The UN’s Sustainable Development Goals, released in 2015, outline 17 urgent objectives to be achieved through global collaboration. Among these, Goal 2 aims to end hunger, achieve worldwide food security, improve nutrition, and promote sustainable agriculture. As the world’s population increases, the demand for food production also rises. This increases the pressure on agricultural systems globally (United Nations, 2015). One important development that will help address these issues is the growing popularity in some countries of plant-based diets as an alternative to traditional meat and dairy consumption.
Chemical fertilizers play a crucial role in meeting the increasing demand for plant-based food by maximizing crop yields (Bhatti et al., 2017; Hera, 1995; Pahalvi et al., 2021). The three primary nutrients in commercial fertilizers, nitrogen (N), phosphate (P), and potassium (K) are used extensively in modern agriculture (McGuire, 2015). However, these fertilizers can also disrupt natural soil processes, leading to reduced water retention, imbalanced soil fertility, declined the agricultural soil quality with the reduction in soil organic matter (Dar et al., 2016; Dinesh et al., 2010; Ongley et al., 2010).
Similarly, the widespread use of pesticides to combat crop diseases poses significant environmental and health risks. Many pesticides are toxic to humans, animals, and non-target organisms, including essential pollinators (Brevik and Burgess, 2012; Geiger et al., 2010; Lee and Choi, 2020; Sponsler et al., 2019). The ecological impacts of many pesticides extend to soil and water systems. These chemicals disrupt microbial communities and reduce soil fertility (Law et al., 2017; Pimentel et al., 1993; Viaene et al., 2016). The loss of beneficial microbial species in the agricultural soil can exacerbate pathogen invasions and compromise ecosystem resilience (Jacobsen and Hjelmsø, 2014; Meena et al., 2020). Since microbial communities are the primary drivers of soil nutrient cycling, due to their various metabolic activities, their relevance in moderating ecosystem function cannot be understated (Balser et al., 2002).
In response to these challenges, there is a growing imperative to explore sustainable alternatives to conventional agricultural practices (Vasilescu et al., 2023). In this regard, microbiological tools, such as biofertilizers and biocontrol agents, have emerged as promising solutions. Biofertilizers, containing beneficial microorganisms, enhance soil fertility and promote plant growth through natural processes (Verma et al., 2019). Biocontrol agents offer non-chemical methods for managing plant diseases by leveraging the antagonistic properties of microorganisms against pathogens (Bhardwaj et al., 2014; Bonaterra et al., 2022; Parani and Saha, 2012).
The bacterial genus Pseudomonas, characterized by its metabolic diversity and abundance in various environments, has garnered particular attention for its potential applications in agriculture (Palleroni, 2015). Members of this genus show considerable metabolic and genetic diversity (Peix et al., 2009); its various secondary metabolites are known to form virulence factors, pigments, and biofilms (Drenkard and Ausubel, 2002; Moissenet and Khedher, 2011; Raio and Puopolo, 2021; Stover et al., 2000). A large number of Pseudomonas strains are known for plant growth-promoting potential by producing various substances such as siderophores, 1-aminocyclopropane-1-carboxylate (ACC) deaminase and lipopeptides (Leontidou et al., 2020; Pršić and Ongena, 2020). Using microbes such as Pseudomonas offers a promising approach to sustainable farming, offering solutions that support plant health while minimizing environmental impacts (Hamid et al., 2021; Khatoon et al., 2020; Nikel et al., 2014; Sharma and Archana, 2016).
Here, we provide a comprehensive overview of Pseudomonas spp. as microbiological tools for sustainable agriculture, examining their status and potential applications in crop production, particularly in cereal crops. We discuss the mechanisms by which Pseudomonas spp. act as biocontrol agents, including direct inhibition of phytopathogens and induction of systemic resistance in plants. Additionally, we explore approaches for optimizing the efficacy of Pseudomonas-based biocontrol strategies, such as improved delivery methods and genetic engineering. Finally, we address the challenges and future directions in harnessing Pseudomonas for sustainable crop protection, emphasizing the importance of ongoing research in advancing environmentally friendly agricultural practices.
2 Diversity and plant interactions of Pseudomonas speciesKey interactions between plants and microbes occur in the soil surrounding plant roots (Berg et al., 2014; Gaiero et al., 2013; Jain et al., 2020). The rhizosphere, the immediate soil layer influenced by root exudates, is a hotspot for microbial colonization, fostering diverse communities of bacteria, fungi, and other microorganisms (Berg et al., 2014; Gaiero et al., 2013; Jain et al., 2020). Pseudomonas spp. are often major components of the rhizosphere microbiome and engage in multifaceted interactions with plants, exerting significant influence on plant health, nutrient cycling, and ecosystem functioning (Botelho and Mendonça-Hagler, 2006; Chaudhary et al., 2021; Raio and Puopolo, 2021).
Pseudomonas spp. display remarkable adaptability to diverse environmental conditions, thriving in various soil types and agricultural settings (Palleroni, 2015). Their metabolic versatility and genetic plasticity enable them to colonize plant roots and establish intricate symbiotic relationships with their host plants (Palleroni, 2015; Peix et al., 2009). Many studies have demonstrated the enrichment of Pseudomonas spp. in the roots and rhizosphere of diverse plants such as Arabidopsis thaliana, potatoes, rice, wheat, and barley (Andreote et al., 2009; Buddrus-Schiemann et al., 2010; Jain et al., 2020; Lawongsa et al., 2008; Persello-Cartieaux et al., 2001; Raio and Puopolo, 2021; Wang et al., 2012). The adaptability of Pseudomonas spp. to different plant environments might have evolved due to selective pressures, leading to the development of metabolic pathways conducive to nutrient acquisition from plant-derived compounds (Imperato et al., 2019; Rainey, 1999).
At the molecular level, plant-Pseudomonas interactions are orchestrated through intricate signaling pathways and molecular dialogs (Girard et al., 2020; Preston, 2004). Root exudates, comprising a diverse array of largely low molecular weight, organic compounds serve as chemoattractants, guiding Pseudomonas migration toward the rhizosphere (Drigo et al., 2009; Jain et al., 2020; Marilley et al., 1999; Waldon et al., 1989; Wang et al., 2017). Upon encountering plant roots, Pseudomonas spp. employ chemotaxis and quorum sensing systems to modulate their behavior and adapt to changing environmental cues (Loh et al., 2002; Schikora et al., 2016).
Pseudomonas species actively participate in nutrient cycling processes, facilitating the uptake and assimilation of essential nutrients by plants (Botelho and Mendonça-Hagler, 2006; Chaudhary et al., 2021; Raio and Puopolo, 2021). Through the production of plant growth-promoting substances, such as phytohormones and siderophores (Leontidou et al., 2020), Pseudomonas species stimulate root growth, enhance nutrient acquisition, and confer resistance to environmental stresses (Raio and Puopolo, 2021; Sun et al., 2022). Moreover, Pseudomonas spp. produce a wide range of antimicrobial secondary metabolites. This diversity enables them to outcompete other microorganisms for niche space and carbon resources provided by plants (Figure 1) (Raio and Puopolo, 2021; Validov et al., 2005).
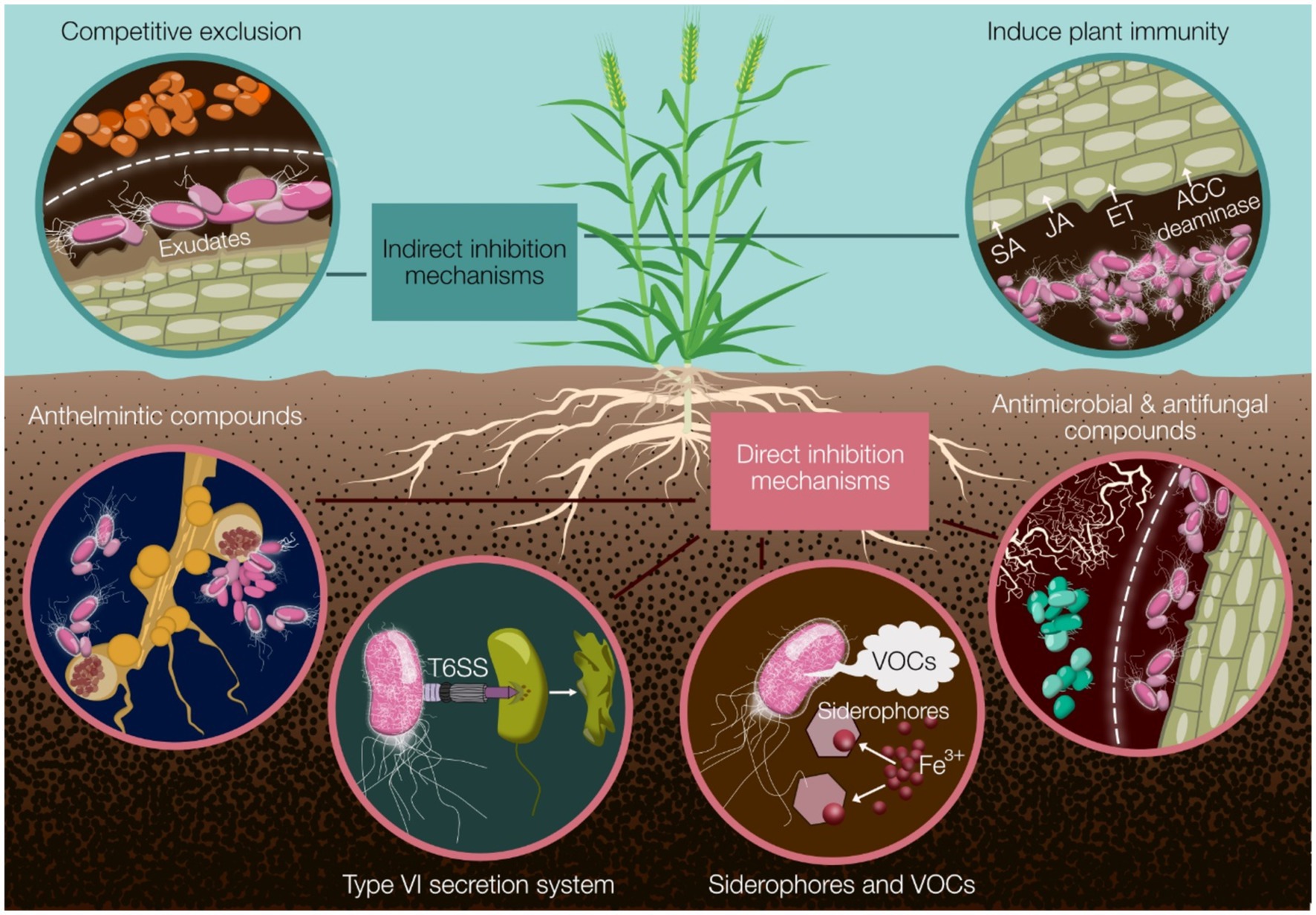
Figure 1. A summary of the major mechanisms by which Pseudomonas biocontrol strains can protect cereal crops from disease. Indirect mechanisms include competitive exclusion and induction of plant immunity through phytohormone modulation (SA, JA, ET) and ACC deaminase activity. Direct mechanisms encompass the production of anthelmintic, antimicrobial, and antifungal compounds; deployment of the type VI secretion system (T6SS); and secretion of siderophores and volatile organic compounds (VOCs). These diverse strategies collectively contribute to effective disease suppression and improved crop health. SA, salicylic acid; JA, jasmonic acid; ET, ethylene; ACC, 1-aminocyclopropane-1-carboxylate; T6SS, type VI secretion system; VOCs, volatile organic compounds.
In addition to promoting plant growth and nutrient acquisition, Pseudomonas spp. play a pivotal role in suppressing plant pathogens and mitigating disease incidence (Hernández-León et al., 2015; Lahlali et al., 2022; Validov et al., 2005; Vivekananthan et al., 2004). Through the production of antimicrobial compounds, lytic enzymes, and volatile organic compounds, Pseudomonas spp. inhibit the proliferation of phytopathogens and confer protection to their host plants (Hernández-León et al., 2015; Mehmood et al., 2023; Raio and Puopolo, 2021; Validov et al., 2005; Vivekananthan et al., 2004). Furthermore, Pseudomonas-mediated induction of systemic resistance within host plants primes the plants for enhanced defense responses, bolstering their resilience against pathogen attacks (Haney et al., 2018; Leeman et al., 1995).
These attributes position Pseudomonas spp. as promising candidates for biocontrol agents in agriculture, as they not only contribute to plant protection but also promote plant growth under various environmental conditions, including high salinity and drought (Cheng et al., 2007; Forni et al., 2017; Orozco-Mosqueda et al., 2019; Rangarajan et al., 2003). However, it is essential to acknowledge that while many Pseudomonas strains act as beneficial or passive colonizers of the plant microbiome, certain species may exhibit phytopathogenic traits (Leontidou et al., 2020; Montes-Osuna et al., 2022; Pršić and Ongena, 2020; Young, 1991). One well-documented example is Pseudomonas syringae, known for causing various plant diseases by producing virulence factors such as coronafacic acid, ice nucleation protein, and the type III secretion system (T3SS) and its associated type III secreted effector (T3SE) proteins (Alattas et al., 2023; Bundalovic-Torma et al., 2022; Büttner, 2016; Weiler et al., 1994; Xin et al., 2018).
3 Pseudomonas as a plant pathogenP. syringae is a highly diverse bacterial species complex known for its ability to cause disease in a wide range of plant hosts. The complex is currently divided into at least 13 phylogenetic groups or phylogroups, with 7 considered primary phylogroups that contain most of the recognized pathogenic strains (Dillon et al., 2021). Although strains are often classified into pathovars based on the host they were isolated from, there is not always a strong correlation between phylogeny and host specificity. Many closely related strains can infect different hosts, while strains isolated from the same host may be phylogenetically diverse (Baltrus et al., 2017; Morris et al., 2019).
Key factors in P. syringae pathogenicity are the T3SS and T3SE proteins. The T3SS protein allows the bacteria to inject effector proteins directly into plant cells, where they can manipulate host processes to promote infection (Büttner, 2016). P. syringae strains typically possess 20–30 different T3SE proteins, with over 70 distinct T3SE families identified across the species complex (Dillon et al., 2019). However, only a small number of core effectors are conserved across most strains. The diversity and composition of effector repertoires plays a major role in determining host range and virulence capabilities (Laflamme et al., 2020).
T3SE proteins have a range of virulence functions in plants, including suppressing immune responses, altering hormone signaling, disrupting cellular processes, and creating favorable conditions for bacterial growth (Khan et al., 2018). Some effectors, like HopZ1a, demonstrate remarkable functional diversity, targeting multiple unrelated plant proteins to suppress immunity, alter phytohormone signaling, and disrupt microtubule integrity (Jiang et al., 2013; Lee et al., 2012; Rufián et al., 2021). However, plants have evolved immune receptors capable of recognizing many effectors, triggering strong defense responses called effector-triggered immunity (ETI) (Jones and Dangl, 2006). This creates an evolutionary arms race, with bacteria evolving new or modified effectors to evade detection, and plants evolving new receptors.
Interestingly, recent research has found that a relatively small number of plant immune receptors are capable of recognizing effectors from a wide range of P. syringae strains, providing broad-spectrum resistance. For example, in Arabidopsis thaliana, the ZAR1 and CAR1 receptors can detect effectors from 95% of P. syringae strains (Laflamme et al., 2020). The ZAR1 receptor in particular shows a remarkably broad recognition profile, capable of detecting at least six unrelated T3SE families through association with multiple kinase proteins (Martel et al., 2020).
The evolution and diversity of P. syringae effector repertoires is shaped by several key processes (Dillon et al., 2019). Horizontal gene transfer allows effectors to move between strains, potentially expanding host range. Gene loss or pseudogenization can occur when effectors are recognized by plant defenses. Mutational changes in effector sequences may alter their function or allow evasion of host recognition (Dillon et al., 2019). Some effectors show functional redundancy, allowing loss of one effector to be compensated for by others (Kvitko et al., 2009). Additionally, some effectors can suppress the immune responses triggered by other effectors, a phenomenon known as meta effector interactions (Wei et al., 2018).
The importance of environmental factors in P. syringae infections should not be overlooked. Temperature, humidity, and leaf wetness play crucial roles in disease development. For example, optimal conditions for infection include temperatures around 15–25°C and periods of high humidity or leaf wetness (Xin et al., 2018). These environmental factors influence both bacterial growth and the plant’s immune responses. P. syringae is not limited to agricultural settings, it has also been isolated from various environmental sources including aquatic habitats, rain, and wild plants (Morris et al., 2013). This environmental ubiquity may contribute to the emergence of new plant diseases as strains adapt to new hosts or environmental conditions change.
Despite the existence of pathogenic strains, the vast majority of isolated and characterized Pseudomonas strains are beneficial or benign (Palleroni, 2015; Peix et al., 2009). Only a fraction of Pseudomonas strains, out of the numerous species identified, are pathogenic (Palleroni, 2015), highlighting the potential for screening diverse strains to identify those suitable for biocontrol applications. Below, we examine the diverse mechanisms through which Pseudomonas spp. contribute to the suppression of cereal crop diseases. Additionally, we explore the practical applications of these strains in agriculture and discuss the factors influencing their competitiveness and efficacy as biocontrol agents.
4 Control of plant diseases in cereal cropsPseudomonas spp. offer significant potential for managing plant diseases in cereal crops. For instance, when rice seeds undergo treatment with Pseudomonas fluorescens PF1 before sowing, at the time of sowing, and again at 30 days after sowing, the resulting seedlings display enhanced resistance to Xanthomonas oryzae pv. pryzae (Vidhyasekaran et al., 2001). This treatment led to a significant decrease in disease incidence from 6.8 to 1.2%. In both greenhouse and field conditions, P. fluorescens strains PF1 and FP7 exhibit the ability to inhibit the mycelial growth of the sheath blight fungus Rhizoctonia solani. This inhibition contributes to improved seedling vigor and increased yield in rice plants (Nandakumar et al., 2001). Moreover, the treatment of rice cv. IR50 with the same Pseudomonas strains induces systematic resistance against R. solani, accompanied by an increase in chitinase and peroxidase activity (Nandakumar et al., 2001).
Field trials conducted with rice across different seasons evaluate the efficacy of P. fluorescens strain Pf-1 in controlling Hirschmanniella gracilis, root endoparasitic nematodes that are common in aquatic environments. The seed treatment with this biocontrol strain leads to a high level of bacterial colonization, suppression of nematodes, and a 13% increase in rice yield (Ramakrishnan et al., 1998).
In the case of wheat (Triticum aestivum L.), studies have shown that two Pseudomonas strains, GRP3 and PRS9, play a role in promoting wheat growth in terms of root-shoot length and plant weight (Sharma et al., 2011). P. fluorescens strain PSR21, when applied as a wheat seed treatment and later as a foliar spray during the spring, results in a significant decrease in the average degree of culm damage (where the culm is the above-ground stem of a grass or sedge) (Kita et al., 2004). Further research has highlighted the efficacy of P. fluorescens in reducing disease incidence, such as that caused by the fungal pathogen Helminthosporium sativum in wheat. Interestingly, following treatment, the bacterial population tends to decrease toward the root tip in wheat plants (Srivastava et al., 1999; Wang et al., 1999). Moreover, a recent study has investigated the effectiveness of eight Pseudomonas strains as biocontrol agents (Clough et al., 2022). Among these, Pseudomonas protegens CHA0 was identified as the most potent strain, significantly inhibiting the growth of Ralstonia solanacearum, a pathogen causing bacterial wilt in plants. This inhibitory activity was linked to the production of key secondary metabolites, including orfamides, pyoluteorin, and 2,4-diacetylphloroglucinol (DAPG) (Clough et al., 2022).
Pseudomonas spp. hold immense potential for combating plant diseases in cereal crops, offering multifaceted strategies for disease management. From rice to wheat, the application of various Pseudomonas strains has shown remarkable results, including reduced disease incidence, enhanced seedling vigor, and increased crop yield. These findings underscore the importance of developing microbial-based solutions in agriculture to mitigate the impact of plant pathogens on crop production.
5 Direct inhibition of phytopathogens 5.1 Antimicrobial and antifungal compoundsIn the pursuit of novel biocontrol agents, Pseudomonas spp. have garnered considerable attention for their potential to inhibit some of the most damaging cereal crop pathogens. Efforts to isolate Pseudomonas strains from diverse environments have revealed promising candidates capable of combating phytopathogens (Chaudhary et al., 2021; Lv et al., 2023). Several studies have identified Pseudomonas species with the ability to inhibit numerous cereal crop pathogens, including Magnaporthe oryzae (causing rice blast), Gaeumannomyces graminis var. tritici (a fungus responsible for wheat take-all disease), Fusarium spp. (associated with head blight, root rot, wilt, and grain contamination), Pyrenophora tritici-repentis (a fungal pathogen that is the cause of tan spot disease), and Rhizoctania solani (a wide-host-range soil-borne pathogen) (Garbeva et al., 2004; Karthikeyan and Gnanamanickam, 2008; Lemanceau and Alabouvette, 1993; Rangarajan et al., 2003; Thomashow and Weller, 1988) (Table 1). These findings mark the initial steps in the quest to identify effective biocontrol agents, demonstrating the ability of isolates to confer plant protection in vivo, through greenhouse experiments and field trials.
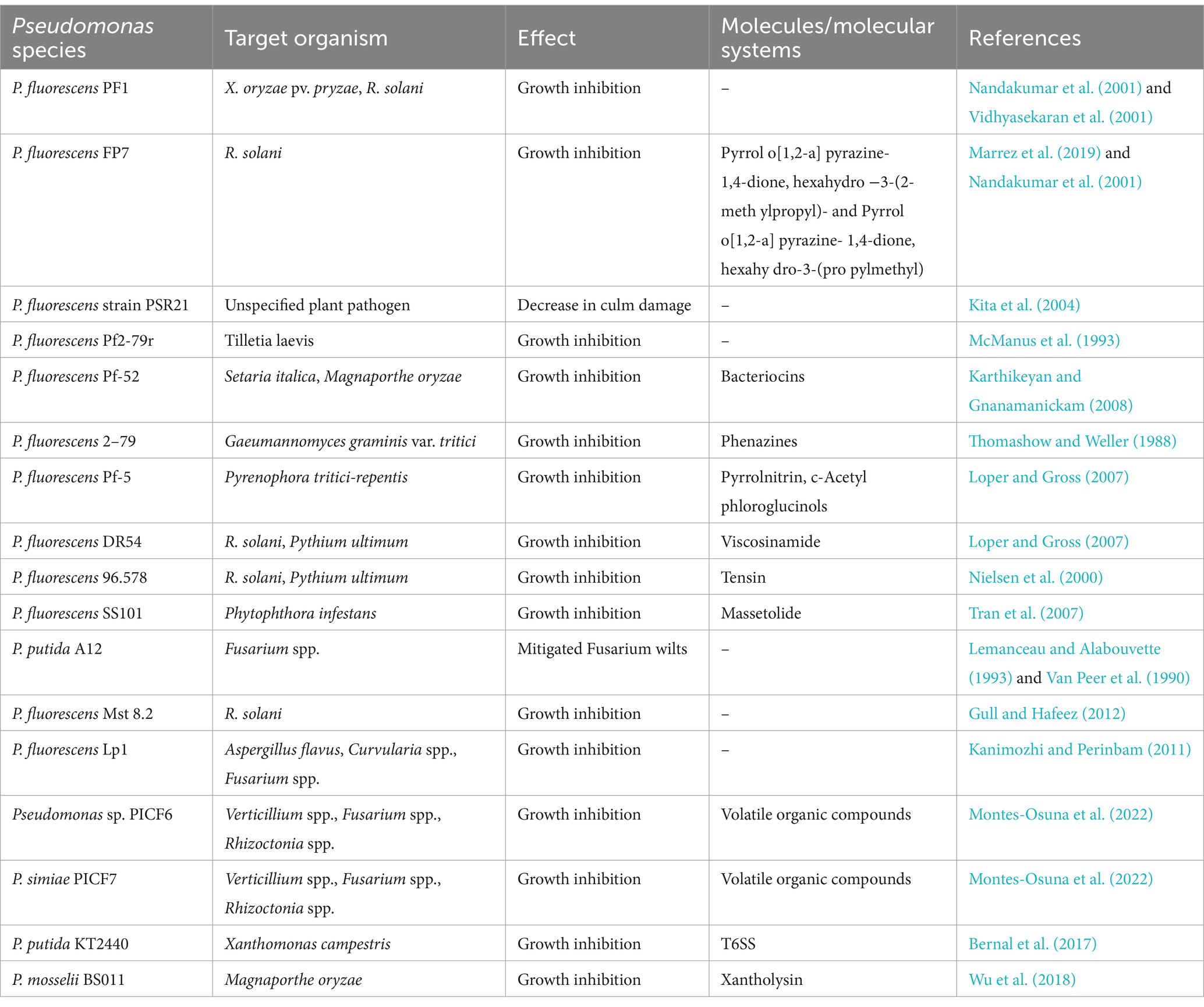
Table 1. Effects of Pseudomonas spp. against plant bacteria and fungi pathogens.
Several greenhouse and growth chamber studies have explored the use of bioactive compounds purified from cultures of Pseudomonas species (Table 1) (Ligon et al., 2000; Wu et al., 2018). For instance, metabolites from Pseudomonas mosselii BS011 exhibited broad-spectrum inhibition against phytopathogenic fungi including M. oryzae (Wu et al., 2018). Importantly, Pseudomonas strains have demonstrated equal inhibition of pathogens compared to standard chemical fungicides (Ligon et al., 2000). For example, culture filtrates of P. fluorescens strain BL915 exhibited equal efficacy against R. solani compared to quintozene, a conventional chemical fungicide (Ligon et al., 2000).
Pseudomonas spp. Metabolites multiple compounds which contribute to their antagonistic properties. For example, the antagonistic properties of P. protegens FD6 were examined against pathogenic fungi such as Botrytis cinerea and Monilinia fructicola (Zhang et al., 2020). Genomic analysis identified 12 gene clusters responsible for the production of key secondary metabolites, including 2,4-diacetylphloroglucinol (2,4-DAPG), pyoluteorin (PLT), and pyrrolnitrin (PRN), all essential for its antifungal activities. Mutant analysis revealed that the pltD mutant, lacking PLT production, exhibited only 30% inhibition of grey mold disease, while the phlC mutant, deficient in 2,4-DAPG production but with a marked increase in PLT, demonstrated 65% inhibition. In contrast, the wild-type FD6 strain showed complete suppression of the pathogen, with no visible disease lesions on tomato fruits after 5 days of treatment. These findings underscore the critical roles of PLT and 2,4-DAPG, with PLT being particularly crucial for the high-level inhibition of fungal pathogens. The inverse relationship between the production of these two metabolites highlights the complexity of the biocontrol mechanisms in P. protegens FD6 (Zhang et al., 2020).
For wheat take-all disease, Pseudomonas spp. have been used as potential alternatives to conventional chemical treatments. Pseudomonas species offer resilience under unfavorable conditions and exhibit promising colonization abilities with cereal crop roots (Al Zadjali et al., 2023; Capper and Higgins, 1993; Dowling and O'Gara, 1994; Thomashow and Weller, 1988; Xu et al., 2021). Thus, the exploration of Pseudomonas-based biocontrol agents holds significant promise in augmenting sustainable agricultural practices, heralding a new era in crop protection strategies.
5.2 SiderophoresSiderophores constitute a group of secondary metabolites synthesized by various bacteria, fungi, yeast, and specific plants in response to iron scarcity (Dwivedi and Johri, 2003). These low molecular weight molecules, typically ranging from 500–1,500 Daltons, exhibit a strong binding affinity for iron (III) (Hider and Kong, 2010). The term “siderophore” is derived from the Greek, meaning “iron carrier” (Neilands, 1995); the first siderophore was isolated during 1949–1952 (Hider and Kong, 2010). The first crystalline form of siderophore was isolated by Neilands (Neilands, 1952), sparking research into these iron-chelating compounds (Chincholkar et al., 2000). Kloepper et al. (1980) provided initial evidence of siderophores from plant growth-promoting bacteria (PGPB) acting as biocontrol agents. The ability of an organism to produce siderophores is closely related to cyanide production, and their absence can impact the biocontrol activity of the microbes and their ability to restrict iron access to target pathogens (Ho et al., 2021; Jha et al., 2011). Iron plays an important role in the life of nearly all living organisms (Krewulak and Vogel, 2008).
Plants rely heavily on iron for various vital processes such as photosynthesis, oxygen metabolism, DNA and RNA synthesis, and more (Aznar et al., 2015; Rout and Sahoo, 2015). However, in many natural environments with aerobic conditions and neutral pH levels, iron exists mainly in its oxidized ferric (Fe3+) state, which is largely insoluble and limits its availability to plants (Colombo et al., 2014). The siderophores act as high-affinity chelating agents to solubilize ferric ions and transport them to the plant or bacterial cell where it is converted to Fe2+ (Kramer et al., 2020). Moreover, restricting the supply of iron to pathogens acts as a strategy to hinder their growth, owing to the insolubility of ferric iron forms (Kramer et al., 2020; Lau et al., 2016).
Siderophores can take up the iron available in the surrounding environment, and subsequently, iron is acquired by the organisms possessing receptors for that siderophore-iron complex, making it unavailable to their competitors (Hakim et al., 2021). Besides being used as iron carriers, bacterial siderophore-iron complexes can also be utilized by certain plants aiding in their better growth (Pathak et al., 2017). Among the Gram-negative bacteria where over 90% of the bacterial population produces siderophores, the most dominant genera are Enterobacter and Pseudomonas (Tian et al., 2009). Among Pseudomonas species, pyoverdine is the predominant siderophore observed (Cornelis and Matthijs, 2002; Ghssein and Ezzeddine, 2022).
Siderophores primarily contribute to the survival and fitness of microbial populations, directly and indirectly enhancing the activity of biocontrol species (Gull and Hafeez, 2012; Kanimozhi and Perinbam, 2011). For example, siderophores from P. fluorescens strain Mst 8.2 were shown to be a potent inhibitor against R. solani, with 70% disease reduction in wheat (Gull and Hafeez, 2012). Similarly, siderophores from P. fluorescens Lp1 have been used to inhibit plant fungal pathogens such as Aspergillus flavus, Curvularia spp., and Fusarium spp. (Kanimozhi and Perinbam, 2011).
5.3 Volatile organic compoundsPseudomonas species are known to produce a wide array of volatile organic compounds (VOCs) with various functions and applications in agricultural settings (Hernández-León et al., 2015). These VOCs are small molecules characterized by low molecular weights and high vapor pressures, allowing them to diffuse effectively through soil pores and influence microbial communities and plant health (Wheatley, 2002).
Pseudomonas strains can generate complex mixtures of VOCs, although the full extent of their functional diversity is still being elucidated (Schmidt et al., 2015). Some VOCs produced by Pseudomonas spp. exhibit antimicrobial properties against phytopathogens, making them potential candidates for biocontrol applications. For instance, studies have identified Pseudomonas-derived VOCs with antifungal activity against a range of plant pathogens, including Verticillium spp., Fusarium spp., and Rhizoctonia spp. (Cordero et al., 2014; Elkahoui et al., 2015; Montes-Osuna et al., 2022). Pseudomonas sp. PICF6 produces 20 VOCs, that include compounds with reported antifungal (e.g., 1-undecene, (methyldisulfanyl) methane and 1-decene) or plant growth promoting (e.g., tridecane, 1-decene) activities (Montes-Osuna et al., 2022). These findings suggest that Pseudomonas-derived VOCs could serve as biofumigants to mitigate the proliferation of pathogenic species and promote plant health.
Further studies have investigated the antifungal activity of VOCs produced by P. fluorescens ZX against postharvest fungal pathogens (Wang et al., 2021; Yue et al., 2023). The research demonstrates that P. fluorescens ZX VOCs effectively inhibit the growth of both Penicillium italicum and Botrytis cinerea, causative agents of blue mold in citrus and gray mold in various fruits, respectively (Wang et al., 2021; Yue et al., 2023). The VOCs significantly suppressed mycelial growth, conidial germination, and sporulation of these pathogens in vitro and reduced disease incidence and lesion size on infected fruits in vivo. Mechanistically, the VOCs primarily act by damaging the pathogens’ cell membrane integrity and permeability, leading to cellular content leakage, decreased ergosterol biosynthesis, and increased malondialdehyde content (Wang et al., 2021; Yue et al., 2023). Additionally, the VOCs interfere with pathogen respiration by inhibiting key enzymes such as ATPase, malate dehydrogenase, and succinate dehydrogenase, causing energy metabolism disruption and reactive oxygen species accumulation. Transcriptomic analysis revealed significant changes in gene expression related to membrane components and amino acid metabolism pathways in treated pathogens (Wang et al., 2021; Yue et al., 2023).
However, to fully harness the potential of Pseudomonas VOCs for practical applications, further investigations are necessary to fully characterize their production within the plant root system and assess their efficacy under natural environmental conditions. By exploring the roles of Pseudomonas-derived VOCs in plant-microbe interactions, researchers can unlock valuable insights into sustainable strategies for crop protection and soil management.
5.4 Anthelmintic compoundsPseudomonas spp., in addition to their antimicrobial activities, have also demonstrated the capability to produce potent anthelmintic compounds, they can destroy parasitic worms and nematodes (Devaraj et al., 2019; Lee C. H. et al., 2000). While not as extensively studied in Pseudomonas as in Streptomyces, the potential for nematode control exists within this bacterial genus (Table 2) (Devaraj et al., 2019; Lee C. H. et al., 2000; Rahanandeh et al., 2024; Spiegel et al., 1991; Wang et al., 2024a; Wang et al., 2024b).
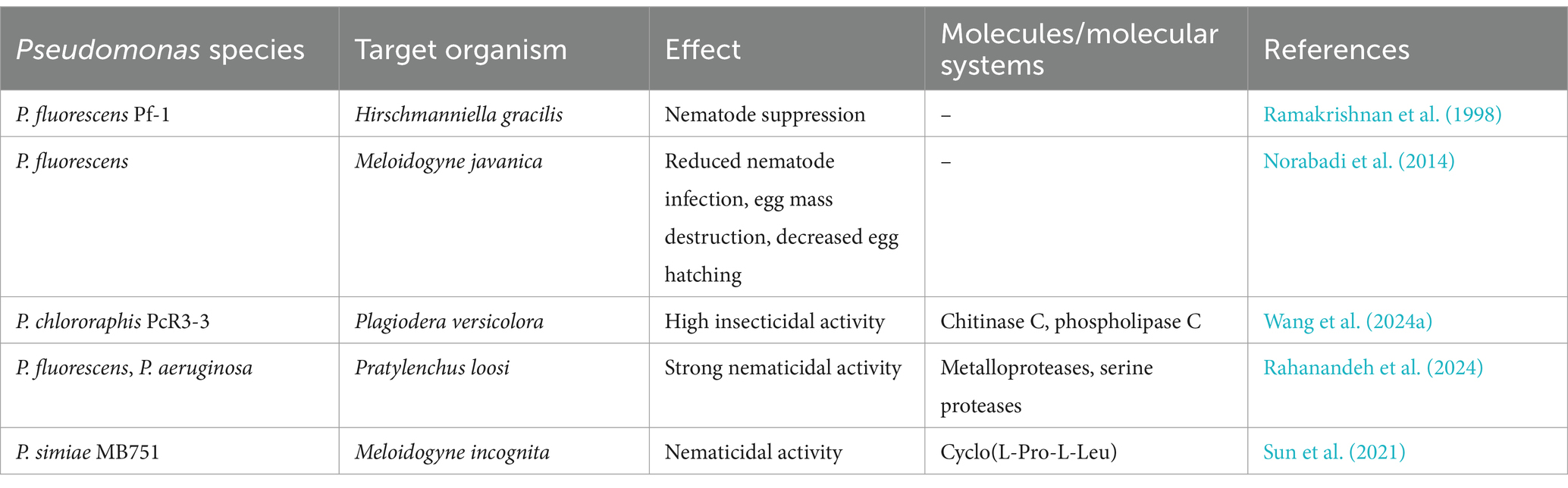
Table 2. Effects of Pseudomonas spp. on nematodes and insects.
Root-knot nematodes (Meloidogyne spp.) are among the most economically damaging plant-parasitic nematodes worldwide, causing significant yield losses in many crops (Sun et al., 2021). These microscopic roundworms infect plant roots, forming characteristic galls or “knots” that disrupt water and nutrient uptake. Meloidogyne incognita is one of the most widespread and studied species, with a broad host range and short generation time that makes it particularly difficult to control.
One study investigated the potential of a strain of P. fluorescens as a biocontrol agent against Meloidogyne javanica, another important root-knot nematode species (Norabadi et al., 2014). In greenhouse and laboratory experiments, P. fluorescens application significantly reduced nematode infection compared to the control group. The bacteria destroyed the nematode egg mass matrix and decreased egg-hatching levels. Additionally, P. fluorescens inoculation led to increased activities of peroxidase (POX) and phenylalanine ammonia lyase (PAL), key enzymes associated with plant defense mechanisms (Norabadi et al., 2014). These observations suggest that P. fluorescens effectively suppress nematode populations by disrupting egg structures and inducing systemic resistance in plants.
Recent research has expanded our understanding of Pseudomonas spp. potential for control of both nematodes and insect pests (Wang et al., 2024a; Wang et al., 2024b). For example, Pseudomonas chlororaphis PcR3-3, isolated from willow roots, exhibited high insecticidal activity against the coleopteran pest Plagiodera versicolora (Wang et al., 2024a). This leaf beetle is a significant pest of willow and poplar trees. While this study primarily focused on insecticidal activity, it highlights the diverse capabilities of Pseudomonas strains against different invertebrates. The genome of P. chlororaphis PcR3-3 was found to contain several genes potentially involved in insect pathogenicity, including those encoding chitinase C and phospholipase C (Wang et al., 2024b). These enzymes have been previously associated with the disruption of insect gut structures and could potentially play a role in nematode control as well.
Other studies have demonstrated the nematicidal potential of Pseudomonas spp. against the root lesion nematode Pratylenchus loosi, a significant pest of tea plants (Rahanandeh et al., 2024). It was found that P. fluorescens and Pseudomonas aeruginosa strains, which were isolated from tea rhizosphere, produced proteases with strong nematicidal activity. These proteases were capable of completely degrading Pratylenchus loosi nematodes within 8 h of exposure (Rahanandeh et al., 2024). Characterization of the proteases revealed them to be metalloproteases and serine proteases, classes of enzymes known to be involved in the degradation of nematode cuticles.
Although the research exploring Pseudomonas spp. as a source of anthelmintic compounds is relatively limited in Pseudomonas compared to other prokaryotes, these studies have indicated the potential of certain Pseudomonas strains to control populations of both plant-parasitic nematodes and insect pests. Given the diverse array of natural products synthesized by Pseudomonas strains and their interactions within soil ecosystems, it is likely that additional anthelmintic compounds may be discovered among Pseudomonas spp. Metabolites (Devaraj et al., 2019). This highlights the potential of Pseudomonas species as versatile biocontrol agents capable of addressing multiple agricultural pest problems.
5.5 Type VI secretion systemThe Type VI Secretion System (T6SS) has emerged as a pivotal weapon in bacterial warfare, allowing bacteria to deliver toxic effectors directly into neighboring cells (Mougous et al., 2006; Pukatzki et al., 2006). T6SSs are present in more than 25% of gram-negative bacteria (Ho et al., 2014). The T6SS is a contact-dependent secretion system that plays a crucial role in interbacterial competition (Mougous et al., 2006; Pukatzki et al., 2006). It provides a selective advantage to producer strains by annihilating competitors either in an indiscriminate manner or in response to danger signals (Basler et al., 2013; Durán et al., 2021; Ho et al., 2014; Hood et al., 2010). The T6SS was originally described in Vibrio cholerae and P. aeruginosa as a proteinaceous nanomachine that translocates specific proteins directly into target cells (Mougous et al., 2006; Pukatzki et al., 2006). It was later observed and analyzed in many other bacterial pathogens (Burtnick et al., 2011; De Pace et al., 2010; Murdoch et al., 2011; Suarez et al., 2008). However, the analytical description of T6SS in non-pathogenic bacteria is underrepresented in the scientific literature (Bernal et al., 2017; Durán et al., 2021; Marchi et al., 2013).
The killing activity conferred by antibacterial T6SSs can be extremely potent during in vitro co-culture experiments, with T6SS-wielding cells often able to virtually eliminate similar numbers of susceptible competitor cells within a few hours (Basler et al., 2013; Durán et al., 2021; Ho et al., 2014). Antibacterial T6SSs are frequently found in both pathogenic and symbiotic or beneficial plant-associated bacteria (Bernal et al., 2018; Durán et al., 2021). This suggests that antibacterial T6SSs may be involved in establishing and protecting beneficial plant-associated communities from invasion of these communities by pathogens. For example, the T6SS-dependent antibacterial activity in the rhizosphere bacterium P. putida KT2440 contributes to reducing colonization and necrosis induced by the phytopathogen Xanthomonas campestris when both are co-infiltrated into Nicotiana benthamiana leaves (Bernal et al., 2017).
Further in planta studies show that T6SS in P. fluorescens MFE01 play crucial role in protecting potato tubers from Pectobacterium atrosepticum infection (Bourigault et al., 2023). Fluorescence microscopy revealed that MFE01 exhibits an aggressive T6SS behavior with continuous and intense firing activity, causing rounding and lysis of target P. atrosepticum cells. The study identified a putative T6SS-secreted amidase effector, Tae3Pf, as a major contributor to MFE01’s antibacterial activity. This effector was found to be toxic when produced in the periplasm of Escherichia coli, with its toxicity neutralized by the inner membrane immunity protein Tai3Pf. While Tae3Pf plays a significant role, the research suggests that other T6SS effectors likely contribute to MFE01’s overall antibacterial activity (Bourigault et al., 2023). These findings highlight the importance of the T6SS in P. fluorescens MFE01’s biocontrol capabilities, offering new perspectives on bacterial antagonism in the context of plant protection and potentially leading to the development of more effective biocontrol strategies in agriculture.
6 Indirect inhibition of phytopathogensIn addition to direct inhibition via the production of antagonistic compounds, Pseudomonas spp. demonstrate a remarkable capacity to indirectly inhibit plant pathogens (Haney et al., 2018; Leeman et al., 1995). Competitive exclusion is one of the primary mechanisms through which Pseudomonas strains exert their inhibitory effects (Archetti et al., 2011; Buddrus-Schiemann et al., 2010; Rainey, 1999). By occupying niche spaces and consuming available resources, these strains effectively thwart the colonization efforts of pathogens, preventing them from establishing robust populations and causing harm to plants (Archetti et al., 2011; Buddrus-Schiemann et al., 2010; Rainey, 1999). This strategy, however, does not operate in isolation; rather, it complements direct antagonism, wherein Pseudomonas spp. secretes antimicrobial compounds that further hinder pathogen growth and proliferation (Haney et al., 2018; Leeman et al., 1995). These antimicrobials may arise as byproducts of interference competition over resources provided via plant root exudates or organic matter in the soil, illustrating the multifaceted nature of Pseudomonas-mediated inhibition.
Pseudomonas spp. possess the ability to activate host resistance pathways, thereby inducing systemic resistance (ISR) in plants (Table 3) (Chen et al., 1999; Elsharkawy et al., 2022; Haney et al., 2018; Hoffland et al., 1996; Leeman et al., 1995; Meziane et al., 2005; Nandakumar et al., 2001). ISR is a state of enhanced defensive capacity developed by a plant reacting to specific biotic or chemical stimuli. Pseudomonas spp. mediate ISR through phytohormone defense signaling pathways such as jasmonic acid/ethylene (JA/ET) or salicylic acid (SA) (Chen et al., 1999; Pangesti et al., 2016). For instance, a study investigated the efficacy of Pseudomonas corrugata strain 13 and Pseudomonas aureofaciens strain 63–28 in inducing systemic resistance against Pythium aphanidermatum in cucumber roots (Chen et al., 1999). Both bacterial strains were found to produce SA in vivo and induced cucumber roots to accumulate endogenous SA within a day of inoculation (Chen et al., 1999). Notably, plants treated with a Pseudomonas strain showed significantly elevated SA levels compared to the control, persisting from 1 to 5 days post-bacterization (Chen et al., 1999). Interestingly, SA inhibited the mycelial growth of the pathogen P. aphanidermatum at higher SA concentrations (Chen et al., 1999).
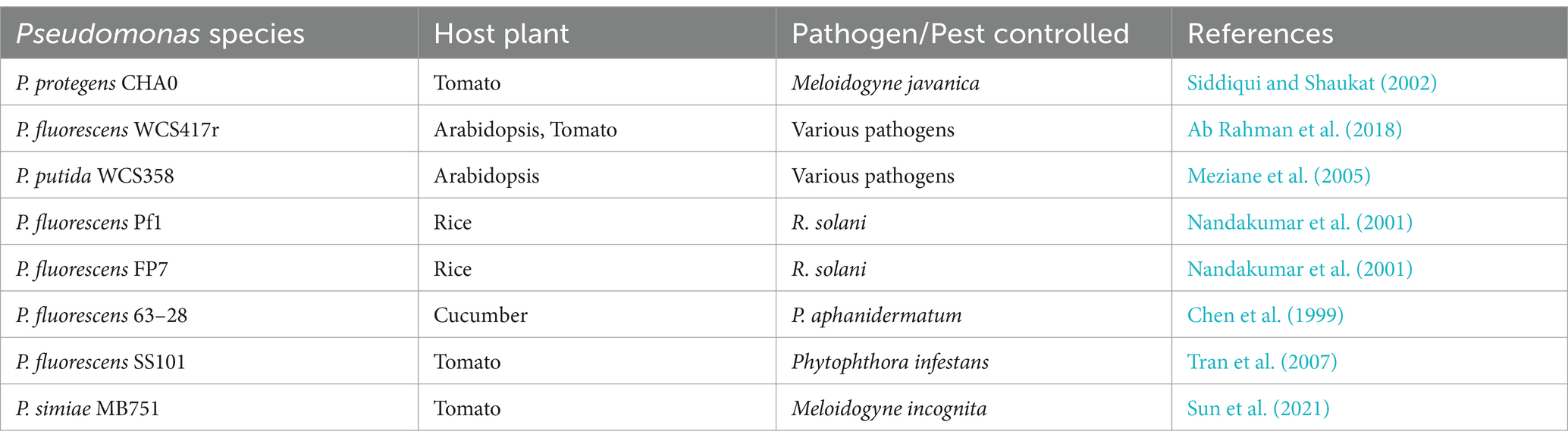
Table 3. Pseudomonas species inducing systemic resistance (ISR) in plants.
In response to infections by various pathogens, such as fungi, bacteria, or nematodes, plants often produce stress ethylene (Abeles et al., 1992; Iqbal et al., 2017). The symptoms observed in an infected plant largely stem from the stress caused by the pathogen (Iqbal et al., 2017; Van Loon, 1984). A significant portion of the damage to infected plants results from the plant’s response to increased levels of stress ethylene (Stearns and Glick, 2003). Exogenous ethylene can exacerbate pathogen infections, while chemical inhibitors of ethylene synthesis can reduce their severity (Stearns and Glick, 2003; Yang et al., 2017). Additionally, pretreating plants with ACC deaminase-containing plant growth-promoting bacteria can provide significant protection against ethylene-induced damage from pathogen infections (Glick et al., 1995; Hao et al., 2011; Toklikishvili et al., 2010; Wang et al., 2000). Some bacteria that promote plant growth encode the enzyme ACC deaminase, which breaks down ACC exudates from plants, the direct precursor of ethylene production, and lowers the amount of ethylene that plants produce in response to different stressors (Glick et al., 1995; Hao et al., 2011). Many other studies showed that the application of ACC-deaminase-producing plant-growth-promoting bacteria was found to inhibit the growth of phytopathogens in crops (Toklikishvili et al., 2010; Wang et al., 2000).
The ability of Pseudomonas spp. to induce plant disease resistance holds significant promise for its application as a biocontrol agent (Haney et al., 2018; Leeman et al., 1995). Strains that exhibit such abilities may prove highly effective at protecting their plant hosts against pathogenic infections in situ, even if they demonstrate poor bioactivity against phytopathogens in vitro. This underscores the importance of screening for biocontrol strains based not only on their performance in traditional in vitro bioactivity assays but also on their ability to elicit host defenses and confer protection to host plant species in vivo. As research in this field progresses, better proxies, and methodologies for evaluating the efficacy of Pseudomonas strains as biocontrol agents should be developed, considering the intricate interplay between bacterial-induced plant defenses and pathogen susceptibility.
7 The potential use of Pseudomonas species as effective biocontrol agentsMany studies demonstrate promise in laboratory settings but exhibit inconsistent efficacy in agricultural settings (Arora et al., 2008; Garbeva et al., 2004; Lee H. S. et al., 2000; Rini and Sulochana, 2007). The inconsistent results of biocontrol treatments show we need to better understand what affects soil and root microbes. Abiotic factors such as soil type, climate, and farming practices, along with biotic factors like host crop species and root exude profiles, profoundly influence microbial assemblages and biocontrol success (Babin et al., 2019; Classen et al., 2015; Edwards et al., 2015; Glick and Gamalero, 2021; Rousk et al., 2010). Efforts such as the Microbiome Stress project aim to understand bacterial community responses to environmental stressors, aiding in the development of robust biocontrol strategies resilient to changing environmental conditions and advancing sustainable agriculture practices (Rocca et al., 2019).
7.1 Abiotic factors influencing biocontrol efficacyNumerous studies have focused on enhancing the effectiveness and consistency of Pseudomonas spp. biocontrol agents through various agricultural practices and interventions (Zhang et al., 2015). This includes the identification of several minerals and carbon sources that have a differential influence on the production of the antibiotics 2,4-diacetylphloroglucinol, pyoluteorin and pyrrolnitrin and the salicylic acid and pyochelin by disease-suppressive strains of P. fluorescens (Duffy and Défago, 1999). For example, the addition of glycerol, zinc and ammonium molybdate to P. protegens CHA0 has shown promise in increasing its biocontrol efficacy against Meloidogyne javanica (Hamid et al., 2003; Siddiqui and Shaukat, 2002). Additionally, adding zinc to soil containing P. fluorescens PfAs1 has enhanced the expression of genes resistant to bacterial leaf blight in rice, while also significantly reducing bacterial leaf blight in rice caused by X. oryzae pv. oryzae (Sharma et al., 2020). Similarly, supplementing P. fluorescens NK2 with the zinc oxide nanoparticle (ZnO-NP) enhances the inhibition efficacy against the pathogenic bacterium Pseudomonas viridiflava NK2 in cucumber (Al-Karablieh et al., 2022).
Beyond strain inoculation, various agricultural practices significantly impact the composition and establishment of species within the plant root microbiome. Practices like irrigation, tillage, and cropping methods can play crucial roles (Babin et al., 2019; Dennert et al., 2018; Mavrodi et al., 2018). Agro-chemicals such as pesticides and fertilizers also shape the plant root and soil microbiome, offering protection against crop diseases (Ding et al., 2013; Shen et al., 2019). For instance, ammonia fumigation effectively suppresses Fusarium wilt disease in bananas (Musa acuminate Cavendish) while inducing shifts in the soil microbial community, notably reducing Fusarium species abundance (Shen et al., 2019). Studies suggest that combining organic fertilizers with biocontrol strains enhances disease suppression further. Thus, the addition of biocontrol strains to organic fertilizers before application creates a more conducive soil environment, fostering nutrient availability, root colonization, and biocontrol effectiveness, a strategy known as bio-organic fertilizer application, widely recognized for its efficacy in disease suppression (Ding et al., 2013; Ketabchi et al., 2016; Watanabe et al., 1987).
Conversely, certain chemical additives and carbon compounds have been found to impede the biocontrol efficacy of Pseudomonas spp. For example, the addition of glucose can hinder the efficacy of P. protegens CHA0 against Meloidogyne javanica (Siddiqui and Shaukat, 2002). However, some Pseudomonas strains exhibit a high tolerance to commonly used fungicidal compounds, paving the way for synergistic approaches where chemical and biological pest control methods can be combined to enhance efficacy while reducing the overall pesticide dose (Anand et al., 2010; Kataria et al., 2002).
While these findings suggest the potential for optimizing farming practices to maximize disease suppression, comprehensive research in this area remains limited. The complexity of agricultural ecosystems and the variability in pathogen dynamics necessitates further investigation to identify the most effective strategies tailored to specific pathogens, climatic conditions, and soil characteristics. Nonetheless, exploring the interplay between Pseudomonas spp. biocontrol agents, agricultural practices, and chemical additives hold promise for sustainable disease management and crop protection.
Optimizing biocontrol delivery systems involving PseudomonasNumerous methods exist for delivering biocontrol strains to crops, each potentially influencing the consistency of biocontrol strategies (Jambhulkar et al., 2016; Preininger et al., 2018). Foliar spraying may seem appealing, especially in developed countries with available spraying equipment, but microbial suspensions can damage or clog machinery due to settling out of solution, and stresses from spraying apparatus (e.g., heat stress, shearing forces) can reduce biocontrol strain viability (Figure 2) (Preininger et al., 2018). Moreover, foliar spray that is suitable for microbial inoculants targeting foliar diseases (Jambhulkar et al., 2016) is often less effective for controlling root diseases like wheat take-all. Soil inoculation, another recommended method, is often employed if biocontrol strains are susceptible to desiccation (Jambhulkar et al., 2016). As discussed earlier, methods such as bio-organic fertilizer application (Ding et al., 2013; Ketabchi et al., 2016; Watanabe et al., 1987) can enhance biocontrol strain success. However, these strategies may increase the expense and complexity of applying disease-suppressive measures, while also potentially altering soil chemistry and microbiome composition with unknown or conflicting effects (Babin et al., 2019; Shen et al., 2019).
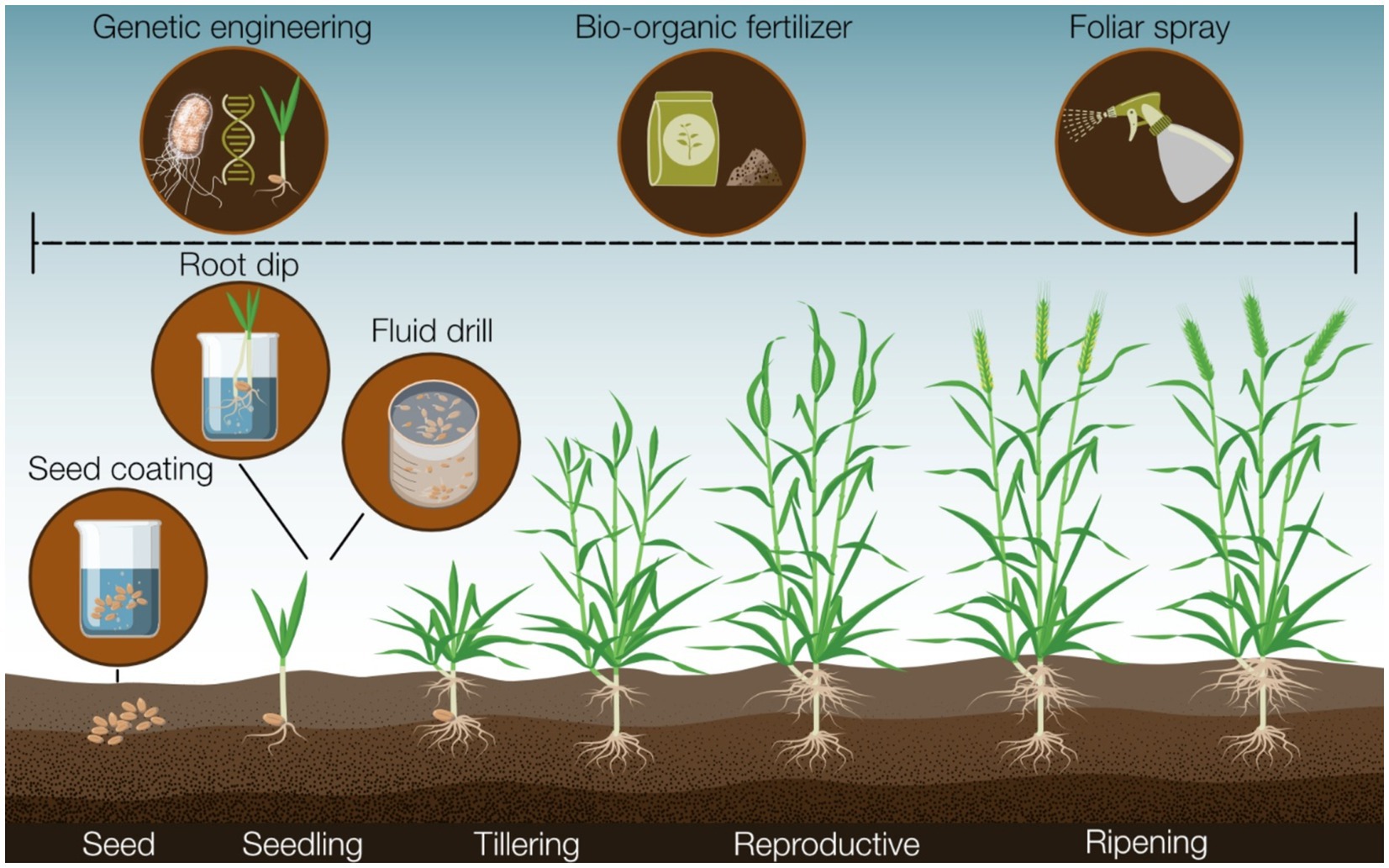
Figure 2. A summary of the major tools and methods available that could facilitate the application of biocontrol strains that can influence biocontrol efficacy in the field.
Direct inoculation of biocontrol strains onto plant roots offers a strategy to bypass challenges associated with soil-survivability as the strain bypasses exposure to an environmental medium before colonizing roots (Jambhulkar et al., 2016; Pill, 1991). Techniques like fluid drill inoculation and root transplant dip exemplify this approach, allowing biocontrol agents to colonize roots under controlled conditions (Jambhulkar et al., 2016; Pill, 1991). In root dip, seedling roots are immersed in a liquid cell suspension before field transfer, while in fluid drill methods, seeds pre-germinate within a gel containing the biocontrol strain (Jambhulkar et al., 2016;
留言 (0)