Gastric cancer (GC) is the fifth most prevalent cancer and ranks fourth in terms of mortality rates in the world (1, 2). Due to the difficulty in the diagnosis of early GC, almost up to 70% of patients were in the advanced stage at initial diagnosis (3). The conventional treatment to GC therapy is radical surgery, followed by chemotherapy, which does not lead to acceptable outcomes in these GC patients (4). Therefore, developing innovative treatment strategies for GC is urgent. Over the past few decades, immunotherapy has become one of the most promising cancer treatment strategies (5). However, the tumor immune microenvironment and drug resistance continue to impact immunotherapy’s effectiveness (6). Therefore, an effective immune-related prognostic biomarker is essential for the treatment of GC.
The tumor microenvironment is a unique heterogeneous environment for the tumorigenesis and development of tumors. The growth of tumors and normal tissue homeostasis are both significantly influenced by the bidirectional communication between cells in the tumor microenvironment (TME) (7). Tumor-infiltrating immune cells (TIICs), which are necessary for tumor progression in addition to stromal cells, are a vital component of the TME (8). Accumulating evidence shows that TIICs participate in immunosuppression and progression of GC (9). Immune checkpoint inhibitors (ICIs) are antibody-based therapy that targets immune cells in TME. Blocking the programmed cell death protein 1 (PD-1) or cytotoxic T-lymphocyte antigen 4 (CTLA-4) receptor-ligand interactions could produce favorable therapeutic effects (10). Due to no response to ICIs, only a few patients can benefit from immunotherapeutic interventions. Nevertheless, the existing biomarkers all have advantages and defects when selecting patients who might benefit from immunotherapy.
In the era of tumor immunotherapy, new methods for judging the clinical prognosis and predicting their response to immunotherapy are constantly being developed. These methods are more accurate than traditional methods in screening the beneficiaries of immunotherapy. It is important to choose the most likely group of patients who will benefit from immunotherapy. In comparison with conventional immunohistochemistry (IHC), multiplex IHC (mIHC) can detect the expression of multiple markers simultaneously in situ, thereby identifying the phenotype of each cell and cell-to-cell interaction in the tissue, and with quantitative pathology and machine-learning algorithms, highly reproducible statistical data can be obtained (11). Up to now, much research has used mIHC to explore specific immune cells in the tumor immune microenvironment and has contributed to clinical prognosis and efficacy prediction (12).
Procalcitonin gamma subfamily A, 10 (PCDHGA10) is a type I transmembrane protein with 6 or 7 extracellular cadherin repeats, containing 53 genes arranged in strings and belongs to the protocadherin gamma gene cluster. The mutation or the aberrant expression of PCDHGA10 could cause intellectual disability and benign recurrent vertigo (13). The protocadherin gene cluster was verified to participate in regulating tumor progression in lung cancer (14), colorectal cancer (15), and GC (16). Recently, it is reported that PCDHGA10 was highly expressed in lung squamous cell carcinoma, and elevated PCDHGA10 levels exhibited a worse prognosis. Moreover, PCDHGA10 was closely related to tumor immune cell infiltration and immune checkpoints (17). However, there are few studies on the functions of PCDHGA10 in GC.
In the present study, we examined the mRNA level of PCDHGA10 by bioinformatic methods and verified the protein expression of PCDHGA10 via tissue microarrays (TMAs). Then, we applied mIHC to evaluate the relationship between PCDHGA10 and TIICS and immune checkpoints. As an independent predictor of poor outcome, high expression of PCDHGA10 might orchestrate tumor immunity and might be a potential treatment target for GC.
2 Materials and methods2.1 Data collection and bioinformatics analysisThe Cancer Genome Atlas (TCGA) (https://portal.gdc.cancer.gov/) datasets were used to collect clinical and RNA-sequence data of patients with GC. Using an online tool Xiantao Academy (https://www.xiantao.love), the mRNA expression of PCDHGA10 from TCGA between the adjacent and tumoral tissues was comparatively analyzed (18). Kaplan-Meier methods were used to assess the survival prognosis of 407 GC samples in TCGA.
2.2 Samples collection and TMAsThe TMAs with 195 GC tissues and 70 normal gastric mucosa tissues was prepared at the Department of Clinical Biobank of the Affiliated Hospital of Nantong University from June 2004 to July 2009. A core on the TMAs represents a sample with a diameter of 2 millimeters. All the GC patients underwent radical surgery and did not receive preoperative radiotherapy, chemotherapy, and immunotherapy. From the date of surgery until death or last follow-up, data were collected retrospectively. The study was approved by the Ethics Committee of the Affiliated Hospital of Nantong University.
2.3 IHCTMA slides were dewaxed with xylene and rehydrated with alcohol. TMA slides were then placed in a sodium citrate buffer solution (10 mM, pH 6.0) to repair the antigen through microwave heating. The slides were blocked for 1 h with 5% bovine serum albumin after being treated with 3% H2O2 for 20 min to eliminate the peroxidase activity. Then rabbit anti-PD-L1 (13684S, Cell Signaling Technology) and mouse anti-CTLA-4 antibodies (NB10064849, NOVUS) were incubated on slides overnight at 4°C. The secondary antibodies were incubated for 2 h and then stained with An EliVision Plus DAB kit (Kit-0015, Maxim Biotechnologie) according to the instructions of the Manufacturers (19). The results of IHC staining were evaluated by the semi-quantitative H-score method with pathologists who were blinded to the patients’ clinical information. The intensity of IHC staining was recorded as 0, 1, 2 and 3, representing no staining, weak staining, moderate staining, and strong staining, respectively. The positivity rate ranges from 0 to 100. The staining intensity and percentage of each sample were evaluated, and the product of each sample was finally calculated as a score ranging from 0 to 300.
2.4 mIHCTMA slides were dewaxed and rehydrated with xylene and alcohol and then were heated in AR6 buffer (210921004, Akoya Bioscience) by microwave to repair the antigen. The blocking buffer (ARD1001EA, Akoya Bioscience) was used for 10 min to block the slides, and the primary and secondary antibodies were added. The mIHC staining was performed after the secondary antibody was added, and then the antigen was repaired through heat induction and cooling. For signal amplification, opal fluorophore-conjugated tyramide signal amplification was used. The nucleus was stained with 4, 6-diamino-2-phenyl indole (DAPI) (F6057, Sigma) and sealed the slides.
The slides were scanned using the Vectra 3.0 Automated Quantitative Pathology Imaging System (PerkinElmer, USA) to detect and measure the positive rate of markers. The cores containing both tumor and stroma were captured with a ×20 Olympus lens objective. With inForm® Cell Analysis software (version 4.1.0, Perkin Elmer), we trained machine-learning algorithms to segment the images into areas of cancerous cells and stromal cells, to segment individual cells by DAPI counterstaining. The pathologists set the threshold of each marker to ensure an accuracy of more than 95%.
This study used the following primary antibodies: rabbit anti-PCDHGA10 (1:50, D1247, Biobyt), rabbit anti-CD11b antibody (1:100, 49420S, CST), rabbit anti-CD8 antibody (1:100, ab83278, Abcam), rabbit anti-CD3 antibody (1:200, 85061S, CST), rabbit anti-CD4 antibody (1:200, ab133616, Abcam), mouse anti-Foxp-3 antibody (1:50, ab20034, Abcam), anti-LAG-3 antibody (1:50, ab52587, Abcam), anti-CD66b antibody (1:500, ab214175, Abcam), anti-cytokeratin antibody (1:8000, orb69073, Biobyt), anti-CD68 antibody (1:500, 797778S, CST). The secondary antibody was Opal™ polymer HRP Ms+Rb (211011069, Akoya Bioscience).
2.5 Statistical analysisThe association between clinicopathological characteristics and PCDHGA10 was validated using Pearson’s χ2. A comparison of mRNA or protein expression between the two groups was performed using the Student’s t-test. Cox regression models were used to identify independent prognostic variables. R software (v.4.0.2), GraphPad Prism (v.8.3), SPSS (v.26.0), and X-tile (3.6.1) were used for data analysis. All results with p < 0.05 were considered significant.
3 Results3.1 Bioinformatics analysis of PCDHGA10 in GCTo investigate the role of the PCDHGA10 in GC and peritumoral tissue, the expression of PCDHGA10 was assessed according to the TCGA dataset, which contained 407 GC cases and 32 peritumoral cases. We observed that PCDHGA10 mRNA expression was increased in GC tissues compared to peritumoral tissues (Figure 1A, p < 0.001). The level of PCDHGA10 was shown to be up-regulated in GC and related to unfavorable clinical outcomes (Figure 1B). The area under the curve (AUC) for PCDHGA10 expression in GC was 0.838 (95% CI = 0.807-0.870) (Figure 1C). Therefore, PCDHGA10 was closely related to GC in the PCDHGAs family and may have a vital role in tumorigenesis and development in GC.
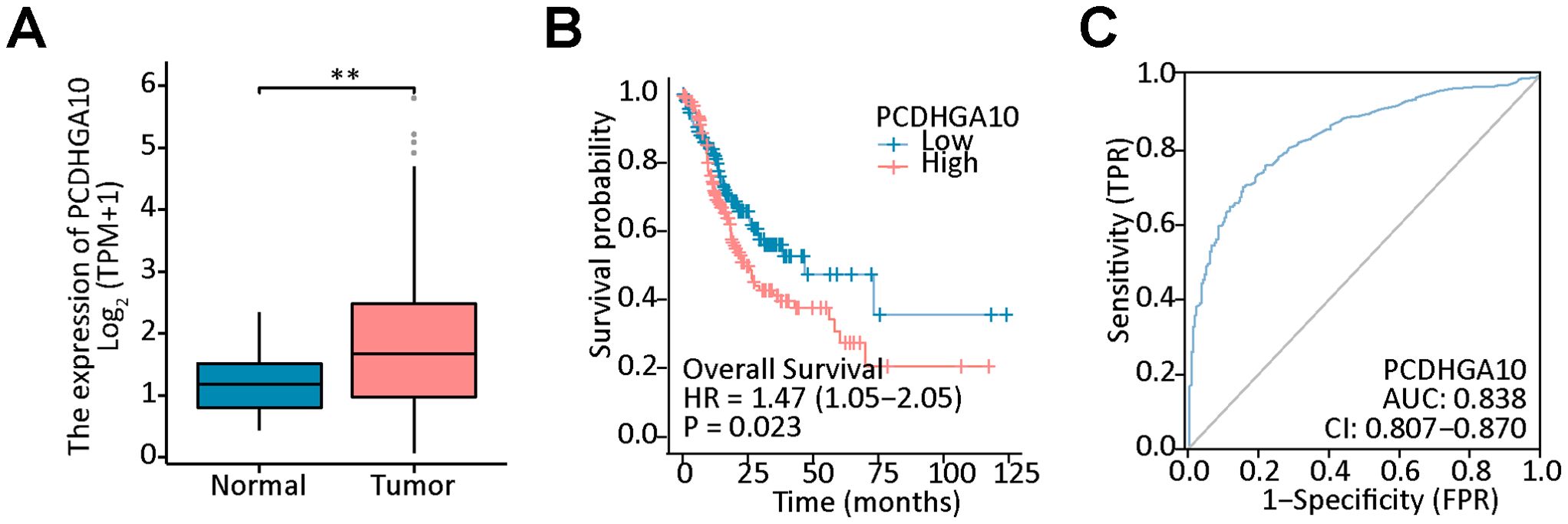
Figure 1. Bioinformatics analysis of PCDHGA10 mRNA expression in gastric cancer (GC). (A) The mRNA levels of PCDHGA10 in gastric cancer tissues was higher than that in benign gastric tissues. (B) High PCDHGA10 mRNA levels correlated with poor overall survival in GC. (C) The receiver operating characteristic curve for PCDHGA10 mRNA levels in GC. ** p < 0.05.
3.2 PCDHGA10 protein expression in GCTo confirm the results from the TCGA dataset, we evaluated PCDHGA10 protein expression in GC and para-cancerous tissues using the mIHC technique. Cytokeratin, a marker of epithelial cells, was used to identify tumors and stroma, and nuclei were stained with DAPI. PCDHGA10, primarily found on the cell membrane of GC mucosal epithelial cells, was considerably increased in GC samples, consistent with that of PCDHGA10 mRNA levels (p < 0.05; Figures 2A, B).
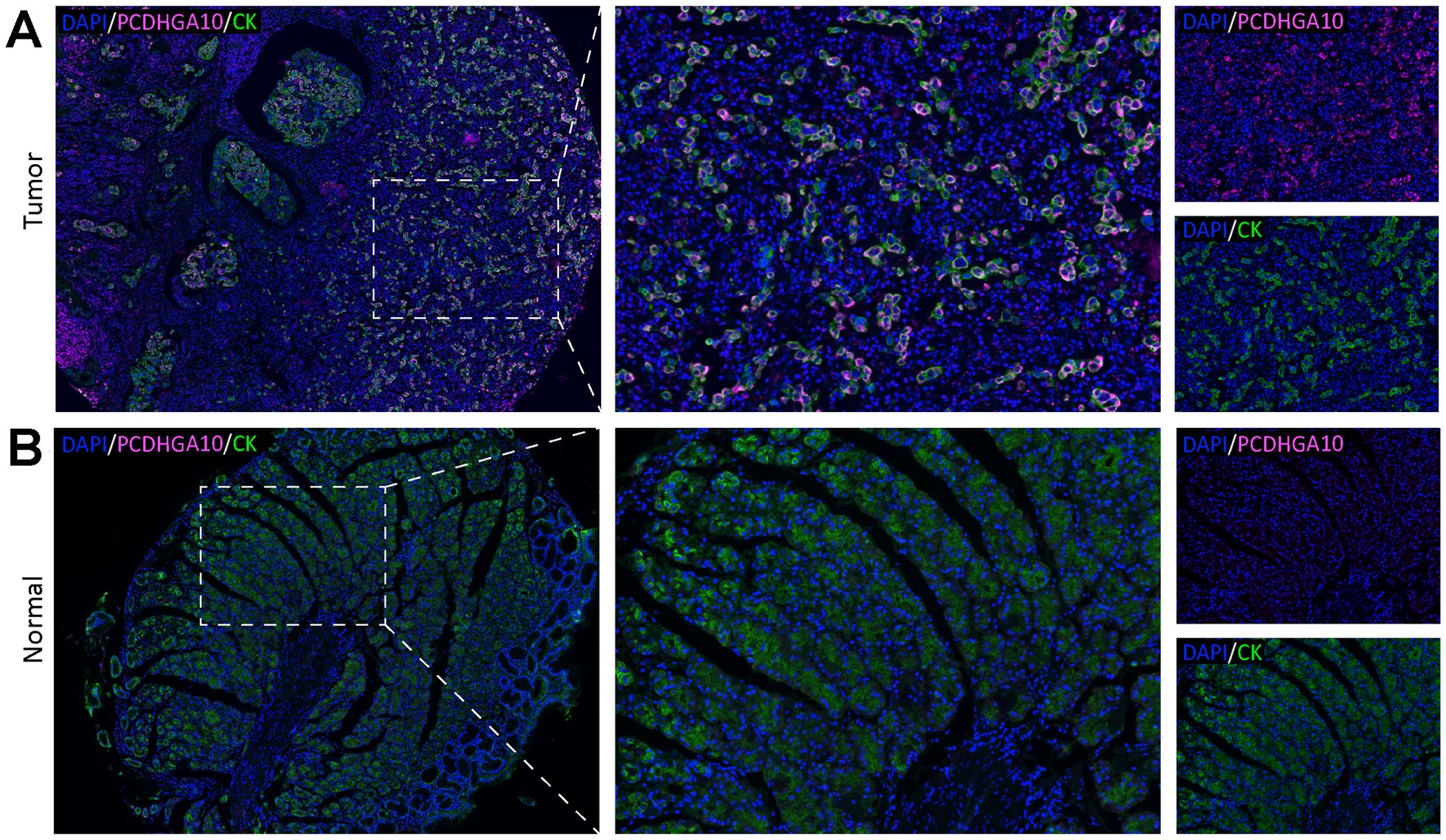
Figure 2. PCDHGA10 protein expression in gastric cancer. PCDHGA10 protein in gastric cancer (A) and peritumoral tissues (B) with fluorescence multiplex immunohistochemistry. Purplish red: PCDHGA10, green: CK, blue: DAPI. Left column magnification ×40; right two column magnification ×200. DAPI: 4, 6-diamino-2-phenyl indole; CK, cytokeratin.
3.3 Association between PCDHGA10 protein expression and GC clinical characteristicsUsing the X-tile software, cutoff points of 61.8 were used to categorize patients in TMAs as high or low, including the PCDHGA10-high group (86 cases) and PCDHGA10-low group (109 cases). Following that, the relationship between PCDHGA10 protein expression and clinicopathological characteristics such as gender, age, Laurén categorization, and differentiation, tumor size (T), lymph node metastasis (N), distant metastasis (M), and TNM stage was analyzed, and we observed that PCDHGA10 expression was associated with tumor size (p < 0.05), N (p < 0.05), M (p = 0.01) and TNM staging significantly (p < 0.001; Supplementary Table 1).
3.4 Prognostic potential of PCDHGA10 protein expression in GCKaplan-Meier curve analysis showed that GC patients with high PCDHGA10 protein expression had poor prognosis (Figure 3A). As shown in Figure 3B, age (p = 0.022), TNM stage (p < 0.0001), differentiation (p = 0.003) and PCDHGA10 expression (p < 0.001) were identified as significant prognostic factors in GC by univariate analysis. Additionally, by the multivariate Cox regression analysis, PCDHGA10 protein expression (p = 0.034), TNM stage (p < 0.001) were found to be an independent risk factor in GC patients.
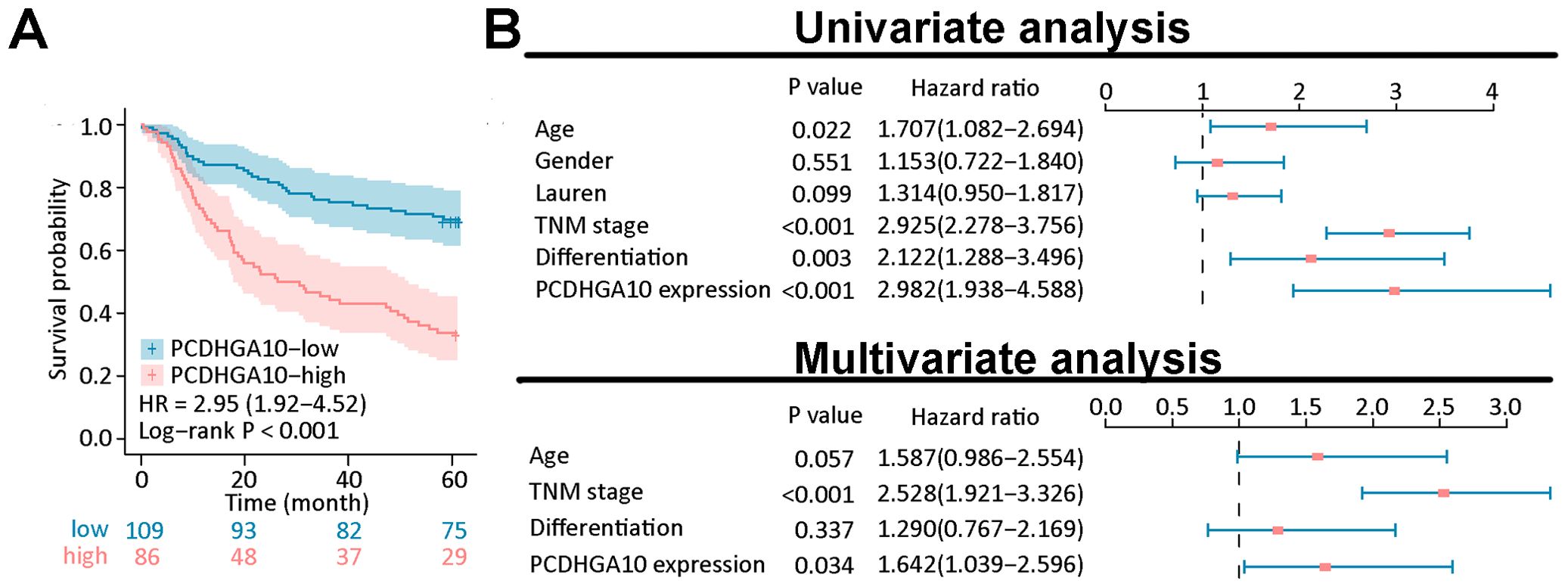
Figure 3. Prognostic analysis of PCDHGA10 protein expression levels. (A) Overall survival analysis of PCDHGA10 protein levels based on gastric cancer tissue microarray. (B) A forest plot visualizing the univariate and multivariate analysis of PCDHGA10 in gastric cancer.
3.5 Relationship between PCDHGA10 protein levels and the tumor microenvironmentThe relationship between the proportion of PCDHGA10 protein expression and the fraction of TIIC in GC tissues was investigated using immune cell-targeted labeling by mIHC. Neutrophils (CD66b+), CD8+ T cells (CD8+), CD4+ T cells (CD4+), regulatory T (Treg) cells (Foxp3+), and macrophages (CD11b+, CD68+) are the different types of targeted staining indicators. In all samples, immune cells infiltrated to varying degrees, and TIICs were generally found within the tumor stroma (Figures 4A–C). Spearman correlation analysis indicated that PCDHGA10 protein level in GC tissues was significantly positively correlated with Foxp3+ Treg cells, CD68+ macrophages, CD4+ T cells, and CD8+ T cells (Figure 4E). However, a significant association between PCDHGA10 expression and CD66b+ neutrophils is not observed in GC tissues.
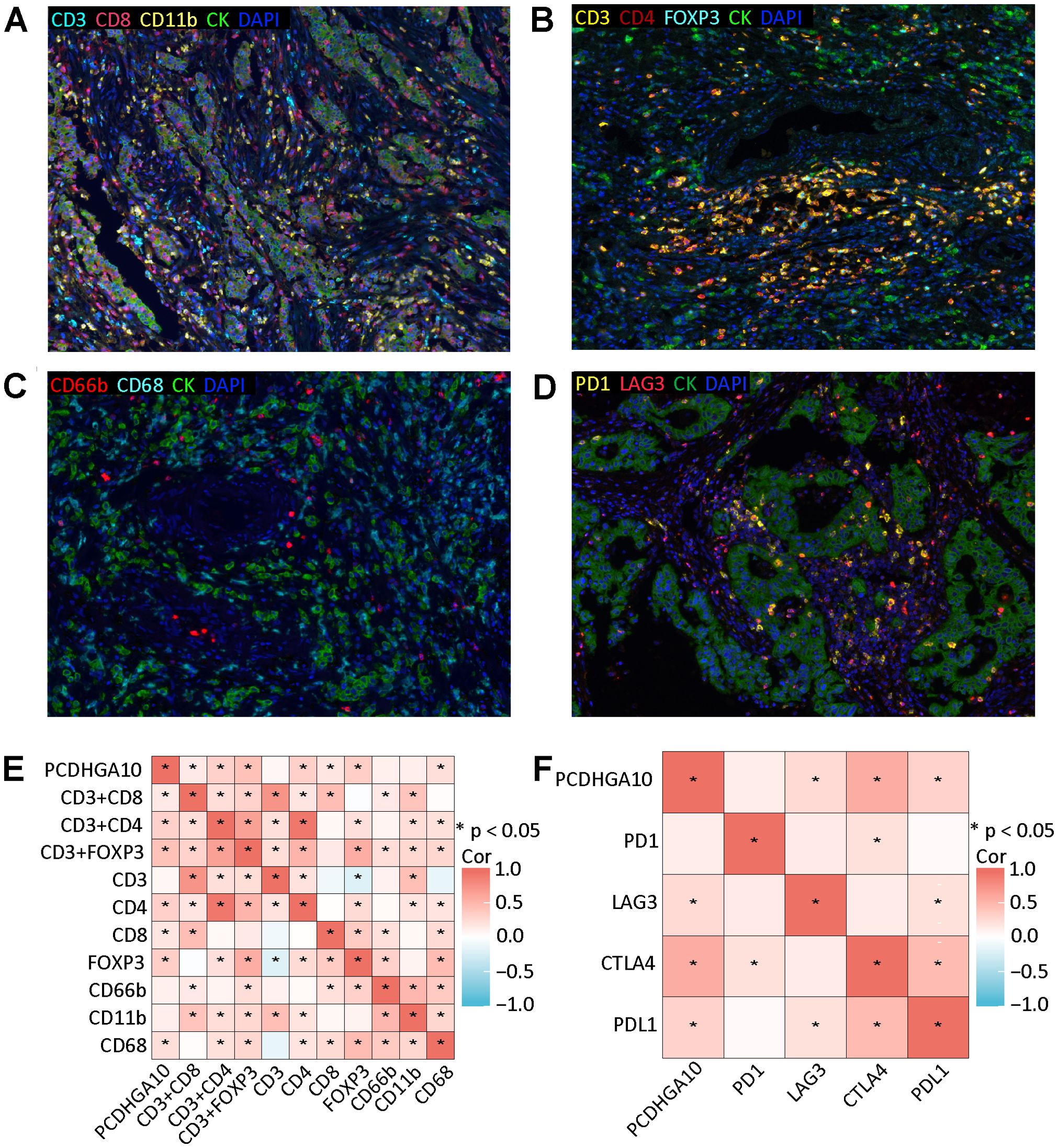
Figure 4. Relationship between PCDHGA10 protein expression and tumor-infiltrating immune cells and immune checkpoints. (A-C) Multispectral composite of CD3, CD4, CD8, CD11b, CD66b, CD68, CK and DAPI, magnification ×200. (D) Four-color multispectral composite of LAG3, PD1, CK and DAPI, magnification ×200. Green: CK, blue: DAPI. (E) Correlation analysis of PCDHGA10 protein expression and tumor-infiltrating immune cells. (F) Correlation analysis of PCDHGA10 protein expression and immune checkpoints. DAPI: 4, 6-diamino-2-phenyl indole; CK, cytokeratin. * p < 0.05.
Then we verified the relationship between PCDHGA10 and immune checkpoints by mIHC and IHC. mIHC staining showed that PD-1 and LAG3 were mainly localized in tumor mesenchyme (Figure 4D). Positive correlations were observed between PCDHGA10 protein expression and LAG3. IHC staining showed that CTLA4 and PD-L1 were also expressed to varying degrees in GC tissues (Supplementary Figure S1). Statistical analysis revealed that the protein presentation of PCDHGA10 was significantly correlated with the protein presentation of CTLA4 and PD-L1. However, no significant association was identified with regard to PD-1 protein expression (Figure 4F).
4 DiscussionThe present study showed that PCDHGA10 mRNA was considerably more expressed in tumor tissues than in normal tissues using the TCGA datasets. In addition, PCDHGA10 was identified as an independent poor prognosis factor. Moreover, it was found that PCDHGA10 expression is associated with clinicopathological features and involved in immune cell infiltration, thus making it a promising target for cancer immunotherapy.
The interactions between tumor and immune cells in the tumor immune microenvironment (TIME) determine the trend of anti-tumor or pro-tumor immunity (9). Tumors appear to evade immune surveillance by gradually shaping the TIME into an immunosuppressive state through recruiting tumor-promoting immune cells, and the balance between pro- and anti-tumor inflammatory mediators may determine tumor progression (20, 21). Our results revealed that PCDHGA10 expression positively correlated with Foxp3+ T cells. Foxp3+ is a specific surface marker for Treg cells, which play a key role in maintaining immune homeostasis and peripheral tolerance (22, 23). Additionally, Tregs inhibit anti-tumor immune response by producing immunosuppressive cytokines, such as TGF-β, IL-10, and IL-35 in the TIME (24). Up to now, many studies have demonstrated the extensive infiltration of Tregs in malignant tumors, including gastric cancer, is associated with poor prognosis (25). In addition, we found that the expression of PCDHGA10 was related to CD68+ tumor-associated macrophages (TAMs). TAMs are divided into anti-tumor M1-type macrophages and tumor-promoting M2-type macrophages. It is accepted that CD68 and F4/80 are the markers of the M2-type TAMs (26). M2 macrophages are usually the dominant cells in TAMs and secrete immunosuppressive cytokines to promote tumor immune escape (27, 28). Chen et al.’s reported that GC patients with a high density of CD68+ TAMs tended to have a bad prognosis (29). These results established a link between PCDHGA10 protein expression and TIICS in GC.
The emergence of ICIs, mainly including PD-1/PD-L1, LAG-3, and CTLA-4 monoclonal antibodies (mAbs), has shaped the therapeutic landscape of some types of cancers (30). It is reported that some cancer patients’ improved survival outcomes are largely due to the improved control of systemic disease provided by ICIs (31, 32). s. Disrupting co-inhibitory signaling pathways enhances clinical outcomes in cancer patients (33, 34). Currently, anti-CTLA-4 agents such as Ipilimumab and Tremelimumab are broadly applied as therapeutic agents in clinical studies of different cancers (35, 36). In a randomized, phase III trial, compared to chemotherapy alone, the PD-1 inhibitor Nivolumab combined with chemotherapy showed superior overall survival (OS) and progression-free survival (PFS) in previously untreated patients with advanced gastric adenocarcinoma (37). Despite immunotherapy having markedly improved the survival rate of patients in certain tumor types, not all patients benefit from checkpoint blockade, and some suffer from notable immunotoxicities (38, 39). Thus, it is crucial to identify potential biomarkers suitable for screening the population who might benefit from immunotherapy (40–42). In the present study, we found that the protein expression levels of PCDHGA10 were correlated with PD-L1, CTLA-4, and LAG-3 with mIHC. Currently, available evidence indicates that PD-L1, tumor mutational burden (TMB), and microsatellite instability (MSI)/mismatched repair-deficient (MMR) have been acknowledged for screening the population in whom immunotherapy is effective of immune drugs (43–45). So, combined with the above-mentioned effective immunotherapy predictors, PCDHGA10 might be a biomarker that predicts immunotherapy responses in GC.
There are several limitations in this study. First and foremost, our study was retrospective research, and additional prospective research is required to strengthen our conclusions. Additionally, the interaction mechanism between PCDHGA10 and immune cells and immune checkpoints in GC needs further experimental verification.
- Our results show that PCDHGA10 is up-regulated dramatically in GC and is an independent prognostic factor. It is revealed that PCDHGA10 is correlated with TIICs as well as immune checkpoint expression in GC. Thus, PCDHGA10 might be a potential biomarker for predicting GC prognosis and provides a perspective on immunotherapeutic strategies for treating GC.
Data availability statementThe data presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
Ethics statementThe studies involving humans were approved by the Ethics Committee of the Affiliated Hospital of Nantong University. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributionsMZ: Formal analysis, Writing – original draft. ZY: Visualization, Writing – review & editing. QW: Data curation, Writing – review & editing. BL: Data curation, Writing – review & editing. PS: Validation, Writing – review & editing. XZ: Validation, Writing – review & editing. LY: Methodology, Writing – review & editing. HW: Conceptualization, Writing – original draft, Writing – review & editing.
FundingThe author(s) declaret financial support was received for the research, authorship, and/or publication of this article. This study was supported by grants from Jiangsu Provincial Research Hospital (YJXYY202204), Jiangsu Province Capability Improvement Project through Science, Technology and Education (ZDXK202234), Postdoctoral Research Funding Plan in Affiliated Hospital of Nantong University (BSH202306) and Nantong Minsheng Science and Technology Plan Project 2022 (MS22022053).
AcknowledgmentsWe express our gratitude to every laboratory members. We appreciate Bullet Edits Limited for revising and reviewing the manuscript with regards to linguistic aspects.
Conflict of interestThe authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s noteAll claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary materialThe Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2024.1500478/full#supplementary-material
Supplementary Figure 1 | Quantified protein expression using the immunohistochemistry score (intensity: brown, intense staining; orange, moderate staining; yellow, weak staining; and blue, no staining. A1: CTLA4 in cancer tissues. A2: CTLA4 expression in cancer cells was scored using software. B3: CTLA4 expression in TILs was scored using software. Magnification ×200.
References2. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: globocan estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2021) 71:209–49. doi: 10.3322/caac.21660
PubMed Abstract | Crossref Full Text | Google Scholar
3. Thrift AP, Wenker TN, El-Serag HB. Global burden of gastric cancer: epidemiological trends, risk factors, screening and prevention. Nat Rev Clin Oncol. (2023) 20:338–49. doi: 10.1038/s41571-023-00747-0
PubMed Abstract | Crossref Full Text | Google Scholar
5. Chen J, Lin Z, Liu L, Zhang R, Geng Y, Fan M, et al. Golm1 exacerbates cd8+ T cell suppression in hepatocellular carcinoma by promoting exosomal pd-L1 transport into tumor-associated macrophages. Signal Transduct Target Ther. (2021) 6:397. doi: 10.1038/s41392-021-00784-0
PubMed Abstract | Crossref Full Text | Google Scholar
6. Lei Z-N, Teng Q-X, Tian Q, Chen W, Xie Y, Wu K, et al. Signaling pathways and therapeutic interventions in gastric cancer. Signal Transduct Target Ther. (2022) 7:358. doi: 10.1038/s41392-022-01190-w
PubMed Abstract | Crossref Full Text | Google Scholar
8. Wang T-T, Zhao Y-L, Peng L-S, Chen N, Chen W, Lv Y-P, et al. Tumour-activated neutrophils in gastric cancer foster immune suppression and disease progression through gm-csf-pd-L1 pathway. Gut. (2017) 66:1900–11. doi: 10.1136/gutjnl-2016-313075
PubMed Abstract | Crossref Full Text | Google Scholar
10. Janjigian YY, Kawazoe A, Yañez P, Li N, Lonardi S, Kolesnik O, et al. The keynote-811 trial of dual pd-1 and her2 blockade in her2-positive gastric cancer. Nature. (2021) 600:727–30. doi: 10.1038/s41586-021-04161-3
PubMed Abstract | Crossref Full Text | Google Scholar
11. Tan WCC, Nerurkar SN, Cai HY, Ng HHM, Wu D, Wee YTF, et al. Overview of multiplex immunohistochemistry/immunofluorescence techniques in the era of cancer immunotherapy. Cancer Commun (Lond). (2020) 40:135–53. doi: 10.1002/cac2.12023
PubMed Abstract | Crossref Full Text | Google Scholar
12. Sheng W, Zhang C, Mohiuddin TM, Al-Rawe M, Zeppernick F, Falcone FH, et al. Multiplex immunofluorescence: A powerful tool in cancer immunotherapy. Int J Mol Sci. (2023) 24:3080. doi: 10.3390/ijms24043086
PubMed Abstract | Crossref Full Text | Google Scholar
13. Anazi S, Maddirevula S, Salpietro V, Asi YT, Alsahli S, Alhashem A, et al. Expanding the genetic heterogeneity of intellectual disability. Hum Genet. (2017) 136:1419–29. doi: 10.1007/s00439-017-1843-2
PubMed Abstract | Crossref Full Text | Google Scholar
14. Ginn L, Maltas J, Baker MJ, Chaturvedi A, Wilson L, Guilbert R, et al. A tiam1-trim28 complex mediates epigenetic silencing of protocadherins to promote migration of lung cancer cells. Proc Natl Acad Sci U.S.A. (2023) 120:e2300489120. doi: 10.1073/pnas.2300489120
PubMed Abstract | Crossref Full Text | Google Scholar
15. Zheng Z, Luan N, Tu K, Liu F, Wang J, Sun J. The roles of protocadherin-7 in colorectal cancer cells on cell proliferation and its chemoresistance. Front Pharmacol. (2023) 14:1072033. doi: 10.3389/fphar.2023.1072033
PubMed Abstract | Crossref Full Text | Google Scholar
16. Zhang N, Gao X, Yuan Q, Fu X, Wang P, Cai F, et al. E3 ubiquitin ligase rnf180 prevents excessive pcdh10 methylation to suppress the proliferation and metastasis of gastric cancer cells by promoting ubiquitination of dnmt1. Clin Epigenet. (2023) 15:77. doi: 10.1186/s13148-023-01492-y
PubMed Abstract | Crossref Full Text | Google Scholar
17. Song S, Lu R, Chen Y, Feng Y. Pcdhga10 as a potential biomarker of lung squamous cell carcinoma based on bioinformatics and experimental verification. Mol Biotechnol. (2024). doi: 10.1007/s12033-024-01178-7
PubMed Abstract | Crossref Full Text | Google Scholar
18. Ma W, Li X, Yang L, Pan J, Chen Y, Lu Y, et al. High vsx1 expression promotes the aggressiveness of clear cell renal cell carcinoma by transcriptionally regulating fkbp10. J Transl Med. (2022) 20:554. doi: 10.1186/s12967-022-03772-2
PubMed Abstract | Crossref Full Text | Google Scholar
19. Sun P, Zhang H, Shi J, Xu M, Cheng T, Lu B, et al. Krtcap2 as an immunological and prognostic biomarker of hepatocellular carcinoma. Colloids Surf B Biointerfaces. (2023) 222:113124. doi: 10.1016/j.colsurfb.2023.113124
PubMed Abstract | Crossref Full Text | Google Scholar
20. Notarangelo G, Spinelli JB, Perez EM, Baker GJ, Kurmi K, Elia I, et al. Oncometabolite D-2hg alters T cell metabolism to impair cd8+ T cell function. Science. (2022) 377:1519–29. doi: 10.1126/science.abj5104
PubMed Abstract | Crossref Full Text | Google Scholar
22. Knorr DA, Blanchard L, Leidner RS, Jensen SM, Meng R, Jones A, et al. Fcγriib is an immune checkpoint limiting the activity of treg-targeting antibodies in the tumor microenvironment. Cancer Immunol Res. (2024) 12:322–33. doi: 10.1158/2326-6066.CIR-23-0389
PubMed Abstract | Crossref Full Text | Google Scholar
25. Kumagai S, Koyama S, Itahashi K, Tanegashima T, Lin Y-T, Togashi Y, et al. Lactic acid promotes pd-1 expression in regulatory T cells in highly glycolytic tumor microenvironments. Cancer Cell. (2022) 40:201–18. doi: 10.1016/j.ccell.2022.01.001
PubMed Abstract | Crossref Full Text | Google Scholar
26. Tang B, Zhu J, Wang Y, Chen W, Fang S, Mao W, et al. Targeted xct-mediated ferroptosis and protumoral polarization of macrophages is effective against hcc and enhances the efficacy of the anti-pd-1/L1 response. Adv Sci (Weinh). (2023) 10:e2203973. doi: 10.1002/advs.202203973
PubMed Abstract | Crossref Full Text | Google Scholar
27. Kerzel T, Giacca G, Beretta S, Bresesti C, Notaro M, Scotti GM, et al. In vivo macrophage engineering reshapes the tumor microenvironment leading to eradication of liver metastases. Cancer Cell. (2023) 41:1892–910. doi: 10.1016/j.ccell.2023.09.014
PubMed Abstract | Crossref Full Text | Google Scholar
28. Chen S, Cui W, Chi Z, Xiao Q, Hu T, Ye Q, et al. Tumor-associated macrophages are shaped by intratumoral high potassium via kir2.1. Cell Metab. (2022) 34:1843–59. doi: 10.1016/j.cmet.2022.08.016
PubMed Abstract | Crossref Full Text | Google Scholar
29. Chen Y, Jia K, Sun Y, Zhang C, Li Y, Zhang L, et al. Predicting response to immunotherapy in gastric cancer via multi-dimensional analyses of the tumour immune microenvironment. Nat Commun. (2022) 13:4851. doi: 10.1038/s41467-022-32570-z
PubMed Abstract | Crossref Full Text | Google Scholar
30. Tang T, Huang X, Zhang G, Hong Z, Bai X, Liang T. Advantages of targeting the tumor immune microenvironment over blocking immune checkpoint in cancer immunotherapy. Signal Transduct Target Ther. (2021) 6:72. doi: 10.1038/s41392-020-00449-4
PubMed Abstract | Crossref Full Text | Google Scholar
31. Samstein RM, Lee C-H, Shoushtari AN, Hellmann MD, Shen R, Janjigian YY, et al. Tumor Mutational Load Predicts Survival after Immunotherapy across Multiple Cancer Types. Nat Genet. (2019) 51:202–6. doi: 10.1038/s41588-018-0312-8
PubMed Abstract | Crossref Full Text | Google Scholar
33. Chen M, Ma P, Zhang Y, Wang D, Yu Z, Fu Y, et al. Divergent tumor and immune cell reprogramming underlying immunotherapy response and immune-related adverse events in lung squamous cell carcinoma. J Immunother Cancer. (2023) 11:e007305. doi: 10.1136/jitc-2023-007305
PubMed Abstract | Crossref Full Text | Google Scholar
34. Chu X, Niu L, Xiao G, Peng H, Deng F, Liu Z, et al. The long-term and short-term efficacy of immunotherapy in non-small cell lung cancer patients with brain metastases: A systematic review and meta-analysis. Front Immunol. (2022) 13:875488. doi: 10.3389/fimmu.2022.875488
PubMed Abstract | Crossref Full Text | Google Scholar
35. Wei SC, Meijers WC, Axelrod ML, Anang N-AAS, Screever EM, Wescott EC, et al. A genetic mouse model recapitulates immune checkpoint inhibitor-associated myocarditis and supports a mechanism-based therapeutic intervention. Cancer Discovery. (2021) 11:614–25. doi: 10.1158/2159-8290.CD-20-0856
PubMed Abstract | Crossref Full Text | Google Scholar
36. Campbell KM, Amouzgar M, Pfeiffer SM, Howes TR, Medina E, Travers M, et al. Prior anti-ctla-4 therapy impacts molecular characteristics associated with anti-pd-1 response in advanced melanoma. Cancer Cell. (2023) 41:791–806. doi: 10.1016/j.ccell.2023.03.010
PubMed Abstract | Crossref Full Text | Google Scholar
37. Janjigian YY, Shitara K, Moehler M, Garrido M, Salman P, Shen L, et al. First-line nivolumab plus chemotherapy versus chemotherapy alone for advanced gastric, gastro-oesophageal junction, and oesophageal adenocarcinoma (Checkmate 649): A randomised, open-label, phase 3 trial. Lancet. (2021) 398:27–40. doi: 10.1016/S0140-6736(21)00797-2
PubMed Abstract | Crossref Full Text | Google Scholar
38. Kendre G, Murugesan K, Brummer T, Segatto O, Saborowski A, Vogel A. Charting co-mutation patterns associated with actionable drivers in intrahepatic cholangiocarcinoma. J Hepatol. (2023) 78:614–26. doi: 10.1016/j.jhep.2022.11.030
PubMed Abstract | Crossref Full Text | Google Scholar
39. Friedman CF, Ravichandran V, Miller K, Vanderbilt C, Zhou Q, Iasonos A, et al. Assessing the genomic landscape of cervical cancers: clinical opportunities and therapeutic targets. Clin Cancer Res. (2023) 29:4660–8. doi: 10.1158/1078-0432.CCR-23-1078
PubMed Abstract | Crossref Full Text | Google Scholar
40. Kong J, Ha D, Lee J, Kim I, Park M, Im S-H, et al. Network-based machine learning approach to predict immunotherapy response in cancer patients. Nat Commun. (2022) 13:3703. doi: 10.1038/s41467-022-31535-6
PubMed Abstract | Crossref Full Text | Google Scholar
41. Shahrajabian MH, Sun W. Survey on multi-omics, and multi-omics data analysis, integration and application. Curr Pharm Anal. (2023) 19:267–81. doi: 10.2174/1573412919666230406100948
Crossref Full Text | Google Scholar
42. Song Z, Yu J, Wang M, Shen W, Wang C, Lu T, et al. CHDTEPDB: transcriptome expression profile database and interactive analysis platform for congenital heart disease. Congenital Heart Dis. (2023) 18:693–701. doi: 10.32604/chd.2024.048081
Crossref Full Text | Google Scholar
43. Wang N, Zhu L, Wang L, Shen Z, Huang X. Identification of Shcbp1 as a Potential Biomarker Involving Diagnosis, Prognosis, and Tumor Immune Microenvironment Across multiple cancers. Comput Struct Biotechnol J. (2022) 20:3106–19. doi: 10.1016/j.csbj.2022.06.039
留言 (0)