Moyamoya disease (MMD) is characterized by chronic progressive stenosis of the terminal internal carotid artery and an abnormal vascular network at the base of the skull (1). The exact cause of this distinctive cerebrovascular condition remains unknown. Predominantly observed in East Asia, patients with MMD present with various clinical symptoms, including ischemia, hemorrhage, and epilepsy (2). The prevalence of asymptomatic MMD patients is increasing due to the widespread use of non-invasive imaging techniques, such as magnetic resonance angiography (MRA) (3). Previous studies have suggested that asymptomatic MMD is not a stable condition, and carries a risk of progressing to symptomatic manifestations, such as ischemia or hemorrhage (3, 4). A historical prospective cohort study in Japan revealed that asymptomatic patients managed conservatively faced an annual stroke risk of 3.2% (3).
The current effective treatment for MMD is cerebral revascularization, including extra-intracranial bypass surgery, indirect revascularizations, and combined revascularization (5). These interventions have shown benefits for symptomatic patients (6, 7). However, there is a lack of clear management options for asymptomatic patients. A study revealed that surgery did not effectively reduce the incidence of stroke progression in North American asymptomatic patients over a 5-year follow-up period (8). This highlights the necessity for a more precise characterization of asymptomatic patients to guide management strategies.
Several recent studies have proposed imaging risk factors for ischemia or hemorrhage in asymptomatic MMD (4, 9, 10). However, the majority of these studies have focused on adult patients, leaving a gap in research regarding pediatric patients, who represent another peak age group for the onset of MMD (11). This study aims to explore the potential contribution of angiographic variations in the increased stroke risk among pediatric patients.
Methods Participants and settingsWe retrospectively collected data on patients diagnosed with moyamoya disease who were consecutively admitted to Beijing Tiantan Hospital Affiliated to Capital Medical University from January 2019 to December 2023.
Enrolled patients in this study met specific criteria: (1) diagnosed with MMD by digital subtraction angiography (DSA) according to the 2021 guidelines (12); (2) aged under 18 years; (3) presented with asymptomatic manifestations, hemorrhagic events, or ischemic patterns, including cerebral infarction and transient ischemic attack (TIA); (4) had not undergone previous cerebral revascularization surgery. Patients diagnosed with unilateral, quasi-moyamoya disease or moyamoya syndrome were excluded from the study. Additionally, patients were excluded if they lacked sufficient angiographic or clinical data, or if they had primary epilepsy, congenital aphasia, or movement disorders. Asymptomatic MMD refers to patients who have not experienced TIA, ischemic stroke, hemorrhagic stroke, seizures or any other neurological deficits (4). These patients are typically identified due to unexplained dizziness, headaches, or incidental findings during physical examination. Two neurosurgeons independently reviewed and evaluated the cases, with any discrepancies resolved through thorough discussion in a roundtable meeting. This study was approved by the Ethics Committee of Beijing Tiantan Hospital Affiliated to Capital Medical University (KY2022-051).
Patients were classified into three groups based on their clinical presentations: hemorrhagic group, ischemic group, and asymptomatic group. The study included hemispheres from these patients, which were further divided into five subgroups based on the patient’s origin and location of symptoms. For instance, in patients with hemorrhagic MMD, the hemispheres were categorized into two groups: those with hemorrhages (hh-hemispheres) and those who are asymptomatic (ah-hemispheres). Similarly, the hemispheres with symptoms in patients with ischemic symptoms (including cerebral infarction and TIA) were designated as ii-hemispheres, while asymptomatic hemispheres were labeled as ai-hemispheres. The hemispheres of asymptomatic patients were identified as aa-hemispheres. The localization of hemorrhagic and ischemic hemispheres was determined based on clinical manifestations and imaging.
Angiographic evaluationPreoperative DSA of the included hemispheres was retrospectively analyzed by an image interpretation team consisting of two neurosurgeons with extensive experience in neuroimaging interpretation. The interpretation involved an assessment of Suzuki’s stage, moyamoya vessels, lenticulostriate artery (LSA), thalamotuberal artery (TTA), thalamoperforating artery (TPA), anterior choroidal arteries (AChA), posterior choroidal arteries (PChA) and posterior cerebral artery (PCA). Each hemisphere was classified into six stages according to Suzuki’s angiographic staging (13). Moyamoya vessels were categorized as absent (grade 0), sparse (grade 1), or dense (grade 2) (14, 15). The assessment of collateral vessels was conducted using the grading system established in supplementary studies of the Japanese Moyamoya Disease in Adults (JAM) trial (9, 16, 17), where grade 0 indicated normal vessels, grade 1 represented dilation with distal branches, and grade 2 represents dilatation with abnormal branch extension to provide blood supply to other areas, considered positive for dilation (Figures 1, 2). The extent of involvement of PCA was categorized into grade 0 for normal and grade 1 for stenosis or occlusion (Figure 2).
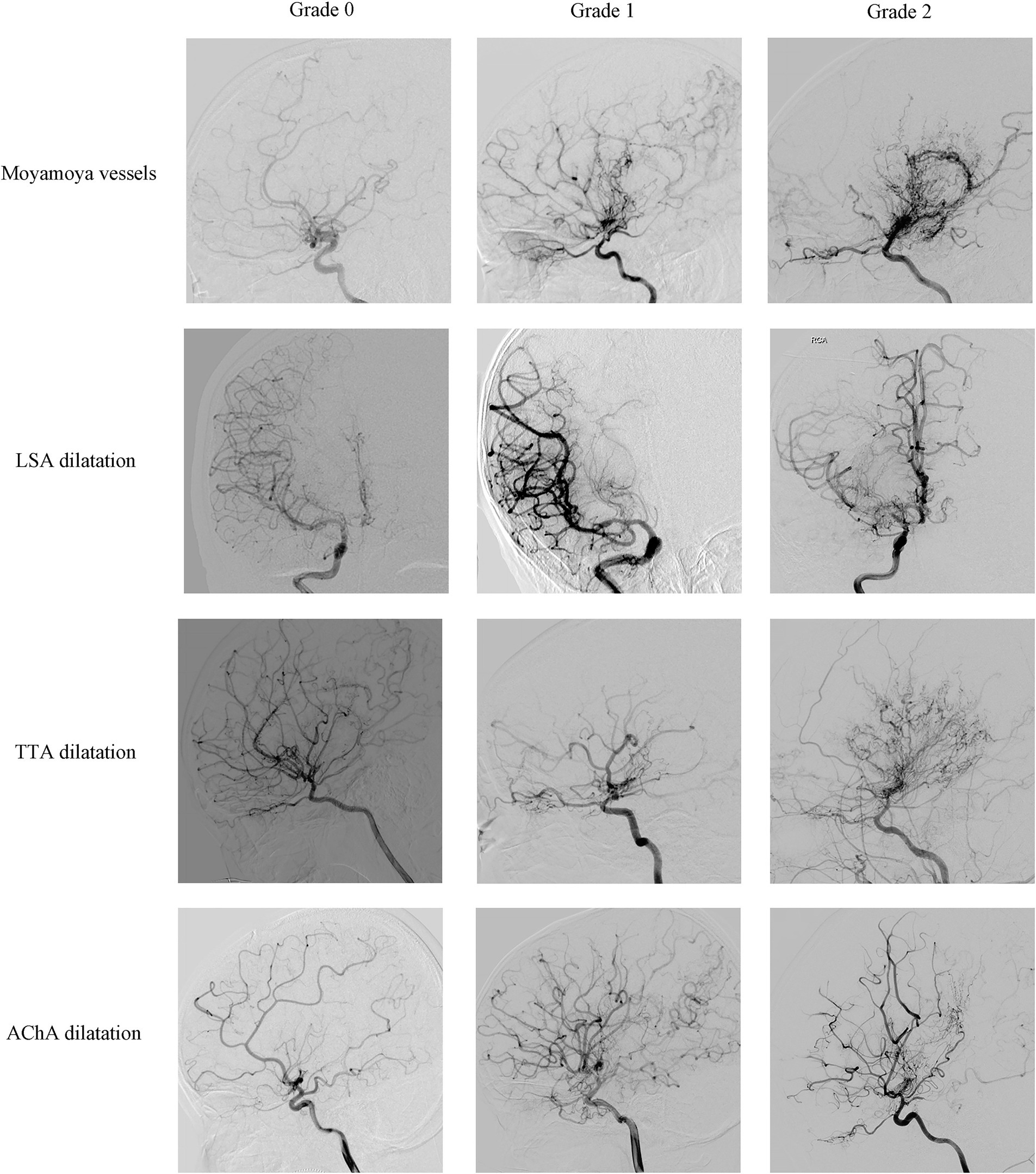
Figure 1. Representative cerebral angiography of the grading of moyamoya vessels, LSA dilatation, TTA dilatation and AChA dilatation. Moyamoya vessels were categorized as absent (grade 0), sparse (grade 1), or dense (grade 2); LSA dilatation, TTA dilatation and AChA dilatation were categorized as normal (grade 0), dilation with distal branches (grade 1), or dilatation with abnormal branch extension to provide blood supply to other areas (grade 2).
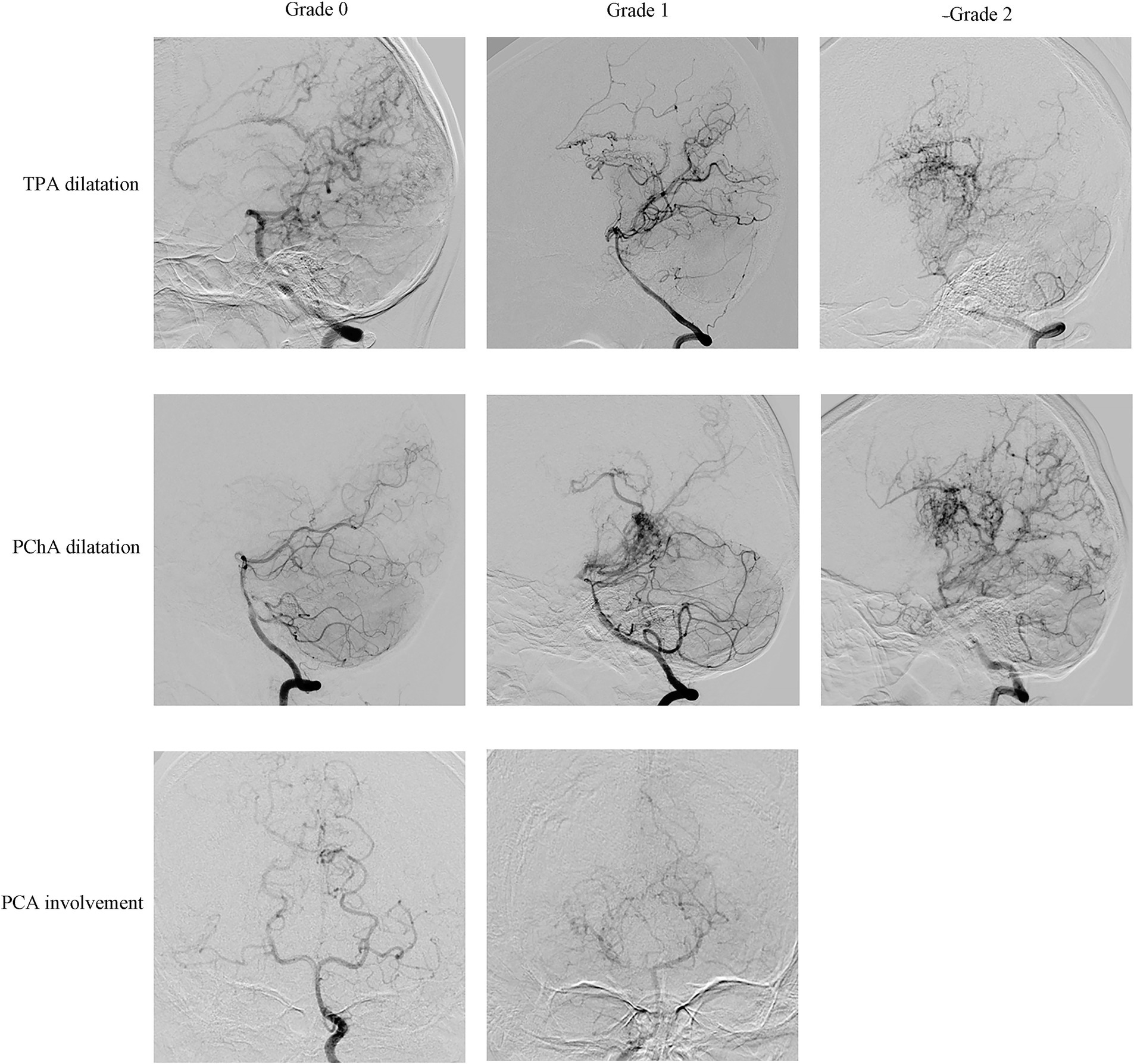
Figure 2. Representative cerebral angiography of the grading of TPA dilatation, PChA dilatation and PCA involvement. TPA dilatation and PChA dilatation were categorized as normal (grade 0), dilation with distal branches (grade 1), or dilatation with abnormal branch extension to provide blood supply to other areas (grade 2); PCA involvement was categorized as normal (grade 0), stenosis or occlusion (grade 1).
Statistical analysisAll statistical analyses were conducted using SPSS version 26.0 (SPSS Inc., Chicago, IL, United States). For continuous variables, either Student’s t-test or the Mann–Whitney U test was employed, while the chi-square test or Fisher’s exact test was utilized for categorical variables to compare baseline differences. Single-factor and multi-factor logistic regression models were used to identify risk factors. A significance level of p < 0.05 was set for the two-tailed tests.
Results Demographic and history informationThis retrospective study analyzed a total of 219 hospitalized patients, comprising 438 cerebral hemispheres. The cohort consisted of 204 consecutive cases from a five-year period and 15 individuals from a previous database, with 4 being asymptomatic and 11 experienced hemorrhages. The detailed inclusion and exclusion process is illustrated in the workflow diagram in Figure 3. Among 219 patients with MMD analyzed, 27 belonged to hemorrhagic group, 165 to the ischemic group, and 27 to the asymptomatic group. Demographic information showed that patients in the asymptomatic and ischemic groups were significantly younger compared to patients in hemorrhagic group (p < 0.001). There was no significant difference in sex distribution among the groups (Table 1). Regarding hemispheres, out of the 438 included, there were 31 hh-hemispheres, 225 ii-hemispheres, 23 ah-hemispheres, 105 ai-hemispheres, and 54 aa-hemispheres examined for angiographic characteristics.
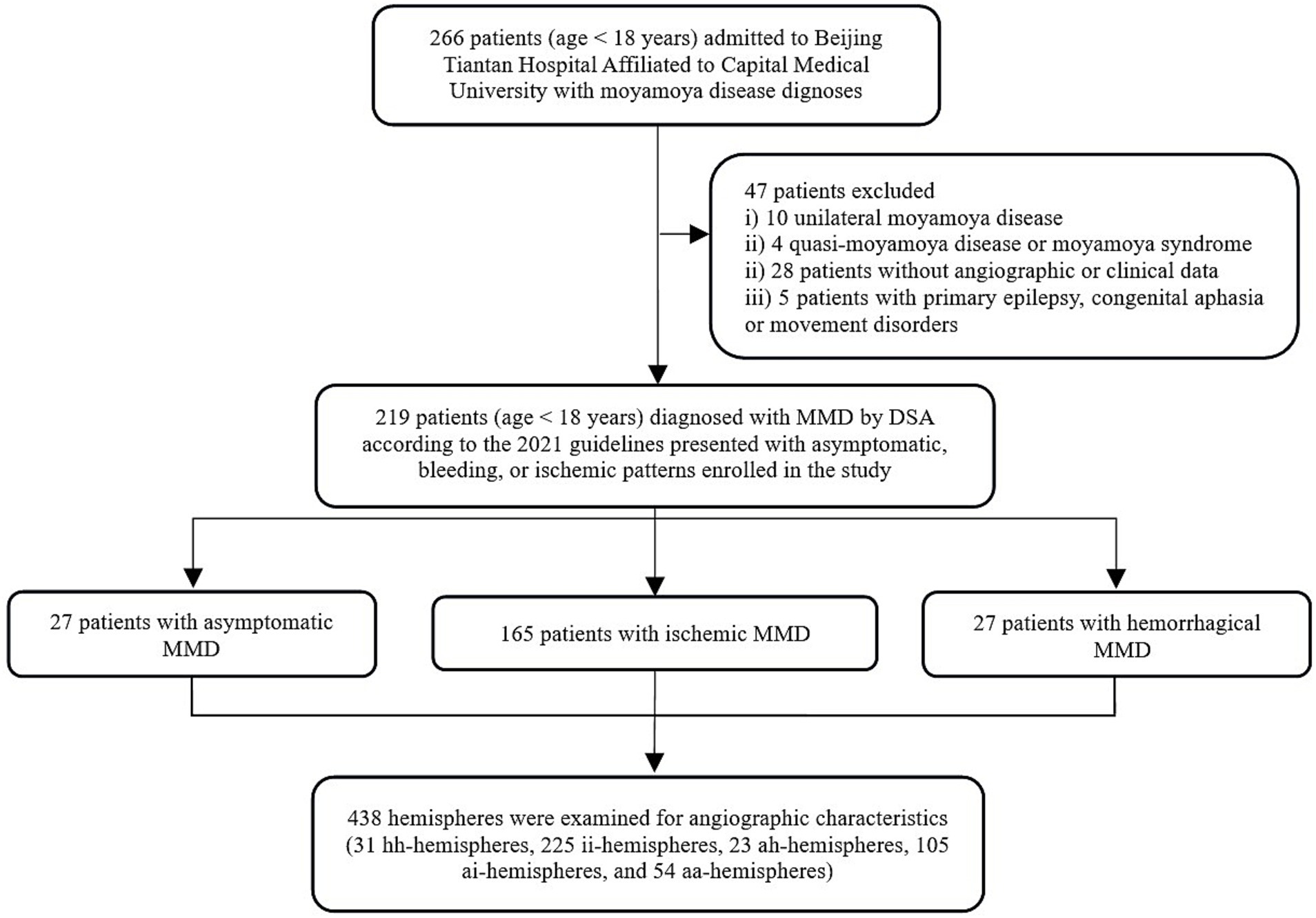
Figure 3. Workflow illustration diagram for study inclusion/exclusion process. MMD, moyamoya disease; DSA, digital subtraction angiography.
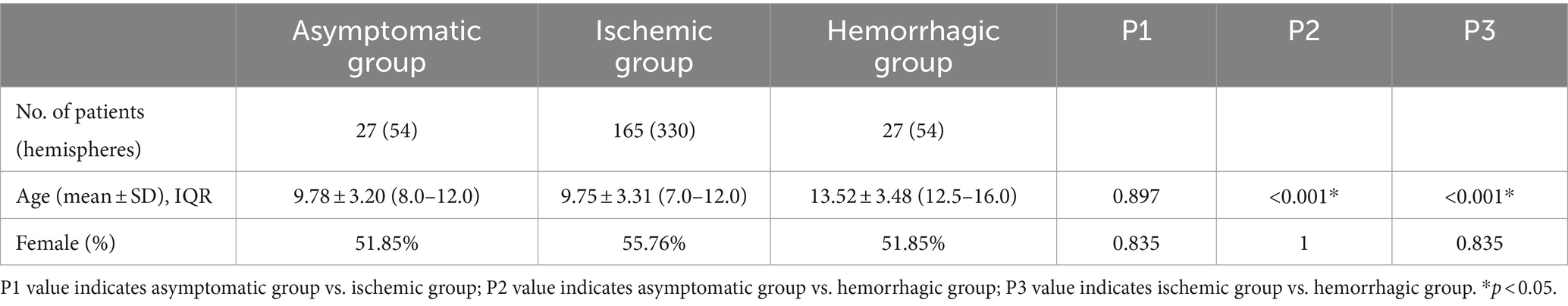
Table 1. Comparison of baseline characteristics of study cases.
Angiographic feature assessmentThe baseline angiographic characteristics of aa-hemispheres group, ii-hemispheres group, and hh-hemispheres group were compared in pairs, including Suzuki’s stage, moyamoya vessels, LSA dilation, TTA dilation, TPA dilation, AChA dilation, PChA dilation, and PCA involvement (Table 2). The positive rates of LSA (p = 0.040) and AChA (p = 0.024) in aa-hemispheres group were significantly higher than those in ii-hemispheres group, while TTA (p = 0.100), TPA (p = 0.619) and PChA (p = 0.280) exhibited no significant differences. However, the five collateral assessment results of aa-hemispheres group did not show significant differences compared to those of hh-hemispheres group. Additionally, the incidence of PCA involvement in aa-hemispheres group was notably lower compared to ii-hemispheres group and hh-hemispheres group (p = 0.026, p = 0.008). Multivariate analysis was conducted separately on aa-hemispheres group, ii-hemispheres group, and hh-hemispheres group, revealing one factor associated with infarction: Suzuki’s stage (p = 0.002, 95% CI 1.261–2.867). In addition, two factors were linked to hemorrhage: age (p < 0.001, 95% CI 0.712–2.014) and PCA involvement (p = 0.037, 95% CI 0.087–13.377) (Tables 3, 4).
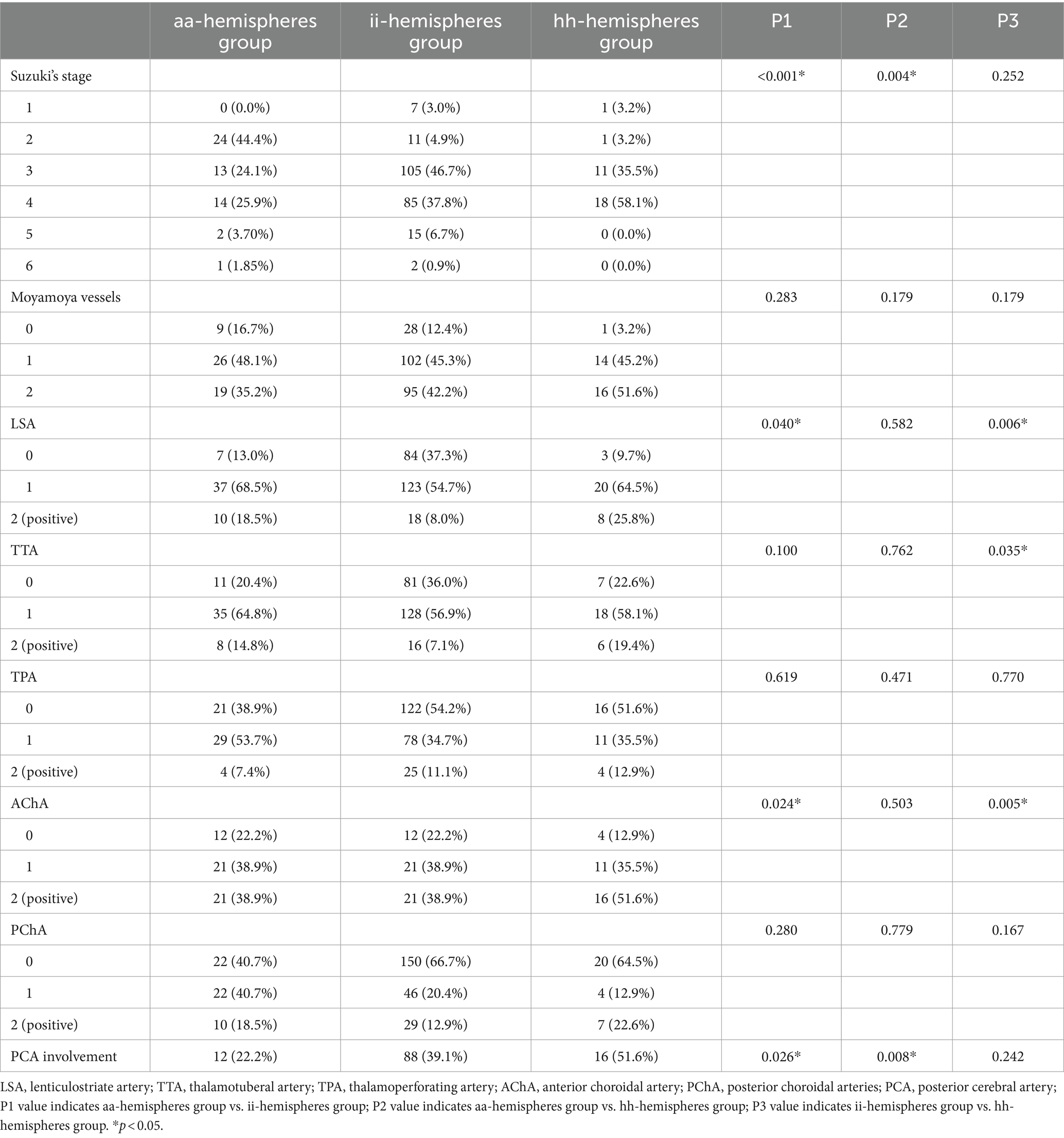
Table 2. Comparison of angiographic characteristics of hemispheres.
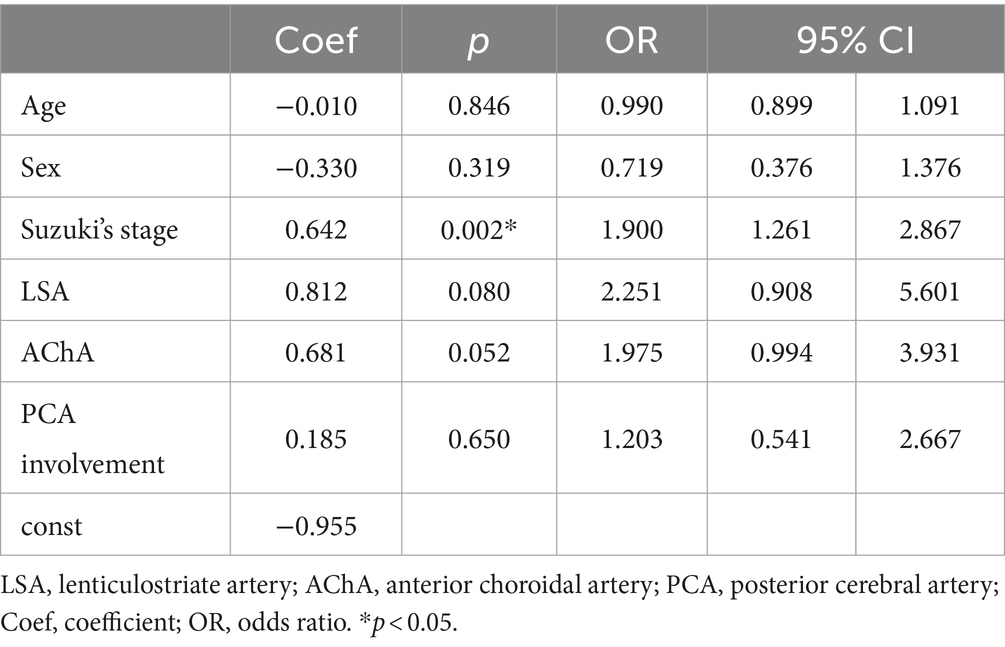
Table 3. Multivariate analysis of ischemia in hemispheres of pediatric patients with asymptomatic moyamoya disease.
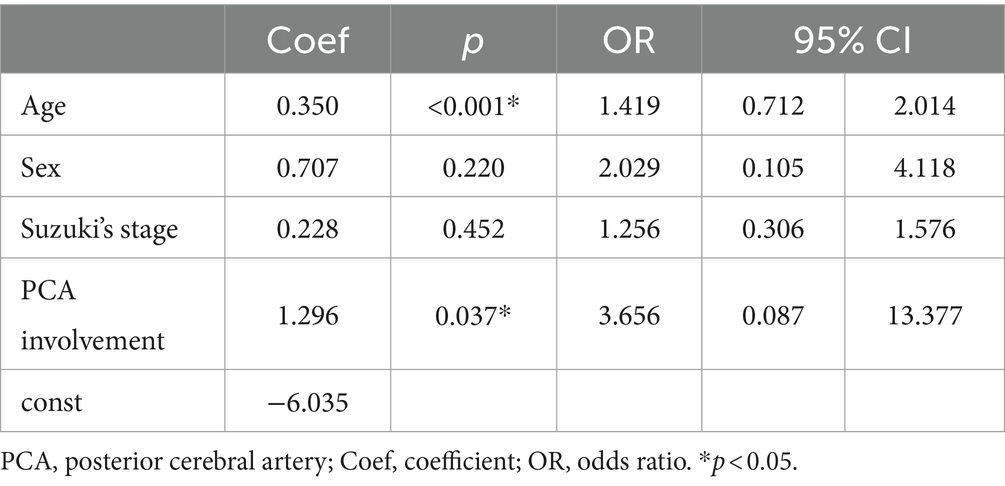
Table 4. Multivariate analysis of hemorrhage in hemispheres of pediatric patients with asymptomatic moyamoya disease.
The angiographic characteristics of aa-hemispheres group, ai/ah-hemispheres group, and ii/hh hemispheres group were further compared. The incidence of PCA involvement of aa-hemispheres group was significantly lower than ah-hemispheres group (p = 0.032). Additionally, Suzuki’s stage of ai-hemispheres group was significantly lower than that of ii-hemispheres group (p < 0.001). There were no significant differences in collateral dilation between ai-hemispheres group and ii-hemispheres group, as well as between ah-hemispheres group and hh-hemispheres group. More details can be found in Supplementary Table S1.
DiscussionOur study found that age and PCA involvement are two significant risk factors for hemorrhage in children with asymptomatic MMD. Previous research has shown that adults are more likely to experience hemorrhagic symptoms than children, suggesting a relationship between age and hemorrhage. However, this correlation had not been established in pediatric patients until our study. We observed that the children who developed hemorrhage were significantly older than those who remained asymptomatic. Age was identified as an independent predictor in the multivariate analysis, suggesting that, when assessing bleeding risk and making clinical decisions for these patients, it is essential to consider age stratification in pediatric patients rather than relying solely on staging based on imaging. Our study revealed that PCA involvement is a significant predictor of hemorrhage in children with asymptomatic MMD, consistent with previous research in adults (18). This link could be due to PCA stenosis and occlusion leading to ischemia in the posterior circulation, increasing hemodynamic stress on collateral vessels such as ChA and increasing the risk of hemorrhage. Furthermore, research suggests that PCA involvement in MMD is associated with a higher risk of negative outcomes, such as increased rates of ischemic perioperative complications, and poorer functional outcomes (19). Therefore, it is essential to focus on the evaluation of PCA involvement in MMD cases. Previous studies have suggested a relationship between ChA dilation and hemorrhage in MMD (20), a study comparing hemorrhagic and ischemic MMD in children found a significant increase in ChA dilation in the hemorrhagic group, suggesting a similar trend in pediatric patients (21). Our study produced comparable findings, indicating a significantly higher incidence of AChA dilation in the hemorrhagic group compared to the ischemic group. In 2012, Japan initiated the AMORE (Asymptomatic Moyamoya Registry) study, a multi-center prospective investigation of asymptomatic MMD. The five-year follow-up results from this study revealed that ChA dilation poses a risk for hemorrhagic stroke in adults with asymptomatic MMD (4). However, our retrospective study did not observe any difference in ChA dilation, including AChA and PChA, between hemorrhagic group and asymptomatic group. Further follow-up is needed to determine if ChA dilation can be considered a risk factor for hemorrhage in children.
In our comparison of aa-hemispheres and ii-hemispheres, we identified Suzuki’s stage as a significant risk factor, with higher Suzuki’s stages correlating with an increased risk of ischemia. This finding is consistent with the established understanding that asymptomatic MMD can be considered an early stage of symptomatic MMD. A study on the progression of asymptomatic MMD suggested that hemodynamic disturbance, rather than vascular morphological changes, play a crucial role in the transition from the asymptomatic hemisphere to the ischemic hemisphere (22). Our multivariate analysis also revealed no significant correlation between angiographic features and ischemia, except for Suzuki’s stage. However, we did observe that the dilation of certain collateral vessels (LSA, TTA, AChA) in ii-hemispheres was less pronounced compared to aa-hemispheres. This difference may be attributed to the abundant blood perfusion provided by the excessively dilated collateral vessels in asymptomatic hemispheres, preventing the manifestation of ischemic symptoms. Moving forward, future analyses should consider incorporating hemodynamic characteristics to further explore this relationship.
In addition, a comparative study between the AMORE and JAM (Japan Adult Moyamoya) cohorts indicated that there was no significant difference in LSA and ChA dilation between asymptomatic adults and those in hemorrhagic group. This finding suggests that asymptomatic adults may have a higher risk of hemorrhage (18). This observation was supported by the 5-year follow-up results of the AMORE prospective study, which revealed a higher incidence of hemorrhagic stroke in asymptomatic adults compared to ischemic stroke (4). Our study produced similar results. When comparing aa-hemispheres and hh-hemispheres, no differences were observed in the five collateral dilation studied. This indicates that asymptomatic pediatric patients may be at a greater risk of hemorrhagic stroke than ischemic stroke. This finding challenges the prevailing belief that children are more prone to ischemic events and will have significant implications for the clinical management of these patients.
Our study evaluated the asymptomatic hemispheres (ai-hemispheres and ah-hemispheres) of symptomatic patients and found that their angiographic characteristics were not similar to aa-hemispheres, but rather showed consistent characteristics with their corresponding symptomatic hemispheres. Previous research suggests that non-bleeding hemispheres in patients with hemorrhagic MMD have a tendency towards hemorrhagic stroke, with an annual risk of new hemorrhage at 2.0% (23). Therefore, it is reasonable to speculate that such hemispheres are at an increased risk of developing symptoms consistent with the contralateral hemispheres, especially for hemorrhagic stroke. Therefore early intervention in these temporarily stable hemispheres is crucial. Based on this assumption, we can consider aa-ai-ii and aa-ah-hh as hemispheres in three different stages. We further aimed to investigate the changes in risk factors across these three distinct stages. Our analysis revealed that Suzuki’s stage exhibited an increasing trend in the comparison of aa-ai-ii hemispheres. In the comparison of aa-ah-hh hemispheres, both age and the incidence of PCA involvement also demonstrated an upward trend (Figure 4). These findings suggest that the progression from asymptomatic to symptomatic hemispheres is identifiable, and the risk factors examined in our study (Suzuki’s stage, age and PCA involvement) are valuable for monitoring this progression. Previous studies have examined the hemodynamics and Suzuki’s stage of these hemispheres, concluding that the progression from aa to ah to hh is associated with changes in angiographic features, while the progression of aa to ai to ii is linked to hemodynamics (22). Our research delved into the angiographic characteristics of pediatric patients and reached similar conclusions. We further found a relationship between PCA involvement and the transition from asymptomatic to hemorrhagic pattern. Therefore, monitoring PCA is valuable, as it can assist in assessing the direction and progression of pediatric patients with asymptomatic MMD.

Figure 4. Comparison of angiographic characteristics of different hemispheres. (A) Box plot for the Suzuki’s angiographic stage of aa-hemispheres, aihemispheres and ii-hemispheres. (B) Stacked bar plot for the PCA involvement of aa-hemispheres, ah-hemispheres and hh-hemispheres. *p < 0.05, **p < 0.01, and **p < 0.001.
LimitationsThis study has several limitations. Firstly, it was a retrospective cross-sectional study with no reported follow-up results. This article is the first to systematically compare the angiographic characteristics of asymptomatic children’s hemispheres with those of children with ischemic and hemorrhagic hemispheres, highlighting the necessity for prospective studies in the future. Secondly, the sample size of this study was relatively small due to practical constraints. Future studies should aim to increase the sample size to validate these findings.
ConclusionHigher Suzuki’s stage correlates with ischemic events in children with MMD, while increased PCA involvement and older age are associated with hemorrhagic events. Furthermore, the angiographic characteristics of children with asymptomatic MMD more closely resembled the hemispheres with hemorrhages. It is crucial to prioritize the potential risk of progression to hemorrhage over progression to ischemia. Monitoring angiographic characteristics like Suzuki’s stage and PCA involvement can help track the transition from asymptomatic to symptomatic hemispheres. The findings of this study provide valuable insights for improving the clinical management of children with asymptomatic MMD, including refining surgical indications, determining optimal timing for surgery, and effectively monitoring patients under conservative observation.
Data availability statementThe data that support the findings of the study are available from the corresponding author upon reasonable request.
Ethics statementThe studies involving humans were approved by the Ethics Committee of Beijing Tiantan Hospital affiliated to Capital Medical University. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin due to the retrospective nature of the study.
Author contributionsJW: Data curation, Investigation, Writing – original draft, Writing – review & editing. QZ: Investigation, Writing – review & editing. FD: Conceptualization, Writing – review & editing. DZ: Conceptualization, Methodology, Writing – review & editing.
FundingThe author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This study was supported by National Key Research and Development Program of China, Grant No. 2021YFC2500500.
AcknowledgmentsThe authors acknowledge all individuals for their participation in this study.
Conflict of interestThe authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The reviewer SH declared a shared affiliation with the authors JW, QZ, FD, and DZ at the time of review.
Publisher’s noteAll claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary materialThe Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2024.1484132/full#supplementary-material
References1. Ihara, M, Yamamoto, Y, Hattori, Y, Liu, W, Kobayashi, H, Ishiyama, H, et al. Moyamoya disease: diagnosis and interventions. Lancet Neurol. (2022) 21:747–58. doi: 10.1016/s1474-4422(22)00165-x
Crossref Full Text | Google Scholar
3. Kuroda, S, Hashimoto, N, Yoshimoto, T, and Iwasaki, YResearch Committee on Moyamoya Disease in Japan. Radiological findings, clinical course, and outcome in asymptomatic moyamoya disease: results of multicenter survey in Japan. Stroke. (2007) 38:1430–5. doi: 10.1161/strokeaha.106.478297
PubMed Abstract | Crossref Full Text | Google Scholar
4. Kuroda, S, Yamamoto, S, Funaki, T, Fujimura, M, Kataoka, H, Hishikawa, T, et al. Five-year stroke risk and its predictors in asymptomatic moyamoya disease: asymptomatic moyamoya registry (AMORE). Stroke. (2023) 54:1494–504. doi: 10.1161/strokeaha.122.041932
PubMed Abstract | Crossref Full Text | Google Scholar
5. Pandey, P, and Steinberg, GK. Neurosurgical advances in the treatment of moyamoya disease. Stroke. (2011) 42:3304–10. doi: 10.1161/strokeaha.110.598565
Crossref Full Text | Google Scholar
6. Nguyen, VN, Motiwala, M, Elarjani, T, Moore, KA, Miller, LE, Barats, M, et al. Direct, indirect, and combined extracranial-to-intracranial bypass for adult moyamoya disease: an updated systematic review and meta-analysis. Stroke. (2022) 53:3572–82. doi: 10.1161/strokeaha.122.039584
PubMed Abstract | Crossref Full Text | Google Scholar
7. Liu, X, Zhang, D, Shuo, W, Zhao, Y, Wang, R, and Zhao, J. Long term outcome after conservative and surgical treatment of haemorrhagic moyamoya disease. J Neurol Neurosurg Psychiatry. (2013) 84:258–65. doi: 10.1136/jnnp-2012-302236
PubMed Abstract | Crossref Full Text | Google Scholar
8. Lai, PMR, Gomez-Paz, S, Patel, NJ, Frerichs, KU, Thomas, AJ, Aziz-Sultan, MA, et al. Asymptomatic moyamoya disease in a North American adult cohort. World Neurosurg. (2022) 161:e146–53. doi: 10.1016/j.wneu.2022.01.076
PubMed Abstract | Crossref Full Text | Google Scholar
9. Funaki, T, Takahashi, JC, Houkin, K, Kuroda, S, Takeuchi, S, Fujimura, M, et al. Angiographic features of hemorrhagic moyamoya disease with high recurrence risk: a supplementary analysis of the Japan Adult Moyamoya trial. J Neurosurg. (2018) 128:777–84. doi: 10.3171/2016.11.Jns161650
Crossref Full Text | Google Scholar
10. Zhang, Q, Zhao, M, Ge, P, Liu, X, Wang, R, Zhang, Y, et al. Hemorrhagic patterns and their risk factors in patients with moyamoya disease. Eur J Neurol. (2020) 27:2499–507. doi: 10.1111/ene.14477
PubMed Abstract | Crossref Full Text | Google Scholar
12. Kuroda, S, Fujimura, M, Takahashi, J, Kataoka, H, Ogasawara, K, Iwama, T, et al. Diagnostic criteria for moyamoya disease—2021 revised version. Neurol Med Chir. (2022) 62:307–12. doi: 10.2176/jns-nmc.2022-0072
PubMed Abstract | Crossref Full Text | Google Scholar
13. Suzuki, J, and Takaku, A. Cerebrovascular “moyamoya” disease. Disease showing abnormal net-like vessels in base of brain. Arch Neurol. (1969) 20:288–99. doi: 10.1001/archneur.1969.00480090076012
Crossref Full Text | Google Scholar
14. Morioka, M, Hamada, J-I, Kawano, T, Todaka, T, Yano, S, Kai, Y, et al. Angiographic dilatation and branch extension of the anterior choroidal and posterior communicating arteries are predictors of hemorrhage in adult moyamoya patients. Stroke. (2003) 34:90–5. doi: 10.1161/01.str.0000047120.67507.0d
PubMed Abstract | Crossref Full Text | Google Scholar
15. Luo, S, Zhan, W, Zhang, L, Zeng, C, Hong, D, Fang, P, et al. Ischemic patterns and their angiographic risk factors in adult patients with moyamoya disease. Ann Clin Transl Neurol. (2023) 10:2386–93. doi: 10.1002/acn3.51927
PubMed Abstract | Crossref Full Text | Google Scholar
16. Baltsavias, G, Khan, N, Filipce, V, and Valavanis, A. Selective and superselective angiography of pediatric moyamoya disease angioarchitecture in the posterior circulation. Interv Neuroradiol. (2014) 20:403–12. doi: 10.15274/inr-2014-10041
PubMed Abstract | Crossref Full Text | Google Scholar
17. Hori, S, Kashiwazaki, D, Yamamoto, S, Acker, G, Czabanka, M, Akioka, N, et al. Impact of interethnic difference of collateral angioarchitectures on prevalence of hemorrhagic stroke in moyamoya disease. Neurosurgery. (2019) 85:134–46. doi: 10.1093/neuros/nyy236
PubMed Abstract | Crossref Full Text | Google Scholar
18. Yamamoto, S, Funaki, T, Fujimura, M, Takahashi, JC, Uchino, H, Houkin, K, et al. Development of hemorrhage-prone anastomoses in asymptomatic moyamoya disease-a comparative study with Japan Adult Moyamoya trial. J Stroke Cerebrovasc Dis. (2019) 28:104328. doi: 10.1016/j.jstrokecerebrovasdis.2019.104328
PubMed Abstract | Crossref Full Text | Google Scholar
19. Tigchelaar, SS, Wang, AR, Vaca, SD, Li, Y, and Steinberg, GK. Incidence and outcomes of posterior circulation involvement in moyamoya disease. Stroke. (2024) 55:1254–60. doi: 10.1161/strokeaha.123.044693
PubMed Abstract | Crossref Full Text | Google Scholar
20. Fujimura, M, Funaki, T, Houkin, K, Takahashi, JC, Kuroda, S, Tomata, Y, et al. Intrinsic development of choroidal and thalamic collaterals in hemorrhagic-onset moyamoya disease: case-control study of the Japan Adult Moyamoya trial. J Neurosurg. (2019) 130:1453–9. doi: 10.3171/2017.11.Jns171990
PubMed Abstract | Crossref Full Text | Google Scholar
21. Liu, P, Han, C, Li, DS, Lv, XL, Li, YX, and Duan, L. Hemorrhagic moyamoya disease in children: clinical, angiographic features, and long-term surgical outcome. Stroke. (2016) 47:240–3. doi: 10.1161/strokeaha.115.010512
PubMed Abstract | Crossref Full Text | Google Scholar
22. Sun, H, Li, W, Xia, C, Ren, Y, Ma, L, Xiao, A, et al. Angiographic and hemodynamic features in asymptomatic hemispheres of patients with moyamoya disease. Stroke. (2022) 53:210–7. doi: 10.1161/strokeaha.121.035296
PubMed Abstract | Crossref Full Text | Google Scholar
23. Funaki, T, Takahashi, JC, Houkin, K, Kuroda, S, Fujimura, M, Tomata, Y, et al. Effect of choroidal collateral vessels on de novo hemorrhage in moyamoya disease: analysis of nonhemorrhagic hemispheres in the Japan Adult Moyamoya trial. J Neurosurg. (2019) 132:408–14. doi: 10.3171/2018.10.Jns181139
留言 (0)