This work may not be copied, distributed, displayed, published, reproduced, transmitted, modified, posted, sold, licensed, or used for commercial purposes. By downloading this file, you are agreeing to the publisher’s Terms & Conditions.
Have you ever been uncertain how to distinguish tardive dyskinesia (TD) from other movement disorders? Have you been unsure about who is at greatest risk for developing TD? Have you wondered whether TD can be prevented or successfully treated? If so, the following case vignette and discussion should prove useful.
CASE VIGNETTEMs P, a 58-year-old woman who developed schizophrenia in her early twenties, was started on the antipsychotic medication haloperidol at the time of her first psychotic episode. Early in her treatment, she developed akathisia, which was managed with propranolol. Due to a partial response of psychosis with a low dose of haloperidol, her dose was gradually increased to 30 mg per day over several years. Ms P remained psychiatrically stable on this dose for many years with occasional auditory hallucinations; however, she displayed a flat affect, poverty of speech, and amotivation.
Over the past 4 months, Ms P developed unusual movements involving her face (with intermittent grimacing) and tongue (which would dart in and out of her mouth). One of her neighbors mentioned that she had seen similar movements in Ms P roughly 1 year earlier, but Ms P had not noticed any abnormal movements. More recently, both the neighbor and Ms P noticed that the movements were increasingly common and more intense. She was unable to control these movements and found them distressing. The movements made it hard for her to eat, as food would often fall from her mouth to the floor. She also thought that people had been staring at her when she was in public; as a result, she stayed home more often. Ms P asked her psychiatrist about these symptoms at her next appointment.
DISCUSSION What Is TD, and What Causes It?TD is a hyperkinetic movement disorder caused by chronic exposure to dopamine receptor–blocking agents, such as antipsychotics1; the involuntary, rhythmic, repetitive, and stereotyped movements typically involve muscles of the face, lips, jaw, tongue, upper extremities, lower extremities, and trunk. Respiratory muscles may also be involved. While patients are often initially unaware of or unbothered by these movements, TD is often irreversible, disfiguring, and societally stigmatized, which likely explains its associations with poor daily functioning and adverse psychosocial outcomes.1 Therefore, providers should be vigilant for the emergence of TD and proactively mitigate symptom progression.
Dopamine receptor blockade (eg, from use of first generation antipsychotics [FGAs], second-generation antipsychotics [SGAs], and certain antiemetics [eg, metoclopramide and prochlorperazine]) can precipitate TD.2 The most widely accepted pathophysiological mechanism for the genesis of TD is through dopamine super-sensitivity: chronic exposure to D2 blockade leads to upregulation of postsynaptic receptors, which heightens sensitivity to dopamine in the basal ganglia, thus creating hyperkinetic movements.2 As such, medications with a greater propensity to block dopamine (such as FGAs, like haloperidol or fluphenazine) confer a greater risk than do low-potency SGAs (eg, quetiapine and clozapine).3 Moreover, larger cumulative doses are associated with a greater risk of TD, whether through higher doses or longer length of time exposed to dopamine blockade.2
How Common Is TD?The risk of TD increases with a greater cumulative exposure to dopamine receptor blockade. At the same time, the incidence and prevalence of TD vary widely across groups.
A 2017 meta-analysis of 41 studies between the years 2000 and 2015 estimated that the global mean prevalence of TD was 25% among patients receiving an antipsychotic.4 Patients with current FGA treatment had a prevalence of 30%, while those with current SGA treatment had a prevalence of 21%; importantly, prior FGA exposure was associated with more than a tripling of the prevalence.4 Studies of TD incidence are somewhat heterogeneous; a 2008 meta-analysis with over 28,000 patients found that the annualized TD incidence was 5.5% among patients on an FGA and 3.9% for those on an SGA.5 Among older adults, annualized incidence rates are substantially higher at 23% and 7%, for those prescribed FGAs and SGAs, respectively.3
How Does It Feel to Have TD, and How Do Others React?The involuntary movements of TD, especially of the orofacial area, can markedly interfere with a patient’s emotional, social, and professional functioning, largely due to the negative reactions of others.6 Studies have shown that TD (especially frowning, tongue thrusting, lip smacking, or puckering) frequently leads to unwanted social attention, embarrassment, and social isolation, as well as difficulty with activities of daily living.7 Moreover, large proportions of TD patients endorse work absenteeism, presenteeism, and overall work impairment, greater than among those with complications associated with lung or breast cancer.6 Broadly, TD is associated with lower quality of life and higher mortality rates than many other medical conditions.7
What Looks Like TD But Is Not?To receive a diagnosis of TD secondary to neuroleptic use, patients must have symptoms (characterized by stereotyped muscle movements of the face, trunk, neck, and/or limbs8) for at least 1 month and must have been exposed to an antipsychotic for at least 3 months. Notably, patients may demonstrate withdrawal dyskinesia after the cessation of a neuroleptic medication; unlike TD, these movements are usually short-lived and resolve spontaneously.
Involuntary movements of other etiologies and non-TD movement disorders are frequently mistaken for TD. For example, edentulous dyskinesia, which occurs in 10%–20% of edentulous individuals and can be correlated with poor denture fit, can mimic the buccolingual movements seen in TD.9 Notably, edentulous dyskinesia is almost never associated with tongue movements, which often accompany the orofacial movements of TD. Huntington disease, a hereditary and progressive neurodegenerative disease, causes choreiform movements that can be mistaken for TD; however, Huntington disease can be differentiated from TD by examining the family history and by genetic testing.10 Another possible mimic is benign essential blepharospasm, a movement disorder that causes involuntary dystonic movements of the eyelid.11 Meige syndrome, a progressive dystonic movement disorder that involves the muscles of the eyelids and oromandibular area, presents much like TD, but may be differentiated by it beginning as a focal dystonia—often with unilateral blepharospasm—before spreading to other muscles of the face, neck, and limbs.12 Finally, tic disorders, restless leg syndrome, Wilson disease, Fahr syndrome, and systemic lupus erythematosus can all cause abnormal movements that can be delineated from those of TD.13
To aid in the diagnosis, patients should be questioned thoroughly about all the medications and supplements they have taken over the last 6 months. It is also crucial to take a complete history of exposure to potential toxins. Providers should consider obtaining an electroencephalogram to rule out complex partial seizures. When there is diagnostic uncertainty, additional testing (eg, imaging and genetic testing) and consultation with a neurologist are recommended to rule out non-TD causes of involuntary movements.
Who Is at Greatest Risk for Developing TD?Identification of patients at greatest risk for developing TD is crucial for its prevention and early treatment. Table 1 summarizes risk factors (eg, older age, female sex, a preexisting mood disorder, and a longer-duration treatment with FGA agents) associated with the development of TD.14–16 A genetic basis for TD has not been fully established. However, some evidence suggests that TD has been linked with polymorphisms of the dopamine D2 receptor, dopamine D3 receptor, Ser9Gly, and serotonin 2A and 2C receptor genes.17,18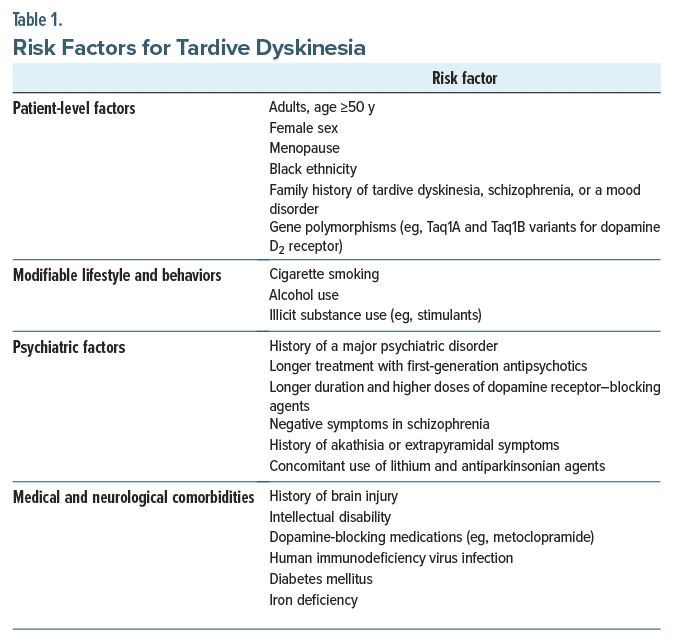

Several psychiatric risk factors contribute to the development of TD, such as schizophrenia and mood disorders that require long-term treatment with antipsychotic medications. In addition, higher severity and prolonged chronicity of psychiatric illnesses often necessitate use of higher antipsychotic dosages, which further potentiates TD risk. Certain medical and neurological conditions also elevate the risk of developing TD; for instance, those with diabetes mellitus who have been treated with antipsychotics are more likely to develop TD than those without diabetes, possibly due to the complex interactions between metabolic factors and the neurological effects of antipsychotic medications.19 Given that the proposed mechanism by which age increases TD risk is an age related decrease in dopaminergic neurons in the substantia nigra,20 it is likely that the presence of a neurodegenerative illness (especially Parkinson disease and dementia with Lewy bodies) increases risk for TD; however, an association between dementias and TD has not been explicitly or consistently demonstrated in the literature.21
What Type of Monitoring Should Be Employed to Identify TD Early In Its Course?TD usually manifests after long-term use of dopamine receptor antagonists, but it can occur as soon as 1 month after such drugs are initiated. The Abnormal Involuntary Movement Scale (AIMS) is a clinician-administered assessment of TD severity that can assist in the early recognition of TD.22,23 The American Psychiatric Association guidelines recommend using the AIMS at the outset of treatment with antipsychotics and then at least annually, although more frequent monitoring (eg, every 6 months) is recommended for patients at high risk of TD, such as older patients and those taking FGAs.24 Unfortunately, TD is often irreversible; thus, it is critical to detect TD early in its course and change medications to those that are less likely to cause TD.
Which Treatments Can Mitigate the Manifestations of TD?TD is an iatrogenic condition that can be caused by use of myriad medications (including neuroleptics, antiemetics, antitussives, calcium channel blockers, lithium, and serotonin reuptake inhibitors).25 When patients present with early symptoms of TD, the medication regimen should be scrutinized for an offending agent—typically a dopamine receptor–blocking agent—and consideration should be given to tapering and discontinuing the offending agent. This is especially true for high-risk patients such as the elderly, where mitigating progression is considered the best option.21 Unfortunately, only a significant minority of individuals with TD (13%) experience remission of tardive symptoms after medication cessation.26
Several pharmacologic and procedural approaches can mitigate the manifestations of TD. The most effective of these is vesicle monoamine transporter-2 (VMAT-2) inhibitors, which deplete presynaptic dopamine levels.27 Deutetrabenazine and valbenazine have been US Food and Drug Administration approved since 2017 for the treatment of TD, and multiple studies have confirmed their effectiveness in decreasing symptoms.28,29 Of note, both these medications have also demonstrated comparable efficacy and tolerability in older adults over the age of 55 years.21 However, adverse effects of VMAT-2 inhibitors include gait disturbance, orthostatic hypotension, parkinsonism, dyskinesias, and, rarely, neuroleptic malignant syndrome. Therefore, caution should be exercised when prescribing VMAT-2 inhibitors to older adults with neurodegenerative illnesses, who may be predisposed to these reactions.30 Tetrabenazine, from which deutetrabenazine and valbenazine are derived, is also effective for reducing symptoms of TD; however, its use has been limited due to its adverse effects.31 Unfortunately, TD symptoms often return when VMAT-2 inhibitors are discontinued.
Benzodiazepines, specifically longer-lasting ones such as clonazepam, have demonstrated efficacy for treating TD, particularly in patients with more dystonia predominant symptoms.32 It is unclear if short-acting benzodiazepines (eg, alprazolam) have efficacy in treating TD.33 Amantadine, an N-methyl-D-aspartate antagonist, has some efficacy in the treatment of TD, though the studies establishing this have been small and based on short-term (<1 month) treatment.34 Gingko biloba extract, a supplement commonly used in herbalist medicine traditions, has also reduced symptoms of TD.35 Research of other pharmacologic treatments (eg, β blockers, baclofen, vitamins B6 and E, zolpidem, acetazolamide, and levetiracetam) has been inconclusive.32
Nonpharmacologic options exist for treatment of TD. Deep brain stimulation, targeting either the globus pallidus or subthalamic nucleus, has been used off label with significant success in patients with severe and disabling TD.36 A few small, uncontrolled trials have been done with injections of botulinum toxin type A; the results have not demonstrated clear efficacy in treatment of TD.32
Case Vignette: What Happened to Ms P?At Ms P’s next appointment, her psychiatrist conducted an AIMS examination. She scored a 3 (moderate severity) in 2 areas (muscles of facial expression and tongue), thus meeting consensus criteria for a diagnosis of TD. She lacked signs and symptoms that might have been suggestive of any other movement disorder. Ms P reviewed the treatment options with her psychiatrist, including switching to an antipsychotic medication with a lower risk of causing or exacerbating TD (such as clozapine) or starting symptomatic treatment with a VMAT-2 inhibitor. Because she had been stable on haloperidol for many years, she was reluctant to change her antipsychotic regimen and therefore decided to start valbenazine, a VMAT-2 inhibitor. Valbenazine was extremely helpful in controlling her abnormal movements, and it did not induce adverse side effects. Ms P became less distressed about her movements, and she could more comfortably eat and leave her home again.
CONCLUSIONTD is an involuntary hyperkinetic movement disorder involving the muscles of the face, lips, jaw, tongue, upper extremities, lower extremities, and trunk, which can markedly interfere with a patient’s emotional, social, and professional functioning. It is caused by chronic exposure to dopamine receptor–blocking agents (such as antipsychotics); the risk of TD increases as the cumulative exposure to dopamine receptor blockade increases. To receive a diagnosis of TD secondary to antipsychotic use, patients must have symptoms for at least 1 month and must have been exposed to a neuroleptic for at least 3 months. Left untreated, TD is typically irreversible, disfiguring, and societally stigmatized; however, several pharmacologic strategies such as switching antipsychotics or VMAT-2 inhibitors can prove efficacious.
Article InformationPublished Online: November 19, 2024. https://doi.org/10.4088/PCC.24f03706
© 2024 Physicians Postgraduate Press, Inc.
Submitted: January 10, 2024; accepted April 24, 2024.
To Cite: Daneshvari NO, Vyas CM, Lim CS, et al. Tardive dyskinesia: etiology, prevention, and management. Prim Care Companion CNS Disord. 2024;26(6):24f03706.
Author Affiliations: Department of Psychiatry, Massachusetts General Hospital and Harvard Medical School, Boston, Massachusetts (all authors).
Corresponding Author: Nicholas O. Daneshvari, MD, Department of Psychiatry, Massachusetts General Hospital, 15 Parkman St, WACC 812, Boston, MA 02114 ([email protected]).
Drs Daneshvari, Vyas, Lim, Donovan, and Lissanu are co-first authors.
Funding/Support: None.
Relevant Financial Relationships: Dr Vyas has received research support from Nestlé-Purina Petcare Company, Mars Edge, and American Foundation for Suicide Prevention.
留言 (0)