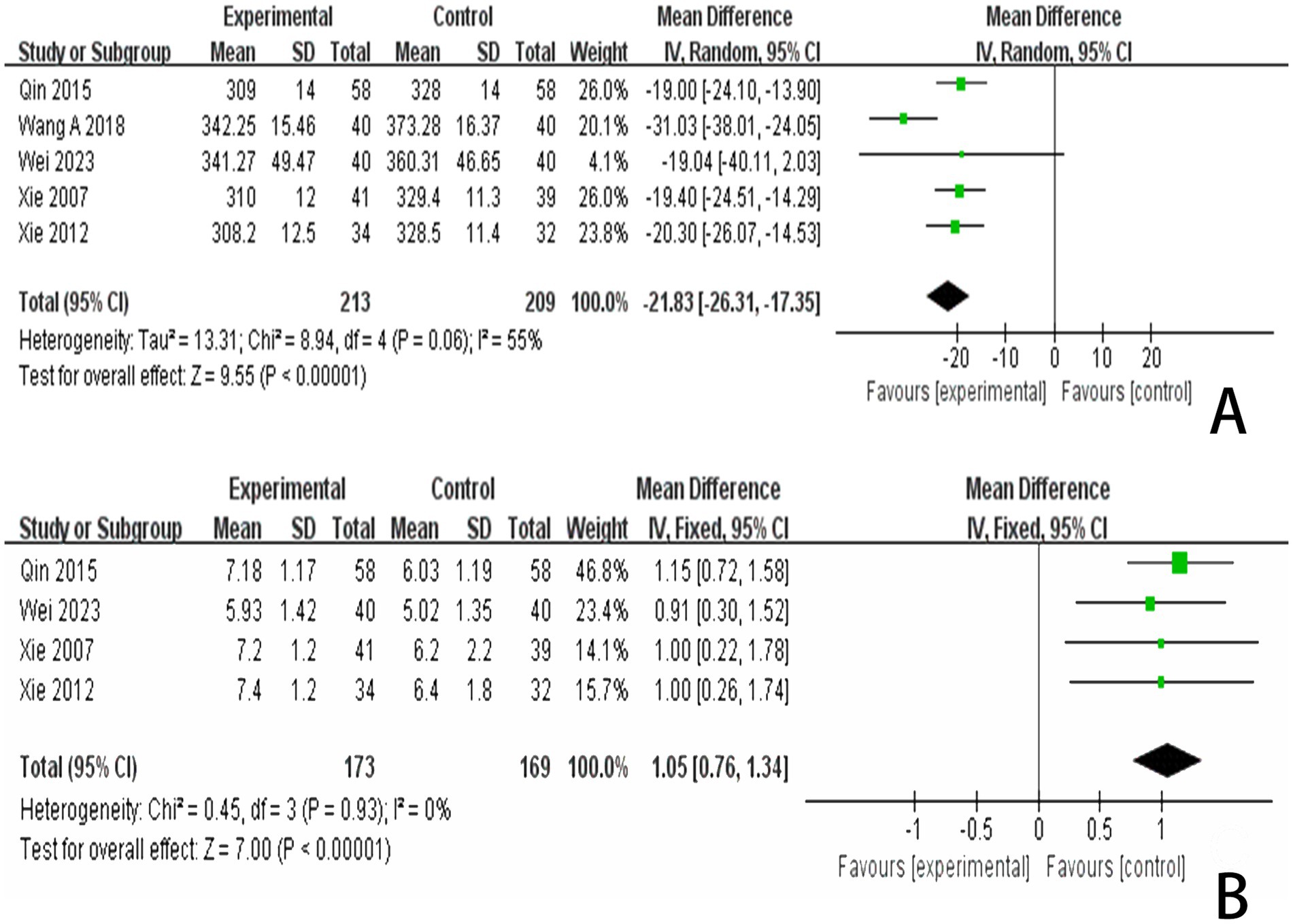
Figure 8. (A) Forest plots of the P300 latency. (B) Forest plots of the P300 latency after removing one study. (C) Forest plots of the P300 amplitude.
3.5 Subgroup analysis 3.5.1 Subgroup analysis of MoCA scoresWe conducted a subgroup analysis of studies that recorded MoCA scores based on the type of treatment administered in the control group (Figure 9). The results indicated that the SA + CT vs. CT subgroup was significantly effective (MD = 4.12, 95% CI: 2.34–5.89, I2 = 89%, 2 studies, 140 participants). Similarly, the SA + R vs. R subgroup also demonstrated significant effectiveness (MD = 3.61, 95% CI: 2.25–4.97, I2 = 86%, 5 studies, 438 participants). The SA + H vs. H subgroup showed significant effectiveness as well (MD = 4.09, 95% CI: 0.55–7.62, I2 = 92%, 2 studies, 97 participants). In the comparison of SA + donepezil hydrochloride vs. donepezil hydrochloride, the results were significant (MD = 2.84, 95% CI: 0.38–5.30, I2 = 94%, 2 studies, 129 participants). Heterogeneity was considered high, as I2 > 75% in four subgroups, indicating that the results were less reliable. Additionally, the subgroup SA + rTMS vs. rTMS showed significant results (MD = 2.42, 95% CI: 1.21–3.63, Z = 3.94, p < 0.0001), and SA + CACT vs. CACT also yielded significant findings (MD = 3.14, 95% CI: 2.15–4.13, Z = 6.22, p < 0.00001), although there is only one study among the three subgroup analyses, the p < 0.05 for each study indicates that the efficacy is evident.
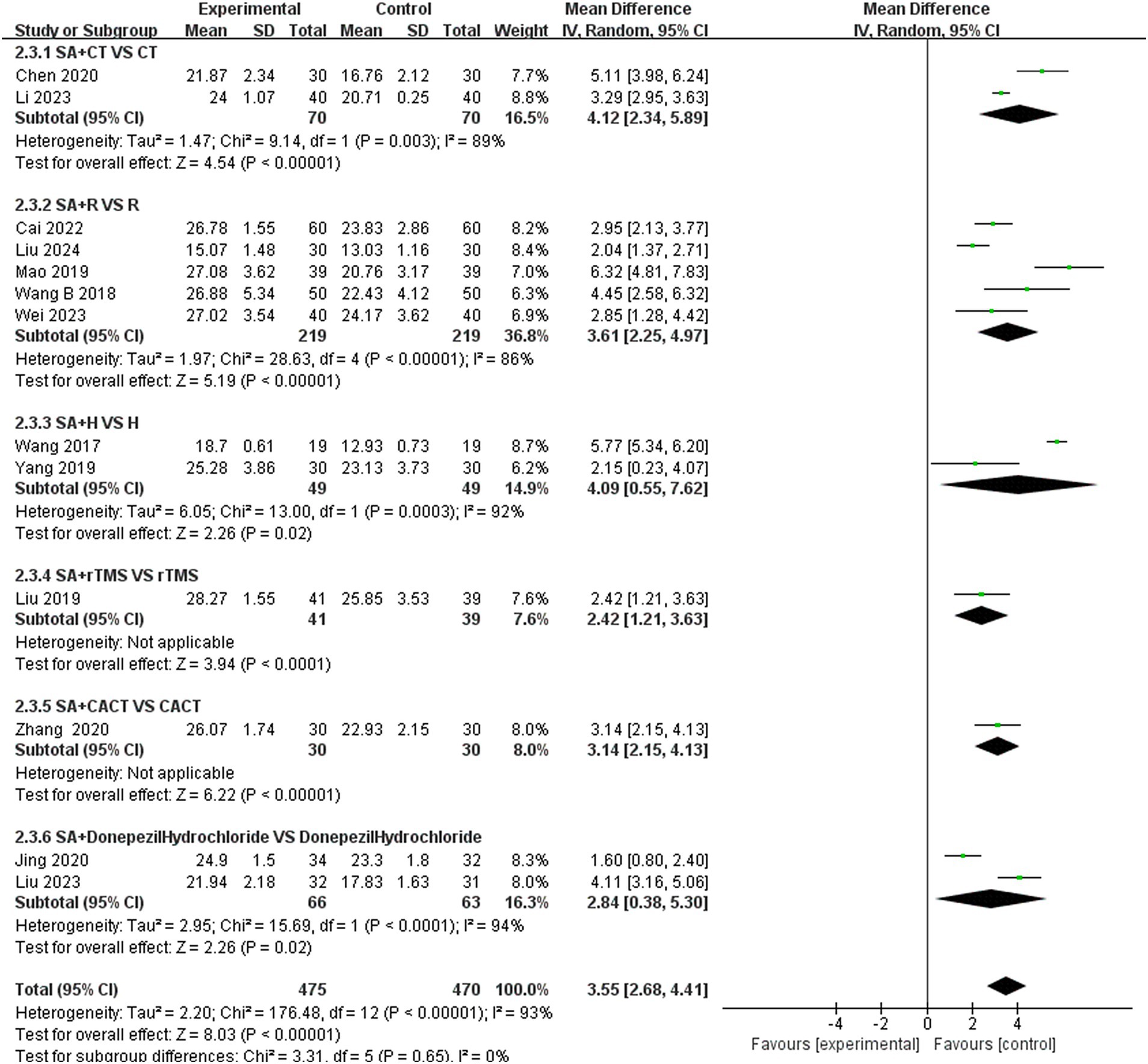
Figure 9. Forest plots of subgroup analysis of MoCA scores.
3.5.2 Subgroup analysis of MMSE scoresWe further conducted a subgroup analysis of the 14 studies that recorded MMSE scores based on the type of treatment (Figure 10). The results indicated that the SA + CT vs. CT subgroup was significantly effective (MD = 1.95, 95% CI: 0.11–3.80, I2 = 78%, 2 studies, 160 participants). Similarly, the SA + R vs. R subgroup demonstrated significant effectiveness (MD = 4.96, 95% CI: 3.27–6.65, I2 = 94%, 7 studies, 602 participants). The SA + rTMS vs. rTMS subgroup also showed significant effectiveness (MD = 3.15, 95% CI: 2.62–3.69, I2 = 0%, 2 studies, 110 participants). Notably, the I2 was >75% in the SA + CT vs. CT and SA + R vs. R subgroups, suggesting high heterogeneity, which may compromise the reliability of these results. In contrast, the SA + rTMS vs. rTMS subgroup analysis, with an I2 = 0% (less than 50%), indicates low heterogeneity, suggesting that scalp acupuncture combined with rTMS treatment is more effective than rTMS alone in treating PSCI, rendering these results highly reliable. Furthermore, in the comparison of SA + donepezil hydrochloride vs. donepezil hydrochloride (MD = 2.88, 95% CI: 1.86–3.90, Z = 5.54, p < 0.00001), SA + H vs. H (MD = 2.08, 95% CI: 0.27–3.89, Z = 2.26, p < 0.02), SA + idebenone vs. idebenone (MD = 3.59, 95% CI: 1.90–5.28, Z = 4.17, p < 0.0001), there is only one study for each of the three subgroup analyses. Nonetheless, the p-values of each study were less than 0.05, indicating that the efficacy was evident.
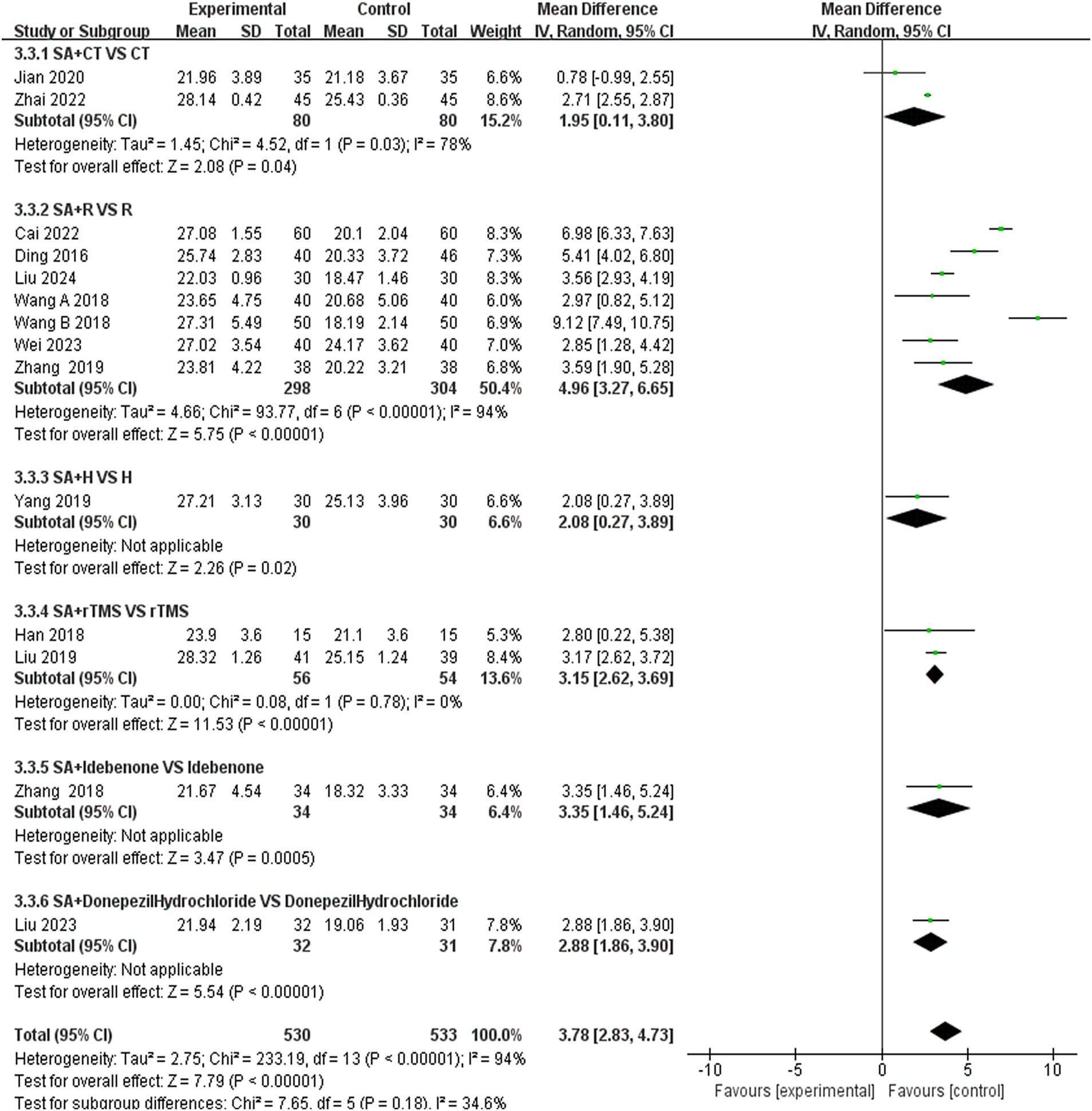
Figure 10. Forest plots of subgroup analysis of MMSE scores.
3.5.3 Subgroup analysis of LOTCA scoresWe further conducted a subgroup analysis on the six studies that recorded LOTCA scores based on the type of treatment administered in the control group (Figure 11). The results indicated that the subgroup comparison of SA + CT vs. CT was significantly effective (MD = 13.58, 95% CI: 10.08–16.37, I2 = 0%, 2 studies, 130 participants). Similarly, the subgroup comparison of SA + R vs. R demonstrated significant effectiveness (MD = 8.37, 95% CI: 6.92–9.81, I2 = 0%, 2 studies, 148 participants). Since I2 = 0 < 50%, heterogeneity is considered low, suggesting that the results are reliable. Additionally, the comparison of SA + CACT vs. CACT (MD = 8.70, 95% CI: 4.69–12.71, Z = 4.25, p < 0.0001), SA + B vs. B (MD = 8.44, 95% CI: 5.03–11.85, Z = 4.85, p < 0.00001) Although each of the two subgroup analyses includes only one study, the p < 0.05 for each indicate that the efficacy is evident.
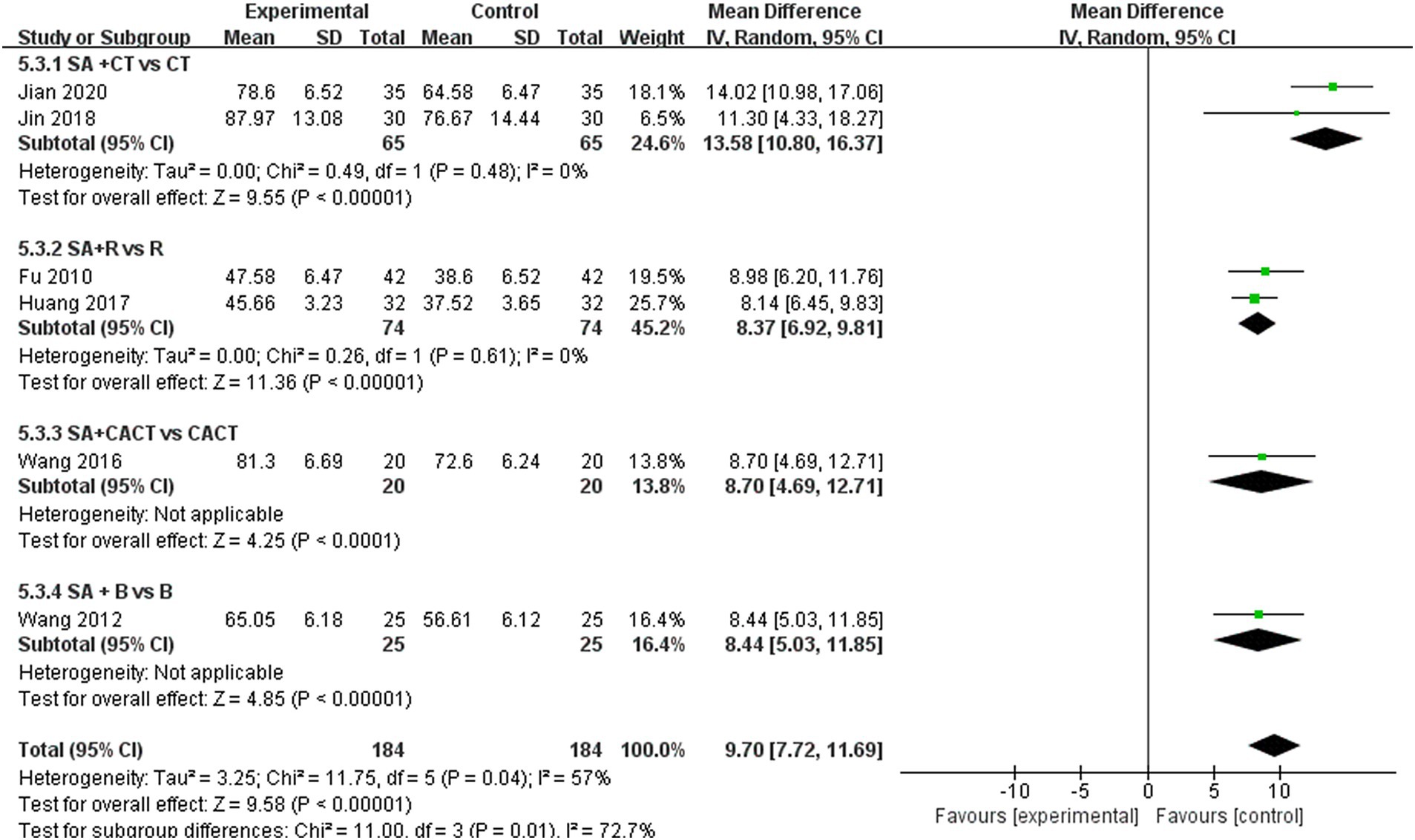
Figure 11. Forest plots of subgroup analysis of LOTCA scores.
3.6 Publication bias and sensitive estimateAn analysis of 13 studies documenting MoCA scores revealed that the funnel plot exhibited asymmetry, with 5 studies falling outside the 95% CI (Figure 12A). After excluding these 5 studies (Wang et al., 2017; Mao and Cheng, 2019; Chen A. Z. et al., 2020; Chen J. et al., 2020; Liu et al., 2024), we observed that I2 = 19 < 50%, indicating that the reliability of scalp acupuncture in improving cognitive dysfunction post-stroke is supported (MD = 3.25, 95% CI: 2.99–3.52, I2 = 19%, 8 studies, 643 participants) (Figure 5B). Nonetheless, the funnel plot still displayed slight asymmetry (Figure 12B). In the analysis of 14 studies that recorded MMSE scores, the funnel plot also showed asymmetry, with 4 studies falling outside the 95% CI (Figure 12C). Upon excluding these 4 studies (Ding and Zhang, 2016; Wang W. et al., 2018; Cai et al., 2022; Liu et al., 2024) (MD = 2.74, 95% CI: 2.59–2.89, I2 = 2%, 10 studies, 697 participants), we found that I2 = 2% < 50% (Figure 6B). At the same time, the funnel plot exhibited slight asymmetry (Figure 12D). Additionally, the analysis of 6 studies that recorded LOTCA scores indicated that the funnel plot was asymmetric, with 1 study falling outside the 95% CI (Figure 12E). After excluding this 1 study (Xiong et al., 2020) (MD = 8.50, 95% CI: 7.26–9.74, I2 = 0%, 5 studies, 298 participants), we determined that I2 = 0% < 50 (Figure 7B) and the funnel plot showed slight asymmetry (Figure 12F). A consistent conclusion was reached through the analysis of studies recording both MMSE and LOTCA scores.
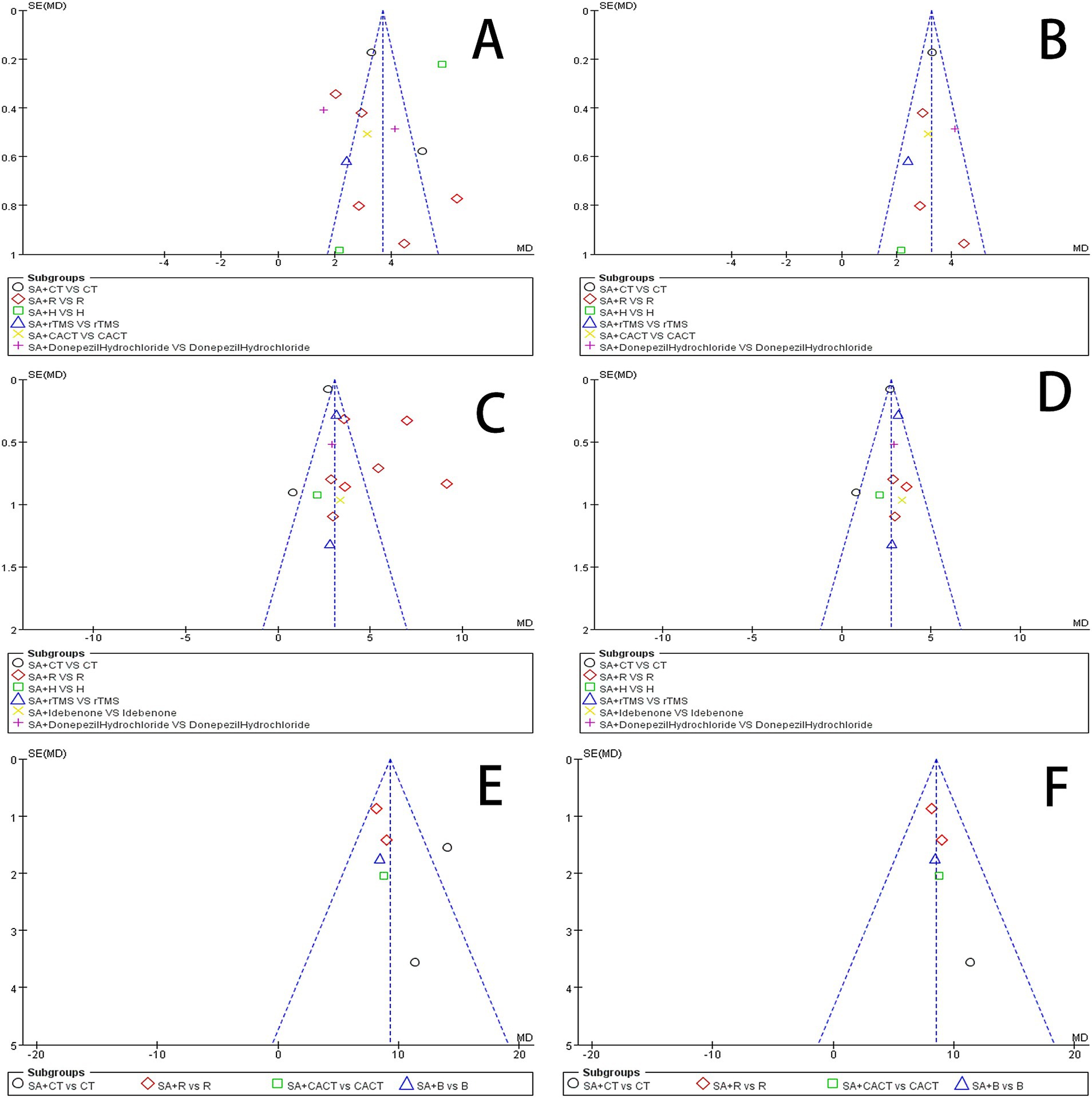
Figure 12. (A) Funnel plot of the MoCA scores. (B) Funnel plot of the MoCA scores after removing four studies. (C) Funnel plot of the MMSE scores. (D) Funnel plot of the MoCA scores after removing one from four studies. (E) Funnel plot of the LOTCA scores. (F) Funnel plot of the MoCA scores after removing one study.
Begg’s test and Egger’s test can detect the symmetry of the funnel plot to determine whether there is publication bias (Jin et al., 2015). The results of the Begg and Egger bias tests are presented in Table 3. The Egger bias tests for the prevalence of MoCA (p = 0.6590), MMSE (p = 0.1790), LOTCA (p = 0.4350), P300 latency (p = 0.7300), and P300 amplitude (p = 0.2590) indicated the absence of publication bias. However, the Egger bias test regarding the incidence rate of effective rate (p = 0.0100 < 0.05) revealed the presence of publication bias.

Table 3. Beeg test and Egger test for publication bias of outcome indicators reported in this study.
3.7 Adverse eventsOne study (Liu Y. F. et al., 2023) observed that some patients did not adhere to the prescribed treatment and subsequently dropped out. The remaining 27 studies (Xie et al., 2007; Fu et al., 2010; Wang et al., 2012; Xie et al., 2012; Qin, 2015; Ding and Zhang, 2016; Wang, 2016; Huang et al., 2017; Wang et al., 2017; Du et al., 2018; Han et al., 2018; Wang W. et al., 2018; Wang Y. X. et al., 2018; Zhang and An, 2018; Yang L. et al., 2019; Liu et al., 2019; Mao and Cheng, 2019; Zhang et al., 2019; Chen A. Z. et al., 2020; Chen J. et al., 2020; Xiong et al., 2020; Zhang et al., 2020; Cai et al., 2022; Zhai et al., 2022; Li and Pan, 2023; Wei et al., 2023; Liu et al., 2024) did not report any adverse events. Consequently, we were unable to assess the safety of scalp acupuncture in the treatment of PSCI, which raises concerns about a potential risk of reporting bias.
4 Discussion 4.1 Main findingsThe study encompassed 28 randomized controlled trials involving a total of 1,995 participants. It evaluated the efficacy of combined scalp acupuncture in treating PSCI by examining multiple outcome indicators, including the MoCA, MMSE, and LOTCA scale scores, as well as P300 latency and amplitude. The findings indicate that the combination of scalp acupuncture with CT, CACT, R, H, and rTMS is more effective than any single treatment modality. Notably, the evidence supporting the effectiveness of scalp acupuncture combined with R in improving P300 latency and amplitude in PSCI patients is considered reliable. Furthermore, there is moderate to high heterogeneity among the studies regarding MoCA, MMSE, and LOTCA scores, along with a moderate to high risk of bias. All included studies focused on the application of scalp acupuncture for patients in the acute stroke phase. Thus, this study concludes that combined scalp acupuncture is more effective in addressing cognitive impairment following acute stroke compared to individual therapies such as CT, CACT, R, H, and rTMS.
Subgroup analyses of studies examining the MoCA and MMSE scores across various intervention approaches revealed a consistent association with moderate to high risk. In contrast, studies focusing on LOTCA scores exhibited lower risks, suggesting that the findings from the subgroup analyses comparing SA + CT versus CT, SA + R versus R, SA + CACT, and SA + donepezil hydrochloride were reliable.
In the context of the scalp acupuncture treatment regimens examined in the studies included in this analysis, the majority utilized the International SA method, with the additional incorporation of Yu SA and Fang SA. The most frequently employed acupoints are the mid-frontal line (MS1), mid-parietal line (MS5), anterior parietotemporal oblique line (MS6), posterior parietotemporal oblique line (MS7), anterior temporal line (MS10), and posterior temporal line (MS11). Other notable acupoints include the Baihui point (DU20), Sishencong point (EX-HN1), Qianding (GV21), and Shenting (GV24). This suggests that SA treatment primarily targets the frontal, temporal, and parietal regions, which correspond to the frontal lobes, temporal lobes, and parietal lobes—areas closely associated with the brain’s learning, memory, executive functions, and visuospatial/structural abilities. However, the underlying mechanisms of action remain to be elucidated in future studies. The most common treatment duration is 30 min per session, with a typical operational frequency of 200 sessions per minute. This clinical treatment protocol may serve as a guide for future clinical applications.
Resting-state functional magnetic resonance imaging (resting-state fMRI) is a technology employed to investigate the treatment mechanisms of PSCI, observing changes in brain function by analyzing changes in resting blood oxygen level signals. This technology primarily evaluates three indicators: amplitude of low-frequency fluctuations (ALFF), regional homogeneity (ReHo), and functional connectivity (Zhang et al., 2021). Studies have confirmed that scalp acupuncture treatment can enhance ALFF values in various regions of the brain, including the hippocampus, cingulate gyrus, splenium cortex, right inferior temporal gyrus, left middle occipital gyrus, left superior occipital gyrus, right superior parietal gyrus, prelimbic cortex, and sensory cortex (Wen et al., 2018; Han et al., 2024). The effectiveness of this treatment is associated with an increase in spontaneous neuronal activity in these areas (Han et al., 2024), which may facilitate the recovery of overall cognitive function, executive abilities, attention, and speech expression. ReHo is a technique utilized for processing local brain functional activity data, with its values closely linked to cognitive functions (Veselinovic et al., 2022). Following PSCI, alterations in functional brain activity are observed. Research indicates that scalp acupuncture can positively influence ReHo values in the brain regions of PSCI patients, particularly in the superior parietal gyrus, right inferior frontal gyrus, right inferior temporal gyrus, precuneus, lentiform nucleus, ventrolateral nucleus of the thalamus, and middle temporal gyrus. Conversely, negative activation was noted in the lentiform nucleus, ventrolateral thalamic nucleus, middle temporal gyrus, and parahippocampal gyrus (Han et al., 2024). These findings indicate that scalp acupuncture treatment may enhance the regeneration and repair of synapses in nerve cells, thereby facilitating the recovery of brain regions associated with cognitive functions in patients with PSCI. Furthermore, PSCI can lead to abnormal functional connections within the brain’s cognitive-related networks, resulting in alterations to the brain’s information integration processes (Xiong et al., 2022). Studies have confirmed that scalp acupuncture can influence the topological properties of brain functional network nodes in patients with PSCI (Wang, 2021), modulating brain networks by increasing functional connectivity between brain regions related to cognitive activities (Tan et al., 2017) and thereby improving the cognitive function of patients to a certain extent.
Diffusion tensor imaging (DTI) is a non-invasive technique that visualizes brain white matter fiber tracts and detects changes in brain white matter microstructure in vivo (Zhu et al., 2019; Li X. T. et al., 2022). Following PSCI, abnormal alterations in white matter fiber tracts occur, with fractional anisotropy (FA) values in the frontal lobe, hippocampus, and corpus callosum typically decreasing. This reduction suggests that the white matter fibers in these regions may have sustained damage or degeneration, thereby impacting the brain’s information integration processes (Yin et al., 2019). Research has demonstrated that scalp acupuncture can enhance the blood supply to white matter tracts in the brain and facilitate the repair of white matter fiber damage, consequently improving cognitive function in PSCI patients (Sun and Zhang, 2020). Additionally, scalp acupuncture may stimulate the release of neurotransmitters and neuropeptides by enhancing cerebral blood circulation, which can slow neuronal apoptosis, activate neural pathways, and further enhance cognitive function (Zhou et al., 2020). Furthermore, scalp acupuncture can improve the metabolic levels of brain functional tissues (Zhang et al., 2020), increase blood circulation, and promote the release of neurotrans
留言 (0)