Enteric fever, encompassing typhoid and paratyphoid fevers, is a protracted systemic illness caused by Gram-negative pathogens, Salmonella enterica serovar Typhi (S. Typhi) and S. enterica serovar Paratyphi A, and rarely by S. Paratyphi B and C. They are transmitted through the fecal-oral route and pose significant threats to global public health, especially in the Southeast Asia and Sub-Saharan Africa’s low-and middle-income countries (LMICs) with poor standards of potable water supply, sanitation and hygiene (WASH)1. This review addresses the need for vaccination against enteric fever in the endemic zones and the journey of the global scientific community, including our own, towards developing affordable and broad-specificity vaccines, capable of providing high level of durable protection.
Disease burden of typhoid and paratyphoid fever: India and the worldThe global burden of disease (GBD) study estimated the worldwide incidence and mortality due to typhoid fever in the year 2017 to the tune of 10.9 million and 116.8 thousand, respectively2. Corresponding figures for paratyphoid fever during the same time were 3.4 million and 19.1 thousand, respectively2. South Asian countries accounted for nearly 70 per cent of all cases and deaths due to enteric fever with children aged 5-9 yr having the highest incidence rates and mortality2. However, GBD data may be an underestimate, especially for LMICs3,4. Outbreak data, which are frequently uncaptured by community-based studies, could be an additional measure for disease burden estimation5. A systematic, global literature search of 303 outbreaks of enteric fever, affecting 1,80,940 individuals between 1990 and 2018 found over 50 per cent of the cases from Asia, but only 46 per cent of the outbreaks reported culture confirmation5.
Typhoid and paratyphoid fevers continue to be major sources of illness and death in India. GBD (2017) estimated more than 50 per cent of the global typhoid burden from India2,6. A systematic review and meta-analysis7 from India, spanning from 1950 to 2015, documented 377 typhoid and 105 paratyphoid cases per 100,000 person-years, with the highest incidence reported in children of 2-4 yr age group. Other studies reported a similar overall picture but a higher incidence in south-western States and northern urban centers7. Interestingly, multiple investigations found the highest incidence in children under five in India, challenging the prevalent notion that typhoid is primarily a disease of older children8,9. However, the disease incidence reported in India underscores the significant urban-rural divide, with 576–1173 cases versus 35 cases per 100,000 child-years in the urban versus rural areas6.
S. Paratyphi A infections comprised only 3–17 per cent of cases in India in the early 1960s10. However, recent data indicate a significant increase in the proportion of total enteric fever cases, which exceeded 55 per cent in 2003 and 200411. Similar results were reported in a semi-urban population of West Bengal and from rural Maharashtra12,13. A retrospective and a prospective study from Delhi and Chandigarh also confirmed the trend of a significant rise in S. Paratyphi A infection. At the same time, the overall number of culture-positive Salmonella Typhi remained stable14,15.
Social and economic cost of enteric fever versus the cost of vaccinationEnteric fever is a costly disease for the suffering individuals and their families as well as for the national health systems of the LMICs because of the high disease burden, prolonged disease course and time to complete recovery as well as the cost of antibiotics, especially for multi-drug resistant (MDR) infections16. A scoping review17 of 13 published studies, mainly from Asia between 2000 and 2024 revealed the total cost of a typhoid episode ranging from US$ 23 in India to US$ 884 in Indonesia (as per US$ in 2022), with nine studies characterizing typhoid-related household expenditure as catastrophic. The cost of illness (CoI) also increases substantially for the treatment of severe complications like intestinal perforations (US$ 551 in Niger to US$ 1,735 in India) and drug-resistant infections; USD 223 for extensively drug-resistant (XDR) typhoid in Pakistan17. Recently, searches of four databases for studies conducted between 2000-2017 identified 11 CoI, five cost-of-delivery (for the vaccine) and 11 cost-effectiveness analyses (CEA) that compared typhoid treatment and vaccination. Analyses revealed that the costs per outpatient and inpatient cases ranged between US$ 16 and 74 and US$ 159 and 636, respectively, in India18,19. However, indirect cost accounted for most of the total CoI, reaching as high as 89 per cent of over US$ 1.3 billion total cost for typhoid fever in LMICs20. The high economic burden of typhoid indicates vaccine introduction as a good-value-for-money approach for disease control. For example, the Vi-PS vaccine produced net benefits for mass vaccination or school-based vaccination but was cost-effective (CE) for preschool vaccination in most analyses. However, all Vi-PS vaccination programmes would be very CE if the indirect expenses were also accounted for20. Despite limited evidence, Typhoid conjugate vaccine (TCV) was generally found CE for infant routine immunization programmes in most countries and could prevent new infections and deaths21.
Typhoid elimination: barriers and opportunities, the vaccine gapEarly and accurate diagnosis of enteric fever remains a challenge to the world, because of the non-specific signs and symptoms. Blood culture is considered the gold standard for diagnosis but of limited usefulness in the clinical setting due to high cost, low yield (40-60% positivity) and prolonged time to get the results. Serological tests (Widal, Typhidot) lack specificity22, while the newer diagnostic methods, such as PCR or multiplex PCR, ELISA, dot immunoassay, immuno-electrophoresis, haem-agglutination and co-agglutination are promising, but unsuitable for routine clinical use due to technical challenges22. Rising antimicrobial resistance, particularly the emergence and spread of multidrug-resistant (MDR - resistant to chloramphenicol, ampicillin, and co-trimoxazole) and extensively drug-resistant (XDR - additional resistance to third-generation cephalosporins) strains posing a major challenge to enteric fever control, especially in the LMICs23. It is most alarming that XDR strains replaced all other Salmonella Typhi strains in Sindh, Pakistan and has started showing resistance to azithromycin, the sole antibiotic left to deal with them24. Although MDR-phenomena is still rare in S. Paratyphi, a healthcare facility-based surveillance from Bangladesh reported increased MIC to ciprofloxacin in more than 99 per cent of strains25. The high disease burden of enteric fever in the LMICs, accompanied by diagnostic challenges and emerging multidrug resistance, leading to potentially severe complications and lethality, and the disproportionately high social and economic cost of illness, call for heightened activities for disease elimination. While elimination is a long-term goal, reduction of incidence to the locally acceptable level could be achieved within a defined time-period with the improvement of WASH and food safety, availability of point of contact water disinfection techniques, improved surveillance tools for disease burden estimation and efficacy of control measures and blood cultures for diagnosis26. However, adoption of the available Ty21a and Vi-PS vaccines in the routine immunization programme of the high endemic countries was poor despite WHO recommendations because of their unsuitability for infants and younger children. These concerns were largely eliminated by the recently commercialized TCVs, which were found to be safe for six-month-old infants and impart higher magnitude and longer duration of protection.
Studies have suggested that TCV introduction into the routine immunization programme in endemic areas at nine months of age with a catch-up campaign to 15 years will be cost-effective, and accounting for the indirect cost of enteric fever would make vaccination even more cost-saving. TCV is not only effective against MDR and XDR strains (95% against culture-confirmed MDR and 97% against XDR S. Typhi) but also could reduce antimicrobial resistance of typhoid by ∼16 per cent27. Several countries, such as Pakistan, Samoa, Liberia, Nepal and Zimbabwe, introduced TCV for routine immunization28. Still, its wider acceptance by countries like India would require additional information, including duration of protection and frequency of booster doses, as well as its role in eliminating infection, reducing faecal shedding of Salmonella in chronic carriers and providing herd protection.
Vaccine development strategies: A historical perspective Typhoid vaccinesIn 1896, Richard Pfeiffer and Almond Wright independently published their work on the first typhoid fever vaccine – a-heat inactivated whole cell vaccine29. This vaccine was successfully and extensively used during World War I29. However, local and systemic reactogenicity in the vaccine recipients resulted in its withdrawal from the list of licensed vaccines and routine immunization programmes. After a long gap, live attenuated, oral typhoid vaccine, Ty21a was developed in the late 1980s by chemical mutagenesis of the S. Typhi Ty2 strain. Ty21a is modestly immunogenic and requires multiple booster doses for optimal immunogenicity30. However, the large capsules make it difficult for children below six years of age to swallow and the need for pre-administration of buffer to neutralize the stomach acid is also a potential delivery challenge29. An additional risk of bacteremia was also reported for live-engineered typhoid vaccines. Around the same time, Robins and Robins from NIH, USA developed an injectable Vi-PS vaccine in 1986, followed by WHO prequalification of the vaccine manufactured by Pasteur31. Despite an acceptable safety profile, Vi-PS, being a T-independent antigen, is poorly immunogenic, especially for younger children.
Further research was directed towards single-dose typhoid vaccine development by attenuation of genetically modified S. Typhi on one hand and conjugation of Vi-PS to carrier proteins to convert it to a T-dependent antigen on the other. A single oral dose, containing 107 viable organisms of CVD 908, an aroC/aroD deletion mutant of S. Typhi Ty2 was immunogenic, but resulted in vaccinemia. The attenuation was further enhanced by deleting a heat-stress protein, htrA that prevented vaccinemia while retaining both humoral and cellular immune responses32. To ensure more consistent serum anti-Vi antibodies, Vi-PS was constitutively expressed in CVD 908 strain, generating CVD 909. However, volunteers receiving one or two oral doses of CVD 909 or a prime boost regimen with oral CVD 909, followed by an injection of Vi-PS vaccine, failed to induce consistent anti-Vi antibody response, although Vi-specific IgA+ memory B cells were significantly raised33.
Chemical conjugation of Vi-PS to a carrier protein significantly augmented immunogenicity. Typbar TCV (Vi-TT, Vi-PS conjugated to tetanus toxoid), launched by Bharat Biotech, India, was the first WHO pre-qualified typhoid conjugate vaccine (TCV) and was approved for administration to infants as young as six months of age34. Recent TyVac trials with Typbar TCV in Nepal, Bangladesh and Malawi showed protective efficacy of 79 per cent, 85 per cent for upto two years35,36 and 80 per cent for up to four years37.
Despite this, the protective antibody titer is still unknown, and in the absence of Salmonella-specific cytotoxic T cells generation, clearance of intracellular bacteria remains uncertain38. Several other Vi conjugate vaccines, carrying recombinant carrier proteins, such as Pseudomonas aeruginosa exotoxin A (rEPA)39, CRM19740-43 and diphtheria toxoid44-46 showed comparable efficacy, but suffer from the same limitations as Vi-TT. Typhoid conjugate vaccines are still under investigations to further improve their efficacy and awaiting approval for wider application (Table30-36,37,39,41-54 & Fig. 1).
Table. Vaccines against typhoid fever
Licensed typhoidal vaccine Nature of the vaccine Name of the vaccine Modifications in vaccine candidates Status of the vaccine Reference Live attenuated vaccineTy21a
Vivotif (Crucell)
gal E mutant. Commercially available 30, 47, 48Vi based
Vaccine
Vi-PS
Typherix (GSK), Typhim Vi (Sanofi), Typbar Vi (Bharat Biotech)
Purified Vi polysaccharide. Commercially available 31, 47Vi based conjugate
Vaccine
Vi-TT, (Typbar TCV, Bharat Biotech, (PedaTyph-Biomed) (ZyVac-TCV, Cadila Healthcare) Vi polysaccharide is conjugated with the Tetanus toxoid Prequalified and Recommended by WHO 34, 35, 36, 37 Typhoidal vaccines under developmentVi based conjugate
Vaccine
Vi-DT, PT Biopharma, Vi polysaccharide is conjugated with diphtheria toxoid Phase III clinical trial. 44 Vi-DT, SK Bioscience WHO Prequalified on 2024 45, 46Vi-CRM197
EuBiologicals
Vi polysaccharide is conjugated with the CRM197, a nontoxic mutant of diphtheria toxin. Phase III clinical trials. 43Vi-CRM197
Biological E
WHO Prequalification on 2020 41, 42Vi-EPA,
Lanzhou Institute of Biological Products
Pseudomonas aeruginosa exotoxin A as a carrier protein. National Licensure 39, 49, 50Live attenuated
Vaccine
CVD 908
University of Maryland
Deletion in the aroC and aroD Showed bacteremia in clinical trials. 51CVD 908-htrA
University of Maryland
Deletion in the aroC, aroD and htrA genes Phase II study with 80 human volunteers. 32CVD 909
University of Maryland
Constitutive expression of Vi polysaccharide Phase I clinical trial 33, 52Ty800
Avant Immunotherapeutics
Deletion in the phoP/phoQ virulence regulatory genes. Phase II clinical trial 53M01ZH09
emergent Biosolutions
Deletion in the aroC and ssaV genes Phase II clinical trial. 54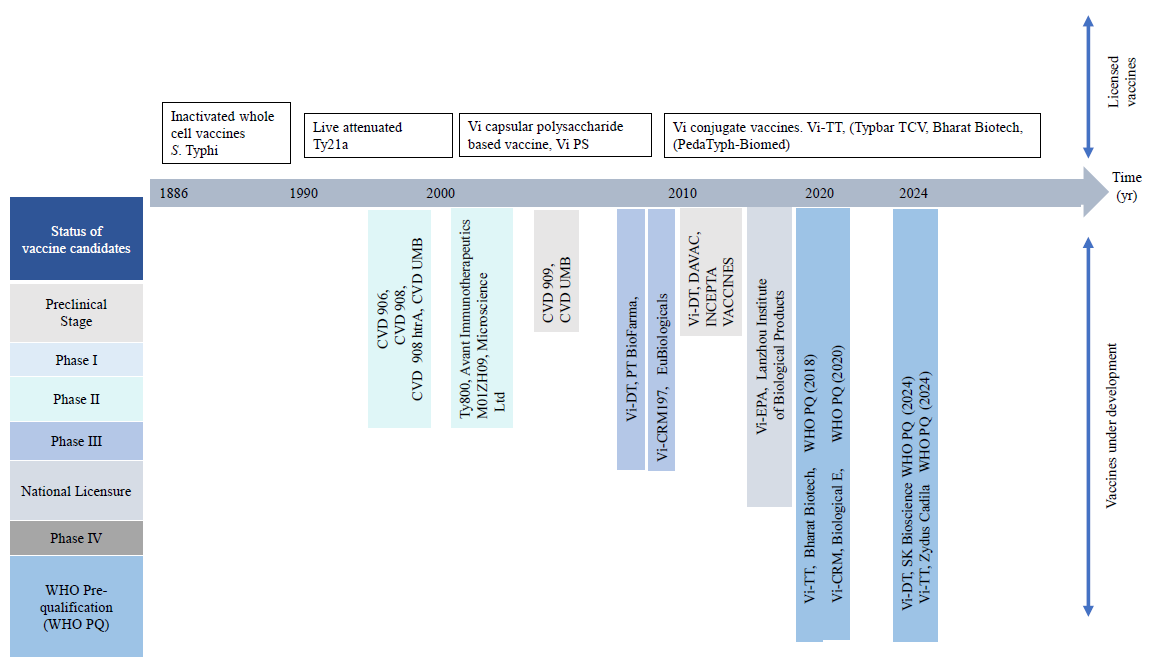
Export to PPT
Paratyphoid vaccinesA similar strategy to typhoid vaccines was adopted to develop paratyphoid vaccines. Genetically engineered, live attenuated S. Paratyphi A strains were generated by mutating critical target genes, such as phoP/phoQ, htrA, ssaV and clpPX. Genetic deletion of phoP/phoQ in S. Paratyphi A by Roland et al55 in 2010 gave rise to an attenuated strain, which was immunogenic and well tolerated in an oral rabbit model. Another study with SPADD01, containing genetic mutation of aroC, critical for amino acid biosynthesis and yncD, encoding a TonB-dependent transporter showed significant attenuation, but excellent humoral and mucosal immune response in a mouse model56. Researchers introduced an additional mutation of the htrA gene in the yncD mutant S. Paratyphi A, further reducing the virulence57. Nasal administration of this double mutant strain protected immunized mice against lethal bacterial challenge57. CVD 1902, which incorporated combined mutations in the guaBA and clpX genes, involved in the de novo synthesis of guanine nucleotides and a chaperon ATPase, respectively, is also an attractive, live attenuated, paratyphoid vaccine candidate. Volunteer trials with single doses of 10^9 or 10^10 CFU of CVD 1902 strain were well tolerated and triggered paratyphi lipopolysaccharide-specific IgG and/or IgA B-memory cells and paratyphi-specific CD8+ and/or CD4+ T effector/memory cells58.
Besides, subunit vaccine candidates for S. Paratyphi, comprising of surface or secretory proteins, such as the outer membrane proteins and O-specific polysaccharide (OSP) exhibited robust immune protection. Systemic immunization with 100 µg to 500 µg of S. Paratyphi A outer membrane proteins PagC, LamB, NmpC, TolCFadL and SpaO conferred 60 per cent to 95 per cent protective efficacy against paratyphoid infection, but requires further detailed evaluation of dose optimization and cross-protection against typhoidal infection59. Instead of Vi-PS, surface OSP as a protective antigen for S. Paratyphi A, which lacks the Vi antigen. OSP conjugates linked to diverse carrier proteins were developed for S. Paratyphi A, following the similar strategy employed for Vi conjugate vaccines48. In 1996, researchers at the US National Institute of Health (NIH) developed OSP-TT60, which documented considerable immunogenicity but no significant vaccination-induced side effects in a Vietnamese trial. However, booster response was not observed in children61. This technology was transferred to the Lanzhou Institute of Biological Products, and its product has progressed through Phase I and II clinical trials49. Other OSP-conjugate vaccines against paratyphoid infection undergoing preclinical evaluation used diphtheria toxoid62 (International Vaccine Institute, Seoul, Korea) and CRM197, a genetically modified diphtheria toxin (Novartis Vaccine Institute of Global Health, Sienna, Italy) as carrier proteins63. Isolation of bacterial OSP requires large-scale fermentation of pathogenic organisms, followed by a detoxification process to eliminate endotoxins. Aiming to bypass the need for detoxification, one research group utilized synthetic oligosaccharides that corresponded to the O-polysaccharide repeating units of S. Paratyphi A to construct a glycoconjugate formulation by linking them to a carrier, bacteriophage Qb64. This conjugate successfully induced high levels of anti-glycan IgG antibodies in mice, and passive immunization with the antisera protected from lethal challenges with S. Paratyphi A64.
Bivalent vaccinesSince endemic areas of Salmonella Typhi and Paratyphi infections largely overlap, bivalent vaccine candidates targeting both organisms are in high demand65. Mass immunization with Vi conjugate vaccines may exert selection pressure on the existing Vi-negative strains, eventually making vaccination ineffective38. A prospective study65 in Guangxi, China, found a significant shift from S. Typhi to S. Paratyphi A outbreaks three years after introducing Vi-based vaccines. Data on the efficacy of the oral Ty21a vaccine against paratyphoid infections are inconsistent66-67. OSP O2-conjugates of S. Paratyphi A were combined with Vi-TT, Vi-CRM197 or Vi-DT for wider protection68. An exciting alternative to the traditional conjugation methods is the Multiple Antigen Presenting System (MAPS), which uses the biotin-rhizavidin affinity pair to create a complex of polysaccharides and proteins. Vaccines based on MAPS generate functional antibodies and Th1/Th17 cell responses. A bivalent vaccine targeting Vi and OSP was developed using the MAPS that contained a fusion of three proteins, CRM197, Pseudomonas rEPA, and pneumococcal SP1500-SP0785 to Rhizavidin. This vaccine demonstrated significantly higher affinity maturation of both Vi and OSP antibodies with minimal cross-interference functionally when compared with the monovalent vaccine69. Recent investigations used an engineered S. Paratyphi A, utilizing pDC5-viaB plasmid to produce GMMA that displayed S. Typhi Vi antigen and the O:2 antigen from Paratyphi A and elicited strong humoral responses and bactericidal activity against both pathogens, supporting its potential use for enteric fever control70,71,72 (Supplementary Table I).
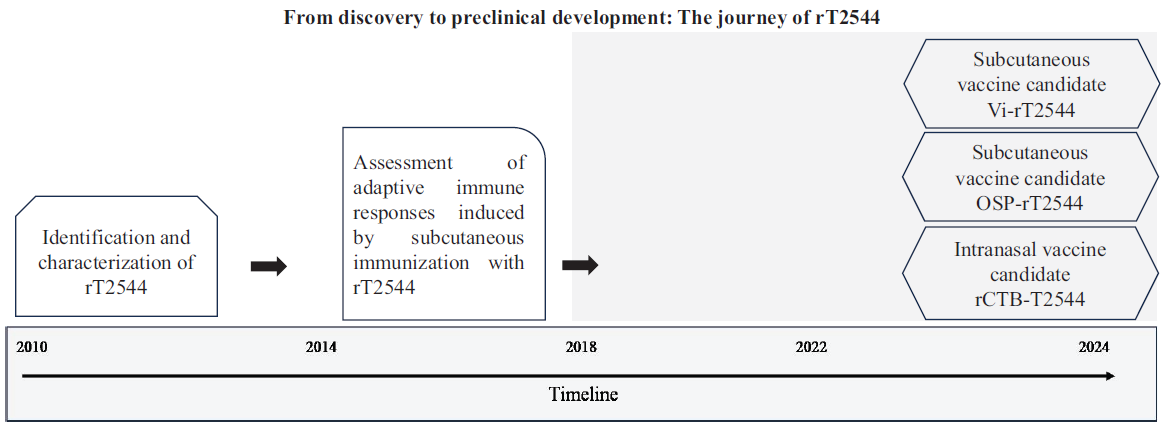
Our vaccine development efforts as a case studyNear the end of the first decade of the new millennium when we started our journey for Salmonella vaccine development, there were only two licensed Salmonella vaccines – live, attenuated Ty21a and injectable Vi-polysaccharide (Vi-PS) vaccines, meant for use against only Salmonella Typhi, although not suitable for young children. Both vaccines offered inconsistent cross-protection against Salmonella Paratyphi A and B73-75. This prompted us to consider protein subunit-based vaccine development that could simultaneously protect against Salmonella Typhi and Paratyphi infections. Through advanced bioinformatics and experimental techniques, our team identified several promising candidates, finally leading to the discovery of a significant protein, called T254476. Computational prediction of the three-dimensional structure of this 27-kDa outer membrane protein revealed membrane embedded β-sheets and externally projected α-helices that bind to the host extracellular matrix protein, laminin. However, that T2544-laminin binding was essential for bacterial virulence and T2544-based subunit vaccine could protect against intestinal Salmonella infection required an animal model that was not available at that moment for the human restricted enteric fever pathogens, except for primates76. Literature searches gave us the impression that the in vivo availability of elemental iron might be the limiting factor for typhoidal Salmonellae to establish rodent infection77. Previous research had shown that host siderophore, NRAMP-1 mutant mouse, was exquisitely susceptible to Salmonella infection78, while systemic iron overload increased the susceptibility of wild-type mouse strain to S. Typhi infection77,79. However, the use of a large dose of iron often results in immunosuppression and lethality due to organ toxicities80, which could be avoided by the co-administration of iron and iron chelator, desferrioxamine that makes the element iron (Fe3+) available to the intracellular bacteria, promoting their survival and growth79. We standardized a paired dose of iron (0.32 mg per gm of body weight) and Desferal (25 mg/Kg body weight) that limited iron toxicity but established infection in wild-type BALB/c mouse after oral gavage with S. Typhi. Similar to humans, liver, spleen and the bone marrow were the primary visceral organs affected in the mouse, suggesting that this might be considered a physiological model for Salmonella Typhi infection. The model developed fulfilled a long-standing demand for a rodent model of typhoid after infection through the natural route, which could serve the dual purpose of studying intestinal pathogenesis and immune response. Immunization of mice with the candidate subunit vaccine indeed induced raised intestinal secretory IgA levels that decreased gut colonization by S. Typhi81. While the induction of intestinal immune response following systemic vaccine administration was reported earlier76, its protective role in vivo was not demonstrated. In addition, immunized mice developed high titers of T2544-specific opsonic antisera, which augmented complement-mediated lysis, phagocytosis by the macrophages and antibody-dependent cellular cytotoxicity (ADCC) of the bacteria82 and conferred protection after passive immunization. Most impressively, acute and convalescent typhoid patients’ sera containing significantly raised titers of T2544-specific bactericidal antibodies could be neutralized by adsorption with T2544, suggesting that it is an immunodominant antigen for the human infection76. Our subsequent studies revealed that the candidate vaccine could also elicit T2544-specific cell-mediated immune response, including the T helper 1 (Th1) cells and cytotoxic T lymphocytes (CTLs)82. Together, our research findings underscored the importance of T2544 in orchestrating an effective immune response to human pathogenic Salmonella spp. This was further supported by significant protection of mice immunized with recombinant T2544-based candidate vaccine or passively administered with T2544 antiserum against S. Typhi82. In addition to considering the apparent advantage of a protein subunit vaccine compared with the polysaccharide-based formulations for younger children and the presumed protection conferred by T2544 against S. Paratyphi A infection, this candidate vaccine was patented by us (Patent no. 283894; dated 09.09.2011) to ensure retention of its intellectual property within India.
However, we failed to identify an interested industrial partner for further development of the candidate vaccine to commercialize it. This was perhaps influenced by intense research to develop Vi-polysaccharide-based typhoid conjugate vaccines (TCVs) during that period. The success of capsular polysaccharide-based conjugate vaccines against Hib, pneumococci and meningococci fuelled this interest. TCVs demonstrated excellent safety profile and robust and durable antibacterial immunity in children as young as 6-9 months of age37,83. Typhi Vi polysaccharide conjugated to tetanus toxoid from Bharat Biotech in December 2017, followed by TYPHIBHEV (Vi polysaccharide from Citrobacter freundii conjugated to CRM) by Biological E in December 2020 and SKY Typhoid (S. Typhi Vi polysaccharide conjugated to diphtheria toxoid) in February 2024 that was marketed by SK Biosciences. However, concerns were raised against the carrier proteins most commonly used for TCVs, namely the tetanus toxoid and diphtheria toxoid, also used as vaccine antigens in the routine immunization programme for children or as carrier proteins for several conjugate vaccines. Simultaneous or sequential use of the same carrier protein as a part of multiple conjugate vaccines or as a vaccine antigen and part of a conjugate vaccine may lead to decreased immunogenicity of the co-administered antigen due to antigenic competition or carrier-induced epitope suppression (CIES)84,85. For example, vaccination with PCV13 and MCV4, 3-4 wk after Tdap vaccine significantly reduced the geometric mean titer to seven of the 13 pneumococcal serotypes in adults86 and priming with DT suppressed the response to DT-MenA conjugates87. Several mechanisms have been implicated for this immune interference, including carrier specific B cells expansion during priming, followed by competition with the co-administered antigen-specific B cells, presentation of the carrier-polysaccharide conjugate by the B-cells as opposed to dendritic cells after pre-immunization, competition for antigen and antigen-bearing cells and the development of carrier-specific suppressor T cells during priming that can induce suppressor T cells specific for the conjugated antigen after immunization88. To overcome such problems, we replaced TT/DT with recombinant T2544 as the provider of the T cell helper epitopes for the new TCV. Given that T2544 is a protective antigen, this approach would add an ‘additional valency’, which is generally neglected for conjugate vaccines and further augment the immune response. To check for the immune adjuvant functions of T2544, we immunized mice with Vi-PS along with recombinant T2544. This led to modest increase in Vi-PS specific serum IgG titers. Several studies had indicated that most adjuvants work better when covalently conjugated to the antigens rather than co-administered as a mixture38,89. However, solubility of T2544 was challenge which we finally succeeded in overcoming. Serum SBA titer was greater for Vi-T2544, which conferred better protection to mice against S. Typhi infection than Vi-TT with a wider coverage that includes paratyphoid infection (Fig. 2 & Supplementary Table II). A patent application for the candidate vaccine formulation containing Vi-T2544 has been filed to the Indian Patent Office (IPO) (Application number 202411074276; filing date: October 1, 2024).
Export to PPT
To further extend vaccine-induced protection to the non-typhoidal Salmonella (NTS) serovars, we considered conjugating recombinant T2544 protein to S. Typhimurium O-specific polysaccharide (OSP)
留言 (0)